Published online Sep 26, 2024. doi: 10.4330/wjc.v16.i9.531
Revised: August 28, 2024
Accepted: September 10, 2024
Published online: September 26, 2024
Processing time: 176 Days and 3.5 Hours
The combination of acute ST-segment elevation myocardial infarction (STEMI) and gastric ulcers poses a challenge to primary percutaneous coronary inter
A 54-year-old male patient presented to the emergency department due to chest pain on June 24, 2019. Within the first 3 minutes of the initial assessment in the emergency room, the electrocardiogram (ECG) showed significant changes. There was atrial fibrillation with ST-segment elevation. Subsequently, atrial fibrillation terminated spontaneously and reverted to sinus rhythm. Soon after, the patient experienced syncope. The ECG revealed torsades de pointes ventricular tachycar
A PPCI without stents may be a viable treatment strategy for select patients with STEMI, and further research is warranted.
Core Tip: The focus of this report is the emergency management of a young patient with ST-segment elevation myocardial infarction and a history of gastric ulcers. Coronary angiography revealed near-total occlusion of the proximal left anterior descending artery. Primary percutaneous coronary intervention are likely difficult. After discussion with the patient, our strategy was to inject a novel thrombolytic agent (recombinant human prourokinase) via the intracoronary route to dissolve or clear the local thrombus in the coronary artery. Then, a conventional balloon combined with a cutting balloon is used for adequate pre-expansion. Finally, paclitaxel drug-eluting balloon angioplasty was performed, achieving satisfactory short-term and long-term results.
- Citation: She LQ, Gao DK, Hong L, Tian Y, Wang HZ, Huang S. Intracoronary thrombolysis combined with drug balloon angioplasty in a young ST-segment elevation myocardial infarction patient: A case report. World J Cardiol 2024; 16(9): 531-541
- URL: https://www.wjgnet.com/1949-8462/full/v16/i9/531.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i9.531
The use of drug-coated balloons (DCBs) in treating de novo coronary lesions is controversial, especially in larger vessels[1]. There are limited data on primary percutaneous coronary intervention (PPCI) with DCBs in patients with ST-segment elevation myocardial infarction (STEMI). A novel, emergency stentless intervention strategy involving intracoronary thrombolysis and a combination of conventional and paclitaxel-coated drug balloon angioplasties achieved good short- and long-term results in a young STEMI patient with proximal left anterior descending artery subtotal occlusion and a history of gastric ulcers.
A 54-year-old male patient presented to the emergency department on June 24, 2019, due to recurrent chest pain for 3 days and continuous chest pain for 1 hour.
In the emergency room, the ECG showed significant changes. Atrial fibrillation with coupled premature ventricular contractions was observed, along with ST-segment depression in leads II, III, and AVF and ST-segment elevation in leads V1-V4. Additionally, there were suspicious ST-segment elevations and electrical alternans in leads aVR and aVL. Atrial fibrillation self-terminated very quickly and sinus rhythm was restored accompanied by second-degree type 1 atrioventricular block. Soon after, the patient experienced syncope. The ECG revealed torsades de pointes and ventricular tachycardia. There was spontaneous conversion to a stable sinus rhythm with a heart rate of 82 beats per minute, with ST-segment elevation observed in leads I, AVL, and V1-V5. High-sensitivity troponin I was 22.7 pg/mL.
On January 29, 2018, multiple ulcers were observed in the gastric antrum via fibrogastroscopy. The results of the HP test were positive. The patient was treated with pantoprazole combined with hydrotalcite, etc. There was no history of gastrointestinal bleeding. The patient did not review gastroscopy as directed by the doctor's orders. The patient denied a history of hypertension, diabetes, atrial fibrillation or syncope.
Divorced, with one child. The patient smoked 40 cigarettes per day, on average, for 20 years. A history of alcohol consum
The patient’s temperature was 36.3 °C, pulse velocity was 82 beats/minute, respiration rate was 20 breaths/minute, and blood pressure was 16/8.66 kPa (120/65 mmHg). His weight was 77 kg, and his height was 172 centimeters. Acute painful facial expressions. There was no jugular vein distention. No dry or moist rales were audible in either lung. The cardiac border was normal. No murmurs were detected in any of the valve auscultation areas. No edema was found in either lower extremity. No other special conditions exist.
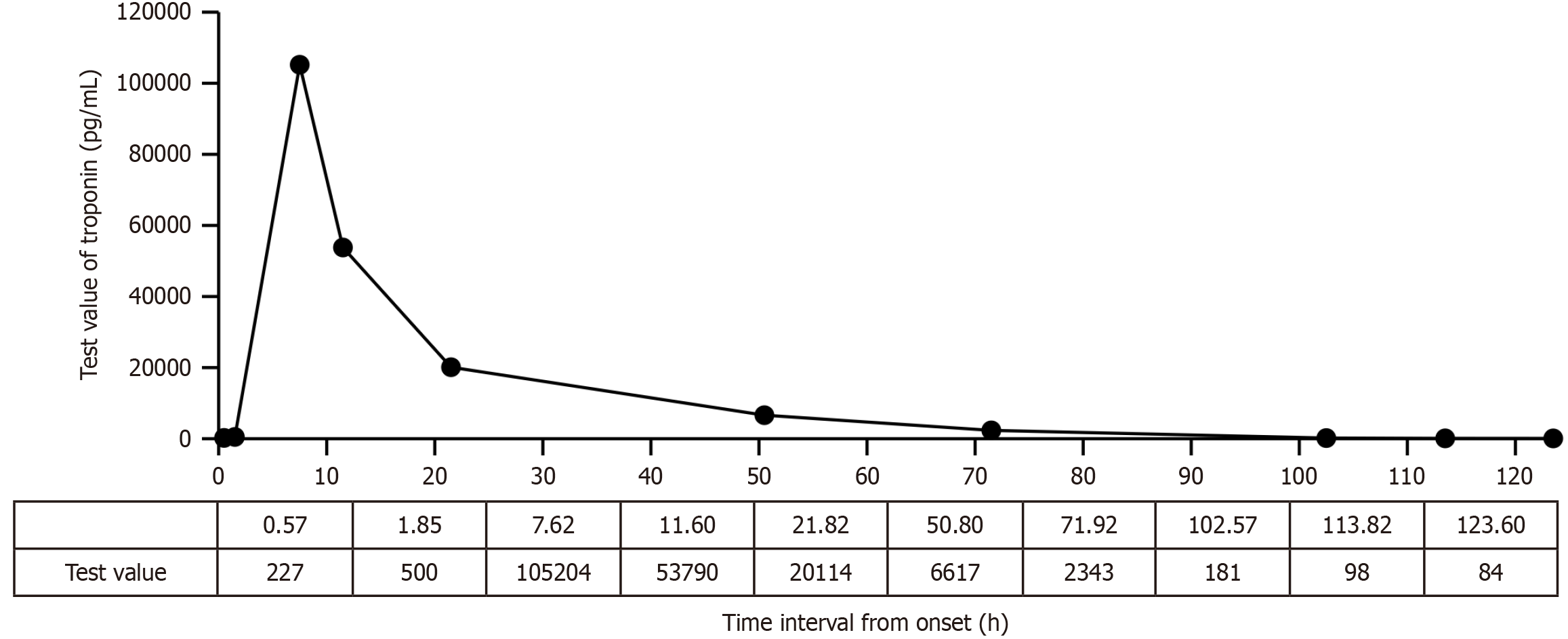
On the day of admission, the following test results were recorded: Plasma D-dimer level, 0.004 µg/mL; NT-proBNP level, 523.2 pg/mL; total cholesterol level, 4.59 mmol/L; triglyceride level, 3.48 mmol/L; high-density lipoprotein cholesterol level, 0.98 mmol/L; low-density lipoprotein cholesterol level, 2.37 mmol/L; potassium level, 3.04 mmol/L; sodium level, 143.7 mmol/L; chloride level, 106.1 mmol/L; total calcium level, 2.24 mmol/L; inorganic phosphorus level, 0.86 mmol/L; magnesium level, 0.92 mmol/L; random blood glucose level, 9.8 mmol/L; white blood cell count, 14.97 × 109/L; red blood cell count, 5.10 × 1012/L; hemoglobin level, 173.0 g/L; platelet level, 174 × 109/L; C-reactive protein level, 0.98 mg/L; urea level, 5.54 mmol/L; creatinine level, 74.6 μmol/L; uric acid level, 290.30 μmol/L; and estimated glomerular filtration rate, 89.64 mL/min/1.73 m2. Liver function and thyroid function were normal. The next day, the electrolyte re-examination was normal, urine glucose was positive (+), and fecal occult blood was weakly positive (±). The peak value of troponin (105204 pg/mL at 7.62 hours after onset) increased (Figure 1).
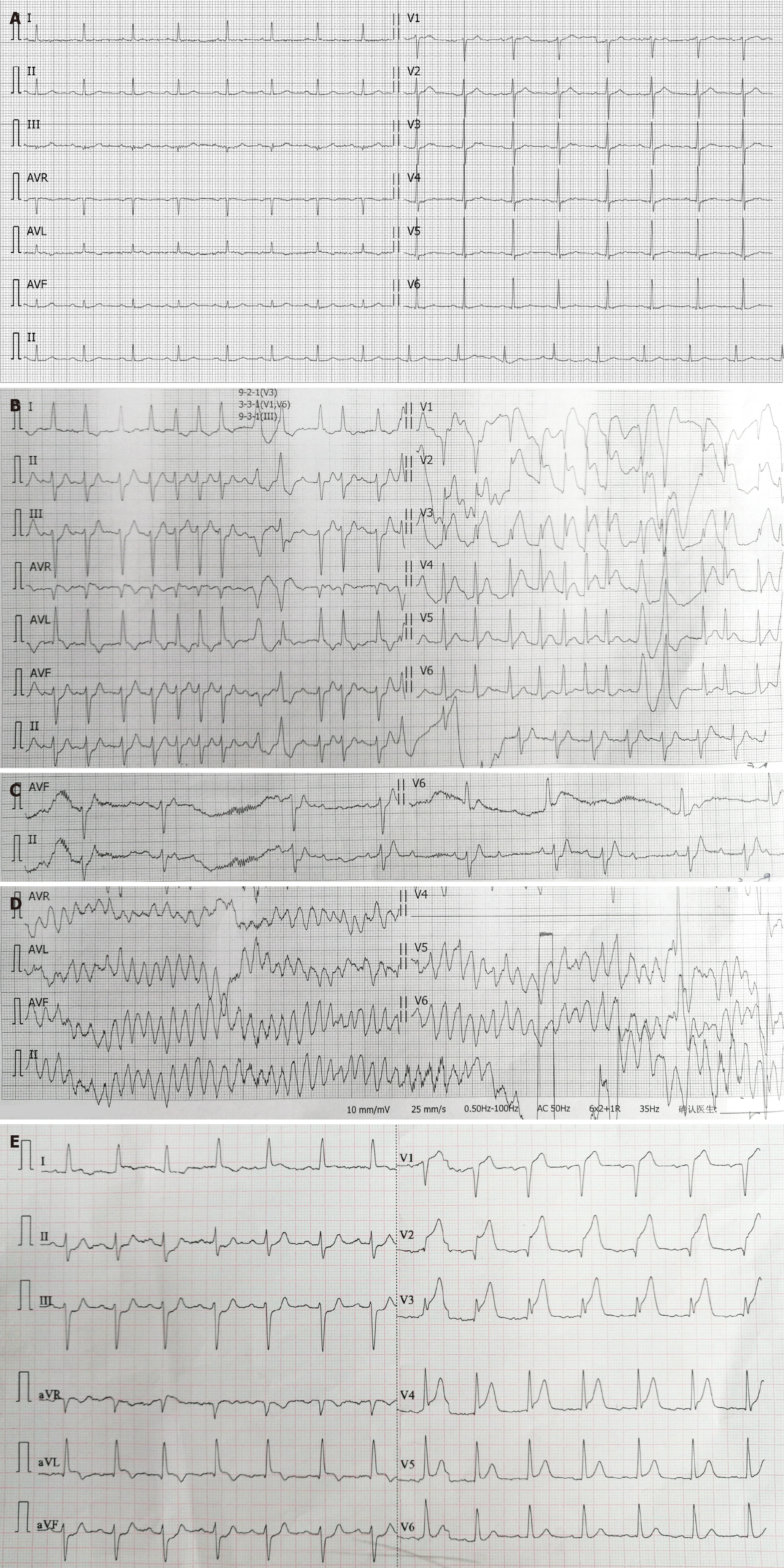
The patient's baseline ECG on January 29, 2018 shows sinus rhythm with a pulse rate of 86 beats per minute. The T waves are flattened in leads I, AVL, and V2 to V6 (Figure 2A). Within the first 3 minutes of the initial assessment in the emergency room, the ECG showed significant changes. Atrial fibrillation with coupled premature ventricular contractions was observed, along with ST-segment depression in leads II, III, and AVF and ST-segment elevation in leads V1-V4. Additionally, there were suspicious ST-segment elevations and electrical alternans in leads aVR and aVL (Figure 2B). The atrial fibrillation self-terminated, and sinus rhythm returned, with second-degree type 1 atrioventricular block (Figure 2C) and torsades de pointes ventricular tachycardia (Figure 2D). There was spontaneous conversion to a stable sinus rhythm with a heart rate of 82 beats per minute, and ST-segment elevation was observed in leads I, AVL, and V1-V5 (Figure 2E).
