Published online Jan 26, 2025. doi: 10.4330/wjc.v17.i1.101491
Revised: November 2, 2024
Accepted: December 17, 2024
Published online: January 26, 2025
Processing time: 124 Days and 1.4 Hours
Sodium-glucose cotransporter-2 (SGLT-2) inhibitors represent a cutting-edge class of oral antidiabetic therapeutics that operate through selective inhibition of glucose reabsorption in proximal renal tubules, consequently augmenting urinary glucose excretion and attenuating blood glucose levels. Extensive clinical investigations have demonstrated their profound cardiovascular efficacy. Parallel basic science research has elucidated the mechanistic pathways through which diverse SGLT-2 inhibitors beneficially modulate pulmonary vascular cells and arterial remodeling. Specifically, these inhibitors exhibit promising potential in enhancing pulmonary vascular endothelial cell function, suppressing pulmonary smooth muscle cell proliferation and migration, reversing pulmonary arterial remodeling, and maintaining hemodynamic equilibrium. This comprehensive review syn
Core Tip: Sodium-glucose cotransporter-2 (SGLT2) inhibitors are innovative antidiabetic agents that lower blood glucose by promoting its excretion via the kidneys. Beyond their glucose-lowering effects, SGLT2 inhibitors have demonstrated significant cardiovascular benefits in clinical trials. Emerging basic science research indicates that SGLT2 inhibitors also positively impact pulmonary vascular health. They enhance the function of pulmonary vascular endothelial cells, inhibit the proliferation and migration of pulmonary smooth muscle cells, reverse pulmonary arterial remodeling, and help maintain hemodynamic stability. This review consolidates current findings on the mechanisms by which SGLT2 inhibitors improve pulmonary vascular cell function and arterial remodeling, suggesting new therapeutic possibilities for pulmonary vascular diseases.
- Citation: Zhang JJ, Ye XR, Liu XS, Zhang HL, Qiao Q. Impact of sodium-glucose cotransporter-2 inhibitors on pulmonary vascular cell function and arterial remodeling. World J Cardiol 2025; 17(1): 101491
- URL: https://www.wjgnet.com/1949-8462/full/v17/i1/101491.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i1.101491
Sodium-glucose cotransporter-2 (SGLT-2) inhibitors, representing an innovative class of hypoglycemic agents, function by impeding renal glucose reabsorption, thereby facilitating blood glucose reduction. The nineteenth century witnessed French chemist C. Pettersen's isolation of phlorizin from apple root bark, revealing its glucose-lowering properties in diabetic animals. However, its limited SGLT1/SGLT2 selectivity and glycosidase-mediated hydrolytic susceptibility precluded its therapeutic implementation[1]. Subsequent SGLT2 research revealed its pivotal role in renal glucose rea
The cardiovascular benefits of SGLT-2 inhibitors have garnered substantial attention, demonstrating efficacy in type 2 diabetes mellitus management independent of direct glucose modulation[4]. These inhibitors have emerged as frontline therapeutics in heart failure management, achieving natriuretic and osmotic diuretic effects through SGLT-2-mediated glucose reabsorption inhibition[5]. They induce autophagy and reduce epicardial adiposity by optimizing cardiomyocyte calcium processing and enhancing cardiac energy metabolism[6]. Through metabolic parameter optimization, including lipid and uric acid profiles, SGLT-2 inhibitors attenuate cardiovascular risk factors, reduce heart failure hospitalization rates, and decrease all-cause mortality[7-10]. Their protective effects against diabetic complications and cardiovascular disease stem from the amelioration of low-grade systemic and tissue inflammation[11]. They demonstrate synergistic cardio-renal protection, reducing diabetic nephropathy proteinuria and optimizing glomerular filtration rates[5,12-14]. A landmark clinical trial by the University of Toronto Health Network[15] and Hulst et al[16] has enhanced understanding of SGLT-2 inhibitor mechanisms and safety across diverse patient populations. Certain SGLT-2 inhibitors have received expanded Food and Drug Administration approval for reducing the risk of type 2 diabetes mellitus and heart disease. Emerging evidence suggests SGLT-2 inhibitors’ therapeutic potential in pulmonary hypertension and right heart failure (RHF). Fundamental studies indicate their capacity to suppress pulmonary smooth muscle proliferation and migration, ameliorate arterial remodeling, induce pulmonary arterial relaxation, and reduce pulmonary arterial pressure across various pulmonary hypertension models[17-19].
SGLT2, a 60 kDa protein predominantly expressed in renal tubules, comprises 565 amino acids arranged in 12 tran
SGLT-2 inhibitors demonstrate multifaceted cardiovascular protection through optimization of cardiomyocyte me
While SGLT2 expression is documented in endothelial cells and cardiomyocytes[35,36], its presence in human cardiac tissue remains predominantly unreported[37,38]. Matrix metalloproteinases (MMPs) are implicated in cardiovascular pathogenesis[39,40], with phloridin demonstrating MMP inhibition. SGLT-1 and SGLT-2 inhibitors, as phloridin deriva
Groundbreaking research by Jiang et al’s team at Tongji University, utilizing various knockout mouse myocardial infarction models, demonstrated empagliflozin’s significant enhancement of post-infarction survival rates, ventricular function, and reduction in cardiac fibrosis and myocardial cell hypertrophy[44]. Their glucose deprivation model re
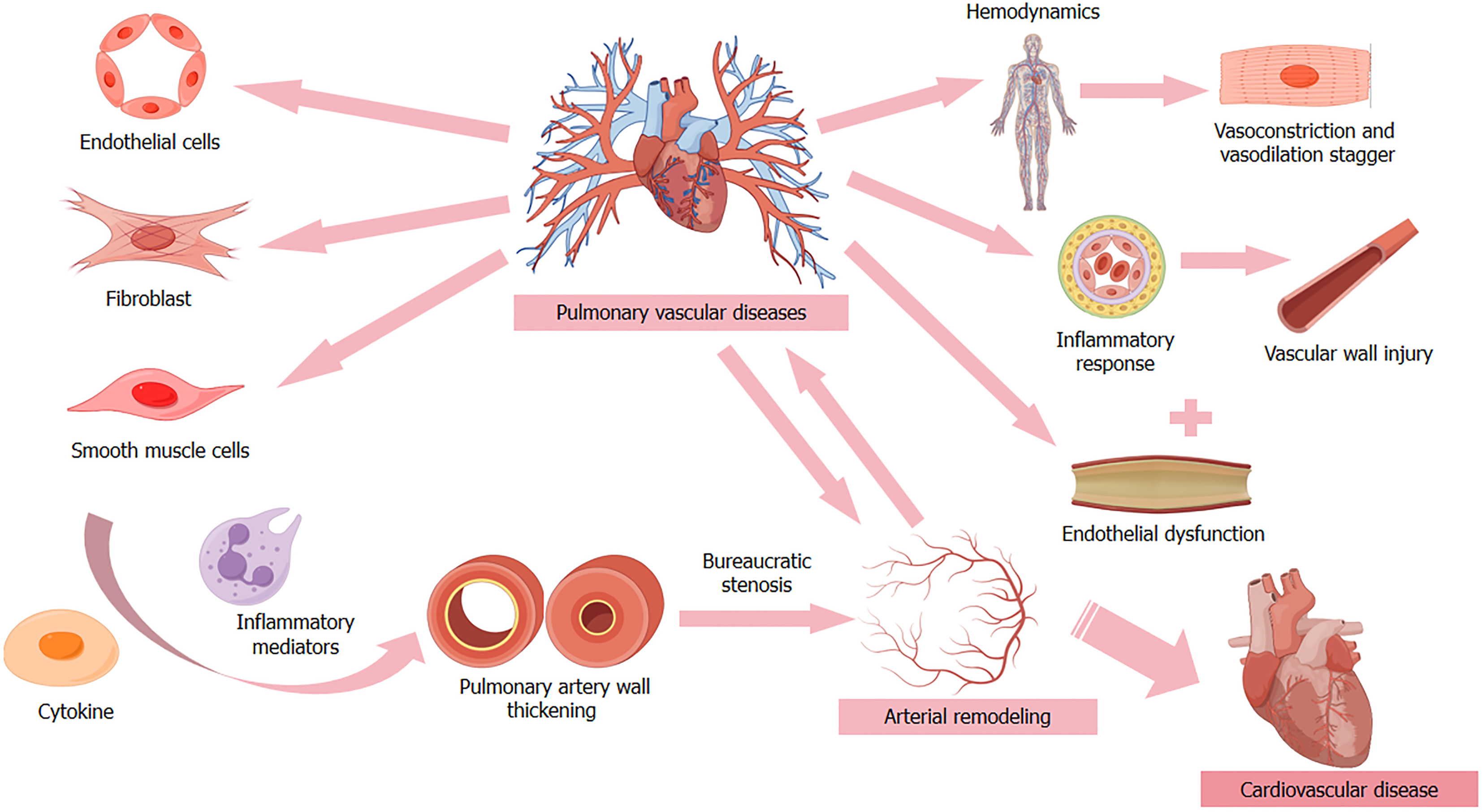
The pulmonary circulatory system serves as a critical interface for oxygen-rich blood transport between the heart and lungs. Pulmonary vascular cells, comprising endothelial cells, smooth muscle cells, and fibroblasts, constitute the vessel walls’ inner, medial, and outer layers, maintaining structural and functional integrity. These cellular components or
Smooth muscle cells, primarily located in the vessel media, regulate vasomotor function. Larger vessels feature pre
Pulmonary vascular cell dysfunction can precipitate diverse pathophysiological cascades, including pulmonary hy
Endothelial dysfunction represents a pivotal factor in pulmonary hypertension and RHF pathogenesis, integrally in
Studies on human immunodeficiency virus (HIV)-associated pulmonary hypertension have revealed elevated vascular endothelial growth factor (VEGF) and platelet-derived growth factor expression in HIV-infected T lymphocytes. The HIV-1 envelope glycoprotein 120 stimulates endothelin-1 (ET-1) and tumor necrosis factor-alpha (TNF-α) production, potentially mediating vasoconstriction, inflammation, and vascular remodeling[49]. ET-1 exhibits vasoconstrictive, pro-inflammatory, and pro-mitotic properties, stimulating free radical formation and platelet activation[50-52].
TNF-α induces endothelial cell apoptosis and barrier dysfunction, reducing extracellular resistance and enhancing apoptotic processes through increased myosin light chain phosphorylation and stress fiber formation[53-56]. EndoMT serves as a key promoter of endothelial dysfunction, while mitochondrial dysfunction significantly contributes to endothelial impairment and pulmonary hypertension. Mitochondrial-targeted interventions and caloric restriction show therapeutic potential[57].
Pulmonary hypertension in sickle cell disease manifests through occlusive vascular smooth muscle hyperplasia, endothelial abnormalities, and in situ thrombosis[58]. Elevated endothelial activation markers, including vascular cellular adhesion molecule-1 (VCAM-1), correlate with decreased nitric oxide (NO) bioavailability[59]. VEGF and VEGF receptor 2 play critical roles in endothelial cell proliferation and differentiation[60]. The activation of nuclear factor of activated T-cells 1 in human pulmonary valve endothelial cells is a specific activation of VEGF signaling mediated by VEGF receptor 2, which may be an important mechanism for maintaining pulmonary valve function by enhancing endothelial cell proliferation[61]. R-spondin3, primarily produced by endothelial cells[62], demonstrates significant regulatory functions. Studies indicate that lung EC-specific R-spondin3 manipulation affects EC proliferation and injury responses, with overexpression promoting endothelial recovery through leucine-rich repeats containing G protein-coupled receptor 4-dependent β-catenin and integrin-linked kinase signaling pathways[63]. The PH-COPD[64] and PERFECT[65] studies continue to advance understanding of pulmonary vascular pathophysiology and therapeutic strategies.
Inflammatory processes play a fundamental role in cardiovascular disease pathogenesis, with pulmonary vascular cell dysfunction potentially mediating cardiovascular pathology through inflammatory pathways. Direct exposure of va
Mast cells demonstrate regulatory effects on pulmonary vascular remodeling in pulmonary hypertension, with pulmonary chymase activity positively correlating with vascular remodeling outcomes[68]. The modulation of immune cell populations, including decreased mast cells, macrophages, and T cells, contributes to improved hemodynamics and vascular remodeling in idiopathic pulmonary hypertension[69]. Transcriptomic analyses reveal G protein-coupled re
C-type natriuretic peptide demonstrates therapeutic potential in monocrotaline-induced pulmonary hypertension, reducing monocyte/macrophage infiltration and enhancing endothelial nitric oxide synthase (eNOS) content[77]. Glucagon-like peptide-1 analogs exhibit multiple beneficial effects, including inflammation suppression, vascular reactive oxygen species (ROS) reduction, and microcirculation improvement[78,79]. Together, these studies confirm the complex interplay between lung blood vessel cells and inflammatory responses in various lung diseases.
Vascular cell dysfunction-induced vasomotor imbalance significantly impacts cardiovascular hemodynamics[80]. Large animal studies have demonstrated SERCA2a gene therapy’s positive effects on chronic retrocapillary pulmonary hy
Porcine ET studies provide valuable insights into pulmonary and systemic vascular bed activity[84]. Pulmonary hy
The pathogenesis of pulmonary hypertension remains incompletely understood, characterized by endothelial cell dy
Pulmonary vasomotor regulation involves complex interactions between endothelial calcium concentrations, NO and prostaglandin I2 release, and endothelium-derived factors. ETB receptor expression in pulmonary vascular endothelial cells mediates crucial regulatory functions, with ET-1 and thromboxane A2 elevation during endothelial dysfunction promoting pulmonary arterial contraction and smooth muscle proliferation[100]. ETB activation induces vasodilator release and ET-1 clearance through Gq/11 signaling pathways[101].
In patients with pulmonary hypertension, eNOS and NO bioavailability in pulmonary vascular endothelial cells is reduced, while ET-1 levels are elevated. Protein kinase C enhances threonine phosphorylation of eNOS, leading to decreased NO levels. β-blockers can inhibit protein kinase C activity, thereby restoring NO bioavailability in endothelial cells[102]. Endogenous NO may activate potassium ion channels on the cell membrane via protein kinase G (PKG), reduce cytoplasmic free calcium concentration, promote membrane hyperpolarization, inhibit Ca²+ influx, and ultimately induce pulmonary vasodilation[103].
In PA-induced human umbilical vein endothelial cells, phloretin partially alleviates endothelial dysfunction by in
Sustaining eNOS phosphorylation and endothelium-dependent relaxation has been shown to improve microvascular density and perfusion[110]. Englaglizin additionally reduces expression levels of p53, p21, p16, tissue factor, VCAM-1, SGLT-1, and SGLT-2, while increasing eNOS expression, thereby enhancing endothelial function, reducing arterial aging, inhibiting cardiac remodeling, and lowering superoxide production in the thoracic aorta of db/db mice with acety
However, the EMBLEM trial demonstrated that enoglizin did not directly improve endothelial dysfunction[113]. Both enoglizin and daglizin restored NO bioavailability and endothelial cell function by reducing mitochondrial oxidative damage and inhibiting ROS production, though they did not impact TNFα-induced eNOS expression or the expression of signaling, barrier, and adhesion molecules in endothelial cells[114-116]. Furthermore, nanomaterials (NPs) substantially upregulated SGLT2 and ENA, while significantly decreasing NPs-induced senescence markers, including β-gal activity, cell cycle arrest, and the aging markers p53 and p21. SGLT2 inhibition prevented NPs-induced endothelial senescence and upregulated eNOS expression, contributing to improved vascular function[117].
Fluid shear stress activates mechanosensitive pathways in pulmonary vascular endothelial cells, regulating vasoconstriction and dilation[118]. Activation of specific cationic channels can lead to calcium inflow and depolarization of cell membrane, which leads to cell contraction and increased pulmonary vascular pressure[118]. Two distinct mechanosensitive channel types - shear-activated potassium channels and stretch-activated cation channels - mediate these re
Daglipzin alleviates endothelial dysfunction and mitigates microvascular injury during cardiac ischemia/reperfusion injury by normalizing the XO-SERCA2-CaMKII-cofilin pathway[121]. Furthermore, daglipzin significantly improves systemic endothelial function, arterial stiffness, and renal resistance index. This effect, which is independent of blood pressure changes, may be mediated by a reduction in oxidative stress in sodium-stable conditions, indicating a rapid and direct beneficial impact on the vascular system[122,123].
Aberrant endothelial cell energy metabolism exacerbates pulmonary vascular remodeling through multiple mechanisms. Oxidative stress plays a central role in this process, with mitochondrial remodeling and autophagy serving as key pa
The DEFENCE study demonstrated that dagaglizin therapy improves endothelial function by reducing urinary 8-hydroxy-2’-deoxyguanosine levels, thereby alleviating oxidative stress[127]. Dagaglizin attenuates TNF-α and hypergly
In endothelial cells under oxidative stress, daglipzin reduces ROS production by restoring eNOS activity, increasing NO bioavailability, and activating sirtuin 1. This reverses endothelial-mesenchymal transformation, regulates mito
Englaglizin’s ability to activate adenosine monophosphate-activated protein kinase (AMPK) plays a crucial role in safeguarding the endothelium from hyperglycemia-induced damage. By inhibiting mitochondrial fission, reducing mito
Caglizin significantly inhibits CD40 upregulation, downregulates Nox organizer 1 gene expression, decreases ICAM-1 and nitrotyrosine, increases platelet endothelial cell adhesion molecule-1 immunoreactivity, and alleviates endothelial dysfunction post-ischemia/reperfusion[135]. Caglipzin further improves apoptosis marker expression (Bax/Bcl-2 ratio) and genes associated with myocardial oxidative stress, significantly boosting endothelium-dependent vasodilation gene expression[136]. Through activation of the α1 AMPK/p38/mitogen-activated protein kinase/heat shock protein 27 pa
Endothelial cells exposed to coronavirus disease 2019 plasma with high cytokine levels experience SGLT2 upregulation via pro-inflammatory cytokines IL-1β, IL-6, and TNF-α, leading to endothelial dysfunction, senescence, nuclear factor-kappaB activation, inflammation, platelet adhesion and aggregation, vasculovascular carcinoma factor secretion, and thrombin production[140,141]. SGLT-2 inhibitors offer clear cardiovascular benefits by ameliorating endothelial dys
Pulmonary artery remodeling represents a critical pathophysiological process in pulmonary hypertension development, particularly evident in paradoxical adipose hyperplasia (PAH) secondary to congenital heart disease. This process manifests through arterial wall thickening, luminal narrowing, and eventual RHF. Pulmonary artery smooth muscle cell (PASMC) proliferation and intimal migration constitute core pathological mechanisms[80,143]. Recent research has identified secreted cysteine-rich acidic proteins as promoters of hypoxic pulmonary hypertension through PASMC activation[144]. Additionally, heterogeneous nuclear ribonucleoprotein A2B1 facilitates pro-proliferative and anti-apo
The cancer theory of pulmonary hypertension suggests environmental stressors promote the development of highly proliferative, apoptosis-resistant cell clones, including smooth muscle cells and fibroblasts[147]. Hypoxia modulates pu
Genetic factors, including variations in vasoactive intestinal peptide genes, may contribute to the development of pulmonary hypertension and vascular remodeling[150]. In hypoxic PASMCs, the activity and expression of glucose-6-phosphate dehydrogenase, an enzyme in the phosphopeptide pathway, is increased[151]. Certain molecules, such as in
A 2005 study from Hebei Medical University demonstrated that combination therapy with spironolactone, captopril, and carvedilol reversed pulmonary artery remodeling in patients with PAH associated with congenital heart disease. Additionally, ET receptor inhibition improved diastolic dysfunction and pulmonary hypertension in heart failure[148]. Sacubitril/valsartan has been shown to lower pulmonary artery pressure in heart failure patients with either preserved or reduced ejection fraction[9,154]. T valentine, a phosphodiesterase-3/4 inhibitor, inhibits smooth muscle cell migration induced by rosaline and reverses pulmonary vascular remodeling in rats[155]. Furthermore, phosphodiesterase 1 in
PASMC regulation plays a pivotal role in PAH pathogenesis. Smooth muscle cell-derived NO modulates cyclic guanosine 3’,5’-monophosphate (cGMP) levels, inducing vascular relaxation through K channel activation[68,157]. NOTCH3 sig
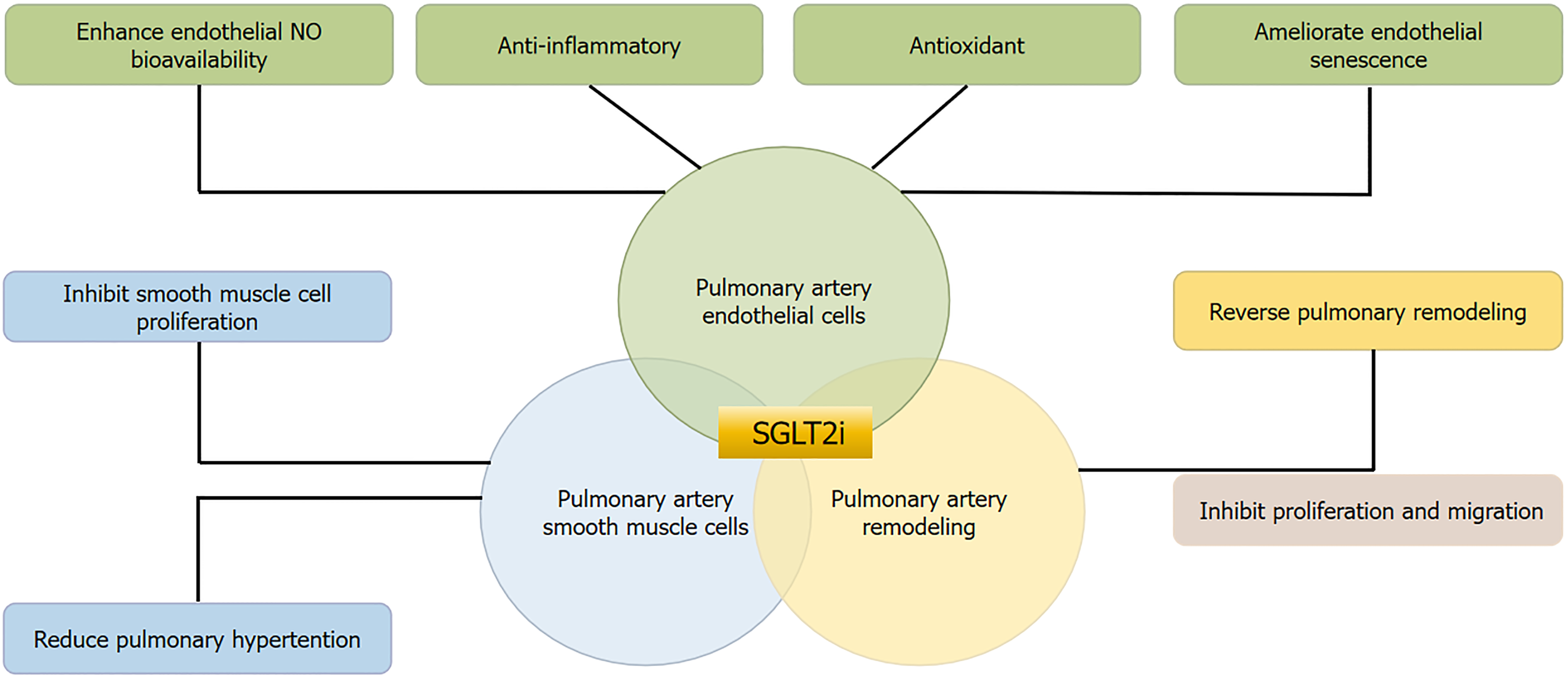
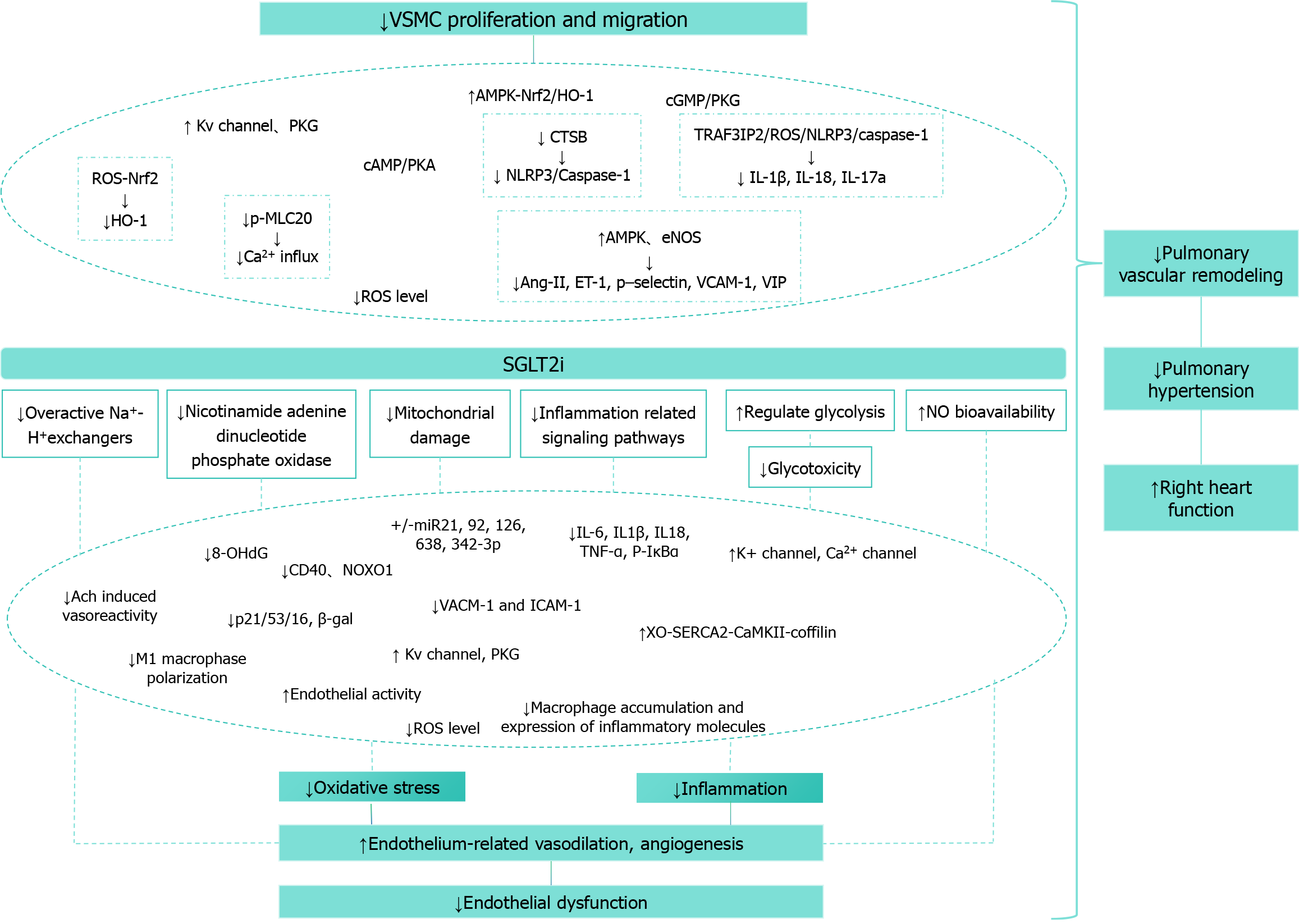
Currently, research on the effects of SGLT-2 inhibitors on PASMCs is limited, though these inhibitors demonstrate significant effects on VSMCs in cardiovascular applications. Englipzin induces vasodilation by activating PKG and Kv channels in vascular smooth muscle, independent of endothelial cells[163]. SGLT-2 inhibitors reduce vascular smooth muscle calcification by inhibiting thioredoxin domain-containing 5-dependent osteogenic reprogramming in the endo
Englipzin also improves smooth muscle cell calcification by inhibiting BHLHE40-dependent nucleotide-binding do
Enoglizin significantly increases AMPK and eNOS levels in aortic tissue, decreases angiotensin II, ET-1, P-selectin, VCAM-1, and vasoactive intestinal peptide, and has a vasodilatory effect[173]. Caraglipzin inhibits VSMC proliferation and migration in the rat aorta in a concentration-dependent manner, halting VSMC growth in the G0/G1 phase and pre
SGLT-2 inhibitors demonstrate significant capacity to reverse or attenuate PAH remodeling[91,179,180]. Empagliflozin reduces mitochondrial ROS and miR-193b levels while restoring nuclear factor Y α subunit/soluble guanylate cyclase activity[181]. Englipzin inhibitors reduced right ventricular systolic pressure and mitigated pulmonary artery remodeling in McT-induced PAH rats, with differential results attributed to PAH severity, duration of treatment, or pleiotropy of SGLT-2 inhibitor[176]. Canagliflozin ameliorates pulmonary artery and right ventricular remodeling in MCT-PAH rats through multiple mechanisms, including reduction of pulmonary arteriole wall thickness and vascular hypertrophy[182].
Caraglipzin inhibits the proliferation and migration of pulmonary smooth muscle cells and improves arterial re
Clinical trials demonstrate canagliflozin’s efficacy in reducing PAP and decreasing time increase, independent of diuretic effects[188]. Novel biomarkers show increasing utility in diagnosing and prognosticating right ventricular dysfunction, potentially offering innovative therapeutic approaches for improving both right and left ventricular function[188,189]. Transcriptomic analyses by Frisk et al[190] have revealed crucial differences between left and right ventricular responses in varying degrees of heart failure. Key biomarkers, including microRNAs, insulin-like growth factor, cardiac troponin, galectin-3, suppression of tumorigenicity 2, and growth differentiation factor-15[191], provide comprehensive assessment capabilities for heart failure, particularly in right ventricular dysfunction.
Dapagliflozin demonstrates promise in reducing cardiac fibrosis and inflammation, potentially benefiting right ventricular function[192]. It effectively reduces susceptibility to PH-induced right ventricular dysfunction by restoring calcium homeostasis in rat models[184,193]. SGLT-2 inhibitors show particular efficacy in ameliorating right ventricular dysfunction due to volume and pressure overload[194], independent of left ventricular remodeling[195-197]. Experimental models and clinical trials consistently demonstrate SGLT-2 inhibitors’ capacity to enhance right ventricular function[19,198,199]. When combined with standard heart failure therapy, these agents prove more effective than conventional PH treatments alone[195,196,200-202]. The addition of SGLT2 inhibition to established PH therapy protocols shows promising improvements in right ventricular function[202,203].
PH and right ventricular dysfunction in HFpEF represent significant hemodynamic complications[204,205]. Right ven
The EMPA-REG OUTCOME trial, encompassing 7020 cardiovascular disease patients, demonstrated empagliflozin’s significant and rapid reduction in pulmonary diastolic blood pressure in heart failure patients[215]. This reduction showed progressive enhancement over time, with pulmonary arterial effects appearing independent of diuretic usage.
Further investigation is crucial in several key areas: (1) Exploring SGLT-2 inhibitors’ therapeutic effects across diverse pulmonary vascular diseases with distinct etiologies and manifestations; (2) Investigating their potential benefits and risks in PH secondary to left heart disease; (3) Evaluating clinical relevance across different stages of pulmonary hy
SGLT-2 inhibitors represent a well-tolerated therapeutic class demonstrating significant efficacy in reducing pulmonary vascular disease incidence and improving patient outcomes. While their precise mechanistic pathways remain under investigation, their beneficial effects on pulmonary vascular cells and arterial remodeling are evident. These agents enhance pulmonary EC function, suppress PASMC proliferation, and attenuate pulmonary arterial remodeling. The pharmacological profile of SGLT-2 inhibitors extends beyond SGLT-2 inhibition and glycemic control, suggesting broader therapeutic potential in pulmonary vascular disease management. Further investigation of the functional, metabolic, and molecular roles of specific SGLT-2 inhibitors in vascular cells may elucidate their mechanisms in preventing pulmonary vascular disease and facilitate the development of targeted therapeutic strategies. However, conflicting data necessitates extensive additional research to validate current findings and identify optimal patient populations for this therapeutic approach.
The authors would like to express their gratitude to the scholars who participated in this study for their invaluable contributions.
| 1. | Ni T, Zhang S, Rao J, Zhao J, Huang H, Liu Y, Ding Y, Liu Y, Ma Y, Zhang S, Gao Y, Shen L, Ding C, Sun Y. Phlorizin, an Important Glucoside: Research Progress on Its Biological Activity and Mechanism. Molecules. 2024;29:741. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Demaris KM, White JR. Dapagliflozin, an SGLT2 inhibitor for the treatment of type 2 diabetes. Drugs Today (Barc). 2013;49:289-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Polychronopoulou E, Bourdon F, Teta D. SGLT2 inhibitors in diabetic and non-diabetic kidney transplant recipients: current knowledge and expectations. Front Nephrol. 2024;4:1332397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 4. | Trujillo JM, Nuffer WA. Impact of Sodium-Glucose Cotransporter 2 Inhibitors on Nonglycemic Outcomes in Patients with Type 2 Diabetes. Pharmacotherapy. 2017;37:481-491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Saisho Y. SGLT2 Inhibitors: the Star in the Treatment of Type 2 Diabetes? Diseases. 2020;8:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 6. | Joshi SS, Singh T, Newby DE, Singh J. Sodium-glucose co-transporter 2 inhibitor therapy: mechanisms of action in heart failure. Heart. 2021;107:1032-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 113] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 7. | Wilson SJ, Miller MR, Newby DE. Effects of Diesel Exhaust on Cardiovascular Function and Oxidative Stress. Antioxid Redox Signal. 2018;28:819-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Fadini GP, Zatti G, Baldi I, Bottigliengo D, Consoli A, Giaccari A, Sesti G, Avogaro A; DARWIN-T2D network. Use and effectiveness of dapagliflozin in routine clinical practice: An Italian multicentre retrospective study. Diabetes Obes Metab. 2018;20:1781-1786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Douros A, Lix LM, Fralick M, Dell'Aniello S, Shah BR, Ronksley PE, Tremblay É, Hu N, Alessi-Severini S, Fisher A, Bugden SC, Ernst P, Filion KB; Canadian Network for Observational Drug Effect Studies (CNODES) Investigators. Sodium-Glucose Cotransporter-2 Inhibitors and the Risk for Diabetic Ketoacidosis: A Multicenter Cohort Study. Ann Intern Med. 2020;173:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 10. | Kappers MH, de Beer VJ, Zhou Z, Danser AH, Sleijfer S, Duncker DJ, van den Meiracker AH, Merkus D. Sunitinib-induced systemic vasoconstriction in swine is endothelin mediated and does not involve nitric oxide or oxidative stress. Hypertension. 2012;59:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Kala P, Vaňourková Z, Škaroupková P, Kompanowska-Jezierska E, Sadowski J, Walkowska A, Veselka J, Táborský M, Maxová H, Vaněčková I, Červenka L. Endothelin type A receptor blockade increases renoprotection in congestive heart failure combined with chronic kidney disease: Studies in 5/6 nephrectomized rats with aorto-caval fistula. Biomed Pharmacother. 2023;158:114157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Abdel-Wahab AF, Bamagous GA, Al-Harizy RM, ElSawy NA, Shahzad N, Ibrahim IA, Ghamdi SSA. Renal protective effect of SGLT2 inhibitor dapagliflozin alone and in combination with irbesartan in a rat model of diabetic nephropathy. Biomed Pharmacother. 2018;103:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Miklovič M, Gawryś O, Honetschlägerová Z, Kala P, Husková Z, Kikerlová S, Vaňourková Z, Jíchová Š, Kvasilová A, Kitamoto M, Maxová H, Puertas-Frias G, Mráček T, Sedmera D, Melenovský V. Renal denervation improves cardiac function independently of afterload and restores myocardial norepinephrine levels in a rodent heart failure model. Hypertens Res. 2024;47:2718-2730. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Oh CM, Cho S, Jang JY, Kim H, Chun S, Choi M, Park S, Ko YG. Cardioprotective Potential of an SGLT2 Inhibitor Against Doxorubicin-Induced Heart Failure. Korean Circ J. 2019;49:1183-1195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 15. | Abraham WT, Lindenfeld J, Ponikowski P, Agostoni P, Butler J, Desai AS, Filippatos G, Gniot J, Fu M, Gullestad L, Howlett JG, Nicholls SJ, Redon J, Schenkenberger I, Silva-Cardoso J, Störk S, Krzysztof Wranicz J, Savarese G, Brueckmann M, Jamal W, Nordaby M, Peil B, Ritter I, Ustyugova A, Zeller C, Salsali A, Anker SD. Effect of empagliflozin on exercise ability and symptoms in heart failure patients with reduced and preserved ejection fraction, with and without type 2 diabetes. Eur Heart J. 2021;42:700-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 147] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 16. | Hulst AH, Hermanides J, DeVries JH, Preckel B. Potential Benefits of Sodium-Glucose Cotransporter-2 Inhibitors in the Perioperative Period. Anesth Analg. 2018;127:306-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Chen X, Quan R, Qian Y, Yang Z, Yu Z, Zhang C, Yang Y, Zhang G, Shen J, Wang Q, Gu Q, Xiong C, Jing X, Han H, He J. 10-year survival of pulmonary arterial hypertension associated with connective tissue disease: insights from a multicentre PAH registry. Rheumatology (Oxford). 2023;62:3555-3564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 18. | Han L, Chen Y, Cheng W, Bai H, Wang J, Yu M. Deep Learning-Based CT Image Characteristics and Postoperative Anal Function Restoration for Patients with Complex Anal Fistula. J Healthc Eng. 2021;2021:1730158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Joki Y, Konishi H, Takasu K, Minamino T. Tofogliflozin, a sodium-glucose cotransporter 2 inhibitor, improves pulmonary vascular remodeling due to left heart disease in mice. J Cardiol. 2023;81:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 20. | Tentolouris A, Vlachakis P, Tzeravini E, Eleftheriadou I, Tentolouris N. SGLT2 Inhibitors: A Review of Their Antidiabetic and Cardioprotective Effects. Int J Environ Res Public Health. 2019;16:2965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 173] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 21. | Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61:2108-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 666] [Article Influence: 95.1] [Reference Citation Analysis (0)] |
| 22. | Anker SD, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, Zannad F, Packer M; EMPEROR-Preserved Trial Committees and Investigators. Evaluation of the effects of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR-Preserved Trial. Eur J Heart Fail. 2019;21:1279-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 188] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 23. | Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab. 2018;20:479-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 353] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 24. | Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium Glucose Cotransporter-2 Inhibition in Heart Failure: Potential Mechanisms, Clinical Applications, and Summary of Clinical Trials. Circulation. 2017;136:1643-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 336] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 25. | Wilcox CS, Shen W, Boulton DW, Leslie BR, Griffen SC. Interaction Between the Sodium-Glucose-Linked Transporter 2 Inhibitor Dapagliflozin and the Loop Diuretic Bumetanide in Normal Human Subjects. J Am Heart Assoc. 2018;7:e007046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 26. | Alshnbari AS, Millar SA, O'Sullivan SE, Idris I. Effect of Sodium-Glucose Cotransporter-2 Inhibitors on Endothelial Function: A Systematic Review of Preclinical Studies. Diabetes Ther. 2020;11:1947-1963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 27. | Qiu M, Ding LL, Zhang M, Zhou HR. Safety of four SGLT2 inhibitors in three chronic diseases: A meta-analysis of large randomized trials of SGLT2 inhibitors. Diab Vasc Dis Res. 2021;18:14791641211011016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 28. | Zou X, Shi Q, Vandvik PO, Guyatt G, Lang CC, Parpia S, Wang S, Agarwal A, Zhou Y, Zhu Y, Tian H, Zhu Z, Li S. Sodium-Glucose Cotransporter-2 Inhibitors in Patients With Heart Failure : A Systematic Review and Meta-analysis. Ann Intern Med. 2022;175:851-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 29. | Lopaschuk GD, Verma S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl Sci. 2020;5:632-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 553] [Article Influence: 110.6] [Reference Citation Analysis (0)] |
| 30. | d'Emden M, Amerena J, Deed G, Pollock C, Cooper ME. SGLT2 inhibitors with cardiovascular benefits: Transforming clinical care in Type 2 diabetes mellitus. Diabetes Res Clin Pract. 2018;136:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Maruyama T, Takashima H, Oguma H, Nakamura Y, Ohno M, Utsunomiya K, Furukawa T, Tei R, Abe M. Canagliflozin Improves Erythropoiesis in Diabetes Patients with Anemia of Chronic Kidney Disease. Diabetes Technol Ther. 2019;21:713-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 32. | Bailey CJ. Uric acid and the cardio-renal effects of SGLT2 inhibitors. Diabetes Obes Metab. 2019;21:1291-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 33. | Herat LY, Matthews VB, Magno AL, Kiuchi MG, Carnagarin R, Schlaich MP. An evaluation of empagliflozin and it's applicability to hypertension as a therapeutic option. Expert Opin Pharmacother. 2020;21:1157-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Horie I, Abiru N, Hongo R, Nakamura T, Ito A, Haraguchi A, Natsuda S, Sagara I, Ando T, Kawakami A. Increased sugar intake as a form of compensatory hyperphagia in patients with type 2 diabetes under dapagliflozin treatment. Diabetes Res Clin Pract. 2018;135:178-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Ng KM, Lau YM, Dhandhania V, Cai ZJ, Lee YK, Lai WH, Tse HF, Siu CW. Empagliflozin Ammeliorates High Glucose Induced-Cardiac Dysfuntion in Human iPSC-Derived Cardiomyocytes. Sci Rep. 2018;8:14872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 36. | Park SH, Belcastro E, Hasan H, Matsushita K, Marchandot B, Abbas M, Toti F, Auger C, Jesel L, Ohlmann P, Morel O, Schini-Kerth VB. Angiotensin II-induced upregulation of SGLT1 and 2 contributes to human microparticle-stimulated endothelial senescence and dysfunction: protective effect of gliflozins. Cardiovasc Diabetol. 2021;20:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 37. | Di Franco A, Cantini G, Tani A, Coppini R, Zecchi-Orlandini S, Raimondi L, Luconi M, Mannucci E. Sodium-dependent glucose transporters (SGLT) in human ischemic heart: A new potential pharmacological target. Int J Cardiol. 2017;243:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 38. | Kondo H, Akoumianakis I, Badi I, Akawi N, Kotanidis CP, Polkinghorne M, Stadiotti I, Sommariva E, Antonopoulos AS, Carena MC, Oikonomou EK, Reus EM, Sayeed R, Krasopoulos G, Srivastava V, Farid S, Chuaiphichai S, Shirodaria C, Channon KM, Casadei B, Antoniades C. Effects of canagliflozin on human myocardial redox signalling: clinical implications. Eur Heart J. 2021;42:4947-4960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 39. | Chan BYH, Roczkowsky A, Cho WJ, Poirier M, Sergi C, Keschrumrus V, Churko JM, Granzier H, Schulz R. MMP inhibitors attenuate doxorubicin cardiotoxicity by preventing intracellular and extracellular matrix remodelling. Cardiovasc Res. 2021;117:188-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 40. | Rempe RG, Hartz AMS, Bauer B. Matrix metalloproteinases in the brain and blood-brain barrier: Versatile breakers and makers. J Cereb Blood Flow Metab. 2016;36:1481-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 486] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 41. | Fatima A, Rasool S, Devi S, Talha M, Waqar F, Nasir M, Khan MR, Ibne Ali Jaffari SM, Haider A, Shah SU, Sapna F, Varrassi G, Khatri M, Kumar S, Mohamad T. Exploring the Cardiovascular Benefits of Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors: Expanding Horizons Beyond Diabetes Management. Cureus. 2023;15:e46243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 42. | Meng L, Uzui H, Guo H, Tada H. Role of SGLT1 in high glucose level-induced MMP-2 expression in human cardiac fibroblasts. Mol Med Rep. 2018;17:6887-6892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Barrios V, Escobar C. Canagliflozin: metabolic, cardiovascular and renal protection. Future Cardiol. 2021;17:443-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Jiang K, Xu Y, Wang D, Chen F, Tu Z, Qian J, Xu S, Xu Y, Hwa J, Li J, Shang H, Xiang Y. Cardioprotective mechanism of SGLT2 inhibitor against myocardial infarction is through reduction of autosis. Protein Cell. 2022;13:336-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 45. | Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, Ishikawa K, Watanabe S, Picatoste B, Flores E, Garcia-Ropero A, Sanz J, Hajjar RJ, Fuster V, Badimon JJ. Empagliflozin Ameliorates Adverse Left Ventricular Remodeling in Nondiabetic Heart Failure by Enhancing Myocardial Energetics. J Am Coll Cardiol. 2019;73:1931-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 455] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 46. | De Luca M, Crisci G, Armentaro G, Cicco S, Talerico G, Bobbio E, Lanzafame L, Green CG, McLellan AG, Debiec R, Caferra P, Scicali R, Cannatà A, Israr MZ, Heaney LM, Salzano A. Endothelial Dysfunction and Heart Failure with Preserved Ejection Fraction-An Updated Review of the Literature. Life (Basel). 2023;14:30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 47. | Huveneers S, Daemen MJ, Hordijk PL. Between Rho(k) and a hard place: the relation between vessel wall stiffness, endothelial contractility, and cardiovascular disease. Circ Res. 2015;116:895-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 148] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 48. | Abe K, Shimokawa H, Morikawa K, Uwatoku T, Oi K, Matsumoto Y, Hattori T, Nakashima Y, Kaibuchi K, Sueishi K, Takeshit A. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ Res. 2004;94:385-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 295] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 49. | Murphy D, Girgis RE. HIV-associated pulmonary arterial hypertension: a clinical problem that is here to stay? Int J Clin Pract Suppl. 2009;19-21. [PubMed] [DOI] [Full Text] |
| 50. | Böhm F, Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res. 2007;76:8-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 353] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 51. | Shao D, Park JE, Wort SJ. The role of endothelin-1 in the pathogenesis of pulmonary arterial hypertension. Pharmacol Res. 2011;63:504-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 52. | Christou H, Khalil RA. Mechanisms of pulmonary vascular dysfunction in pulmonary hypertension and implications for novel therapies. Am J Physiol Heart Circ Physiol. 2022;322:H702-H724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 53. | Mustroph J, Neef S, Maier LS. CaMKII as a target for arrhythmia suppression. Pharmacol Ther. 2017;176:22-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 54. | Santulli G, Xie W, Reiken SR, Marks AR. Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci U S A. 2015;112:11389-11394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 429] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 55. | Xie W, Santulli G, Reiken SR, Yuan Q, Osborne BW, Chen BX, Marks AR. Mitochondrial oxidative stress promotes atrial fibrillation. Sci Rep. 2015;5:11427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 226] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 56. | Yu T, Sheu SS, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res. 2008;79:341-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 387] [Cited by in RCA: 376] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 57. | Peinado VI, Pizarro S, Barberà JA. Pulmonary vascular involvement in COPD. Chest. 2008;134:808-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 58. | Souza R, Fernandes CJ, Jardim CV. Other causes of PAH (schistosomiasis, porto-pulmonary hypertension and hemolysis-associated pulmonary hypertension). Semin Respir Crit Care Med. 2009;30:448-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 59. | Le Cras TD, Markham NE, Tuder RM, Voelkel NF, Abman SH. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am J Physiol Lung Cell Mol Physiol. 2002;283:L555-L562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 287] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 60. | Goto H, Nishikawa T, Sonoda K, Kondo T, Kukidome D, Fujisawa K, Yamashiro T, Motoshima H, Matsumura T, Tsuruzoe K, Araki E. Endothelial MnSOD overexpression prevents retinal VEGF expression in diabetic mice. Biochem Biophys Res Commun. 2008;366:814-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 61. | Johnson EN, Lee YM, Sander TL, Rabkin E, Schoen FJ, Kaushal S, Bischoff J. NFATc1 mediates vascular endothelial growth factor-induced proliferation of human pulmonary valve endothelial cells. J Biol Chem. 2003;278:1686-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 62. | McDonald RA, Hata A, MacLean MR, Morrell NW, Baker AH. MicroRNA and vascular remodelling in acute vascular injury and pulmonary vascular remodelling. Cardiovasc Res. 2012;93:594-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 63. | Zhang H, Liu D, Xu QF, Wei J, Zhao Y, Xu DF, Wang Y, Liu YJ, Zhu XY, Jiang L. Endothelial RSPO3 mediates pulmonary endothelial regeneration by LGR4-dependent activation of β-catenin and ILK signaling pathways after inflammatory vascular injury. Int J Biol Macromol. 2024;269:131805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 64. | Weiss T, Near AM, Zhao X, Ramey DR, Banerji T, Xie H, Nathan SD. Healthcare resource utilization in patients with pulmonary hypertension associated with chronic obstructive pulmonary disease (PH-COPD): a real-world data analysis. BMC Pulm Med. 2023;23:455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 65. | Nathan SD, Argula R, Trivieri MG, Aziz S, Gay E, Medarov B, Parambil J, Raina A, Risbano MG, Thenappan T, Soto JS, Bell H, Lacasse V, Sista P, Di Marino M, Smart A, Hawkes B, Nelson E, Bull T, Tapson V, Waxman A. Inhaled treprostinil in pulmonary hypertension associated with COPD: PERFECT study results. Eur Respir J. 2024;63:2400172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 66. | Gojova A, Guo B, Kota RS, Rutledge JC, Kennedy IM, Barakat AI. Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: effect of particle composition. Environ Health Perspect. 2007;115:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 318] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 67. | Peinado VI, Barberá JA, Abate P, Ramírez J, Roca J, Santos S, Rodriguez-Roisin R. Inflammatory reaction in pulmonary muscular arteries of patients with mild chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:1605-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 173] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 68. | Archer SL, Huang JM, Hampl V, Nelson DP, Shultz PJ, Weir EK. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1994;91:7583-7587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 576] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 69. | Savai R, Pullamsetti SS, Kolbe J, Bieniek E, Voswinckel R, Fink L, Scheed A, Ritter C, Dahal BK, Vater A, Klussmann S, Ghofrani HA, Weissmann N, Klepetko W, Banat GA, Seeger W, Grimminger F, Schermuly RT. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:897-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 308] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 70. | Parikh RV, Ma Y, Scherzer R, Heringer AS, Macgregor JS, Martin JN, Deeks SG, Ganz P, Hsue PY. Endothelin-1 Predicts Hemodynamically Assessed Pulmonary Arterial Hypertension in HIV Infection. PLoS One. 2016;11:e0146355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | Dong L, Li Z, Leffler NR, Asch AS, Chi JT, Yang LV. Acidosis activation of the proton-sensing GPR4 receptor stimulates vascular endothelial cell inflammatory responses revealed by transcriptome analysis. PLoS One. 2013;8:e61991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 72. | Lee IT, Yang CM. Inflammatory signalings involved in airway and pulmonary diseases. Mediators Inflamm. 2013;2013:791231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 73. | Stenmark KR, Davie N, Frid M, Gerasimovskaya E, Das M. Role of the adventitia in pulmonary vascular remodeling. Physiology (Bethesda). 2006;21:134-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 169] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 74. | Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, Sdrimas K, Fernandez-Gonzalez A, Kourembanas S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601-2611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 631] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 75. | Miller MR, Shaw CA, Langrish JP. From particles to patients: oxidative stress and the cardiovascular effects of air pollution. Future Cardiol. 2012;8:577-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 76. | Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99:675-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 781] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 77. | Itoh T, Nagaya N, Murakami S, Fujii T, Iwase T, Ishibashi-Ueda H, Yutani C, Yamagishi M, Kimura H, Kangawa K. C-type natriuretic peptide ameliorates monocrotaline-induced pulmonary hypertension in rats. Am J Respir Crit Care Med. 2004;170:1204-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 78. | Sodhi M, Rezaeianzadeh R, Kezouh A, Etminan M. Risk of Gastrointestinal Adverse Events Associated With Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss. JAMA. 2023;330:1795-1797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 208] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 79. | Mehdi SF, Pusapati S, Anwar MS, Lohana D, Kumar P, Nandula SA, Nawaz FK, Tracey K, Yang H, LeRoith D, Brownstein MJ, Roth J. Glucagon-like peptide-1: a multi-faceted anti-inflammatory agent. Front Immunol. 2023;14:1148209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 113] [Reference Citation Analysis (0)] |
| 80. | Thenappan T, Ormiston ML, Ryan JJ, Archer SL. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ. 2018;360:j5492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 642] [Article Influence: 91.7] [Reference Citation Analysis (0)] |
| 81. | Aguero J, Ishikawa K, Hadri L, Santos-Gallego CG, Fish KM, Kohlbrenner E, Hammoudi N, Kho C, Lee A, Ibáñez B, García-Alvarez A, Zsebo K, Maron BA, Plataki M, Fuster V, Leopold JA, Hajjar RJ. Intratracheal Gene Delivery of SERCA2a Ameliorates Chronic Post-Capillary Pulmonary Hypertension: A Large Animal Model. J Am Coll Cardiol. 2016;67:2032-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 82. | van Voorthuizen EL, van Helvoort HAC, Peters JB, van den Heuvel MM, van den Borst B. Persistent Exertional Dyspnea and Perceived Exercise Intolerance After Mild COVID-19: A Critical Role for Breathing Dysregulation? Phys Ther. 2022;102:pzac105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 83. | Machado RF, Mack AK, Martyr S, Barnett C, Macarthur P, Sachdev V, Ernst I, Hunter LA, Coles WA, Nichols JP, Kato GJ, Gladwin MT. Severity of pulmonary hypertension during vaso-occlusive pain crisis and exercise in patients with sickle cell disease. Br J Haematol. 2007;136:319-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 84. | Lippton HL, Hauth TA, Summer WR, Hyman AL. Endothelin produces pulmonary vasoconstriction and systemic vasodilation. J Appl Physiol (1985). 1989;66:1008-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 90] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 85. | Ameri P, Mercurio V, Pollesello P, Anker MS, Backs J, Bayes-Genis A, Borlaug BA, Burkhoff D, Caravita S, Chan SY, de Man F, Giannakoulas G, González A, Guazzi M, Hassoun PM, Hemnes AR, Maack C, Madden B, Melenovsky V, Müller OJ, Papp Z, Pullamsetti SS, Rainer PP, Redfield MM, Rich S, Schiattarella GG, Skaara H, Stellos K, Tedford RJ, Thum T, Vachiery JL, van der Meer P, Van Linthout S, Pruszczyk P, Seferovic P, Coats AJS, Metra M, Rosano G, Rosenkranz S, Tocchetti CG. A roadmap for therapeutic discovery in pulmonary hypertension associated with left heart failure. A scientific statement of the Heart Failure Association (HFA) of the ESC and the ESC Working Group on Pulmonary Circulation & Right Ventricular Function. Eur J Heart Fail. 2024;26:707-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 86. | Bondarev AD, Attwood MM, Jonsson J, Chubarev VN, Tarasov VV, Liu W, Schiöth HB. Recent developments of phosphodiesterase inhibitors: Clinical trials, emerging indications and novel molecules. Front Pharmacol. 2022;13:1057083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 87. | Kawakami T, Matsubara H, Shinke T, Abe K, Kohsaka S, Hosokawa K, Taniguchi Y, Shimokawahara H, Yamada Y, Kataoka M, Ogawa A, Murata M, Jinzaki M, Hirata K, Tsutsui H, Sato Y, Fukuda K. Balloon pulmonary angioplasty versus riociguat in inoperable chronic thromboembolic pulmonary hypertension (MR BPA): an open-label, randomised controlled trial. Lancet Respir Med. 2022;10:949-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 88. | Ge T, Yang Y, Zhao Y. A study of the efficacy of sacubitril/valsartan plus dapagliflozin combination treatment in pulmonary arterial hypertension due to left heart disease. Perfusion. 2023;38:1697-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 89. | De Stefano A, Tesauro M, Di Daniele N, Vizioli G, Schinzari F, Cardillo C. Mechanisms of SGLT2 (Sodium-Glucose Transporter Type 2) Inhibition-Induced Relaxation in Arteries From Human Visceral Adipose Tissue. Hypertension. 2021;77:729-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 90. | Ugusman A, Kumar J, Aminuddin A. Endothelial function and dysfunction: Impact of sodium-glucose cotransporter 2 inhibitors. Pharmacol Ther. 2021;224:107832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 91. | Durante W, Behnammanesh G, Peyton KJ. Effects of Sodium-Glucose Co-Transporter 2 Inhibitors on Vascular Cell Function and Arterial Remodeling. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 92. | Giannattasio S, Citarella A, Trocchianesi S, Filardi T, Morano S, Lenzi A, Ferretti E, Crescioli C. Cell-Target-Specific Anti-Inflammatory Effect of Empagliflozin: In Vitro Evidence in Human Cardiomyocytes. Front Mol Biosci. 2022;9:879522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 93. | Steven S, Oelze M, Hanf A, Kröller-Schön S, Kashani F, Roohani S, Welschof P, Kopp M, Gödtel-Armbrust U, Xia N, Li H, Schulz E, Lackner KJ, Wojnowski L, Bottari SP, Wenzel P, Mayoux E, Münzel T, Daiber A. The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol. 2017;13:370-385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 231] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 94. | Sawada T, Uzu K, Hashimoto N, Onishi T, Takaya T, Shimane A, Taniguchi Y, Yasaka Y, Ohara T, Kawai H. Empagliflozin's Ameliorating Effect on Plasma Triglycerides: Association with Endothelial Function Recovery in Diabetic Patients with Coronary Artery Disease. J Atheroscler Thromb. 2020;27:644-656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 95. | Lunder M, Janić M, Japelj M, Juretič A, Janež A, Šabovič M. Empagliflozin on top of metformin treatment improves arterial function in patients with type 1 diabetes mellitus. Cardiovasc Diabetol. 2018;17:153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 96. | Dou L, Burtey S. Reversing endothelial dysfunction with empagliflozin to improve cardiomyocyte function in cardiorenal syndrome. Kidney Int. 2021;99:1062-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 97. | Salvatore T, Galiero R, Caturano A, Vetrano E, Loffredo G, Rinaldi L, Catalini C, Gjeloshi K, Albanese G, Di Martino A, Docimo G, Sardu C, Marfella R, Sasso FC. Coronary Microvascular Dysfunction in Diabetes Mellitus: Pathogenetic Mechanisms and Potential Therapeutic Options. Biomedicines. 2022;10:2274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 49] [Reference Citation Analysis (0)] |
| 98. | Jahn LA, Hartline LM, Nguyen T, Aylor K, Horton WB, Liu Z, Barrett EJ. Empagliflozin improves vascular insulin sensitivity and muscle perfusion in persons with type 2 diabetes. Am J Physiol Endocrinol Metab. 2024;326:E258-E267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 99. | Sano R, Shinozaki Y, Ohta T. Sodium-glucose cotransporters: Functional properties and pharmaceutical potential. J Diabetes Investig. 2020;11:770-782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 100. | Baretella O, Vanhoutte PM. Endothelium-Dependent Contractions: Prostacyclin and Endothelin-1, Partners in Crime? Adv Pharmacol. 2016;77:177-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 101. | Yu Q, Li K, Zhao A, Wei M, Huang Z, Zhang Y, Chen Y, Lian T, Wang C, Xu L, Zhang Y, Xu C, Liu F. Sorafenib not only impairs endothelium-dependent relaxation but also promotes vasoconstriction through the upregulation of vasoconstrictive endothelin type B receptors. Toxicol Appl Pharmacol. 2021;414:115420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 102. | Garland CJ, Dora KA. Endothelium-Dependent Hyperpolarization: The Evolution of Myoendothelial Microdomains. J Cardiovasc Pharmacol. 2021;78:S3-S12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 103. | Deng JT, Bhaidani S, Sutherland C, MacDonald JA, Walsh MP. Rho-associated kinase and zipper-interacting protein kinase, but not myosin light chain kinase, are involved in the regulation of myosin phosphorylation in serum-stimulated human arterial smooth muscle cells. PLoS One. 2019;14:e0226406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 104. | Li CY, Wang LX, Dong SS, Hong Y, Zhou XH, Zheng WW, Zheng C. Phlorizin Exerts Direct Protective Effects on Palmitic Acid (PA)-Induced Endothelial Dysfunction by Activating the PI3K/AKT/eNOS Signaling Pathway and Increasing the Levels of Nitric Oxide (NO). Med Sci Monit Basic Res. 2018;24:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 105. | El-Daly M, Pulakazhi Venu VK, Saifeddine M, Mihara K, Kang S, Fedak PWM, Alston LA, Hirota SA, Ding H, Triggle CR, Hollenberg MD. Hyperglycaemic impairment of PAR2-mediated vasodilation: Prevention by inhibition of aortic endothelial sodium-glucose-co-Transporter-2 and minimizing oxidative stress. Vascul Pharmacol. 2018;109:56-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 106. | Tai S, Zhou Y, Fu L, Ding H, Zhou Y, Yin Z, Yang R, Liu Z, Zhou S. Dapagliflozin impedes endothelial cell senescence by activating the SIRT1 signaling pathway in type 2 diabetes. Heliyon. 2023;9:e19152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 107. | Cappetta D, De Angelis A, Ciuffreda LP, Coppini R, Cozzolino A, Miccichè A, Dell'Aversana C, D'Amario D, Cianflone E, Scavone C, Santini L, Palandri C, Naviglio S, Crea F, Rota M, Altucci L, Rossi F, Capuano A, Urbanek K, Berrino L. Amelioration of diastolic dysfunction by dapagliflozin in a non-diabetic model involves coronary endothelium. Pharmacol Res. 2020;157:104781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 108. | Schmidt K, Schmidt A, Groß S, Just A, Pfanne A, Fuchs M, Jordan M, Mohr E, Pich A, Fiedler J, Thum T. SGLT2 inhibitors attenuate endothelial to mesenchymal transition and cardiac fibroblast activation. Sci Rep. 2024;14:16459. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 109. | Borriello G, Buonincontri V, de Donato A, Della Corte M, Gravina I, Iulianiello P, Joshi R, Mone P, Cacciola G, Viggiano D. The interplay between sodium/glucose cotransporter type 2 and mitochondrial ionic environment. Mitochondrion. 2024;76:101878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 110. | Zhou H, Wang S, Zhu P, Hu S, Chen Y, Ren J. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol. 2018;15:335-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 437] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 111. | Park SH, Farooq MA, Gaertner S, Bruckert C, Qureshi AW, Lee HH, Benrahla D, Pollet B, Stephan D, Ohlmann P, Lessinger JM, Mayoux E, Auger C, Morel O, Schini-Kerth VB. Empagliflozin improved systolic blood pressure, endothelial dysfunction and heart remodeling in the metabolic syndrome ZSF1 rat. Cardiovasc Diabetol. 2020;19:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 112. | Barraclough JY, Patel S, Yu J, Neal B, Arnott C. The Role of Sodium Glucose Cotransporter-2 Inhibitors in Atherosclerotic Cardiovascular Disease: A Narrative Review of Potential Mechanisms. Cells. 2021;10:2699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 113. | Tanaka A, Shimabukuro M, Machii N, Teragawa H, Okada Y, Shima KR, Takamura T, Taguchi I, Hisauchi I, Toyoda S, Matsuzawa Y, Tomiyama H, Yamaoka-Tojo M, Ueda S, Higashi Y, Node K. Secondary analyses to assess the profound effects of empagliflozin on endothelial function in patients with type 2 diabetes and established cardiovascular diseases: The placebo-controlled double-blind randomized effect of empagliflozin on endothelial function in cardiovascular high risk diabetes mellitus: Multi-center placebo-controlled double-blind randomized trial. J Diabetes Investig. 2020;11:1551-1563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 114. | Uthman L, Homayr A, Juni RP, Spin EL, Kerindongo R, Boomsma M, Hollmann MW, Preckel B, Koolwijk P, van Hinsbergh VWM, Zuurbier CJ, Albrecht M, Weber NC. Empagliflozin and Dapagliflozin Reduce ROS Generation and Restore NO Bioavailability in Tumor Necrosis Factor α-Stimulated Human Coronary Arterial Endothelial Cells. Cell Physiol Biochem. 2019;53:865-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 115. | Juni RP, Al-Shama R, Kuster DWD, van der Velden J, Hamer HM, Vervloet MG, Eringa EC, Koolwijk P, van Hinsbergh VWM. Empagliflozin restores chronic kidney disease-induced impairment of endothelial regulation of cardiomyocyte relaxation and contraction. Kidney Int. 2021;99:1088-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 116. | He X, Yuan D. A review regarding the article 'Targeting inflammatory signaling pathways with SGLT2 inhibitors: Insights into cardiovascular health and cardiac cell improvement'. Curr Probl Cardiol. 2024;49:102563. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 117. | Dhakal B, Shiwakoti S, Park EY, Kang KW, Schini-Kerth VB, Park SH, Ji HY, Park JS, Ko JY, Oak MH. SGLT2 inhibition ameliorates nano plastics-induced premature endothelial senescence and dysfunction. Sci Rep. 2023;13:6256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 118. | Barbeau S, Gilbert G, Cardouat G, Baudrimont I, Freund-Michel V, Guibert C, Marthan R, Vacher P, Quignard JF, Ducret T. Mechanosensitivity in Pulmonary Circulation: Pathophysiological Relevance of Stretch-Activated Channels in Pulmonary Hypertension. Biomolecules. 2021;11:1389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 119. | Duan HY, Barajas-Martinez H, Antzelevitch C, Hu D. The potential anti-arrhythmic effect of SGLT2 inhibitors. Cardiovasc Diabetol. 2024;23:252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 120. | AbouEzzeddine OF, Kemp BJ, Borlaug BA, Mullan BP, Behfar A, Pislaru SV, Fudim M, Redfield MM, Chareonthaitawee P. Myocardial Energetics in Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2019;12:e006240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 121. | Ma L, Zou R, Shi W, Zhou N, Chen S, Zhou H, Chen X, Wu Y. SGLT2 inhibitor dapagliflozin reduces endothelial dysfunction and microvascular damage during cardiac ischemia/reperfusion injury through normalizing the XO-SERCA2-CaMKII-coffilin pathways. Theranostics. 2022;12:5034-5050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 83] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 122. | Solini A, Giannini L, Seghieri M, Vitolo E, Taddei S, Ghiadoni L, Bruno RM. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol. 2017;16:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 284] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 123. | Cinakova A, Krenek P, Klimas J, Kralova E. Adding SGLT2 Cotransporter Inhibitor to PPARγ Activator Does Not Provide an Additive Effect in the Management of Diabetes-Induced Vascular Dysfunction. Pharmacology. 2023;108:565-575. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 124. | Rafikova O, Srivastava A, Desai AA, Rafikov R, Tofovic SP. Recurrent inhibition of mitochondrial complex III induces chronic pulmonary vasoconstriction and glycolytic switch in the rat lung. Respir Res. 2018;19:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 125. | Eelen G, Treps L, Li X, Carmeliet P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ Res. 2020;127:310-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 329] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 126. | Alhayaza R, Haque E, Karbasiafshar C, Sellke FW, Abid MR. The Relationship Between Reactive Oxygen Species and Endothelial Cell Metabolism. Front Chem. 2020;8:592688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 127. | Shigiyama F, Kumashiro N, Miyagi M, Ikehara K, Kanda E, Uchino H, Hirose T. Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc Diabetol. 2017;16:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 190] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 128. | Gaspari T, Spizzo I, Liu H, Hu Y, Simpson RW, Widdop RE, Dear AE. Dapagliflozin attenuates human vascular endothelial cell activation and induces vasorelaxation: A potential mechanism for inhibition of atherogenesis. Diab Vasc Dis Res. 2018;15:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 129. | Fadini GP, Bonora BM, Zatti G, Vitturi N, Iori E, Marescotti MC, Albiero M, Avogaro A. Effects of the SGLT2 inhibitor dapagliflozin on HDL cholesterol, particle size, and cholesterol efflux capacity in patients with type 2 diabetes: a randomized placebo-controlled trial. Cardiovasc Diabetol. 2017;16:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 130. | He L, Li Y, Zhang D, Song H, Xu D, Song Z. Dapagliflozin improves endothelial cell dysfunction by regulating mitochondrial production via the SIRT1/PGC-1α pathway in obese mice. Biochem Biophys Res Commun. 2022;615:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 131. | Wang W, Li Y, Zhang Y, Ye T, Wang K, Li S, Zhang Y. SIRT1 mediates the inhibitory effect of Dapagliflozin on EndMT by inhibiting the acetylation of endothelium Notch1. Cardiovasc Diabetol. 2023;22:331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 132. | Zhou Y, Tai S, Zhang N, Fu L, Wang Y. Dapagliflozin prevents oxidative stress-induced endothelial dysfunction via sirtuin 1 activation. Biomed Pharmacother. 2023;165:115213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 133. | Zou R, Shi W, Qiu J, Zhou N, Du N, Zhou H, Chen X, Ma L. Empagliflozin attenuates cardiac microvascular ischemia/reperfusion injury through improving mitochondrial homeostasis. Cardiovasc Diabetol. 2022;21:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 86] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 134. | Khemais-Benkhiat S, Belcastro E, Idris-Khodja N, Park SH, Amoura L, Abbas M, Auger C, Kessler L, Mayoux E, Toti F, Schini-Kerth VB. Angiotensin II-induced redox-sensitive SGLT1 and 2 expression promotes high glucose-induced endothelial cell senescence. J Cell Mol Med. 2020;24:2109-2122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 135. | Korkmaz-Icöz S, Kocer C, Sayour AA, Kraft P, Benker MI, Abulizi S, Georgevici AI, Brlecic P, Radovits T, Loganathan S, Karck M, Szabó G. The Sodium-Glucose Cotransporter-2 Inhibitor Canagliflozin Alleviates Endothelial Dysfunction Following In Vitro Vascular Ischemia/Reperfusion Injury in Rats. Int J Mol Sci. 2021;22:7774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 136. | Sayour AA, Korkmaz-Icöz S, Loganathan S, Ruppert M, Sayour VN, Oláh A, Benke K, Brune M, Benkő R, Horváth EM, Karck M, Merkely B, Radovits T, Szabó G. Acute canagliflozin treatment protects against in vivo myocardial ischemia-reperfusion injury in non-diabetic male rats and enhances endothelium-dependent vasorelaxation. J Transl Med. 2019;17:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 137. | Angé M, De Poortere J, Ginion A, Battault S, Dechamps M, Muccioli GG, Roumain M, Morelle J, Druart S, Mathivet T, Bertrand L, Castanares-Zapatero D, Horman S, Beauloye C. Canagliflozin protects against sepsis capillary leak syndrome by activating endothelial α1AMPK. Sci Rep. 2021;11:13700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 138. | Nandula SR, Kundu N, Awal HB, Brichacek B, Fakhri M, Aimalla N, Elzarki A, Amdur RL, Sen S. Role of Canagliflozin on function of CD34+ve endothelial progenitor cells (EPC) in patients with type 2 diabetes. Cardiovasc Diabetol. 2021;20:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 139. | Li X, Römer G, Kerindongo RP, Hermanides J, Albrecht M, Hollmann MW, Zuurbier CJ, Preckel B, Weber NC. Sodium Glucose Co-Transporter 2 Inhibitors Ameliorate Endothelium Barrier Dysfunction Induced by Cyclic Stretch through Inhibition of Reactive Oxygen Species. Int J Mol Sci. 2021;22:6044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 140. | Mroueh A, Fakih W, Carmona A, Trimaille A, Matsushita K, Marchandot B, Qureshi AW, Gong DS, Auger C, Sattler L, Reydel A, Hess S, Oulehri W, Vollmer O, Lessinger JM, Meyer N, Pieper MP, Jesel L, Bäck M, Schini-Kerth V, Morel O. COVID-19 promotes endothelial dysfunction and thrombogenicity: role of proinflammatory cytokines/SGLT2 prooxidant pathway. J Thromb Haemost. 2024;22:286-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 141. | Alshnbari A, Idris I. Can sodium-glucose co-transporter-2 (SGLT-2) inhibitor reduce the risk of adverse complications due to COVID-19? - Targeting hyperinflammation. Curr Med Res Opin. 2022;38:357-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 142. | Tanaka A, Shimabukuro M, Okada Y, Sugimoto K, Kurozumi A, Torimoto K, Hirai H, Node K; PROCEED trial investigators. Rationale and design of an investigator-initiated, multicenter, prospective open-label, randomized trial to evaluate the effect of ipragliflozin on endothelial dysfunction in type 2 diabetes and chronic kidney disease: the PROCEED trial. Cardiovasc Diabetol. 2020;19:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 143. | Wang HL, Tang FQ, Jiang YH, Zhu Y, Jian Z, Xiao YB. AMPKα2 deficiency exacerbates hypoxia-induced pulmonary hypertension by promoting pulmonary arterial smooth muscle cell proliferation. J Physiol Biochem. 2020;76:445-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 144. | Veith C, Vartürk-Özcan I, Wujak M, Hadzic S, Wu CY, Knoepp F, Kraut S, Petrovic A, Gredic M, Pak O, Brosien M, Heimbrodt M, Wilhelm J, Weisel FC, Malkmus K, Schäfer K, Gall H, Tello K, Kosanovic D, Sydykov A, Sarybaev A, Günther A, Brandes RP, Seeger W, Grimminger F, Ghofrani HA, Schermuly RT, Kwapiszewska G, Sommer N, Weissmann N. SPARC, a Novel Regulator of Vascular Cell Function in Pulmonary Hypertension. Circulation. 2022;145:916-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 145. | Ruffenach G, Medzikovic L, Aryan L, Li M, Eghbali M. HNRNPA2B1: RNA-Binding Protein That Orchestrates Smooth Muscle Cell Phenotype in Pulmonary Arterial Hypertension. Circulation. 2022;146:1243-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 146. | Southgate L, Machado RD, Gräf S, Morrell NW. Molecular genetic framework underlying pulmonary arterial hypertension. Nat Rev Cardiol. 2020;17:85-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 194] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 147. | Boucherat O, Vitry G, Trinh I, Paulin R, Provencher S, Bonnet S. The cancer theory of pulmonary arterial hypertension. Pulm Circ. 2017;7:285-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 158] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 148. | Mauban JR, Remillard CV, Yuan JX. Hypoxic pulmonary vasoconstriction: role of ion channels. J Appl Physiol (1985). 2005;98:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 149. | Leopold JA, Maron BA. Molecular Mechanisms of Pulmonary Vascular Remodeling in Pulmonary Arterial Hypertension. Int J Mol Sci. 2016;17:761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 150. | Said SI, Hamidi SA, Dickman KG, Szema AM, Lyubsky S, Lin RZ, Jiang YP, Chen JJ, Waschek JA, Kort S. Moderate pulmonary arterial hypertension in male mice lacking the vasoactive intestinal peptide gene. Circulation. 2007;115:1260-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 151. | Chettimada S, Gupte R, Rawat D, Gebb SA, McMurtry IF, Gupte SA. Hypoxia-induced glucose-6-phosphate dehydrogenase overexpression and -activation in pulmonary artery smooth muscle cells: implication in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2015;308:L287-L300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 152. | Mao SZ, Fan XF, Xue F, Chen R, Chen XY, Yuan GS, Hu LG, Liu SF, Gong YS. Intermedin modulates hypoxic pulmonary vascular remodeling by inhibiting pulmonary artery smooth muscle cell proliferation. Pulm Pharmacol Ther. 2014;27:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 153. | Zhou L, Zhang J, Jiang XM, Xie DJ, Wang JS, Li L, Li B, Wang ZM, Rothman AMK, Lawrie A, Chen SL. Pulmonary Artery Denervation Attenuates Pulmonary Arterial Remodeling in Dogs With Pulmonary Arterial Hypertension Induced by Dehydrogenized Monocrotaline. JACC Cardiovasc Interv. 2015;8:2013-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 154. | De Simone V, Guarise P, Zanotto G, Morando G. Reduction in pulmonary artery pressures with use of sacubitril/valsartan. J Cardiol Cases. 2019;20:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 155. | Pullamsetti S, Krick S, Yilmaz H, Ghofrani HA, Schudt C, Weissmann N, Fuchs B, Seeger W, Grimminger F, Schermuly RT. Inhaled tolafentrine reverses pulmonary vascular remodeling via inhibition of smooth muscle cell migration. Respir Res. 2005;6:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 156. | Schermuly RT, Pullamsetti SS, Kwapiszewska G, Dumitrascu R, Tian X, Weissmann N, Ghofrani HA, Kaulen C, Dunkern T, Schudt C, Voswinckel R, Zhou J, Samidurai A, Klepetko W, Paddenberg R, Kummer W, Seeger W, Grimminger F. Phosphodiesterase 1 upregulation in pulmonary arterial hypertension: target for reverse-remodeling therapy. Circulation. 2007;115:2331-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 157. | Morita T, Perrella MA, Lee ME, Kourembanas S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci U S A. 1995;92:1475-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 545] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 158. | Li X, Zhang X, Leathers R, Makino A, Huang C, Parsa P, Macias J, Yuan JX, Jamieson SW, Thistlethwaite PA. Notch3 signaling promotes the development of pulmonary arterial hypertension. Nat Med. 2009;15:1289-1297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 281] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 159. | Cowan KN, Heilbut A, Humpl T, Lam C, Ito S, Rabinovitch M. Complete reversal of fatal pulmonary hypertension in rats by a serine elastase inhibitor. Nat Med. 2000;6:698-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 258] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 160. | Chowdhury B, Luu AZ, Luu VZ, Kabir MG, Pan Y, Teoh H, Quan A, Sabongui S, Al-Omran M, Bhatt DL, Mazer CD, Connelly KA, Verma S, Hess DA. The SGLT2 inhibitor empagliflozin reduces mortality and prevents progression in experimental pulmonary hypertension. Biochem Biophys Res Commun. 2020;524:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 161. | Nassif ME, Nguyen D, Spertus JA, Gosch KL, Tang F, Windsor SL, Jones P, Khariton Y, Sauer AJ, Kosiborod MN. Association Between Change in Ambulatory Pulmonary Artery Pressures and Natriuretic Peptides in Patients with Heart Failure: Results From the EMBRACE-HF Trial. J Card Fail. 2023;29:1324-1328. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 162. | Tang L, Cai Q, Wang X, Li X, Li X, Chen L, Yang Y. Canagliflozin ameliorates hypobaric hypoxia-induced pulmonary arterial hypertension by inhibiting pulmonary arterial smooth muscle cell proliferation. Clin Exp Hypertens. 2023;45:2278205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 163. | Seo MS, Jung HS, An JR, Kang M, Heo R, Li H, Han ET, Yang SR, Cho EH, Bae YM, Park WS. Empagliflozin dilates the rabbit aorta by activating PKG and voltage-dependent K(+) channels. Toxicol Appl Pharmacol. 2020;403:115153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 164. | Wu S, Luo X, Chen Y, Wang Z, Liu X, Sun N, Zhao J, Luo W, Zhang J, Tong X, Huang L, Liu C, Qin Z. Sodium-glucose cotransporter 2 inhibitors attenuate vascular calcification by suppressing endoplasmic reticulum protein thioredoxin domain containing 5 dependent osteogenic reprogramming. Redox Biol. 2024;73:103183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 165. | Li J, Li C, Huang Z, Huang C, Liu J, Wu T, Xu S, Mai P, Geng D, Zhou S, Zhang K, Liu Z. Empagliflozin alleviates atherosclerotic calcification by inhibiting osteogenic differentiation of vascular smooth muscle cells. Front Pharmacol. 2023;14:1295463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 166. | Lu CW, Lee CJ, Hsieh YJ, Hsu BG. Empagliflozin Attenuates Vascular Calcification in Mice with Chronic Kidney Disease by Regulating the NFR2/HO-1 Anti-Inflammatory Pathway through AMPK Activation. Int J Mol Sci. 2023;24:10016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 167. | Li XX, Chen ZD, Sun XJ, Yang YQ, Jin H, Liu NF. Empagliflozin ameliorates vascular calcification in diabetic mice through inhibiting Bhlhe40-dependent NLRP3 inflammasome activation. Acta Pharmacol Sin. 2024;45:751-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Reference Citation Analysis (0)] |
| 168. | Woźniak E, Świstek M, Broncel M, Bukowska B, Gorzelak-Pabiś P. The protective effects of empagliflozin on DNA oxidative changes in a model of vascular endothelial and smooth muscle cells damaged by oxidized cholesterol. Biomed Pharmacother. 2024;170:116065. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 169. | Choi S, Haam CE, Byeon S, Oh EY, Choi SK, Lee YH. Investigating the Cardiovascular Benefits of Dapagliflozin: Vasodilatory Effect on Isolated Rat Coronary Arteries. Int J Mol Sci. 2023;24:16873. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 170. | Li H, Shin SE, Seo MS, An JR, Choi IW, Jung WK, Firth AL, Lee DS, Yim MJ, Choi G, Lee JM, Na SH, Park WS. The anti-diabetic drug dapagliflozin induces vasodilation via activation of PKG and Kv channels. Life Sci. 2018;197:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 171. | Li H, Zhao Q, Liu D, Zhou B, Gong C, Zhao G. Dapagliflozin Attenuates NLRP3/Caspase-1 Signaling Pathway-Mediated Pyroptosis of Vascular Smooth Muscle Cells by Downregulating CTSB. Altern Ther Health Med. 2023;29:384-392. [PubMed] |
| 172. | Sukhanov S, Higashi Y, Yoshida T, Mummidi S, Aroor AR, Jeffrey Russell J, Bender SB, DeMarco VG, Chandrasekar B. The SGLT2 inhibitor Empagliflozin attenuates interleukin-17A-induced human aortic smooth muscle cell proliferation and migration by targeting TRAF3IP2/ROS/NLRP3/Caspase-1-dependent IL-1β and IL-18 secretion. Cell Signal. 2021;77:109825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 173. | Moustafa Ahmed Y, Shehata Messiha BA, El-Sayed El-Daly M, Abo-Saif AA. Effects of ticagrelor, empagliflozin and tamoxifen against experimentally-induced vascular reactivity defects in rats in vivo and in vitro. Pharmacol Rep. 2019;71:1034-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 174. | Liu L, Ni YQ, Zhan JK, Liu YS. The Role of SGLT2 Inhibitors in Vascular Aging. Aging Dis. 2021;12:1323-1336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 175. | Chen A, Lan Z, Li L, Xie L, Liu X, Yang X, Wang S, Liang Q, Dong Q, Feng L, Li Y, Ye Y, Fu M, Lu L, Yan J. Sodium-glucose cotransporter 2 inhibitor canagliflozin alleviates vascular calcification through suppression of nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 inflammasome. Cardiovasc Res. 2023;119:2368-2381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 176. | Behnammanesh G, Durante GL, Khanna YP, Peyton KJ, Durante W. Canagliflozin inhibits vascular smooth muscle cell proliferation and migration: Role of heme oxygenase-1. Redox Biol. 2020;32:101527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 177. | Seo MS, An JR, Kang M, Heo R, Park H, Han ET, Han JH, Chun W, Park WS. Mechanisms underlying the vasodilatory effects of canagliflozin in the rabbit thoracic aorta: Involvement of the SERCA pump and Kv channels. Life Sci. 2021;287:120101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 178. | Park M, Mun SY, Zhuang W, Jeong J, Kim HR, Park H, Han ET, Han JH, Chun W, Li H, Park WS. The antidiabetic drug ipragliflozin induces vasorelaxation of rabbit femoral artery by activating a Kv channel, the SERCA pump, and the PKA signaling pathway. Eur J Pharmacol. 2024;972:176589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 179. | Frantz RP. Group 2 pulmonary hypertension: from diagnosis to treatment. Curr Opin Pulm Med. 2023;29:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 180. | Munteanu MA, Swarnkar S, Popescu RI, Lungu A, Ciobotaru L, Nicolae C, Tufanoiu E, Nanea IT. SGLT2 Inhibitor: an Emerging Pillar in Heart Failure Therapeutics? Maedica (Bucur). 2023;18:102-110. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 181. | Satoh T, Wang L, Espinosa-Diez C, Wang B, Hahn SA, Noda K, Rochon ER, Dent MR, Levine AR, Baust JJ, Wyman S, Wu YL, Triantafyllou GA, Tang Y, Reynolds M, Shiva S, Hilaire CS, Gomez D, Goncharov DA, Goncharova EA, Chan SY, Straub AC, Lai YC, McTiernan CF, Gladwin MT. Metabolic Syndrome Mediates ROS-miR-193b-NFYA-Dependent Downregulation of Soluble Guanylate Cyclase and Contributes to Exercise-Induced Pulmonary Hypertension in Heart Failure With Preserved Ejection Fraction. Circulation. 2021;144:615-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 182. | Chen X, Yu X, Lian G, Tang H, Yan Y, Gao G, Huang B, Luo L, Xie L. Canagliflozin inhibits PASMCs proliferation via regulating SGLT1/AMPK signaling and attenuates artery remodeling in MCT-induced pulmonary arterial hypertension. Biomed Pharmacother. 2024;174:116505. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 183. | Mori K, Tsuchiya K, Nakamura S, Miyachi Y, Shiba K, Ogawa Y, Kitamura K. Ipragliflozin-induced adipose expansion inhibits cuff-induced vascular remodeling in mice. Cardiovasc Diabetol. 2019;18:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 184. | Wu J, Liu T, Shi S, Fan Z, Hiram R, Xiong F, Cui B, Su X, Chang R, Zhang W, Yan M, Tang Y, Huang H, Wu G, Huang C. Dapagliflozin reduces the vulnerability of rats with pulmonary arterial hypertension-induced right heart failure to ventricular arrhythmia by restoring calcium handling. Cardiovasc Diabetol. 2022;21:197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 185. | Luo LL, Han JX, Wu SR, Kasim V. Intramuscular injection of sotagliflozin promotes neovascularization in diabetic mice through enhancing skeletal muscle cells paracrine function. Acta Pharmacol Sin. 2022;43:2636-2650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 186. | Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, Grimminger F. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115:2811-2821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 780] [Cited by in RCA: 786] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 187. | Ntokou A, Dave JM, Kauffman AC, Sauler M, Ryu C, Hwa J, Herzog EL, Singh I, Saltzman WM, Greif DM. Macrophage-derived PDGF-B induces muscularization in murine and human pulmonary hypertension. JCI Insight. 2021;6:e139067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 188. | Nassif ME, Qintar M, Windsor SL, Jermyn R, Shavelle DM, Tang F, Lamba S, Bhatt K, Brush J, Civitello A, Gordon R, Jonsson O, Lampert B, Pelzel J, Kosiborod MN. Empagliflozin Effects on Pulmonary Artery Pressure in Patients With Heart Failure: Results From the EMBRACE-HF Trial. Circulation. 2021;143:1673-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 188] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 189. | Alcidi G, Pugliese R, Ioannoni S, Romano M, Palmieri G, Tabella E, Correale M, Di Biase M, Brunetti ND, Iacoviello M. Improvement in Left and Right Ventricular Function after Introduction of SGLT2 Inhibitors in Heart Failure Outpatients with Reduced Ejection Fraction. Clin Pract. 2023;13:1303-1312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 190. | Frisk C, Das S, Eriksson MJ, Walentinsson A, Corbascio M, Hage C, Kumar C, Ekström M, Maret E, Persson H, Linde C, Persson B. Cardiac biopsies reveal differences in transcriptomics between left and right ventricle in patients with or without diagnostic signs of heart failure. Sci Rep. 2024;14:5811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 191. | Alzaabi MA, Abdelsalam A, Alhammadi M, Bani Hani H, Almheiri A, Al Matrooshi N, Al Zaman K. Evaluating Biomarkers as Tools for Early Detection and Prognosis of Heart Failure: A Comprehensive Review. Card Fail Rev. 2024;10:e06. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 192. | Yang Z, Li T, Xian J, Chen J, Huang Y, Zhang Q, Lin X, Lu H, Lin Y. SGLT2 inhibitor dapagliflozin attenuates cardiac fibrosis and inflammation by reverting the HIF-2α signaling pathway in arrhythmogenic cardiomyopathy. FASEB J. 2022;36:e22410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 193. | Qin T, Kong B, Dai C, Xiao Z, Fang J, Shuai W, Huang H. Protective effects of Dapagliflozin on the vulnerability of ventricular arrhythmia in rats with pulmonary artery hypertension induced by monocrotaline. Bioengineered. 2022;13:2697-2709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 194. | Lopez L, Cohen MS, Anderson RH, Redington AN, Nykanen DG, Penny DJ, Deanfield JE, Eidem BW. Unnatural history of the right ventricle in patients with congenitally malformed hearts. Cardiol Young. 2010;20 Suppl 3:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 195. | Axelsen JS, Nielsen-Kudsk AH, Schwab J, Ringgaard S, Nielsen-Kudsk JE, de Man FS, Andersen A, Andersen S. Effects of empagliflozin on right ventricular adaptation to pressure overload. Front Cardiovasc Med. 2023;10:1302265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 196. | Mustapic I, Bakovic D, Susilovic Grabovac Z, Borovac JA. Impact of SGLT2 Inhibitor Therapy on Right Ventricular Function in Patients with Heart Failure and Reduced Ejection Fraction. J Clin Med. 2022;12:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 197. | Fusco F, Scognamiglio G, Sorice D, Abbate M, Altobelli I, Sarubbi B. Biventricular performance in adults with a systemic right ventricle: new insights from myocardial work analysis. Int J Cardiovasc Imaging. 2024;40:1067-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 198. | Agasthi P, Van Houten HK, Yao X, Jain CC, Egbe A, Warnes CA, Miranda WR, Dunlay SM, Stephens EH, Johnson JN, Connolly HM, Burchill LJ. Mortality and Morbidity of Heart Failure Hospitalization in Adult Patients With Congenital Heart Disease. J Am Heart Assoc. 2023;12:e030649. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 199. | Fusco F, Scognamiglio G, Abbate M, Merola A, Grimaldi N, Ciriello GD, Sarubbi B. Dapagliflozin in Patients With a Failing Systemic Right Ventricle: Results From the DAPA-SERVE Trial. JACC Heart Fail. 2024;12:789-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 200. | Clements RT, Vang A, Fernandez-Nicolas A, Kue NR, Mancini TJ, Morrison AR, Mallem K, McCullough DJ, Choudhary G. Treatment of Pulmonary Hypertension With Angiotensin II Receptor Blocker and Neprilysin Inhibitor Sacubitril/Valsartan. Circ Heart Fail. 2019;12:e005819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 201. | Sarak B, Verma S, David Mazer C, Teoh H, Quan A, Gilbert RE, Goodman SG, Bami K, Coelho-Filho OR, Ahooja V, Deva DP, Garg V, Gandhi S, Connelly KA, Yan AT. Impact of empagliflozin on right ventricular parameters and function among patients with type 2 diabetes. Cardiovasc Diabetol. 2021;20:200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 202. | Zhao QH, Chen J, Chen FD, Ruan HY, Zhang W, Zhou YL, Wang QQ, Xu XL, Feng KF, Guo JZ, Gong SG, Zhang RF, Wang L. Evaluating the efficacy and safety of oral triple sequential combination therapy for treating patients with pulmonary arterial hypertension: A multicenter retrospective study. Pulm Circ. 2024;14:e12351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 203. | Chaumais MC, Djessas MRA, Thuillet R, Cumont A, Tu L, Hebert G, Gaignard P, Huertas A, Savale L, Humbert M, Guignabert C. Additive protective effects of sacubitril/valsartan and bosentan on vascular remodelling in experimental pulmonary hypertension. Cardiovasc Res. 2021;117:1391-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 204. | Sanders JL, Koestenberger M, Rosenkranz S, Maron BA. Right ventricular dysfunction and long-term risk of death. Cardiovasc Diagn Ther. 2020;10:1646-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 205. | Bosch L, Lam CSP, Gong L, Chan SP, Sim D, Yeo D, Jaufeerally F, Leong KTG, Ong HY, Ng TP, Richards AM, Arslan F, Ling LH. Right ventricular dysfunction in left-sided heart failure with preserved versus reduced ejection fraction. Eur J Heart Fail. 2017;19:1664-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 251] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 206. | Baandrup JD, Markvardsen LH, Peters CD, Schou UK, Jensen JL, Magnusson NE, Ørntoft TF, Kruhøffer M, Simonsen U. Pressure load: the main factor for altered gene expression in right ventricular hypertrophy in chronic hypoxic rats. PLoS One. 2011;6:e15859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 207. | Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, Carson P, Anand I, Doehner W, Haass M, Komajda M, Miller A, Pehrson S, Teerlink JR, Schnaidt S, Zeller C, Schnee JM, Anker SD. Effect of Empagliflozin on Worsening Heart Failure Events in Patients With Heart Failure and Preserved Ejection Fraction: EMPEROR-Preserved Trial. Circulation. 2021;144:1284-1294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 235] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 208. | Iglesias-Garriz I, Olalla-Gómez C, Garrote C, López-Benito M, Martín J, Alonso D, Rodríguez MA. Contribution of right ventricular dysfunction to heart failure mortality: a meta-analysis. Rev Cardiovasc Med. 2012;13:e62-e69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 209. | Surkova E, Kovács A, Tokodi M, Lakatos BK, Merkely B, Muraru D, Ruocco A, Parati G, Badano LP. Contraction Patterns of the Right Ventricle Associated with Different Degrees of Left Ventricular Systolic Dysfunction. Circ Cardiovasc Imaging. 2021;14:e012774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 210. | Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 921] [Cited by in RCA: 917] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 211. | Damy T, Kallvikbacka-Bennett A, Goode K, Khaleva O, Lewinter C, Hobkirk J, Nikitin NP, Dubois-Randé JL, Hittinger L, Clark AL, Cleland JG. Prevalence of, associations with, and prognostic value of tricuspid annular plane systolic excursion (TAPSE) among out-patients referred for the evaluation of heart failure. J Card Fail. 2012;18:216-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 212. | Dini FL, Carluccio E, Simioniuc A, Biagioli P, Reboldi G, Galeotti GG, Raineri C, Gargani L, Scelsi L, Mandoli GE, Cannito A, Rossi A, Temporelli PL, Ghio S; Network Labs Ultrasound (NEBULA) in Heart Failure Study Group. Right ventricular recovery during follow-up is associated with improved survival in patients with chronic heart failure with reduced ejection fraction. Eur J Heart Fail. 2016;18:1462-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 213. | Linde C, Ekström M, Eriksson MJ, Maret E, Wallén H, Lyngå P, Wedén U, Cabrera C, Löfström U, Stenudd J, Lund LH, Persson B, Persson H, Hage C; Stockholm County/Karolinska Institutet 4D heart failure investigators. Baseline characteristics of 547 new onset heart failure patients in the PREFERS heart failure study. ESC Heart Fail. 2022;9:2125-2138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 214. | Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452-3462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 487] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 215. | Fitchett D, Inzucchi SE, Cannon CP, McGuire DK, Scirica BM, Johansen OE, Sambevski S, Kaspers S, Pfarr E, George JT, Zinman B. Empagliflozin Reduced Mortality and Hospitalization for Heart Failure Across the Spectrum of Cardiovascular Risk in the EMPA-REG OUTCOME Trial. Circulation. 2019;139:1384-1395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 238] [Article Influence: 39.7] [Reference Citation Analysis (0)] |