Published online Aug 26, 2024. doi: 10.4330/wjc.v16.i8.469
Revised: June 24, 2024
Accepted: July 23, 2024
Published online: August 26, 2024
Processing time: 112 Days and 3.2 Hours
Mesenchymal stem cells (MSCs), as living biodrugs, have entered advanced phases of clinical assessment for cardiac function restoration in patients with myocardial infarction and heart failure. While MSCs are available from diverse tissue sources, bone-marrow-derived MSCs (BM-MSCs) remain the most well-studied cell type, besides umbilical-cord-derived MSCs (UC-MSCs). The latter offers advantages, including noninvasive availability without ethical considerations.
To compare the safety and efficacy of BM-MSCs and UC-MSCs in terms of left ventricular ejection fraction (LVEF), 6-min walking distance (6MWD), and major adverse cardiac events (MACEs).
Five databases were systematically searched to identify randomized controlled trials (RCTs). Thirteen RCTs (693 patients) were included using predefined eligibi
UC-MSCs significantly improved LVEF vs controls by 5.08% [95% confidence interval (CI): 2.20%-7.95%] at 6 mo and 2.78% (95%CI: 0.86%-4.70%) at 12 mo. However, no significant effect was observed for BM-MSCs vs controls. No significant changes were observed in the 6MWD with either of the two cell types. Also, no differences were observed for MACEs, except rehospitalization rates, which were lower only with BM-MSCs (odds ratio 0.48, 95%CI: 0.24-0.97) vs controls.
UC-MSCs significantly improved LVEF compared with BM-MSCs. Their advantageous characteristics position them as a promising alternative to MSC-based therapy.
Core Tip: Mesenchymal stem cells (MSCs) are fast emerging as living biodrugs to repair and replace dysfunctional myo
- Citation: Safwan M, Bourgleh MS, Aldoush M, Haider KH. Tissue-source effect on mesenchymal stem cells as living biodrugs for heart failure: Systematic review and meta-analysis. World J Cardiol 2024; 16(8): 469-483
- URL: https://www.wjgnet.com/1949-8462/full/v16/i8/469.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i8.469
Cardiovascular diseases (CVDs) remain the most common cause of morbidity and mortality worldwide despite recent advances in pharmacological disease management[1]. The akinetic fibrous scar that develops as part of the inefficient intrinsic repair mechanism in the heart after recurrent myocardial infarction (MI) is one of the critical factors responsible for putting the heart into the vicious cycle of remodeling, leading to heart failure (HF). Most contemporary therapeutic options, at best, can only provide symptomatic relief. In this regard, mesenchymal stem cell (MSC)-based therapy to repair and replace dysfunctional myocardium is fast emerging as a viable option and has progressed to advanced phases of clinical assessment[2].
MSCs were identified as a unique cell group characterized by preferential plastic surface adherence, specific surface marker expression, and trilineage differentiation potential[3]. They showed high proliferation and exceptional abilities to generate proangiogenic and anti-inflammatory paracrine factors[4]. Preclinical studies have demonstrated that MSCs possess a nonimmunogenic phenotype and the capacity to evade immunosurveillance[5]. These characteristics render them a choice for a cell-based therapy approach, and they are being reckoned as prototypes of the living biodrug family with some products already approved for different clinical conditions, such as Prochymal and Lomecel.
While MSCs are available from diverse adult and fetal tissues[6], bone-marrow-derived MSCs (BM-MSCs; adult tissue source) and umbilical-cord-derived (UC-MSCs; fetal tissue source) remain the most well-studied types during recent clinical trials. As of April 20, 2024, 59 clinical trials assessing MSCs for cardiac disease are registered on ClinicalTrials.gov, with 25 focusing explicitly on BM-MSCs. Nevertheless, most of these studies have reported less-than-expected results than the preclinical, experimental data[7]. The modest outcome may be attributed to various confounding factors, encompassing treatment-related factors, such as route of administration and cell dose [8,9], to the quality of cell preparation, such as donor age and health status[10,11]. However, UC-MSCs are readily accessible from medical waste for clinical applications without moral and ethical concerns[12]. Since the first reports of UC-MSCs, they have been extensively studied in experimental animal models of myocardial injury[13,14]. UC-MSCs have recently garnered more attention in clinical settings because of their advantages, including ready-to-use “off-the-shelf” availability, noninvasive collection, lack of ethical issues, younger age origin, and embryonic-cell-like characteristics[12,15,16]. Building upon near-ideal features and promising preclinical data, UC-MSCs have recently advanced to phase II pivotal trials for heart therapy.
We have conducted a rigorous systematic review comparing the clinical performance of BM-MSCs with UC-MSCs, which may be crucial to establishing a more reliable guide for designing future MSCs-based clinical trials. The primary aim of this systematic review and meta-analysis was to evaluate the comparative safety and effectiveness of BM-MSC- and UC-MSC-based therapy in HF patients by analyzing left ventricular ejection fraction (LVEF), 6-min walking distance (6MWD) as the primary functional and clinical outcomes. We examined the safety profile of the two cell types using the major adverse cardiovascular events (MACEs), i.e., cardiac death, rehospitalization for HF, recurrent MI, infract-vessel revascularization procedure, arrhythmias, and stroke as the secondary outcomes.
A search strategy was conducted to identify relevant trials based on the Preferred Reporting Item for Systematic Reviews and Meta-Analysis guidelines[17]. The protocol was registered at the International Prospective Register of Systematics Reviews (PROSPERO; CRD4202348206). Our search strategy encompassed PubMed, Cochrane database, ClinicalTrials.
To be eligible for inclusion, a study met the following criteria: (1) It should be a phase I/II randomized controlled clinical trial that investigates the efficacy and safety of UC-MSCs and BM-MSCs; (2) The study involved patients diagnosed with MI, HF, or cardiomyopathy; (3) The intervention group should be treated with UC-MSCs or BM-MSCs; (4) There should be a control group; (5) The study should report at least one of the following clinical outcomes: LVEF, 6MWD test, death, readmission for HF, and MACEs (arrhythmia, recurrent MI, and stroke); and (6) The follow-up duration should be > 6 mo. Only studies that met the inclusion criteria and were complete or had available full text were included. All other randomized controlled trials (RCTs) were excluded from the study.
Three coauthors independently assessed the eligibility of the studies for meta-analysis using the inclusion/exclusion criteria and a predefined data-extraction sheet. Each included study was examined, and the following variables were extracted: (1) First author; (2) year of publication; (3) trial location (country); (4) intervention type (BM-MSCs or UC-MSCs); (5) source of stem cells (autologous vs allogenic); (6) sample size; (7) sex; (8) mean sample age; (9) comorbidities; (10) follow-up period for key endpoint measurements; (11) dose (number of cells transferred in millions); (12) cell delivery mode (e.g., intravenous, intramyocardial, or intracoronary infusion); (13) cell status (fresh or frozen); (14) New York Heart Association classification of study participants at baseline; (15) study end-point assessment method/tools
The methodological quality of the included RCTs was evaluated using the Cochrane Collaboration tool, which assessed the risk of bias based on the following criteria: sequence generation randomness, allocation concealment, blinding of participants and outcome assessors, incomplete outcome data, selective reporting, and other potential sources of bias. Each study was categorized as having low risk, high risk, or unclear risk of bias for each criterion. The overall risk of bias was determined by considering all the criteria and presenting it as a risk of bias graph.
Two intervention groups from one of the UC-MSC trials were combined into one group[18]. One group received human UC-MSCs (hUC-MSCs) encapsulated in a collagen hydrogel, while the other received only hUC-MSCs. We calculated and presented fixed-effects Peto odds ratios (OR) with 95% confidence interval (CI) for dichotomous data of adverse events, including death, MACEs, and rehospitalization. We chose the Peto OR method due to the anticipated rarity of adverse events reported across the included studies[19]. This method adds a continuity correction factor of 0.5 for any cells containing zero events, allowing for better estimating rare events. We calculated random-effect mean difference pooled effects for continuous data, presented with 95%CI. This included the change in LVEF and 6MWD from baseline to 6 and 12 mo of follow-up. We used a random-effect model due to expected differences in the study samples and countries. We conducted a weighted mean difference (WMD) meta-analysis as LVEF and 6MWD were reported in the same units across the studies (i.e., percentages and meters).
For continuous outcomes, the data reported in CIs or SE were converted to SD using the Cochrane Handbook equations[20]. When examining the mean ± SD difference from baseline to 6 and 12 mo of follow-up, we found that only one of the UC-MSCs studies[21] provided the mean ± SD change from baseline values. To ascribe the missing change in SD for LVEF, we applied a correlation coefficient of 0.65 derived from the study by Gao et al[21] as recommended by the Cochrane Handbook[20,21]. None of the UC-MSCs studies reported the change in mean ± SD for 6MWD; hence, we used a conservative value of 0 as the correlation coefficient to calculate the change in SD[22]. For the BM-MSCs studies that did not report the change in mean ± SD, we used a correlation coefficient value of 0.75 derived from Bolli et al[23] for both LVEF and 6MWD changes in SD calculations.
Subgroup analysis was conducted on studies involving BM-MSCs to explore the impact of patients’ conditions on the significance of the pooled effects of our primary outcome, LVEF. The analysis focused on two subgroups: HF and MI. However, the same subgrouping could not be performed on the UC-MSCs studies due to the limited number of studies available. Only one study focused on MI patients, while the remaining focused on HF patients.
A new methodological approach was implemented to compare MSCs from the two tissue sources and mitigate any potential overestimation of the effect of the control arm in some studies compared to others. The data from the control arm across all included RCTs were consolidated to derive a unified mean (± SD). Using a similar strategy, the intervention arms (UC-MSCs and BM-MSCs) were analyzed, combining the means (± SDs) reported in the relevant RCTs for each cell type into a single combined mean (± SD). Subsequently, the combined mean (± SD) for each cell type was compared with the unified control, providing insights into the performance of both cell types in the unified control group. This methodology facilitated a comprehensive evaluation of the effectiveness of UC-MSCs and BM-MSCs compared to the same established control. All calculations used were according to the formulas provided by the Cochrane Handbook[20].
The risk of publication bias was assessed by creating funnel plots of our primary functional outcome, LVEF, in UC-MSCs and BM-MSCs studies. We then evaluated asymmetry, indicating the publication bias.
Statistical heterogeneity was assessed using the I² statistic. I² < 25% was considered unimportant. A 25%-75% value indicated moderate heterogeneity, and 75%-100% considerable heterogeneity[20]. All statistical data analysis was performed using RevMan 5.4.1 software[24]. P ≤ 0.05 was considered statistically significant.
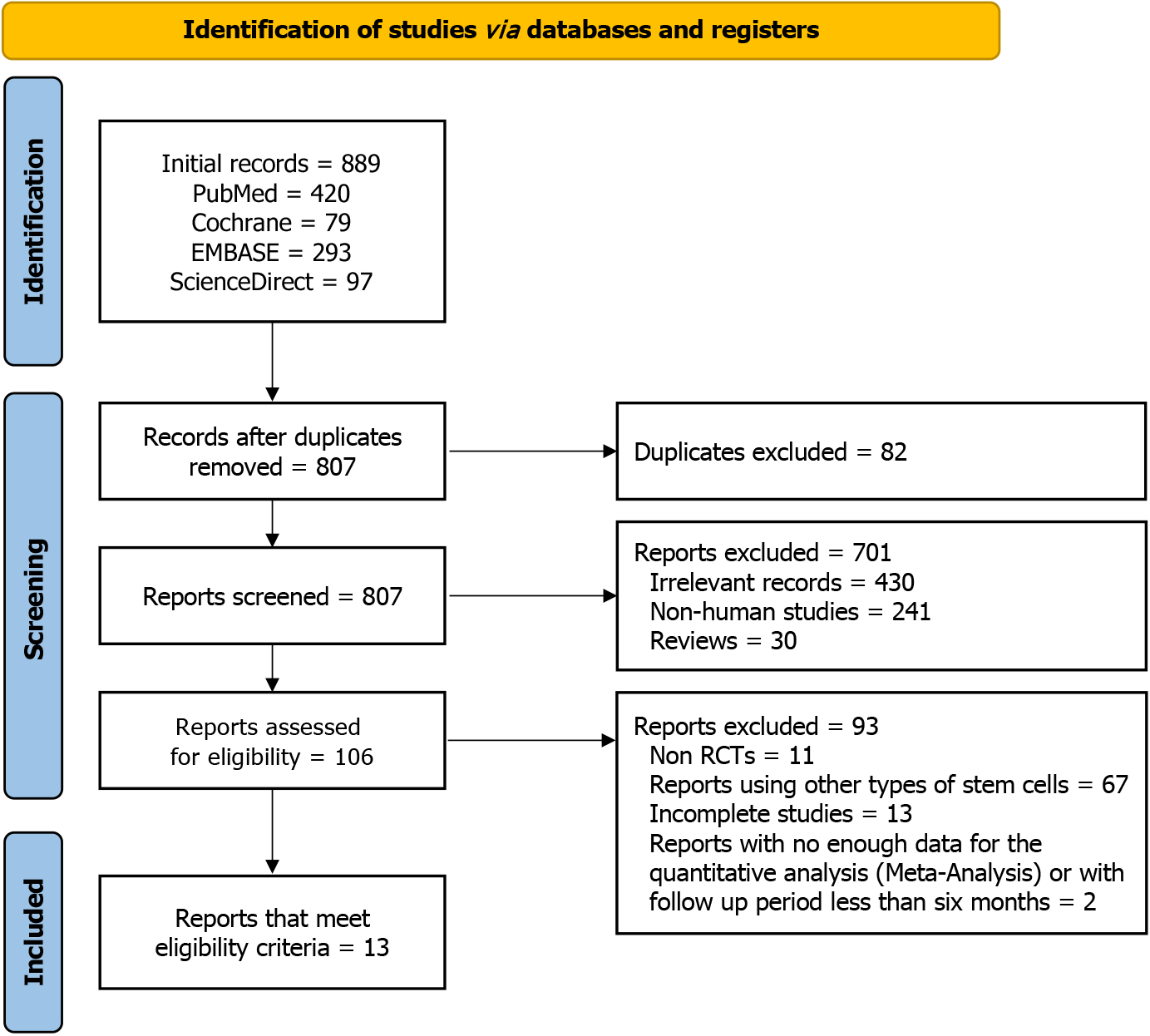
Figure 1 summarizes the process of including studies for the meta-analysis. The literature search across multiple databases yielded 807 potentially relevant studies. Title and abstract screening retained 106 studies, of which 93 were excluded for reasons given in Figure 1, leaving 13 eligible RCTs for analysis.
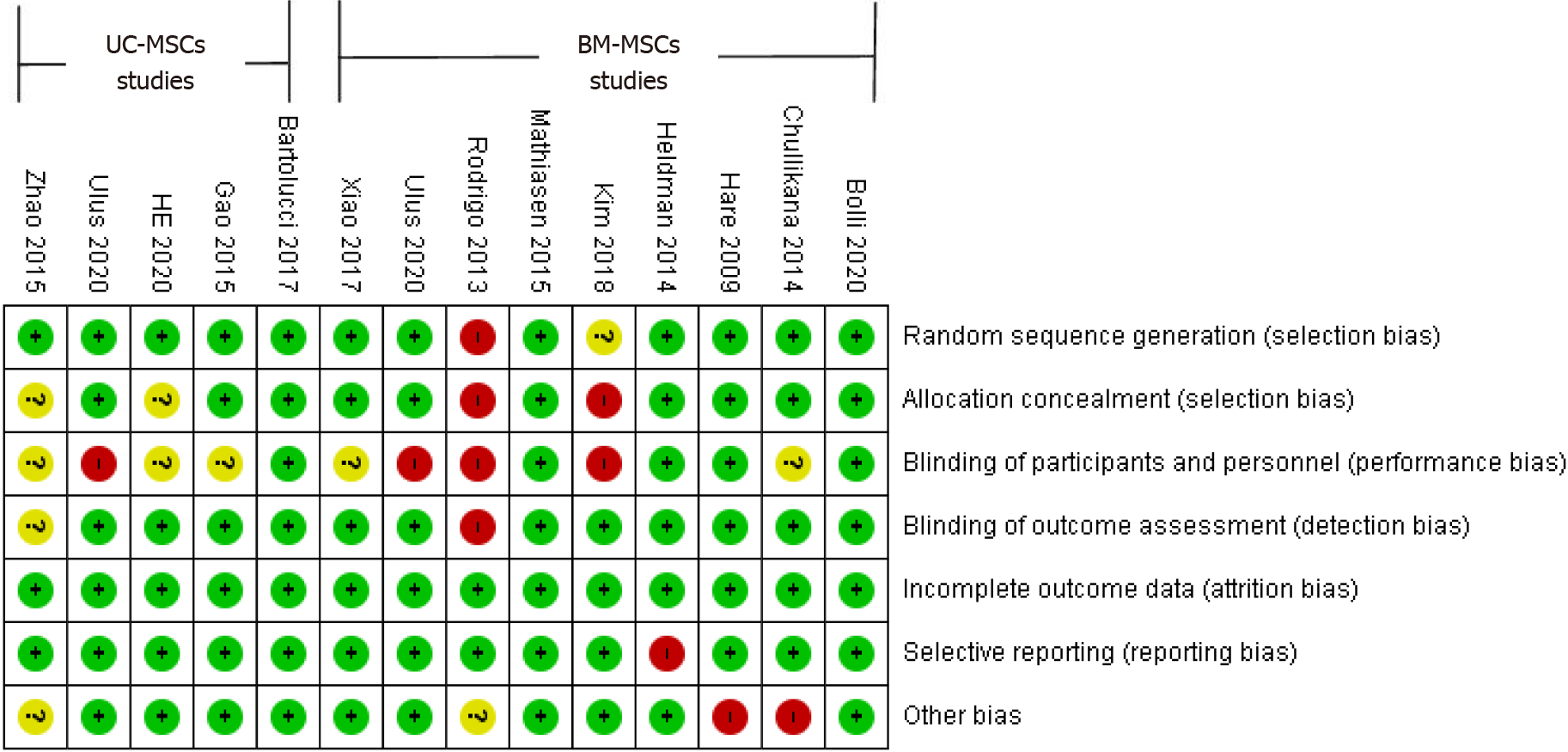
The risk of bias included in the studies was assessed using the Cochrane Collaboration risk-of-bias tool. The studies were evaluated for selection, performance, detection, attrition, and reporting biases. A risk of bias graph was generated to present the review authors’ judgments for each domain in the included studies (Figure 2).