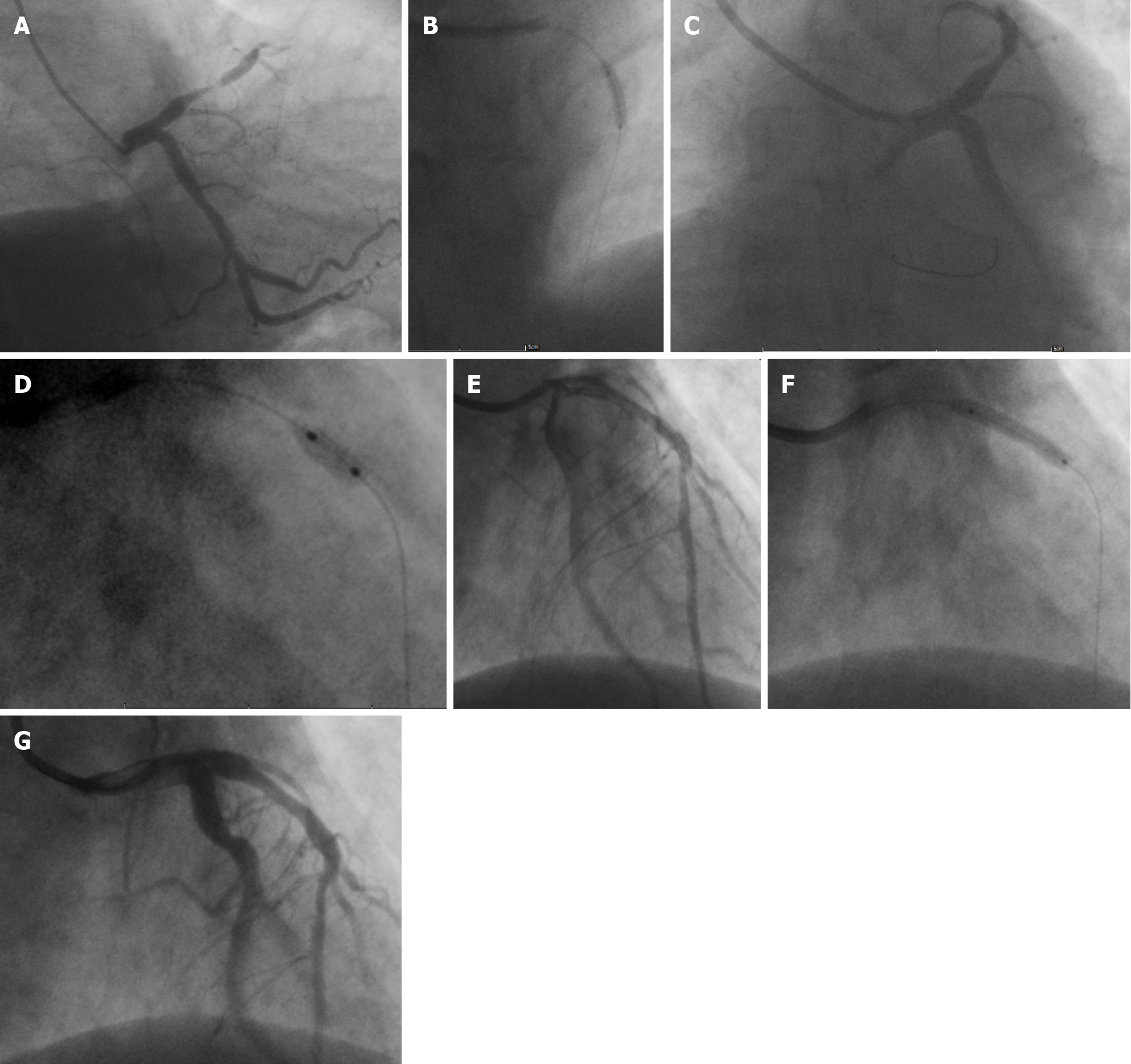
Coronary angiography revealed subtotal occlusion of the proximal segment of the anterior descending artery (Figure 3A). After conventional balloon angioplasty (Figure 3B), the target lesion exhibited elastic retraction with approximately 70% residual stenosis (Figure 3C). Cutting balloon angioplasty (CBA) was performed (Figure 3D), and the postoperative residual stenosis was approximately 20% (Figure 3E). Eventually, drug balloon dilation was administered (Figure 3F), and the postoperative residual stenosis rate was approximately 40% (Figure 3G, Figure 4A).
The chest X-ray on June 27 showed enhanced lung markings in both lungs and a tortuous aorta, and the echocardiogram revealed a slightly enlarged left ventricle (LVDd 53 mm) and decreased left ventricular diastolic function, normal left ventricular systolic function (EF 58.57%).
Based on the patient’s medical history, the alterations noted on the ECG, the variations in the troponin level and the results of coronary angiography, a diagnosis of acute anterolateral STEMI was confirmed.
The patient was administered the following medications: 300 mg of enteric-coated aspirin, 180 mg of ticagrelor, 40 mg of atorvastatin calcium, and 4000 units of intravenous unfractionated heparin.
Systemic heparinization (5000 IU of unfractionated heparin) was performed, and the patient received 20 milligrams of recombinant human prourokinase (rhPro-UK), a thrombolytic agent, via a 5-Fr Tiger (TIG) diagnostic catheter and then underwent predilation with a TREK 2.5 mm × 15 mm balloon for the lesion in the proximal segment of the anterior descending artery. The balloon was inflated to pressures of 12-18 atm for 55-100 seconds to ensure full dilation. However, subsequent imaging revealed significant recoiling at the stenotic site and approximately 70% residual stenosis, indicating the requirement for a cutting balloon (Boston Scientific FlextomeTM Cutting Balloon, 3.0 mm × 6 mm) at pressures of 6-8 atm for 55-100 seconds to reduce the residual stenosis to approximately 20%. Finally, a paclitaxel drug-eluting balloon (PDEB) (QINGZHOU Bingo DEB3020) was inflated to a pressure of 16 atm for 98 seconds. In the end, the residual stenosis was approximately 40%. The immediate net gain was 2.8 mm after pretreatment and 2.3 mm after the procedure, increasing the minimum diameter of the lumen. After each balloon dilation, 200 micrograms of sodium nitroprusside were promptly administered to prevent no-reflow, and the total dose was 1600 micrograms. At the end of the PPCI, there was no significant target vessel dissection, and the TIMI flow was grade 3 (Figure 3G, Figure 4A).
Starting two hours after the PPCI, 0.6 mL of enoxaparin was injected subcutaneously, and then twice a day thereafter. Subsequently, enteric-coated aspirin at 100 mg/d, clopidogrel bisulfate at 75 mg/d, benazepril hydrochloride at 2.5 mg/d, metoprolol tartrate extended release at 12.5 mg/d, and rosuvastatin calcium at 10 mg/d were administered.
Metoprolol tartrate sustained-release tablets at 25 mg/d, and atorvastatin calcium at 10 mg/d were administered. The remaining oral medications were approximately the same as those used during hospitalization.
The patient’s chest pain was significantly relieved after the completion of conventional balloon predilation. The ECG showed a normal rhythm after the operation. No complications, such as arrhythmia, heart failure, or bleeding, were observed. The troponin level peaked earlier. The patient was discharged on the 10th day after the operation.
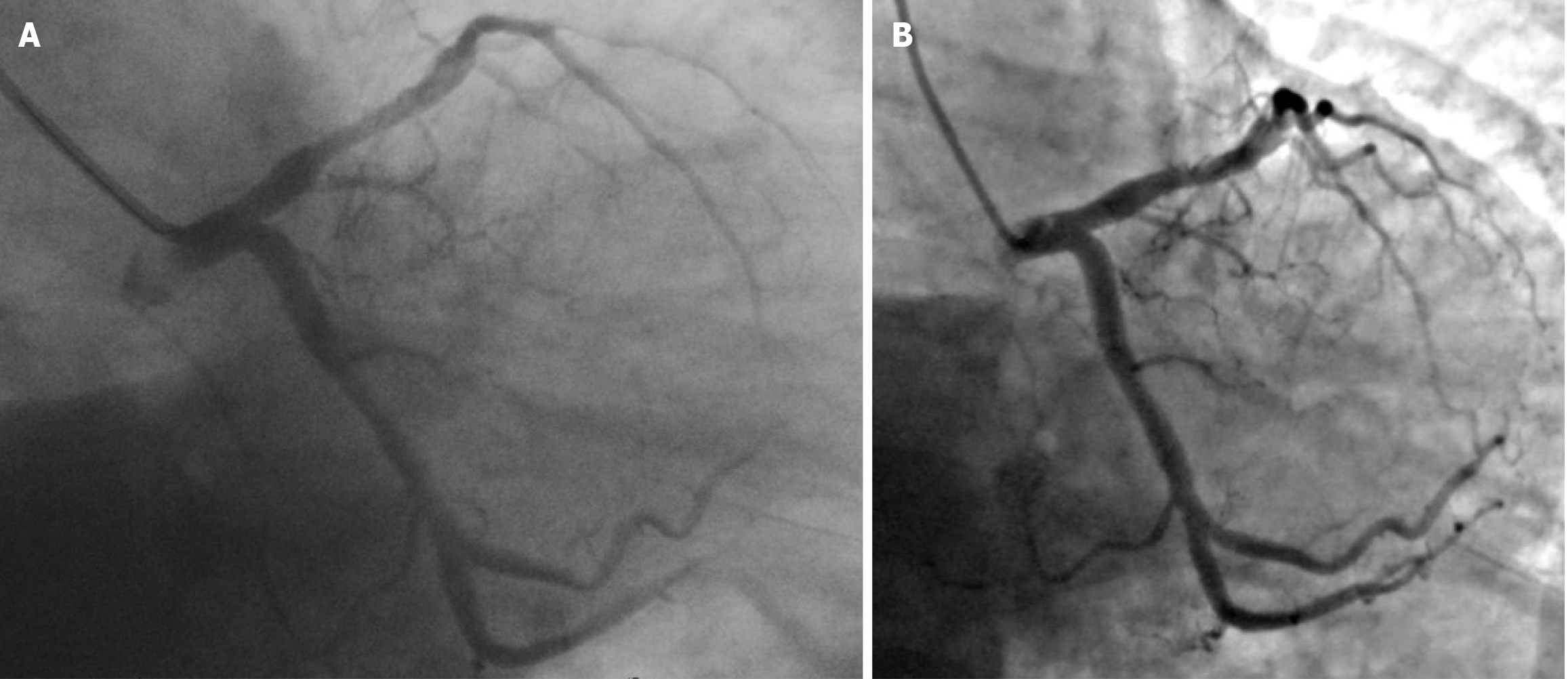
After discharge, the patient took the medicine as prescribed. The patient quit smoking after coronary intervention but resumed smoking 4 months later. Occasionally, there was discomfort in the precordial region. The patient stopped taking the drugs prescribed for coronary heart disease in February 2023. On June 16, 2023, reexamination disclosed a low-density lipoprotein cholesterol level of 1.63 mmol/L. On June 19, the echocardiogram indicated an enlarged left ventricle (LVDd 55 mm) and decreased left ventricular diastolic function, normal left ventricular systolic function (EF 57.73%). At the 4-year follow-up, the residual stenosis of the target lesion was approximately 25%, with a long-term net luminal gain of 3.00 mm, and no adverse events occurred, indicating excellent long-term outcomes (Figure 4B).
Our patient was a 54-year-old individual who necessitated a PPCI. The parameters needed to evaluate the patient’s risk of bleeding due to dual antiplatelet therapy could not be assessed before the operation; however, the patient reported a history of gastric ulcers. For patients with gastric ulcers, the risk of bleeding due to dual antiplatelet therapy increases significantly. We predicted that this patient had a high risk of bleeding. Therefore, after discussion with the patient, we decided to use a stentless technique (Leave Nothing Behind).
In cases of STEMI, the thrombi burden differs. The presence of thrombi limits the contact between the drug coating (paclitaxel) on the surface of the balloon and the vascular endothelium, thus decreasing the efficacy of the DCB, increasing the risk of no-reflow, and ultimately influencing the long-term efficacy of treatment. Therefore, the application of DCBs in the treatment of STEMI patients is limited, and data on the use of DCBs[2-5] in PPCIs are limited. Researchers use DCBs after thrombus aspiration. However, thrombus aspiration cannot improve clinical outcomes[6,7] and may increase the risk of stroke[8]. Therefore, on the basis of conventional dual antiplatelet and anticoagulant therapy, we injected a small dose of a new thrombolytic agent (rhPro-UK) into the target side vessel through a TIG catheter before the percutaneous coronary intervention (PCI) to diminish the thrombus burden.
rhPro-UK is a specific plasminogen activator. In China, it has been approved for the treatment of acute myocardial infarction. rhPro-UK is the precursor of urokinase. rhPro-urokinase is inert in plasma and does not form covalent complexes with protease inhibitors in plasma. It is a non-tissue-type plasminogen activator. Its structure is a single peptide chain with a relatively long half-life, and it can continuously exert thrombolytic effects. At present, there is no guideline or consensus on the optimal method of intracoronary thrombolysis. Judging from limited data (Table 1), the intracoronary administration of a low dose of rhPro-UK is safe and effective and does not increase the risk of bleeding in patients with STEMI undergoing PPCIs. In the literature[9-16], predilated balloons with punctured membranes, microcatheters, mother-child catheters, and aspiration catheters are used to deliver rhPro-UK (10-20 mg) to the distal and proximal segments of the target lesion as well as the target point. These tools are used mostly during the operation (after the guide wire passes through and before stent implantation) (Table 1). This approach seems to be more accurate and efficient. However, the half-life of rhPro-UK is approximately 1.9 hours. In addition to taking effect immediately when the thrombus is in direct contact with the drug, a large part of the drug will inevitably participate in countless systemic circulations. Therefore, there may be no need for "targeted" drug administration. Administering the drug before the PCI instead of after the guidewire has passed through and the balloon has been dilated, is the most time-saving approach. For patients with STEMI, we exclude any contraindications for thrombolysis before the PCI, prepare for intracoronary thrombolysis, and once a high thrombus burden is confirmed during coronary angiography, we start the intracoronary thrombolysis procedure on the target side of the coronary artery and deliver the drug through the TIG catheter. Due to the presence of side holes, some rhPro-UK enters the bloodstream immediately after being injected through the TIG catheter. The TIG catheter allows quick drug delivery, so the onset of action is shortened. Moreover, it may also reduce the risks of thrombus displacement and distal embolization caused by the delivery of guide wires and catheters. When a TIG catheter is used, no additional consumables are needed for drug delivery, and the reperfusion time is shortened. In accordance with our previous application experience, 20 mg of rhPro-UK can be used to reopen acutely occluded blood vessels within 3 minutes at the shortest. Therefore, during the period of preparation for the PCI after coronary angiography is completed, the blood flow in the target vessels of some patients is restored. When the rhPro-UK is utilized in coronary arteries, thrombus aspiration is rarely needed and tirofiban is almost never required. We believe that compared with the other methods in Table 1, the theoretical advantages are obvious, but the short-term and long-term efficacies need to be further clarified by relevant randomized controlled studies.
| Ref. | Infusion route of rhPro-UK | Timing of infusion of rhPro-UK | rhPro-UK dosage (mg) | rhPro-UK input target lesion location | rhPro-UK Input possible additional consumables |
| Wu et al[9] | Thrombus aspiration via a catheter | After thrombus aspiration | 10 | D | Thrombus aspiration |
| Jiang et al[10] | Puncture the balloon catheter membrane with a needle | After balloon catheter dilation | 10 | D | No |
| Geng et al[11] | Puncture the balloon catheter membrane with a needle | After balloon catheter dilation | 10 | D | No |
| Huang et al[12] | Intracoronary catheter | After thrombus aspiration and predilation and before stent implantation | 20 | P | Thrombus aspiration et al |
| Wang et al[13] | Thrombus aspiration via a catheter | After thrombus aspiration | 20 | P | Thrombus aspiration |
| Cao et al[14] | Thrombus aspiration via a catheter | After thrombus aspiration | 10 | T | Thrombus aspiration |
| Ma et al[15] | Microcatheter via a catheter | After thrombus aspiration and before stent implantation | 20 | P | Microcatheter |
| Fu et al[16] | Microcatheter/child-in-mother catheter and/or pierced balloon via a catheter | After the guide wire passes | 10-20+1 | T | Microcatheter/child-in-mother catheter and/or pierced balloon |
In the early stages, a significant drawback of percutaneous transluminal coronary angioplasty (PTCA) was acute occlusion and constrictive remodeling of the target vessel after PCI. Drug-coated balloon angioplasty (DBA) is linked to the same pain points, so comprehensive improvements in drugs, consumables, and techniques are needed. Moreover, researchers have limited experience in using DBA for de novo coronary lesions and large vessel lesions and particularly limited experience in using DBA for treating STEMI, as most cases represent the tentative use of DBA in a small number of patients with a high risk of bleeding. We believe that modern PTCA is significantly different from traditional PTCA. Specifically, alterations in dual antiplatelet drugs and standardized anticoagulation measures have reduced the risk of occlusion caused by thrombosis. However, there remains a risk of acute and chronic occlusion caused by elastic recoil and constrictive remodeling. There are limited measures to decrease this risk. The use of nitrates and calcium channel antagonists may have a preventive effect. Other factors that may induce coronary artery spasm need to be evaded.
CBA can effectively reduce elastic recoil after balloon inflation[17], reduce high-risk dissection and residual stenosis, and lower the target vessel revascularization rate[18], thereby overcoming acute elastic recoil after traditional balloon angioplasty (TBA). Especially in cases of complex lesions such as calcification and fibrosis, CBA may increase the lumen diameter and more effectively restore blood flow. CBA can neatly cut the vascular endothelium and subendothelial tissue, thereby reducing or controlling the progression of dissection and increasing the likelihood that the DCB will pass and therefore make direct contact with the drug on the surface of DCBs and the subendothelial tissue of blood vessels, playing a synergistic role in exerting the effect of DCBs and reducing the long-term risk of restenosis. On the basis of our clinical practice, the results of different PTCA and stentless techniques for different target lesions in the same patient significantly differ. TBA + CBA + DBA is superior to TBA + DBA, which is superior to TBA only.
The application of DCBs in the treatment of de novo coronary artery lesions remains controversial[1], with most studies focusing on the application of such balloons in the treatment of lesions in coronary vessels with diameters less than 2.75 mm. Clinical trials[19] have demonstrated that DCBs are noninferior to drug-eluting stents (DESs) in terms of the incidence of major cardiovascular events within the first 12 months after treatment. However, the safety and efficacy of DCBs in the treatment of new coronary lesions with diameters greater than 3 mm remain unclear[20]. Nakamura et al[21] compared the feasibility of DESs and paclitaxel-coated balloons (PCBs) angioplasty in treating large coronary vessels with de novo coronary lesions [reference vessel diameter (RVD) ≥ 2.75 mm pre- or post-procedure] and reported a late lumen gain of 44.1% in the PCBs group over a 4-year follow-up period.
Herein, we have reported the case of a young patient with STEMI and a history of gastric ulcers. The initial ECG indicated notable instability. For patients with new coronary artery lesions, a high risk of bleeding, and a RVD > 3 mm, a PPCI is regarded as challenging. In particular, whether patients can tolerate long-term dual antiplatelet therapy after surgery is a question that must be considered. After discussion with the patient, we administered a low dose of a third-generation thrombolytic agent (rhPro-UK) to clear the coronary artery thrombus[9,22]. By using a cutting balloon to reduce elastic recoil after conventional predilation of the target vessel combined with PDEB angioplasty, satisfactory short-term and long-term effects were achieved. The minimum diameter of the lumen increased by 2.8 mm before treatment and 2.3 mm after the operation. No complications emerged during or after the operation. Although the incidence of immediate residual stenosis was approximately 40%, the 4-year follow-up revealed good long-term effects, and no late lumen loss occurred[23].
Positive remodeling at the proximal and distal ends of the target lesion, along with negative remodeling of the target lesion itself, resulted in an unnatural and incongruous appearance of the proximal segment of the anterior descending artery after the procedure. Nevertheless, at the 4-year follow-up, significant positive remodeling of the target lesion was observed[24], and mild positive remodeling at both ends made the target lesion and its surrounding areas appear smooth. The vascular lumens at the proximal end of the target lesion, the target point, and the distal end of the target lesion were compared, and the net gains after 4 years were 0.26 mm, 3.00 mm, and 0.17 mm, respectively. The morphology of the target vessel was almost normal (Figure 4). This may be an important reason why the patient stopped taking medicine and smoked again in the later stage without adverse events. Since the patient did not undergo stent implantation and the morphology and function of the target vessel returned to normal, relevant drug treatment can be reduced, thereby reducing the risk of dual antiplatelet therapy required for stents and drug withdrawal in the later stage[25]. Notably, DCBs should not be used in target lesions with significant thrombi in myocardial infarction patients, as doing so may inhibit drug delivery to the vessel wall[26]. We employed rhPro-UK to clear the thrombus in the target lesion as a preparatory step for further treatment. Previously, DCBs were only recommended for the treatment of small vessel lesions, in-stent restenosis, patients at high risk of bleeding, and other special populations. However, more indications have been added[26] despite limited knowledge on the use of DCBs for de novo coronary lesions in large vessels. In cases of elastic recoil or severe dissection, stent implantation was performed[27]. In this case, with the occurrence of elastic recoil after predilation, the residual stenosis was < 30% after a cutting balloon was used. Although elastic recoil recurred after PDEB, the post-procedure residual stenosis was > 30%[26,28], thus alternative interventional treatment should be contemplated. However, in this case, no further intervention was considered. At the 4-year follow-up, stenosis of the target lesion was approximately 25%, indicating a favorable late net increase in the lumen diameter. While the clinical outcomes at 2 years after nonstent DCB angioplasty are excellent for patients with residual stenosis < 50%[3], we believe that patients with 30–50% residual stenosis should undergo functional assessment. Unfortunately, neither FFR nor iFR was measured to further confirm whether residual stenosis was significant[29]. Evidence obtained from our clinical practice shows that standardized DCB treatment is beneficial for bifurcation lesions, large coronary artery PCI, and complex coronary interventions. We expect that precise and functional assessments of the coronary artery can be used to guide DCB treatment, especially for large vessel lesions.
The utilization of a new-generation intracoronary thrombolytic agent for thrombus clearance in target lesions, followed by thorough predilation (including CBA) and PDEB angioplasty, is a feasible PPCI strategy for young STEMI patients at high risk of bleeding (including patients with large vessel lesions). This combination has a synergistic effect and is worthy of further study.
We thank Gang Luo from the Department of Science and Education and the Medical Record Management Department of our hospital for supporting this study in terms of material preparation and other work and Liu Jian from the Department of Medical Laboratory for supporting the revision of the article.
| 1. | Zilio F, Verdoia M, De Angelis MC, Zucchelli F, Borghesi M, Rognoni A, Bonmassari R. Drug Coated Balloon in the Treatment of De Novo Coronary Artery Disease: A Narrative Review. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Nishihira K, Asano Y, Shibata Y. Efficacy of paclitaxel-coated balloon angioplasty combined with intensive lipid-lowering therapy for ST-segment elevation myocardial infarction: insights from near-infrared spectroscopy. Eur Heart J. 2024;45:1576. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 3. | Niehe SR, Vos NS, Van Der Schaaf RJ, Amoroso G, Herrman JR, Patterson MS, Slagboom T, Vink MA. Two-Year Clinical Outcomes of the REVELATION Study: Sustained Safety and Feasibility of Paclitaxel-Coated Balloon Angioplasty Versus Drug-Eluting Stent in Acute Myocardial Infarction. J Invasive Cardiol. 2022;34:E39-E42. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Vos NS, Fagel ND, Amoroso G, Herrman JR, Patterson MS, Piers LH, van der Schaaf RJ, Slagboom T, Vink MA. Paclitaxel-Coated Balloon Angioplasty Versus Drug-Eluting Stent in Acute Myocardial Infarction: The REVELATION Randomized Trial. JACC Cardiovasc Interv. 2019;12:1691-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 137] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 5. | Vos NS, van der Schaaf RJ, Amoroso G, Herrman JP, Patterson MS, Slagboom T, Vink MA. REVascularization with paclitaxEL-coated balloon angioplasty versus drug-eluting stenting in acute myocardial infarcTION-A randomized controlled trial: Rationale and design of the REVELATION trial. Catheter Cardiovasc Interv. 2016;87:1213-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Jolly SS, James S, Džavík V, Cairns JA, Mahmoud KD, Zijlstra F, Yusuf S, Olivecrona GK, Renlund H, Gao P, Lagerqvist B, Alazzoni A, Kedev S, Stankovic G, Meeks B, Frøbert O. Thrombus Aspiration in ST-Segment-Elevation Myocardial Infarction: An Individual Patient Meta-Analysis: Thrombectomy Trialists Collaboration. Circulation. 2017;135:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 221] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 7. | Lagerqvist B, Fröbert O, Olivecrona GK, Gudnason T, Maeng M, Alström P, Andersson J, Calais F, Carlsson J, Collste O, Götberg M, Hårdhammar P, Ioanes D, Kallryd A, Linder R, Lundin A, Odenstedt J, Omerovic E, Puskar V, Tödt T, Zelleroth E, Östlund O, James SK. Outcomes 1 year after thrombus aspiration for myocardial infarction. N Engl J Med. 2014;371:1111-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 276] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 8. | Jolly SS, Cairns JA, Yusuf S, Rokoss MJ, Gao P, Meeks B, Kedev S, Stankovic G, Moreno R, Gershlick A, Chowdhary S, Lavi S, Niemela K, Bernat I, Cantor WJ, Cheema AN, Steg PG, Welsh RC, Sheth T, Bertrand OF, Avezum A, Bhindi R, Natarajan MK, Horak D, Leung RC, Kassam S, Rao SV, El-Omar M, Mehta SR, Velianou JL, Pancholy S, Džavík V; TOTAL Investigators. Outcomes after thrombus aspiration for ST elevation myocardial infarction: 1-year follow-up of the prospective randomised TOTAL trial. Lancet. 2016;387:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 157] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 9. | Wu Y, Fu X, Feng Q, Gu X, Hao G, Fan W, Jiang Y. Efficacy and safety of intracoronary prourokinase during percutaneous coronary intervention in treating ST-segment elevation myocardial infarction patients: a randomized, controlled study. BMC Cardiovasc Disord. 2020;20:308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Jiang W, Xiong X, Du X, Ma H, Li W, Cheng F. Safety and efficacy study of prourokinase injection during primary percutaneous coronary intervention in acute ST-segment elevation myocardial infarction. Coron Artery Dis. 2021;32:25-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Geng W, Zhang Q, Liu J, Tian X, Zhen L, Song D, Yang Y, Meng H, Wang Y, Chen J. A randomized study of prourokinase during primary percutaneous coronary intervention in acute ST-segment elevation myocardial infarction. J Interv Cardiol. 2018;31:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 12. | Huang D, Qian J, Liu Z, Xu Y, Zhao X, Qiao Z, Fang W, Jiang L, Hu W, Shen C, Liang C, Zhang Q, Ge J. Effects of Intracoronary Pro-urokinase or Tirofiban on Coronary Flow During Primary Percutaneous Coronary Intervention for Acute Myocardial Infarction: A Multi-Center, Placebo-Controlled, Single-Blind, Randomized Clinical Trial. Front Cardiovasc Med. 2021;8:710994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Wang X, Liu H, Wu H, Xiao Y, Bai S, Li X, Li X, Zhang L, Chen T, Li H, Liu J, Du R. Safety and efficacy of intracoronary prourokinase administration in patients with high thrombus burden. Coron Artery Dis. 2020;31:493-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Cao M, Wang Z, Meng X, Xu Z, Gao J, Zhu W, Yu S, Zhang H. Effects of intracoronary low-dose prourokinase administration on ST-segment elevation in patients with myocardial infarction and a high thrombus burden: a randomized controlled trial. J Int Med Res. 2022;50:3000605221139723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Ma FH, Qiao ZY. [Effect of thrombus aspiration combined with microcatheter targeted application of recombinant human prourokinase on myocardial blood perfusion in patients with ST elevated acute myocardial infarction]. Linchuang Xinxueguanbing Zazhi. 2020;36:1088-1092. [DOI] [Full Text] |
| 16. | Fu Y, Gu XS, Hao GZ, Jiang YF, Fan WZ, Fan YM, Wei QM, Fu XH, Li YJ. Comparison of myocardial microcirculatory perfusion after catheter-administered intracoronary thrombolysis with anisodamine versus standard thrombus aspiration in patients with ST-elevation myocardial infarction. Catheter Cardiovasc Interv. 2019;93:839-845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Li B, Ding Y, Tian F, Chen W, Han T, Chen Y. Assessment of a Drug-Eluting Balloon for the Treatment of de novo Coronary Lesions Guided by Optical Coherence Tomography: Study Protocol for a Randomized Controlled Trial. Cardiology. 2017;136:252-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Izumi M, Tsuchikane E, Funamoto M, Kobayashi T, Sumitsuji S, Otsuji S, Sakurai M, Kobayashi T, Awata N. Final results of the CAPAS trial. Am Heart J. 2001;142:782-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Jeger RV, Farah A, Ohlow MA, Mangner N, Möbius-Winkler S, Leibundgut G, Weilenmann D, Wöhrle J, Richter S, Schreiber M, Mahfoud F, Linke A, Stephan FP, Mueller C, Rickenbacher P, Coslovsky M, Gilgen N, Osswald S, Kaiser C, Scheller B; BASKET-SMALL 2 Investigators. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): an open-label randomised non-inferiority trial. Lancet. 2018;392:849-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 306] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 20. | Rosenberg M, Waliszewski M, Krackhardt F, Chin K, Wan Ahmad WA, Caramanno G, Milazzo D, Nuruddin AA, Liew HB, Maskon O, Bento A, Macia JC, Frey N. Drug Coated Balloon-Only Strategy in De Novo Lesions of Large Coronary Vessels. J Interv Cardiol. 2019;2019:6548696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Nakamura H, Ishikawa T, Mizutani Y, Yamada K, Ukaji T, Kondo Y, Shimura M, Aoki H, Hisauchi I, Itabashi Y, Nakahara S, Kobayashi S, Taguchi I. Clinical and Angiographic Outcomes of Elective Paclitaxel-Coated Balloon Angioplasty in Comparison with Drug-Eluting Stents for De Novo Coronary Lesions in Large Vessels. Int Heart J. 2023;64:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Fan G, Wu XG, Jiao WP, Zhang HK, Guo DL. Safety and efficacy of intracoronary recombinant human prourokinase administration in patients with acute myocardial infarction and STsegment elevation: A metaanalysis of randomized controlled trials. Exp Ther Med. 2023;25:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Wang Z, Yin Y, Li J, Qi W, Yu B, Xu Z, Zhu W, Yang F, Cao M, Zhang H. New Ultrasound-Controlled Paclitaxel Releasing Balloon vs. Asymmetric Drug-Eluting Stent in Primary ST-Segment Elevation Myocardial Infarction - A Prospective Randomized Trial. Circ J. 2022;86:642-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Her AY, Ann SH, Singh GB, Kim YH, Okamura T, Garg S, Koo BK, Shin ES. Serial Morphological Changes of Side-Branch Ostium after Paclitaxel-Coated Balloon Treatment of De Novo Coronary Lesions of Main Vessels. Yonsei Med J. 2016;57:606-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Elgendy IY, Gad MM, Elgendy AY, Mahmoud A, Mahmoud AN, Cuesta J, Rivero F, Alfonso F. Clinical and Angiographic Outcomes With Drug-Coated Balloons for De Novo Coronary Lesions: A Meta-Analysis of Randomized Clinical Trials. J Am Heart Assoc. 2020;9:e016224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Jeger RV, Eccleshall S, Wan Ahmad WA, Ge J, Poerner TC, Shin ES, Alfonso F, Latib A, Ong PJ, Rissanen TT, Saucedo J, Scheller B, Kleber FX; International DCB Consensus Group. Drug-Coated Balloons for Coronary Artery Disease: Third Report of the International DCB Consensus Group. JACC Cardiovasc Interv. 2020;13:1391-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 312] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 27. | Kleber FX, Rittger H, Bonaventura K, Zeymer U, Wöhrle J, Jeger R, Levenson B, Möbius-Winkler S, Bruch L, Fischer D, Hengstenberg C, Pörner T, Mathey D, Scheller B. Drug-coated balloons for treatment of coronary artery disease: updated recommendations from a consensus group. Clin Res Cardiol. 2013;102:785-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 28. | Cox DA, Stone GW, Grines CL, Stuckey T, Cohen DJ, Tcheng JE, Garcia E, Guagliumi G, Iwaoka RS, Fahy M, Turco M, Lansky AJ, Griffin JJ, Mehran R; CADILLAC Investigators. Outcomes of optimal or "stent-like"balloon angioplasty in acutemyocardial infarction: the CADILLAC trial. J Am Coll Cardiol. 2003;42:971-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Chung JH, Shin ES, Her AY, Lee JM, Doh JH, Nam CW, Koo BK. Instantaneous wave-free ratio-guided paclitaxel-coated balloon treatment for de novo coronary lesions. Int J Cardiovasc Imaging. 2020;36:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |