INTRODUCTION
Atrial fibrillation (AF) is the most common type of cardiac arrhythmia, affecting approximately 12 million people worldwide[1]. AF is a leading risk factor for ischaemic stroke, systemic thromboembolic events, new cases of heart failure and coronary events, and is associated with a significant economic burden, all-cause mortality, morbidity and hospitalization[2,3]. Radiofrequency catheter ablation (RFCA) is by far the most commonly performed cardiac ablation procedure for AF worldwide[4]. It has become an important rhythm control strategy in symptomatic patients with paroxysmal or persistent AF who are refractory or intolerant to antiarrhythmic drugs, and may be considered as a first-line approach in selected asymptomatic patients[5].
RECURRENCE OF AF
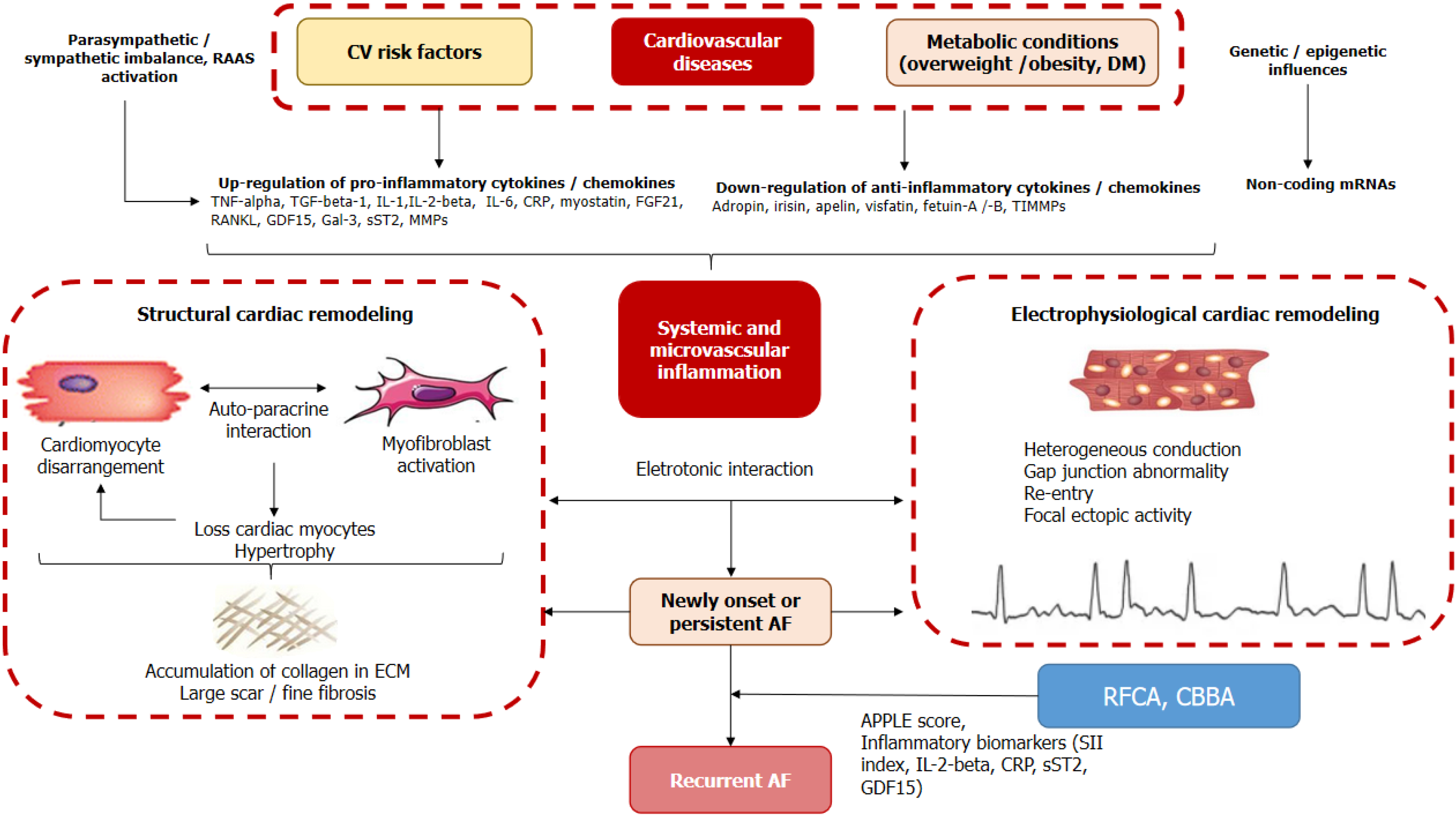
There are many risk factors associated with AF recurrence, such as older age, male sex, concomitant heart failure, chronic obstructive pulmonary disease, pulmonary hypertension, obstructive sleep apnea, hyperthyroidism, smoking, diabetes mellitus and obesity, as well as several hemodynamic parameters, including increased left atrial (LA) size, reduced LA function and left ventricular myocardial strain[6]. In addition, recent observational and clinical studies have suggested a plausible role for inflammation as a key component of the pathophysiological processes leading to the development of AF[7]. Indeed, systemic and microvascular inflammation contributes not only to structural cardiac remodeling associated with cardiac fibrosis, altered myocyte architecture and excessive extracellular matrix accumulation, but also to electrophysiological remodeling characterized by impaired atrial ion currents, intracellular calcium handling, action potential waveform and conduction (Figure 1). In addition, inflammatory changes may link the etiology of AF, associated comorbidities and risk factors with electrical and structural cardiac remodeling, cardiac damage, myocardial fibrotic changes, microvascular dysfunction and altered reparative response, which in turn are considered possible triggers of recurrent AF after ablation[8,9]. In this context, biomarkers reflecting the different stages of AF pathogenesis deserve to be thoroughly investigated, while the levels of specific inflammatory biomarkers, such as C-reactive protein, interleukin (IL)-2-beta, tumor necrosis factor-alpha, have shown conflicting associations with the risk of AF recurrence[10]. In addition, the role of anti-inflammatory treatment in AF remains uncertain[11]. In this context, new predictive approaches are needed to further clarify the risk of AF recurrence using low-grade inflammatory biomarkers with a higher degree of reproducibility, more precise accuracy and affordable economic burden compared to conventional inflammatory indicators.
Figure 1 Plausible role of inflammation as key component of pathophysiological processes leading to AF development and recurrence.
AF: Atrial fibrillation; CBBA: Cryo-balloon-based ablation; CRP: C-reactive protein; CV: Cruciferous vegetables; DM: Diabetes mellitus; ECM: Extracellular matrix; FGF: Fibroblast growth factor; GDF: Growth differential factor; IL: Interleukin; Gal-3: Galectin-3; MMPs: Matrix metalloproteinases; RAAS: Renin-angiotensin-aldosterone system; RANKL: Receptor activator of the NF-kappa B ligand; RFCA: Radiofrequency catheter ablation; SII: Systemic immune-inflammation index; SST2: Soluble suppression of tumorigenicity-2; TIMMPs: Tissue inhibitor of matrix metalloproteinases; TGF-beta: Transforming growth factor-beta; TNF: Tumour necrosis factor.
SYSTEMIC IMMUNE-INFLAMMATION INDEX
The systemic immune inflammation (SII) index-the SII index level, calculated as platelet count × neutrophil/lymphocyte count-was initially developed as an integrated inflammatory indicator of the inflammatory response[12]. It has subsequently been proposed as a prognostic biomarker for several diseases whose pathogenesis includes abnormal inflammatory status, such as coronary artery disease, acute stroke, diabetes mellitus and solid cancer[13-15].
The SII index is currently one of the inflammatory biomarkers positively associated with the presence of a wide range of cardiovascular (AF, acute myocardial infarction, heart failure), metabolic (obesity, diabetes mellitus), neurological (ischaemic stroke, intracranial haemorrhage), respiratory (pneumonia, chronic obstructive pulmonary disease), chronic kidney disease and cancer diseases. It is also associated with clinical outcomes such as mortality, economic burden and risk of hospitalisation[16-19].
It should be noted that the SII index is a complex parameter that mediates the effect of inflammation-related indicators, including leukocytes, neutrophils and lymphocytes, on clinical outcomes. Despite the significant differences in the pathogenesis of the above diseases and the extent to which different pathogenetic mechanisms are involved, the SII index provides a relatively simple and cost-effective way of assessing the inflammatory response. However, there are a large number of clinical studies investigating its predictive value for clinical outcomes in patients with a wide range of diseases in which the inflammatory response is implicated in pathogenesis through a variety of underlying mechanisms.
THE PREDICTIVE ROLE OF SII INDEX IN AF RECURRENCE
Recent clinical studies have demonstrated the plausible role of the SII index in predicting AF recurrence. Indeed, the SII index was found to be a better independent predictor of AF recurrence after successful DC cardioversion with a sensitivity of 96.9% and a specificity of 55.2%[20]. However, the predictive value of the SII index before and after ablation may not only vary significantly between studies, but may also be difficult to determine. Indeed, a higher pre-procedural SII value (> 532) was an independent predictor of 2-year AF recurrence after cryoballoon-based ablation[21]. However, another study showed no significant role of baseline SII in predicting AF recurrence after cryoablation in individuals after mitral valve surgery, whereas a post-procedural SII index ≥ 1696 independently predicted AF recurrence at 1 week follow-up[22]. Interestingly, in a cohort of cryoablated AF patients, the SII index was significantly inferior to other indices of inflammatory activation, such as C-reactive protein/albumin ratio and pan-immune inflammation, in its predictive value for AF recurrence[23,24]. However, a baseline SII index ≥ 444.77 was independently and positively associated with AF recurrence in patients with AF and diabetes mellitus who underwent RFCA[25]. Overall, the predictive value of the SII index alone and in combination with conventional predictive scores such as APPLE for early and late AF recurrence remains controversial[26].
APPLE SCORE METHODOLOGY
APPLE score is essential for individualized AF management through predicting AF recurrence. It includes the following categories: (1) One point for age > 65 years; (2) Persistent AF; (3) Impaired estimated glomerular filtration rate (< 60 mL/minute/1.73 m2); (4) LA diameter ≥ 43 mm; and (5) Left ventricular ejection fraction < 50%[27]. However, the regular APPLE score does not accurately reflect atrial myopathy with higher risk of recurrent AF. A recent clinical study showed that both the regular APPLE score and the modified APPLE [(m)APPLE] score, which includes LA volume and LA emptying fraction, showed strict similarity in predicting AF recurrence[28]. Finally, both the regular APPLE score and the (m)APPLE score can be used to predict AF before ablation, whereas their discriminative power for AF recurrence after ablation is sufficiently limited.
SYSTEMIC IMMUNE-INFLAMMATION INDEX IN IMPROVING APPLE SCORE FOR PREDICTING AF RECURRENCE AFTER RFCA
The study by Wang et al[29], published in this issue of the World Journal of Cardiology, first reported that SII index, APPLE score and their combination had sufficient predictive value for 1-year AF recurrence after effective treatment with RFCA. However, a combined model based on both predictive factors (SII index + APPLE score) was superior to SII and APPLE score alone in this issue. Although these findings are based on a retrospective evaluation of 457 patients with non-valvular AF who underwent initial RFCA, they open new perspectives in the prediction of recurrent AF and post-RFCA events, including mortality, thromboembolic events, heart failure, and major cardiovascular events. In addition, these findings have strong practical implications, as the SII index improved the predictive ability of the APPLE score, which was developed specifically for patients undergoing repeat AF ablation[30].
Despite these promising results, this study[29] had a number of limitations. It was based solely on data from a single retrospective study conducted in a single tertiary hospital with a relatively small sample size. It can be assumed that the one-year follow-up interval and the lack of long-term continuous cardiac rhythm monitoring after ablation, including intra-cardiac monitoring, create conditions for possible underdiagnosis of asymptomatic AF episodes after radiofrequency ablation (RFA) in patients with a low recurrence rate. Another aspect that would be useful to evaluate in future studies is whether the timing of SII index assessment corresponds to dynamic changes in the inflammatory profile of patients over the follow-up period in relation to recurrence rates.
In fact, non-specific inflammatory biomarkers such as high-sensitivity C-reactive protein, IL-6 and growth differential factor-15 have been associated with poor prognosis in patients with AF and at risk of recurrent AF[31]. Elevated biomarkers of myocardial injury (cardiac troponins) and biomechanical stress (N-terminal brain natriuretic pro-peptide) have been implicated in risk stratification for thromboembolic events and cardiovascular death[32]. In addition, biomarkers of fibrosis, such as galectin-3, fibroblast growth factor-21 and soluble suppressor of tumorigenicity-2, which indicate atrial remodeling, have also been associated with AF recurrence[33]. Nevertheless, their discriminatory power in predicting recurrent AF after ablation has not been clearly established, whereas they were found to be better predictor of incident AF in heart failure patients[34,35]. However, the results obtained suggest further face-to-face comparison of the predictive value of SII index and traditionally used biomarkers of biomechanical stress, inflammation and fibrosis, especially in patients with concomitant heart failure, pulmonary hypertension, stable coronary artery disease, and chronic kidney disease.
Another practical aspect concerns the economic burden of biomarker use. In this context, the SII index is promising because its calculation is based on routine blood count measurement and does not require specific kits, blood sample preparation and a highly qualified team. Overall, based on the predictive value demonstrated by the SII index, the development of specific treatment strategies and interventions could be initiated. This would facilitate more individualized and effective clinical management of AF. Further validation by multicentre prospective studies is needed to address these shortcomings and improve the generalizability of the results.
CONCLUSION
The SII index may serve as a valuable indicator of recurrent AF in post-carassius RFA patients and may be a biomarker with plausible predictive value for poor clinical outcomes.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country of origin: Austria
Peer-review report’s classification
Scientific Quality: Grade A, Grade A, Grade A, Grade C
Novelty: Grade A, Grade A, Grade A, Grade C
Creativity or Innovation: Grade A, Grade B, Grade B, Grade B
Scientific Significance: Grade A, Grade A, Grade A, Grade B
P-Reviewer: Dong WK; Liu J; Wang W S-Editor: Luo ML L-Editor: A P-Editor: Zhao YQ