Details of the characteristics of included trials are presented in Tables 1, 2 and 3 for UC-MSCs-based and BM-MSCs-based trials, respectively. The 13 RCTs used for meta-analysis spanned from 2009 to 2020, including four RCTs evaluating UC-MSCs, eight RCTs assessing BM-MSCs, and one RCT utilizing both cell types[25]. Locations of the RCTs were Türkiye[25], China[18,22,26,27], Chile[28], India[29], USA[24,30], Denmark[31], Netherlands[32], and South Korea[33].
| Gao et al[21], 2015 (China) | He et al[18], 2020 (China) | Zhao et al[26], 2015 (China) | Bartolucci et al[28], 2017 (Chile) | Ulus et al[25], 2020 (Türkiye) | ||
| Study type | RCT | RCT | RCT | RCT | Open-label RCT | |
| Phase | I/II | I | I/II | I/II | I/II | |
| Sample size | Total | 116 | 50 | 59 | 30 | 41 |
| Intervention (male) | 58 (94.8) | 32 (78.12) | 30 (80.0) | 15 (80.0) | 25 (100) | |
| Control (male) | 58 (87.9) | 12 (58.30) | 29 (65.5) | 15 (93.3) | 16 (100) | |
| Mean age (mean ± SD) | Intervention | 57.3 ± 9.90 | 59.6 (7.9)/63.6 (8.6) | 52.90 ± 16.32 | 57.33 ± 10.05 | 61.8 ± 10 |
| Control | 56.7 ± 12.95 | 65.2 (7.9) | 53.21 ± 11.46 | 57.20 ± 11.64 | 65.3 ± 6.8 | |
| Mean BMI (mean ± SD) | Intervention | 24.9 ± 2.28 | 25.5 ± 3.3 /24.4 ± 3.3 | N/A | 29.12 ± 2.88 | 26.5 ± 4.5 |
| Control | 25.4 ± 2.28 | 23.59 ± 2.28 | N/A | 29.52 ± 4.00 | 26.6 ± 4.8 | |
| Number of smokers | Intervention | 34 (58.6) | 4 (25.0)/7 (43.8) | N/A | 7 (46.7) | 21 (84) |
| Control | 32 (55.2) | 3 (25.0) | N/A | 4 (26.7) | 15 (88.2) | |
| HTN | Intervention | 33 (56.9) | 10 (62.5)/14 (87.5) | N/A | 7 (46.7) | 15 (60) |
| Control | 26 (44.8) | 9 (75.0) | N/A | 8 (53.3) | 11 (64.7) | |
| DM | Intervention | 17 (29.3) | 8 (50.0)/4 (25.0) | N/A | 5 (33.3) | 16 (66.7) |
| Control | 14 (24.1) | 8 (66.7) | N/A | 7 (46.7) | 9 (52.9) | |
| NYHA; I (n), II (n), III (n), IV (n) | Intervention | N/A | III (4 / 8), IV (12 / 8) | N/A | 2.03 ± 0.61 | 1.9 ± 0.44 |
| Control | N/A | III (7) IV (5) | N/A | 1.67 ± 0.49 | 2.1 ± 0.37 | |
| Comparison | Placebo | CABG only | HF drugs only | Placebo | CABG only | |
| Follow-up, months | 1, 4, 12 and 18 mo | 3, 6 and 12 mo | 1 and 6 mo | 3, 3, 6 and 12 mo | 1, 3, 6 and 12 mo | |
| Assessment modality (yes/no) | ECG | Yes | - | Yes | Yes | Yes |
| Echo | Yes | - | Yes | Yes | Yes | |
| MRI | No | Yes - CMR | - | Yes - CMR | Yes | |
| Cardiac CT | Yes | - | - | - | No | |
| SPECT | Yes | - | - | - | Yes | |
| Measured outcomes | Safety and adverse event (primary), efficacy, and LV functions LVEF (secondary) | Serious adverse events at 12 mo (primary), the efficacy of hUC-MSCs and collagen scaffold assessed according to the CV-CMR-based LVEF and infarct size at 3, 6 and 12 mo after treatment, and NYHA (secondary) | Changes in LVEDD, LVEF, BNP, 6MWD, symptoms of HF, death, and adverse events | Safety: Adverse events after IV infusion -/-. Efficacy: (primary). Change in LVEF in ECHO, changes in - (LVESV) & (LVEDV) at ECHO; LVEF, LVESV, and LVEDV in CMR; NYHA score (secondary) | LVEF, LV remodeling, myocardial mass, 6MWD, NYHA score change | |
| Chullikana et al[29], 2015 (India) | Hare et al[30], 2009 (USA) | Heldman et al[34], 2014 (USA) | Mathiasen et al[31], 2015 (Denmark) | Xiao et al[27], 2017 (China) | ||
| Study type | RCT | RCT | Open label RCT | RCT | Open label RCT | |
| Phase | I/II | I | I/II | I/II | I/II | |
| Condition | MI | MI | HF | HF | HF | |
| Sample size | Total | 20 | 53 | 30 | 60 | 37 |
| Intervention (male) | 10 (100) | 34 (82.4) | 19 (94.7) | 40 (90) | 17 (70) | |
| Control (male) | 10 (80) | 19 (78.9) | 11 (90.9) | 20 (70) | 20 (70) | |
| Mean age (mean ± SD) | Intervention | 47.31 ± 12.10 | 59 ± 12.3 | 57.1 ± 10.6 | 66.1 ± 7.7 | 51.6 ± 12.2 |
| Control | 47.79 ± 6.48 | 55 ± 10.2 | 60.0 ± 12.0 | 64.2 ± 10.6 | 54.4 ± 11.6 | |
| Mean BMI (mean ± SD) | Intervention | 23.32 ± 3.74 | 29.8 ± 6.7 | N/A | 29.8 + 4.7 | N/A |
| Control | 24.86 ± 1.88 | 30.3 ± 4.3 | N/A | 28.7 ± 5.3 | N/A | |
| Number of smokers | Intervention | N/A | 3 (8.8) | 14 (73) | 7 (17) | N/A |
| Control | N/A | 2 (10.5) | 9 (81.9) | 1 (5) | N/A | |
| HTN | Intervention | N/A | 16 (17.6) | 12 (63.2) | 0 | 4 (23) |
| Control | N/A | 9 (47.4) | 6 (54.5) | 0 | 7 (35) | |
| DM | Intervention | N/A | 6 (17.6) | 3 (15.8) | 15 (37) | 5 (29.4) |
| Control | N/A | 1 (5.3) | 3 (27.3) | 3 (15) | 6 (30) | |
| NYHA; I (n), II (n), III (n), IV (n) | Intervention | N/A | N/A | I (5)/II (12)/III (2) | II (11)/III (29) | II |
| Control | N/A | N/A | I (2)/II (5)/III (3) | II (5)/III (15) | II | |
| Comparison | Placebo (multiple electrolytes injection) | Placebo | HF treatments | HF treatments | HF treatments | |
| Follow-up | 6 mo to 2 yr | 6 mo | 12 mo | 6 mo | 12 mo | |
| Assessment modality (yes/no) | ECG | No | Yes | Yes | No | Yes |
| Echo | Yes | Yes | No | No | Yes | |
| MRI | Yes | Yes | Yes | Yes | No | |
| Cardiac CT | No | Yes | Yes | Yes | No | |
| SPECT | Yes | No | No | No | Yes | |
| Measured outcomes | Adverse events, LVEF (Echo and SPECT), total perfusion score, and total infarct volume | Safety, adverse events, LVEF (Echo), and 6MWD | Adverse events (primary), 6MWD, NYHA, and LV parameters (secondary) | LVESV (primary), LVEF, NYHA, 6MWD, and LV parameters (secondary) | LVEF, NYHA, LVEDV, and MAE are primary endpoints | |
| Ulus et al[25], 2020 (Türkiye) | Rodrigo et al[32], 2013 (Netherlands) | Kim et al[16], 2018 (South Korea) | Bolli et al[23], 2020 (USA) | ||
| Study type | Open-label RCT | RCT | RCT | RCT | |
| Phase | I/II | I/II | I | I | |
| Condition | CIC | MI | MI | HF | |
| Sample size | Total | 28 | 54 | 26 | 31 |
| Intervention (male) | 12 (100) | 9 (78) | 14 (100) | 14 (43) | |
| Control (male) | 16 (100) | 45 (78) | 12 (100) | 17 (24) | |
| Mean age (mean ± SD) | Intervention | 56.9 ± 5.20 | 56 ± 8 | 55.3 ± 8.6 | 54.7 ± 12.8 |
| Control | 65.3 ± 6.8 | 61 ± 11 | 57.8 ± 8.9 | 58.2 ± 11.2 | |
| Mean BMI (mean ± SD) | Intervention | 26.2 ± 3.12 | N/A | N/A | 30.2 ± 9.0 |
| Control | 26.6 ± 4.8 | N/A | N/A | 30.4 ± 6.5 | |
| Number of smokers | Intervention | 11 (91.6) | 6 (67) | 5 (35.7) | 5 (36) |
| Control | 15 (88.2) | 19 (42) | 5 (41.7) | 3 (18) | |
| HTN | Intervention | 6 (50) | 4 (44) | 5 (35.7) | 6 (43) |
| Control | 11 (64.7) | 18 (40) | 5 (41.7) | 10 (59) | |
| DM | Intervention | 4 (33.3) | 1 (11) | 3 (21.4) | 3 (21) |
| Control | 9 (52.9) | 5 (11) | 2 (16.7) | 5 (29) | |
| NYHA; I (n), II (n), III (n), IV (n) | Intervention | 2.2 ± 0.6 | N/A | N/A | II (13), III (1) |
| Control | 2.1 ± 0.37 | N/A | N/A | II (13), III (4) | |
| Comparison | CABG only | No placebo (optimal MI treatment) | No placebo (optimal MI treatment) | HF treatments | |
| Follow-up duration | 1, 3, 6, and 12 mo | 3, 6, 12 mo, 4, 5 years | 4 and 12 mo | 6 and 12 mo | |
| Assessment modality (Yes/no) | ECG | Yes | Yes - Holter | No | Yes |
| Echo | Yes | Yes | Yes | No | |
| MRI | Yes | No | No | Yes - CMR | |
| Cardiac CT | No | No | No | No | |
| SPECT | Yes | Yes | Yes | No | |
| Measured outcomes | LVEF, LV remodeling, myocardial mass, 6MWD, NYHA score | Safety and feasibility of IM delivery after PCI for MI (primary). Efficacy regarding change in infarct size, LVEF, LVEDV, and LVESV (secondary) | Absolute changes in global LVEF from baseline to 4 months after PCI using SPECT, Echo changes in global LVEF at 12 mo (primary). Changes in LVEDV, LVESV, and MACE (secondary) | Safety and feasibility of allogenic MSC in population (primary). Effects of allogenic MSC on LV function (LVEF, LVEDV, LVESV, scar), morphology, and functional status (6MWD, MLHFQ) (secondary) | |
In the five UC-MSC RCTs (296 patients), 160 patients were in the intervention group, while 130 were in the control group. Male percentages ranged from 78%-100% (intervention group) to 58%-100% (control group). Similarly, in the eight BM-MSC-based RCTs (397 patients), 197 patients were in the intervention group, and 200 patients were in the control group. Males comprised 43%-100% (intervention) and 24%-100% (control). The follow-up duration in the RCTs ranged from 1 to 18 mo (Tables 1, 2 and 3).
Regarding cell characteristics, six RCTs used frozen MSCs, and seven used fresh MSCs. BM-MSCs were obtained from allogeneic[24,29,30], or autologous[25,27,31-33] tissue sources. Diverse routes of administration were used, including intravenous[29,30], intramyocardial[24,25,31,32], intracoronary[27,33] (Tables 4 and 5).
| Refs | Cell type | Cell condition | MSCs dose and volume | Route of delivery | Concurrent procedure (if any) |
| He et al[18] | WJUC-MSCs | Frozen | 1 × 108/1.5-2.5 mL +/- 1 mL collagen scaffold | IM | CABG for all groups |
| Zhao et al[26] | UC-MSCs | N/S | N/S | IC | N/A |
| Bartolucci et al[28] | WJUC-MSCs | Frozen | 1 × 106/kg in 100 mL | IV | N/A |
| Ulus et al[25] | UC-MSCs | Frozen | 23 × 106 | IM | CABG for all groups |
| Gao et al[21] | WJUC- MSCs | Fresh | 6 × 106 | IC | N/A |
| Refs | Cell type | Cell condition | Cell source | MSCs dose and volume | Route of delivery | Concurrent procedure (if any) |
| Chullikana et al[29] | BM-MSCs | Frozen | Allogenic | 2 million cells/kg, 0.5 mL/kg | IV | N/A |
| Hare et al[30] | BM-MSCs | Frozen | Allogenic | 0.5, 1.6, and 5.0 × 106 | IV | N/A |
| Heldman et al [34] | BM-MSCs | Fresh | Autologous | N/A | IC | PCI |
| Mathiasen et al[31] | BM-MSCs | Fresh | Autologous | 77.5 ± 67.9 × 106 in 10-15 injections | IM | N/A |
| Xiao et al[27] | BM-MSCs | Fresh | Autologous | 4.9 × 108 | IC | N/A |
| Ulus et al[25] | BM-MSCs | Fresh | Autologous | 70 × 107 | IM | CABG |
| Rodrigo et al[32] | BM-MSCs | Fresh | Autologous | 31 ± 2 × 106 IN 10-12 injections | IM | N/A |
| Kim et al[16] | BM-MSCs | Fresh | Autologous | 7.2 ± 0.90 × 107 | IC | N/A |
| Bolli et al[23] | BM-MSCs | Frozen | Allogenic | 1 × 108 via 20 TC injections | IM | N/A |
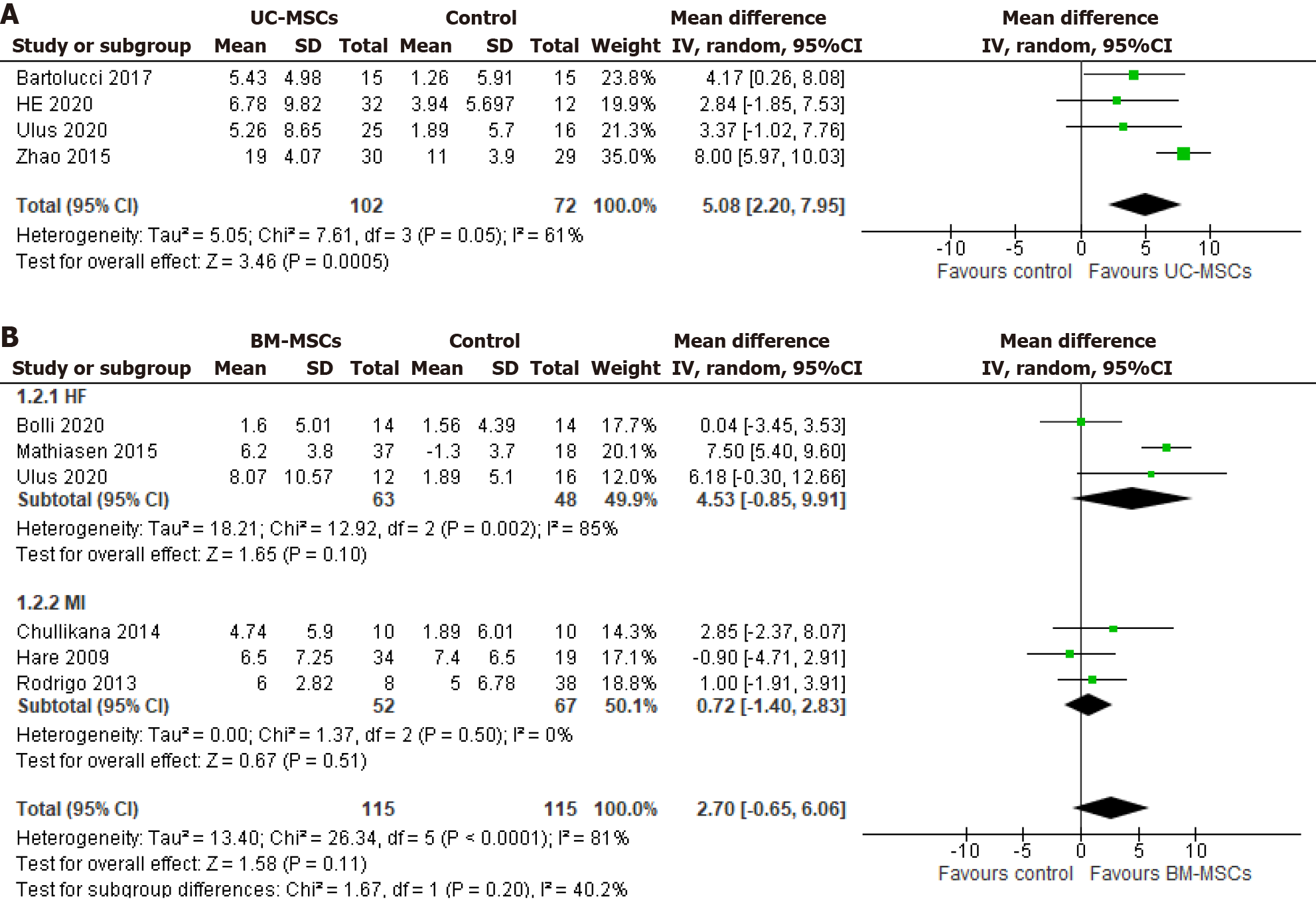
Four of five UC-MSCs studies (intervention group = 102; control group = 72) and six of nine BM-MSCs studies (in
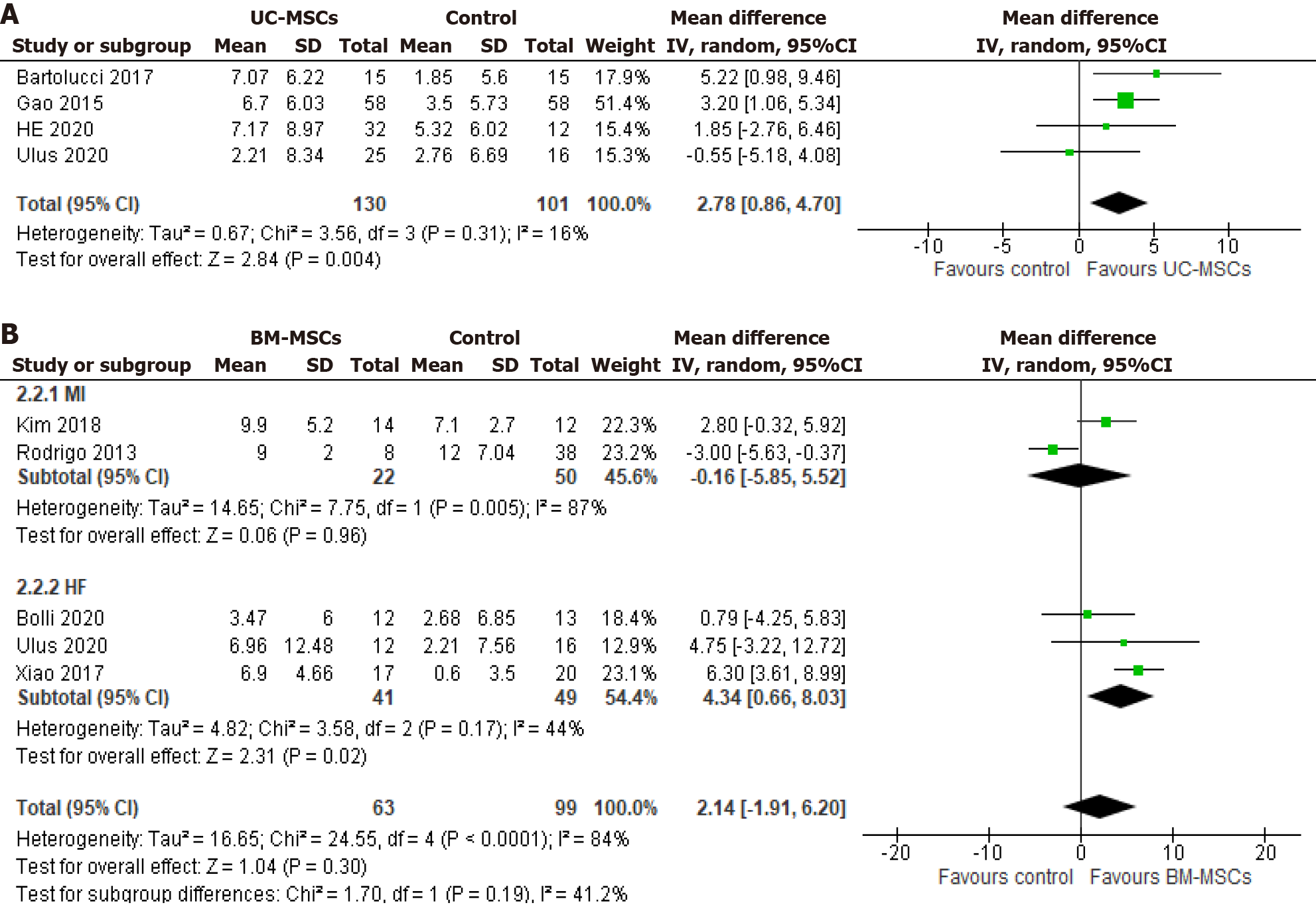
Four of five UC-MSCs studies with a total of 130 patients in the intervention group and 101 in the control group were followed up for 12 mo[18,22,25,28]. The pooled effect of their mean LVEF showed a significant improvement of 2.78% of LVEF in the intervention group compared to its control group (MD 2.78, 95%CI: 0.86-4.70; P = 0.004; I2 = 16%) (Figure 4A). On the contrary, after 12 mo of follow-up, the five BM-MSC studies showed that 63 patients in the BM-MSC intervention group experienced a 4.35% improvement in LVEF within the HF subgroup. This improvement was significantly greater compared to the control group (99 patients) with moderate heterogeneity (MD 4.34, 95%CI: 0.66-8.03; P = 0.02; I2 = 44%). In contrast to the HF group, no significant LVEF change was observed with BM-MSCs in the MI subgroup (MD -0.16, 95%CI: -5.85 to 5.52; P = 0.96; I2 = 87%) (Figure 4B).
When the combined means and SDs of each cell type were compared with the unified control, UC-MSCs improved LVEF by 1.18% (MD 1.18%, 95%CI: -0.43 to 2.79, P = 0.15), but without reaching the level of statistical significance. Combined means and SDs of BM-MSCs showed a significant improvement in LVEF by 2.38% compared to the unified control group (MD 2.38%, 95%CI: 0.38-4.38 P = 0.02) (Supplementary Figure 2). A funnel plot of LVEF was plotted to assess publication bias. The distribution of the studies showed asymmetry, suggesting a potential publication bias (Supplementary Figure 3).
Two of five studies on UC-MSCs, with 55 patients in the intervention group and 45 patients in the control group, were followed up[25,26]. Similarly, three of nine BM-MSCs studies with 60 patients in the intervention group and 49 patients in the control group = 49) reported 6MWD data[24,25,30]. The pooled analysis found no significant difference in 6MWD between the intervention and its respective control groups for either UC-MSCs or BM-MSCs. For BM-MSCs, the mean difference was -6.08 m (95%CI: -46.56 to 34.38; P = 0.77; I2 = 51%) (Supplementary Figure 4A). Similarly, for UC-MSCs, the mean difference was 53.25 m (95%CI: -81.61 to 188.11, P = 0.44, I2 = 83%) (Supplementary Figure 4B). Both results indicate no significant treatment effect of either stem cell type on 6MWD compared to their control group.
When compared with the unified control group, UC-MSCs showed a non significant improvement of 7.47 m (MD 7.47, 95%CI: -20.69 to 35.63, P = 0.60). However, the comparison between the combined means and SDs of BM-MSCs and the unified control group resulted in a significant improvement of 49.74 m in the BM-MSCs group (MD 49.74, 95%CI: 5.53-93.95, P = 0.03) (Supplementary Figure 4C).
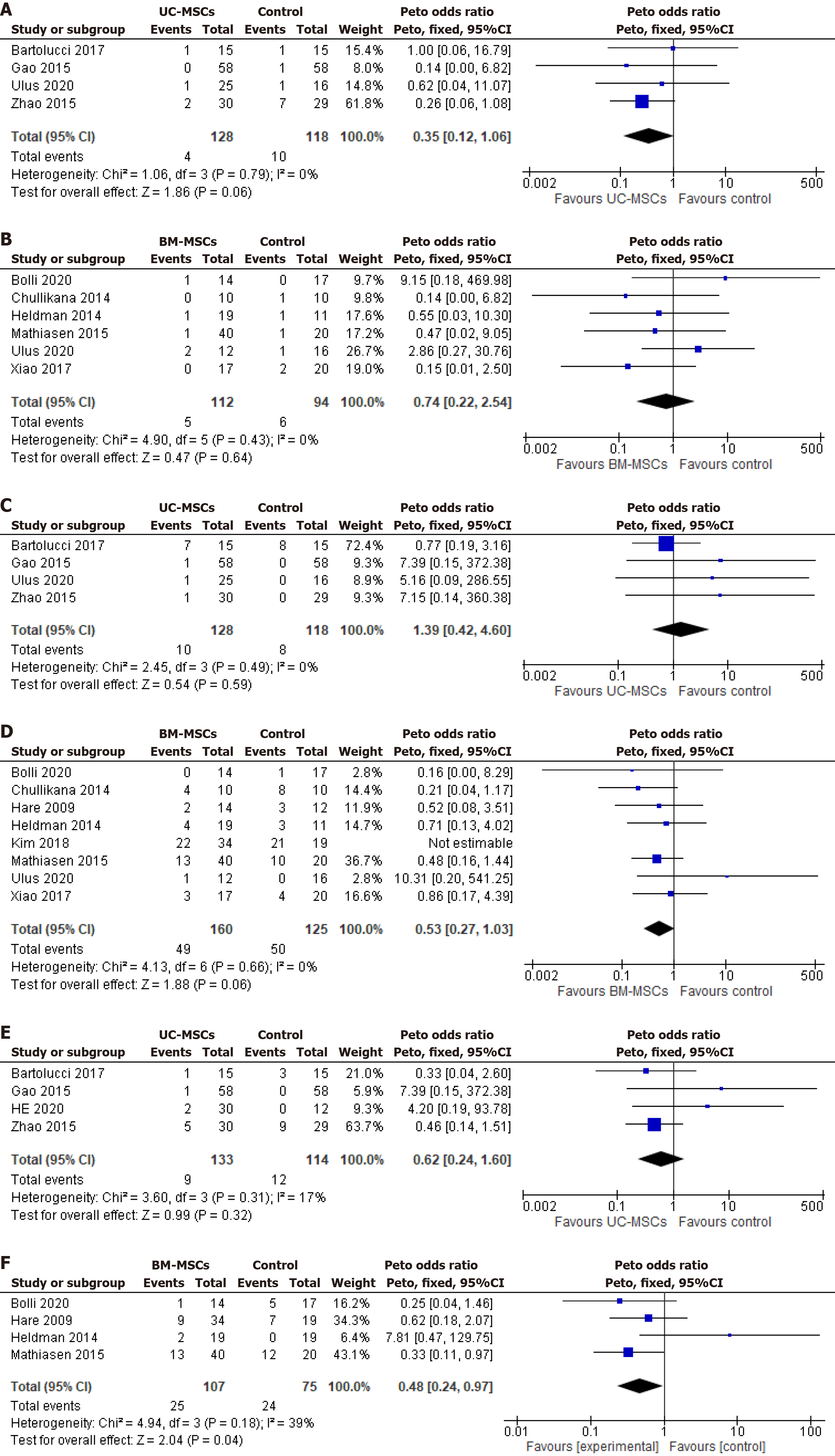
Mortality: Four of five UC-MSCs studies (n = 246)[22,25,26,28] and six of nine BM-MSCs studies (n = 206)[24,25,27,29,31,34] reported on mortality during the follow-up period. No significant difference in the OR of mortality between the intervention and respective control group of UC-MSCs studies (Peto OR 0.35, 95%CI: 0.27-1.03; P = 0.06; I2 = 0%) (Figure 5A) and BM-MSCs studies (Peto OR 0.74; 95%CI: 0.22-2.54; P = 0.64; I2 = 0%) (Figure 5B). Similarly, both cell types did not significantly improve the mortality rate compared to the unified control.
MACEs: Four of five UC-MSCs studies (n = 246)[22,25,26,28] and eight of nine BM-MSCs studies (n = 285)[24,25,27,29-31,33,34] reported the incidence of MACEs, including angina, supraventricular tachycardia, ventricular tachycardia, and revascularization of MI. No significant effect was observed in the pooled OR of UC-MSCs studies (Peto OR 1.39; 95%CI: 0.42-4.60; P = 0.59; I2 = 0%) (Figure 5C) and BM-MSCs studies (Peto OR 0.53; 95%CI: 0.27-1.03; P = 0.06; I2 = 0%) between the intervention and control groups (Figure 5D).
When both cell types are compared with the unified control arm, the UC-MSCs studies demonstrated a significant reduction in the incidence of MACEs by an OR of 0.27 (0.27, 95%CI: 0.13-0.55, P = 0.0003). In contrast, the BM-MSCs studies did not significantly affect the MACE OR (1.41, 95%CI: 0.90-2.20, P = 0.13) (Supplementary Figure 5A).
Four of five UC-MSCs studies (n = 247)[18,22,26,28], and four of nine BM-MSCs studies (n = 182)[24,30,31,34], reported data on rehospitalization of the enrolled patients. UC-MSCs studies reported a nonsignificant difference between the intervention and control groups with a Peto OR of 0.62 (95%CI: 0.24-1.60; P = 0.31; I2 = 17%) (Figure 5E). Analysis of BM-MSCs studies showed a significant reduction of rehospitalization rates by 52% and Peto OR of 0.48 (95%CI: 0.24-0.97; P = 0.04; I2 = 39%) (Figure 5F). These findings suggest that BM-MSCs demonstrated a protective effect in the intervention group, resulting in a lower rehospitalization rate than their respective control group.
Compared to the unified control group, the UC-MSCs studies showed a significant reduction in the rehospitalization rate with an OR of 0.31 (95%CI: 0.14-0.66, P = 0.003). However, the BM-MSCs significant reduction in rehospitalization rate compared to its respective control was not maintained with the unified control (Peto OR 1.30, 95%CI: 0.73-2.31, P = 0.38) (Supplementary Figure 5B).
This systematic review and meta-analysis of MSC-based therapy evaluated the efficacy and safety of MSCs sourced from two different tissues as living biodrugs for treating CVD patients. Besides safety endpoints, the performance of the two cell types used was assessed for functional and clinical indicators, i.e., LVEF and 6MWD. Our significant findings include: (1) UC-MSCs RCTs reported significant improvement in LVEF during 6 and 12 mo follow-up compared to controls and their BM-MSCs counterparts; (2) both cell types did not show a significant improvement in 6MWD compared to the baseline; and (3) both cell types exhibited no disparity in adverse events including MACEs, except for rate of rehospitalization, which showed significant reduction with BM-MSCs group compared to the UC-MSCs and control groups.
Comparing both cell types in MI and HF patients based on the above parameters, UC-MSC-treated patients had a significant pooled increase of 5.08% and 2.78% in LVEF during 6 and 12 mo follow-up, respectively, compared to the nonsignificant 2.70% and 2.14% improvement in BM-MSC-treated patients during 6 wk and 12 mo follow-up, respec
The adverse events reported and analyzed in this review included patient mortality, rehospitalization rate, and MACEs. There was no notable disparity between the intervention and their respective control groups in the UC-MSCs and BM-MSCs RCTs, indicating the clinical safety of MSCs-based therapy. Similarly, no significant impact was observed in the UC-MSCs and BM-MSCs RCTs between the intervention and respective control groups for MACEs, which included angina, supraventricular tachycardia, ventricular tachycardia, and revascularization. Although the point estimate of the Peto OR suggested a higher incidence of MACEs in the UC-MSC group than in its control group, this difference was insignificant. The 95%CI for the Peto OR included the null value of 1.0, indicating no significant difference between the UC-MSC group and its control (95%CI: 0.42-4.60).
When analyzing the rehospitalization rates for cardiac causes following the treatment with both BM-MSCs and UC-MSCs compared to the control group, a significant 52% reduction was reported only in the BM-MSCs group. In contrast, the UC-MSCs group did not experience a significant reduction compared to the control group.
A comprehensive comparison method between the two cell types was used while mitigating the impact of control group variations across studies. The means (± SDs) of the control group from the included studies of UC-MSCs and BM-MSCs were combined using Cochrane formulas, resulting in a unified control group. A similar approach was applied to the means (± SDs) of UC-MSCs and BM-MSCs derived from the included RCTs. Subsequently, each cell type’s combined means (± SDs) were compared to the unified control group. This method thoroughly evaluated the effectiveness of UC-MSCs and BM-MSCs vs the unified control group. After applying this method, noteworthy findings emerged. Both cell types demonstrated the ability to improve LVEF at the 6-mo follow-up. However, only BM-MSCs exhibited a significant improvement at the 12-mo follow-up. While no cell type significantly affected 6MWT compared to their respective control groups, BM-MSCs demonstrated a considerable improvement compared to the unified control group. Addition
This review focuses on phase I and II RCTs that have evaluated the safety and efficacy of MSCs derived from bone marrow and umbilical cord in patients with cardiac pathologies. The primary rationale for only including phase I and II RCTs was that all published UC-MSCs studies are limited to these early clinical trial phases. Therefore, only phase I/II RCTs utilizing BM-MSCs were incorporated to ensure a precise cell comparison.
According to the data obtained from ClinicalTrials.gov, eleven ongoing RCTs investigating the use of UC-MSCs and seven RCTs studying BM-MSCs in patients with HF and MI are currently underway. These clinical trials encompass phase I to phase III.
Irrespective of the tissue source, MSCs possess low immunogenicity due to reduced expression of MHC-II molecule, lack of MHC-I expression, and the absence of co-stimulatory signals[35,36]. UC-MSCs are gaining popularity in clinical settings due to their advantages, which include noninvasive collection methods, minimal bioethical concerns, possible widespread “off-the-shelf” availability, and being rich in primitive cell populations. Additionally, like other MSC types, UC-MSCs have the added benefit of being cryopreserved for extended periods. Bárcia et al[36] reported successful cryopreservation of UC-MSCs using the conventional cryopreservation protocol, i.e., 10% DMSO and 90% fetal bovine serum) for 3 years with a high viability rate upon thawing. Their availability without infection risk and the lack of influence from donor morbidities and aging factors put them in a position of advantage over their counterparts[37]. On the contrary, the less-than-expected results from BM-MSC-based RCTs compared to their respective control may be because most of these trials used autologous cells (Table 5). Autologous MSCs from cardiac patients are significantly affected by a plethora of comorbidities, including hypertension, diabetes mellitus, and age-related cellular changes, that compromise their therapeutic potential[10,11]. Additionally, our data showed that BM-MSCs obtained from HF patients led to a statistically significant 4.35% improvement in LVEF at the 12-mo follow-up compared to the control group. In contrast, no significant effect was observed with BM-MSCs derived from MI patients. These findings highlight the importance of the patient’s clinical status in determining the therapeutic efficacy of MSC treatments.
While MSCs for cell-based therapy hold potential and have significantly affected clinical and functional study endpoints, the reported moderate improvement is also attributed to the inhospitable microenvironment in the ischemic myocardium that causes poor survival of the transplanted cells besides significantly affecting the stemness characteristics of MSCs. Various strategies are being explored, encompassing quality preconditioning of donor cells to protect them against apoptosis and ferroptosis to develop super stem cells with improved stemness and cell biology[38,39]. Based on the translational data, Xu et al[40] designed a multicenter phase II RCT using atorvastatin-preconditioned MSCs for patients with acute MI. This trial aimed to investigate the potential benefits of the preconditioning approach in enhancing the therapeutic effects of MSCs[40]. Additionally, optimizing cell dose and administering multiple doses of MSCs at different times may improve the outcomes[8,41].
Although RCT data from UC-MSCs in the present systematic review are encouraging, it is crucial to acknowledge that the sample size in the included studies is relatively small. Therefore, there is a need for more extensive RCTs to validate these findings. Additionally, standardization of optimal isolation and biobanking methods, time and route of administration, and cell dose are necessary for better clinical outcomes. In conclusion, our study indicated that UC-MSCs significantly improve LVEF and patient prognosis compared to their counterpart BM-MSCs. UC-MSCs may be considered a promising alternative source of MSCs for use, suggesting that they are a promising alternative for MSC-based heart therapy.
| 1. | Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Ferguson JF, Generoso G, Ho JE, Kalani R, Khan SS, Kissela BM, Knutson KL, Levine DA, Lewis TT, Liu J, Loop MS, Ma J, Mussolino ME, Navaneethan SD, Perak AM, Poudel R, Rezk-Hanna M, Roth GA, Schroeder EB, Shah SH, Thacker EL, VanWagner LB, Virani SS, Voecks JH, Wang NY, Yaffe K, Martin SS. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation. 2022;145:e153-e639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2995] [Cited by in RCA: 3254] [Article Influence: 1084.7] [Reference Citation Analysis (0)] |
| 2. | Al-Khani AM, Khalifa MA, Haider KH. Mesenchymal stem cells: How close we are to their routine clinical use? In: Haider KH, editor. Handbook of Stem Cell Therapy. Singapore: Springer, 2022. [DOI] [Full Text] |
| 3. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12669] [Article Influence: 703.8] [Reference Citation Analysis (2)] |
| 4. | Li Z, Hu X, Zhong JF. Mesenchymal Stem Cells: Characteristics, Function, and Application. Stem Cells Int. 2019;2019:8106818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 819] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 6. | Berebichez-Fridman R, Montero-Olvera PR. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ Med J. 2018;18:e264-e277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 276] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 7. | Kalou Y, Al-Khani AM, Haider KH. Bone Marrow Mesenchymal Stem Cells for Heart Failure Treatment: A Systematic Review and Meta-Analysis. Heart Lung Circ. 2023;32:870-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Reference Citation Analysis (0)] |
| 8. | Ahmed ZT, Zain Al-Abeden MS, Al Abdin MG, Muqresh MA, Al Jowf GI, Eijssen LMT, Haider KH. Dose-response relationship of MSCs as living Bio-drugs in HFrEF patients: a systematic review and meta-analysis of RCTs. Stem Cell Res Ther. 2024;15:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 9. | Jihwaprani MC, Sula I, Charbat MA, Haider KH. Establishing delivery route-dependent safety and efficacy of living biodrug mesenchymal stem cells in heart failure patients. World J Cardiol. 2024;16:339-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 10. | Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21:1424-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1079] [Cited by in RCA: 1617] [Article Influence: 179.7] [Reference Citation Analysis (0)] |
| 11. | Safwan M, Bourgleh MS, Alshakaki H, Molhem A, Haider KH. Morbid Cell Status and Donor Age Significantly Alter Mesenchymal Stem Cell Functionality and Reparability. In: Haider KH, editor. Handbook of Stem Cell Applications. Singapore: Springer, 2023. [DOI] [Full Text] |
| 12. | Mebarki M, Abadie C, Larghero J, Cras A. Human umbilical cord-derived mesenchymal stem/stromal cells: a promising candidate for the development of advanced therapy medicinal products. Stem Cell Res Ther. 2021;12:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 13. | Subramanian A, Fong CY, Biswas A, Bongso A. Comparative Characterization of Cells from the Various Compartments of the Human Umbilical Cord Shows that the Wharton's Jelly Compartment Provides the Best Source of Clinically Utilizable Mesenchymal Stem Cells. PLoS One. 2015;10:e0127992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Li T, Xia M, Gao Y, Chen Y, Xu Y. Human umbilical cord mesenchymal stem cells: an overview of their potential in cell-based therapy. Expert Opin Biol Ther. 2015;15:1293-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 15. | Troyer DL, Weiss ML. Wharton's jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26:591-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 596] [Cited by in RCA: 551] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 16. | Kim SH, Cho JH, Lee YH, Lee JH, Kim SS, Kim MY, Lee MG, Kang WY, Lee KS, Ahn YK, Jeong MH, Kim HS. Improvement in Left Ventricular Function with Intracoronary Mesenchymal Stem Cell Therapy in a Patient with Anterior Wall ST-Segment Elevation Myocardial Infarction. Cardiovasc Drugs Ther. 2018;32:329-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 17. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47102] [Article Influence: 2943.9] [Reference Citation Analysis (0)] |
| 18. | He X, Wang Q, Zhao Y, Zhang H, Wang B, Pan J, Li J, Yu H, Wang L, Dai J, Wang D. Effect of Intramyocardial Grafting Collagen Scaffold With Mesenchymal Stromal Cells in Patients With Chronic Ischemic Heart Disease: A Randomized Clinical Trial. JAMA Netw Open. 2020;3:e2016236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 19. | Brockhaus AC, Grouven U, Bender R. Performance of the Peto odds ratio compared to the usual odds ratio estimator in the case of rare events. Biom J. 2016;58:1428-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 updated March 2011. The Cochrane Collaboration. 2011. [cited 3 July 2024]. Available from: www.handbook.cochrane.org. |
| 21. | Gao LR, Chen Y, Zhang NK, Yang XL, Liu HL, Wang ZG, Yan XY, Wang Y, Zhu ZM, Li TC, Wang LH, Chen HY, Chen YD, Huang CL, Qu P, Yao C, Wang B, Chen GH, Wang ZM, Xu ZY, Bai J, Lu D, Shen YH, Guo F, Liu MY, Yang Y, Ding YC, Yang Y, Tian HT, Ding QA, Li LN, Yang XC, Hu X. Intracoronary infusion of Wharton's jelly-derived mesenchymal stem cells in acute myocardial infarction: double-blind, randomized controlled trial. BMC Med. 2015;13:162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 159] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 22. | Pearson MJ, Smart NA. Reported methods for handling missing change standard deviations in meta-analyses of exercise therapy interventions in patients with heart failure: A systematic review. PLoS One. 2018;13:e0205952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Bolli R, Perin EC, Willerson JT, Yang PC, Traverse JH, Henry TD, Pepine CJ, Mitrani RD, Hare JM, Murphy MP, March KL, Ikram S, Lee DP, O'Brien C, Durand JB, Miller K, Lima JA, Ostovaneh MR, Ambale-Venkatesh B, Gee AP, Richman S, Taylor DA, Sayre SL, Bettencourt J, Vojvodic RW, Cohen ML, Simpson LM, Lai D, Aguilar D, Loghin C, Moyé L, Ebert RF, Davis BR, Simari RD; Cardiovascular Cell Therapy Research Network (CCTRN). Allogeneic Mesenchymal Cell Therapy in Anthracycline-Induced Cardiomyopathy Heart Failure Patients: The CCTRN SENECA Trial. JACC CardioOncol. 2020;2:581-595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 24. | Cochrane Training. Review Manager (RevMan) Computer program. Version 5.4. The Cochrane Collaboration. 2020. [cited 3 July 2024]. Available from: https://training.cochrane.org/online-learning/core-software/revman. |
| 25. | Ulus AT, Mungan C, Kurtoglu M, Celikkan FT, Akyol M, Sucu M, Toru M, Gul SS, Cinar O, Can A. Intramyocardial Transplantation of Umbilical Cord Mesenchymal Stromal Cells in Chronic Ischemic Cardiomyopathy: A Controlled, Randomized Clinical Trial (HUC-HEART Trial). Int J Stem Cells. 2020;13:364-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Zhao XF, Xu Y, Zhu ZY, Gao CY, Shi YN. Clinical observation of umbilical cord mesenchymal stem cell treatment of severe systolic heart failure. Genet Mol Res. 2015;14:3010-3017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Xiao W, Guo S, Gao C, Dai G, Gao Y, Li M, Wang X, Hu D. A Randomized Comparative Study on the Efficacy of Intracoronary Infusion of Autologous Bone Marrow Mononuclear Cells and Mesenchymal Stem Cells in Patients With Dilated Cardiomyopathy. Int Heart J. 2017;58:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Bartolucci J, Verdugo FJ, González PL, Larrea RE, Abarzua E, Goset C, Rojo P, Palma I, Lamich R, Pedreros PA, Valdivia G, Lopez VM, Nazzal C, Alcayaga-Miranda F, Cuenca J, Brobeck MJ, Patel AN, Figueroa FE, Khoury M. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ Res. 2017;121:1192-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 335] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 29. | Chullikana A, Majumdar AS, Gottipamula S, Krishnamurthy S, Kumar AS, Prakash VS, Gupta PK. Randomized, double-blind, phase I/II study of intravenous allogeneic mesenchymal stromal cells in acute myocardial infarction. Cytotherapy. 2015;17:250-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277-2286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1006] [Cited by in RCA: 996] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 31. | Mathiasen AB, Qayyum AA, Jørgensen E, Helqvist S, Fischer-Nielsen A, Kofoed KF, Haack-Sørensen M, Ekblond A, Kastrup J. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial). Eur Heart J. 2015;36:1744-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 240] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 32. | Rodrigo SF, van Ramshorst J, Hoogslag GE, Boden H, Velders MA, Cannegieter SC, Roelofs H, Al Younis I, Dibbets-Schneider P, Fibbe WE, Zwaginga JJ, Bax JJ, Schalij MJ, Beeres SL, Atsma DE. Intramyocardial injection of autologous bone marrow-derived ex vivo expanded mesenchymal stem cells in acute myocardial infarction patients is feasible and safe up to 5 years of follow-up. J Cardiovasc Transl Res. 2013;6:816-825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 33. | Kim DW, Staples M, Shinozuka K, Pantcheva P, Kang SD, Borlongan CV. Wharton's jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci. 2013;14:11692-11712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 220] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 34. | Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, Ghersin E, Soto V, Lopera G, Miki R, Willens H, Hendel R, Mitrani R, Pattany P, Feigenbaum G, Oskouei B, Byrnes J, Lowery MH, Sierra J, Pujol MV, Delgado C, Gonzalez PJ, Rodriguez JE, Bagno LL, Rouy D, Altman P, Foo CW, da Silva J, Anderson E, Schwarz R, Mendizabal A, Hare JM. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311:62-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 425] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 35. | Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 773] [Cited by in RCA: 1246] [Article Influence: 207.7] [Reference Citation Analysis (0)] |
| 36. | Bárcia RN, Santos JM, Teixeira M, Filipe M, Pereira ARS, Ministro A, Água-Doce A, Carvalheiro M, Gaspar MM, Miranda JP, Graça L, Simões S, Santos SCR, Cruz P, Cruz H. Umbilical cord tissue-derived mesenchymal stromal cells maintain immunomodulatory and angiogenic potencies after cryopreservation and subsequent thawing. Cytotherapy. 2017;19:360-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | Alessio N, Acar MB, Demirsoy IH, Squillaro T, Siniscalco D, Di Bernardo G, Peluso G, Özcan S, Galderisi U. Obesity is associated with senescence of mesenchymal stromal cells derived from bone marrow, subcutaneous and visceral fat of young mice. Aging (Albany NY). 2020;12:12609-12621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 38. | Zineldeen DH, Mushtaq M, Haider KH. Cellular preconditioning and mesenchymal stem cell ferroptosis. World J Stem Cells. 2024;16:64-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (8)] |
| 39. | Haider KH. Priming mesenchymal stem cells to develop "super stem cells". World J Stem Cells. 2024;16:623-640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 40. | Xu JY, Qian HY, Huang PS, Xu J, Xiong YY, Jiang WY, Xu Y, Leng WX, Li XD, Chen GH, Tang RJ, Huang CR, Hu MJ, Jin C, Wu Y, Zhang J, Qian J, Xu B, Zhao SH, Lu MJ, Shen R, Fang W, Wu WC, Chen X, Wang Y, Li W, Lu XF, Jiang XF, Ma CC, Li JW, Geng YJ, Qiao SB, Gao RL, Yang YJ. Transplantation efficacy of autologous bone marrow mesenchymal stem cells combined with atorvastatin for acute myocardial infarction (TEAM-AMI): rationale and design of a randomized, double-blind, placebo-controlled, multi-center, Phase II TEAM-AMI trial. Regen Med. 2019;14:1077-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Wysoczynski M, Khan A, Bolli R. New Paradigms in Cell Therapy: Repeated Dosing, Intravenous Delivery, Immunomodulatory Actions, and New Cell Types. Circ Res. 2018;123:138-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |