Published online Aug 26, 2024. doi: 10.4330/wjc.v16.i8.458
Revised: July 24, 2024
Accepted: August 6, 2024
Published online: August 26, 2024
Processing time: 87 Days and 21.4 Hours
Cardio-oncology has received increasing attention especially among older patients with colorectal cancer (CRC). Cardiovascular disease (CVD)-specific mortality is the second-most frequent cause of death. The risk factors for CVD-specific mortality among older patients with CRC are still poorly understood.
To identify the prognostic factors and construct a nomogram-based model to predict the CVD-specific mortality among older patients with CRC.
The data on older patients diagnosed with CRC were retrieved from The Sur
A total of 141251 eligible patients with CRC were enrolled, of which 41459 patients died of CRC and 12651 patients died of CVD. The age at diagnosis, sex, marital status, year of diagnosis, surgery, and chemotherapy were independent prognostic factors associated with CVD-specific mortality among older patients with CRC. We used these variables to develop a model to predict CVD-specific mortality. The calibration curves for CVD-specific mortality probabilities showed that the model was in good agreement with actual observations. The C-index value of the model in the training cohort and testing cohort for predicting CVD-specific mortality was 0.728 and 0.734, respectively.
The proposed nomogram-based model for CVD-specific mortality can be used for accurate prognostic prediction among older patients with CRC. This model is a potentially useful tool for clinicians to identify high-risk patients and develop personalized treatment plans.
Core Tip: For older patients with colorectal cancer (CRC), cardiovascular disease (CVD)-specific mortality is the second-most frequent cause of death. Herein, we analyzed data from the Surveillance, Epidemiology, and End Results program. The age at diagnosis, sex, marital status, year of diagnosis, surgery, and chemotherapy were independent prognostic factors associated with CVD-specific mortality among older patients with CRC. Six variables were independent prognostic factors. Subsequently, we proposed a nomogram-based model of the CVD-specific mortality that could be used for accurate prognostic prediction of older patients with CRC.
- Citation: Tan JY, Shen SH. Nomogram predicting the cardiovascular disease mortality for older patients with colorectal cancer: A real-world population-based study. World J Cardiol 2024; 16(8): 458-468
- URL: https://www.wjgnet.com/1949-8462/full/v16/i8/458.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i8.458
Colorectal cancer (CRC) is the third-most deadly cancer worldwide. In 2020, there were 1.9 million new CRC cases[1,2]. Moreover, the incidence rates of CRC have been steadily increasing; the projected increase by 2035 is 2.5 million[2,3]. Cancer-specific mortality is known to be the most common cause for CRC patients[1,3]. With improved treatment options such as endoscopy, surgical local excision, radiotherapy, systemic therapy, chemotherapy, targeted therapy, immunotherapy[1], altered CRC risk factor patterns, and screening, the CRC mortality rates have declined[4]. Thus, the non-cancer causes of death among CRC patients have increased with increasing survival time. Many researchers have been increasingly concerned regarding non-cancer deaths, especially due to cardiovascular disease (CVD)[5-8]. Cardio-oncology has developed as a relatively new discipline and received increasing attention in clinical treatment. Baraghoshi et al[9] showed CRC survivors had almost double the risk of CVD than the general population. Among older patients with CRC, deaths due to cancer and CVD-specific factors were the first and second-most frequent cause of deaths, respectively[8,10].
Therefore, risk factors for cancer-specific mortality and CVD-specific mortality among older patients with CRC merits further analysis. Until now, the risk factors and cardio-oncological factors in older patients with CRC have been poorly understood. Furthermore, there is no predictive model yet that can estimate the CVD risk in older patients with CRC. In this study, we characterized the risk factors for cancer-specific mortality and CVD-specific mortality and established a risk predictive model for CVD-specific mortality, aiming to provide a contemporary and valuable resource for car
In this retrospective study, we used data from The Surveillance, Epidemiology, and End Result (SEER) program which is a public tumor registry that covers 34.6% of the US population with cancer.
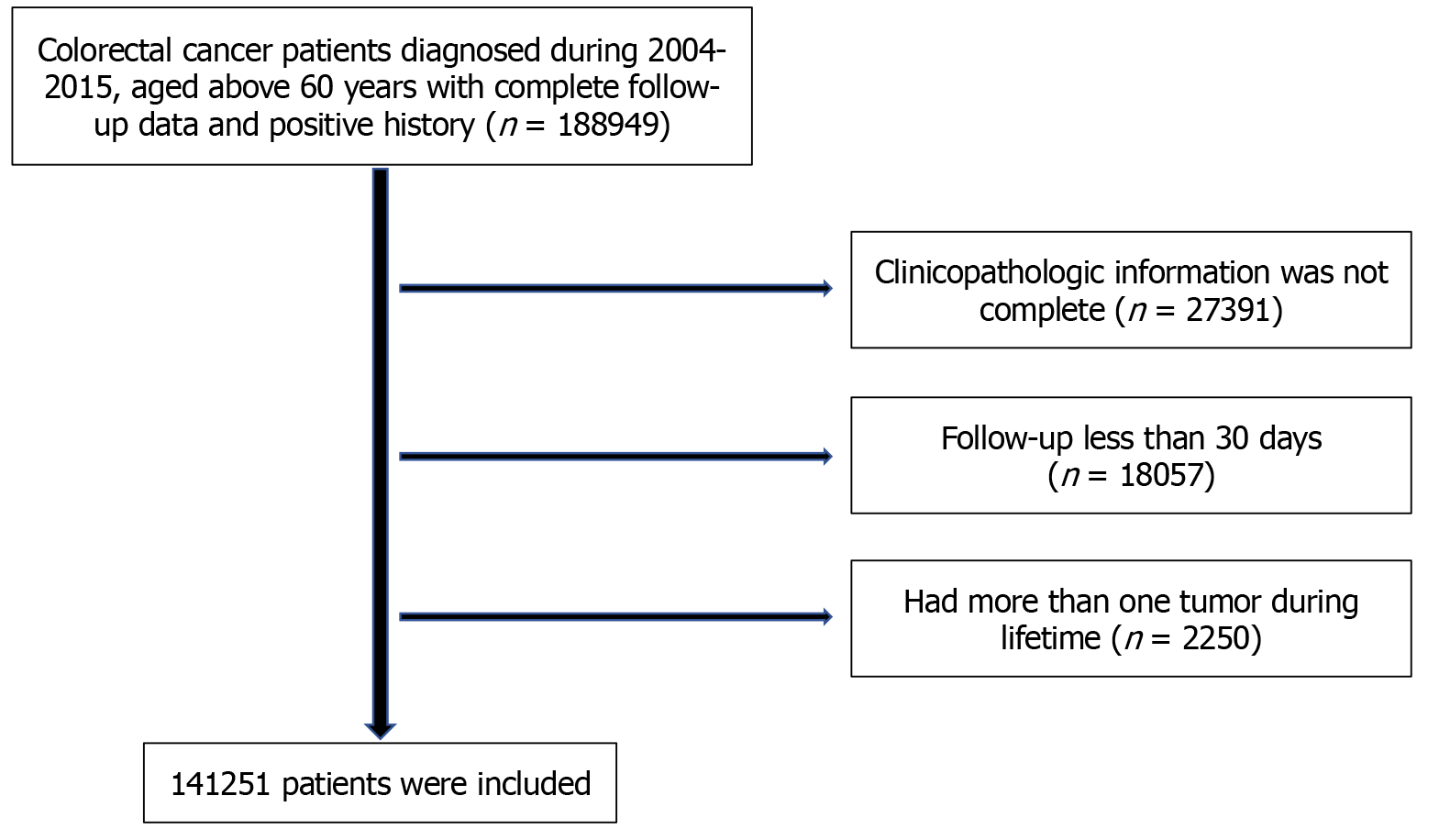
In total, the clinical data of patients diagnosed with CRC in the SEER program from January 1, 2004 to December 31, 2015 were retrospectively included in this study. All patients aged > 60 years with complete follow-up data and positive malignant histological examination were included. Patients whose clinicopathological information was incomplete were excluded. To further decrease the potential bias, we also excluded patients with < 30 days of follow-up and patients with more than one lifetime history of cancer. Finally, 141251 patients were enrolled in the study (Figure 1).
This observational study used de-identified and publicly available data from the SEER registry and thus did not require formal consent or institutional review board approval. This study was conducted in accordance with the tenets of the Helsinki Declaration.
We collected the basic information of patients, namely age at diagnosis; sex; marital status (single, married, or others); race (White, Black, or others); year of diagnosis; insurance status; primary site (right half colon, left half colon, or rectum); TNM stage; histological grade (well, moderate, poorly, or undifferentiated); histology type (adenocarcinoma, mucinous adenocarcinoma, and signet-ring cell carcinoma, or others); surgery; radiotherapy; and chemotherapy.
The cause of death classification was recorded from the variable "COD to site recode" in the SEER database. Cancer-specific mortality was defined by the cause of death recorded as CRC. We considered deaths due to heart disease, atherosclerosis, aortic aneurysm and dissection, cerebrovascular disease, hypertension without heart disease, and the diseases of arteries, arterioles, and capillaries as CVD-specific mortality.
Univariate and multivariate Cox regression analyses were used to determine the survival risk factors of cancer-specific mortality and CVD-specific mortality in the training cohort. Least absolute shrinkage and selection operator (LASSO) method is a commonly used method for regression with high-dimensional predictors[11]. The LASSO Cox regression model was used to select the most valuable prognostic variables of all CRC and CVD-specific mortality in our study. We constructed the nomogram of CVD-specific mortality according to the LASSO results.
The C-index, calibration curves, and prognostic decision curve analysis (DCA) were created to assess the predictive accuracy and discriminative ability of the nomogram-based model in the training and testing cohorts. R software (version 3.6.1) was used for statistical analysis. P < 0.05 was considered to indicate statistically significant differences.
A total of 141251 patients with CRC (48.7% male and 51.3% female) were included in this study. The proportion of patients aged 60-69 years was 38.2%, that of patients aged 70-79 years was 34.1%, and that of patients aged ≥ 80 years was 27.7%. In the entire cohort, 41459 patients died of CRC, 12651 patients died of CVD, 73035 patients were alive, and 14106 patients died of other causes. The clinicopathologic characteristics of patients with CRC are summarized in Table 1.
| Variables | Cancer-specific mortality (n = 41459) | CVD-specific mortality (n = 12651) | Others-specific mortality (n = 14106) | Survivors (n = 73035) | Total (n = 141251) |
| Age at diagnosis (years) | |||||
| 60-69 | 14188 (34.2) | 1911 (15.1) | 2750 (19.5) | 35112 (48.1) | 53961 (38.2) |
| 70-79 | 13969 (33.7) | 3971 (31.4) | 4895 (34.7) | 25384 (34.8) | 48219 (34.1) |
| ≥ 80 | 13302 (32.1) | 6769 (53.5) | 6461 (45.8) | 12539 (17.1) | 39071 (27.7) |
| Sex | |||||
| Female | 21112 (50.9) | 6395 (50.5) | 7294 (51.7) | 37631 (51.5) | 72432 (51.3) |
| Male | 20347 (49.1) | 6256 (49.5) | 6812 (48.3) | 35404 (48.5) | 68819 (48.7) |
| Marital status | |||||
| Single | 4908 (11.8) | 1296 (10.2) | 1431 (10.1) | 7802 (10.7) | 15437 (10.9) |
| Married | 19950 (48.1) | 5414 (42.8) | 6312 (44.7) | 41601 (57) | 73277 (51.9) |
| Others | 16601 (40.1) | 5941 (47) | 6363 (45.2) | 23632 (32.3) | 52537 (37.2) |
| Race | |||||
| White | 33337 (80.4) | 10670 (84.3) | 11818 (83.8) | 58562 (80.2) | 114387 (81) |
| Black | 4928 (11.9) | 1232 (9.8) | 1318 (9.3) | 7158 (9.8) | 14636 (10.4) |
| Others | 3197 (7.7) | 749 (5.9) | 970 (6.9) | 7315 (10) | 12228 (8.6) |
| Year of diagnosis | |||||
| 2004-2007 | 16825 (40.6) | 6651 (52.6) | 7135 (50.6) | 16055 (22) | 46666 (33) |
| 2008-2011 | 14971 (36.1) | 4215 (33.3) | 4873 (34.5) | 23245 (31.8) | 47304 (33.5) |
| 2011-2016 | 9663 (23.3) | 1785 (14.1) | 2098 (14.9) | 33735 (46.2) | 47281 (33.5) |
| Insurance status | |||||
| Insured | 27713 (66.8) | 7290 (57.6) | 8303 (58.9) | 59691 (81.7) | 102997 (72.9) |
| Uninsured | 576 (1.4) | 45 (0.4) | 78 (0.6) | 1030 (1.4) | 1729 (1.2) |
| Unknown | 13170 (31.8) | 5316 (42) | 5725 (40.5) | 12314 (16.9) | 36525 (25.9) |
| Primary site | |||||
| Right half colon | 20201 (48.7) | 6787 (53.6) | 7596 (53.8) | 35512 (48.6) | 20096 (49.6) |
| Left half colon | 10320 (24.9) | 3254 (25.7) | 3530 (25) | 19442 (26.6) | 36546 (25.9) |
| Rectum | 10938 (26.4) | 2610 (20.6) | 2980 (21.2) | 18081 (24.8) | 34609 (24.5) |
| TNM stage | |||||
| I | 3391 (8.2) | 4101 (32.4) | 4414 (31.3) | 24426 (33.4) | 36332 (25.7) |
| II | 7956 (19.2) | 4755 (37.6) | 5207 (36.9) | 25190 (34.5) | 43108 (30.5) |
| III | 14313 (34.5) | 3216 (25.4) | 3657 (25.9) | 20502 (28.1) | 41688 (29.5) |
| IV | 15799 (38.1) | 579 (4.6) | 828 (5.9) | 2917 (4) | 20123 (14.3) |
| Grade | |||||
| Well | 2399 (5.8) | 1314 (10.4) | 1459 (10.3) | 8479 (11.6) | 13651 (9.7) |
| Moderate | 26863 (64.8) | 9080 (71.8) | 10068 (71.4) | 53387 (73.1) | 99398 (70.4) |
| Poorly | 10709 (25.8) | 2043 (16.1) | 2297 (16.3) | 9734 (13.3) | 24783 (17.5) |
| Undifferentiated | 1488 (3.6) | 214 (1.7) | 282 (2) | 1435 (2) | 3419 (2.4) |
| Histology | |||||
| Adenocarcinoma | 32932 (79.4) | 9709 (76.7) | 10920 (77.4) | 56050 (76.7) | 109611 (77.6) |
| Mucinous adenocarcinoma and signet-ring cell carcinoma | 4075 (9.8) | 1073 (8.5) | 112 (7.9) | 5174 (7.1) | 11434 (8.1) |
| Others | 4452 (10.8) | 1869 (14.8) | 2074 (14.7) | 11811 (16.2) | 20206 (14.3) |
| Surgery | |||||
| Yes | 36485 (88) | 12112 (95.7) | 13507 (95.8) | 71367 (97.7) | 133471 (94.5) |
| No | 4974 (12) | 539 (4.3) | 599 (4.2) | 1668 (2.3) | 7780 (5.5) |
| Radiotherapy | |||||
| Yes | 6094 (14.7) | 929 (7.3) | 1202 (8.5) | 9064 (12.4) | 17289 (12.2) |
| No | 35365 (85.3) | 11722 (92.7) | 12769 (91.5) | 63971 (87.6) | 123962 (87.8) |
| Chemotherapy | |||||
| Yes | 19038 (45.9) | 2036 (16.1) | 2593 (18.4) | 24410 (33.4) | 48077 (34) |
| No | 22421 (54.1) | 10615 (83.9) | 11513 (81.6) | 48625 (66.6) | 93174 (66) |
Patients were randomly divided into a training cohort (98876 patients) and a test cohort (42375 patients) in a ratio of 7:3 based on the “caret” package on the outcome of “dead.” Univariate analysis on the cancer-specific mortality and CVD-specific mortality was performed in the training cohort.
As shown in Table 2, univariate analysis revealed that age was both associated with cancer-specific mortality and CVD-specific mortality. The risk of cancer-specific mortality in patients aged ≥ 80 years was 1.63-times that of patients aged 60-69 years, while the risk of CVD-specific mortality in patients aged ≥ 80 years was 7.31-times that of patients aged 60-69 years. TNM stage was positively associated with cancer-specific mortality. However, the TNM stage was negatively associated with CVD-specific mortality, and the risk of CVD-specific mortality in TNM stage IV was 0.73-times that of TNM stage I. Absence of chemotherapy was associated with cancer-specific mortality, but presence of chemotherapy was associated with CVD-specific mortality.
| Variables | Univariate analysis (cancer-specific mortality) | Univariate analysis (CVD-specific mortality) | Multivariate analysis (cancer-specific mortality) | Multivariate analysis (CVD-specific mortality) | ||||||||
| HR | 95%CI | P value | HR | 95%CI | P value | HR | 95%CI | P value | HR | 95%CI | P value | |
| Age at diagnosis (years) | ||||||||||||
| 60-69 | Reference | Reference | Reference | Reference | ||||||||
| 70-79 | 1.17 | 1.14-1.2 | < 0.001 | 2.52 | 2.36-2.69 | < 0.001 | 1.34 | 1.3-1.37 | < 0.001 | 2.44 | 2.29-2.61 | < 0.001 |
| ≥ 80 | 1.63 | 1.58-1.67 | < 0.001 | 7.31 | 6.88-7.77 | < 0.001 | 1.94 | 1.88-2 | < 0.001 | 6.43 | 6.02-6.85 | < 0.001 |
| Sex | ||||||||||||
| Female | Reference | Reference | Reference | |||||||||
| Male | 1.03 | 1.1-1.05 | < 0.01 | 1.07 | 1.02-1.11 | < 0.01 | 1.16 | 1.13-1.19 | < 0.001 | 1.58 | 1.51-1.65 | < 0.001 |
| Marital status | ||||||||||||
| Single | Reference | Reference | Reference | Reference | ||||||||
| Married | 0.77 | 0.74-0.80 | < 0.001 | 0.72 | 0.67-0.77 | < 0.001 | 0.83 | 0.80-0.86 | < 0.001 | 0.68 | 0.63-0.73 | < 0.001 |
| Others | 0.98 | 0.94-1.02 | 0.31 | 1.28 | 1.19-1.38 | < 0.001 | 0.98 | 0.94-1.01 | 0.21 | 0.93 | 0.87-1.00 | 0.06 |
| Race | ||||||||||||
| Black | Reference | Reference | Reference | Reference | ||||||||
| White | 0.82 | 0.79-0.85 | < 0.001 | 1.05 | 0.98-1.12 | 0.2 | 0.84 | 0.81-0.88 | < 0.001 | 0.89 | 0.83-0.96 | < 0.001 |
| Others | 0.71 | 0.68-0.75 | < 0.001 | 0.66 | 0.50-0.74 | < 0.001 | 0.73 | 0.69-0.77 | < 0.001 | 0.63 | 0.57-0.71 | < 0.001 |
| Year of diagnosis | ||||||||||||
| 2004-2007 | Reference | Reference | Reference | Reference | ||||||||
| 2008-2011 | 0.92 | 0.9-0.95 | < 0.001 | 0.86 | 0.82-0.91 | < 0.001 | 0.89 | 0.87-0.91 | < 0.001 | 0.89 | 0.85-0.94 | < 0.001 |
| 2012-2015 | 0.85 | 0.83-0.88 | < 0.001 | 0.73 | 0.68-0.78 | < 0.001 | 0.82 | 0.8-0.85 | < 0.001 | 0.79 | 0.74-0.85 | < 0.001 |
| Primary site | ||||||||||||
| Right half colon | Reference | Reference | Reference | Reference | ||||||||
| Left half colon | 0.93 | 0.9-0.95 | < 0.001 | 0.84 | 0.80-0.89 | < 0.001 | 0.99 | 0.96-1.02 | 0.39 | 1.02 | 0.97-1.07 | 0.44 |
| Rectum | 1.07 | 1.04-1.1 | < 0.001 | 0.76 | 0.72-0.80 | < 0.001 | 1.08 | 1.04-1.12 | < 0.001 | 1.06 | 0.99-1.13 | 0.08 |
| TNM stage | ||||||||||||
| I | Reference | Reference | Reference | Reference | ||||||||
| II | 2.09 | 1.99-2.19 | < 0.001 | 1.05 | 1.00-1.11 | 0.05 | 1.39 | 1.29-1.49 | < 0.001 | 1.03 | 0.98-1.09 | 0.26 |
| III | 4.39 | 4.19-4.59 | < 0.001 | 0.86 | 0.81-0.90 | < 0.001 | 2.46 | 2.28-2.65 | < 0.001 | 0.78 | 0.63-0.98 | 0.04 |
| IV | 18.42 | 17.61-19.27 | < 0.001 | 0.73 | 0.65-0.81 | < 0.001 | 9.26 | 8.61-9.95 | < 0.001 | 0.87 | 0.72-1.06 | 0.17 |
| Grade | ||||||||||||
| Well | Reference | Reference | Reference | Reference | ||||||||
| Moderate | 1.64 | 1.56-1.72 | < 0.001 | 1.05 | 0.98-1.13 | 0.14 | 1.26 | 1.2-1.33 | < 0.001 | 1.05 | 0.99-1.13 | 0.15 |
| Poorly | 3.11 | 2.95-3.28 | < 0.001 | 1.14 | 1.05-1.24 | < 0.001 | 1.65 | 1.56-1.74 | < 0.001 | 1.08 | 0.98-1.13 | 0.07 |
| Undifferentiated | 3.48 | 3.22-3.76 | < 0.001 | 1.11 | 0.93-1.31 | 0.25 | 1.81 | 1.68-1.96 | < 0.001 | 1.11 | 0.93-1.32 | 0.26 |
| Histology | ||||||||||||
| Adenocarcinoma | Reference | Reference | Reference | Reference | ||||||||
| Mucinous adenocarcinoma and signet-ring cell carcinoma | 1.26 | 1.21-1.31 | < 0.001 | 1.14 | 1.06-1.23 | < 0.001 | 1.05 | 1.01-1.09 | 0.01 | 1.13 | 1.05-1.22 | < 0.01 |
| Others | 0.71 | 0.68-0.74 | < 0.001 | 1 | 0.94-1.06 | 0.95 | 1.01 | 0.97-1.04 | 0.77 | 1.01 | 0.95-1.07 | 0.77 |
| Surgery | ||||||||||||
| Yes | Reference | Reference | Reference | Reference | ||||||||
| No | 4.24 | 4.09-4.4 | < 0.001 | 1.74 | 1.57-1.94 | < 0.001 | 3.46 | 3.32-3.62 | < 0.001 | 2.07 | 1.86-2.32 | < 0.001 |
| Radiotherapy | ||||||||||||
| Yes | Reference | Reference | Reference | Reference | ||||||||
| No | 1.22 | 1.18-1.26 | < 0.001 | 0.57 | 0.52-0.61 | < 0.001 | 1.17 | 1.12-1.22 | < 0.001 | 0.99 | 0.89-1.10 | 0.9 |
| Chemotherapy | ||||||||||||
| Yes | Reference | Reference | Reference | Reference | ||||||||
| No | 1.66 | 1.62-1.7 | < 0.001 | 0.39 | 0.37-0.41 | < 0.001 | 1.66 | 1.64-1.68 | < 0.001 | 0.51 | 0.47-0.55 | < 0.001 |
On multivariate analysis, we found that the age at diagnosis ( ≥ 80 years vs 60-69, HR: 6.43;70-79 vs 60-69, HR: 2.44); sex (male vs female, HR: 1.58); marital status (married vs single, HR: 0.68); year of diagnosis (2008-2011 vs 2004-2007, HR: 0.89; 2012-2015 vs 2004-2007, HR: 0.79); surgery (no vs yes, HR: 2.07); and chemotherapy (no vs yes, HR: 0.51) was associated with CVD-specific mortality (Table 2).
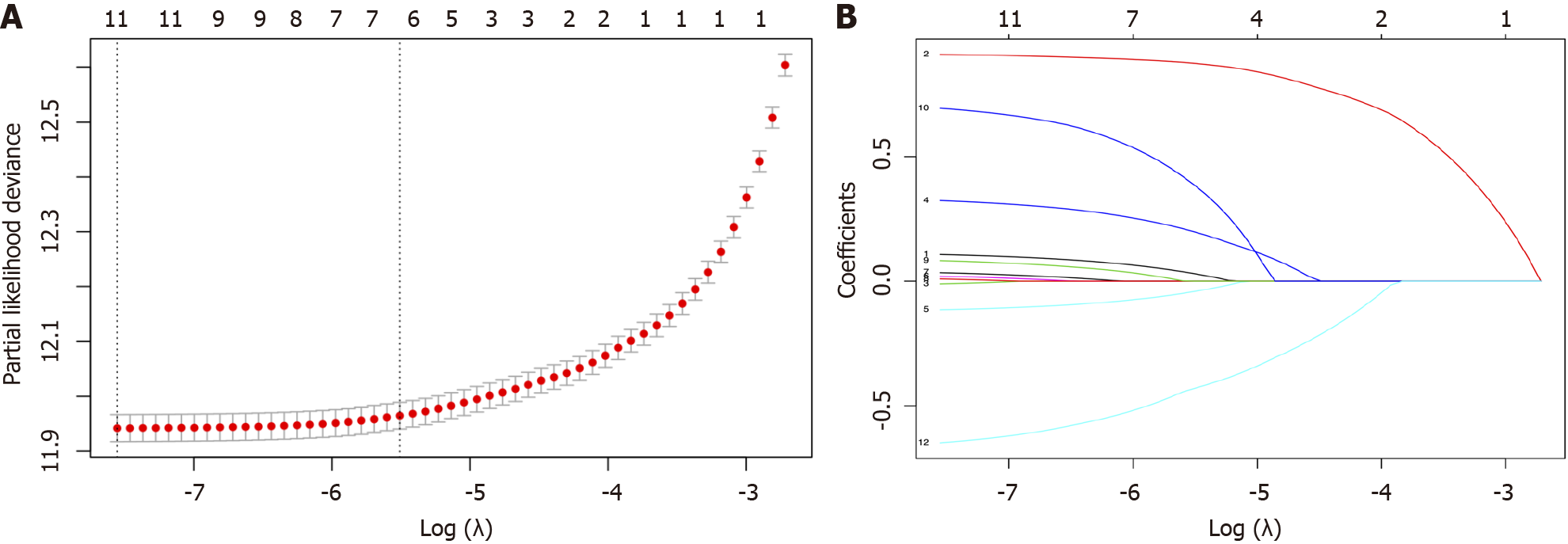
We performed LASSO regression analysis to reduce the risk of over-fitting of our model by compressing the partial factorial regression coefficient to zero[12]. After primary filtration, we used penalty parameter tuning performed via 10-fold cross-validation to further narrow the variables, which requires the selected variables to appear more than 900 times in a total of 1000 times 10-fold cross-validation repetitions (Figure 2).
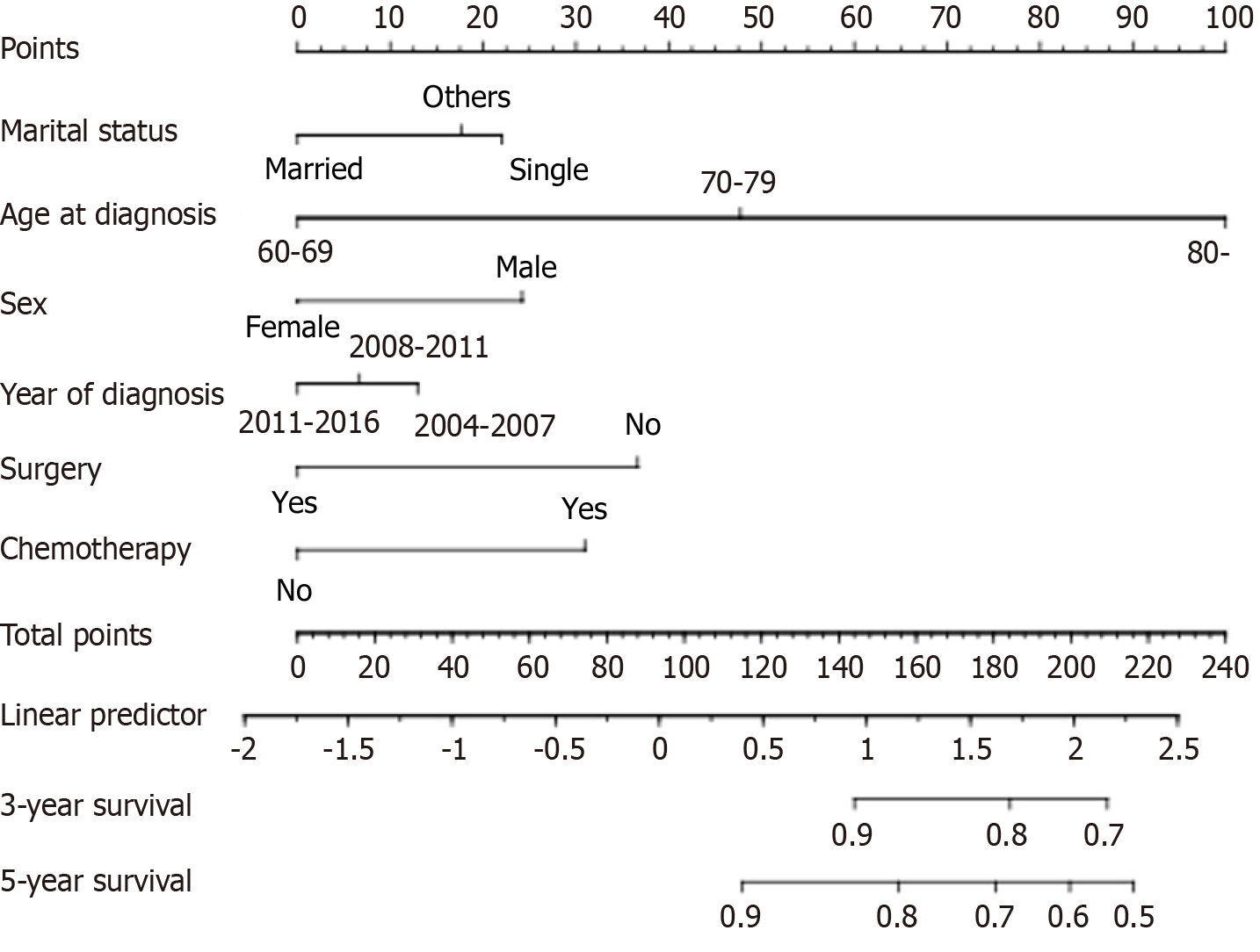
Finally, six variables-age at diagnosis, sex, marital status, year of diagnosis, surgery, and chemotherapy-were selected to construct the nomogram-based model, which was used to estimate the 3-year and 5-year CVD-specific mortality (Figure 3).
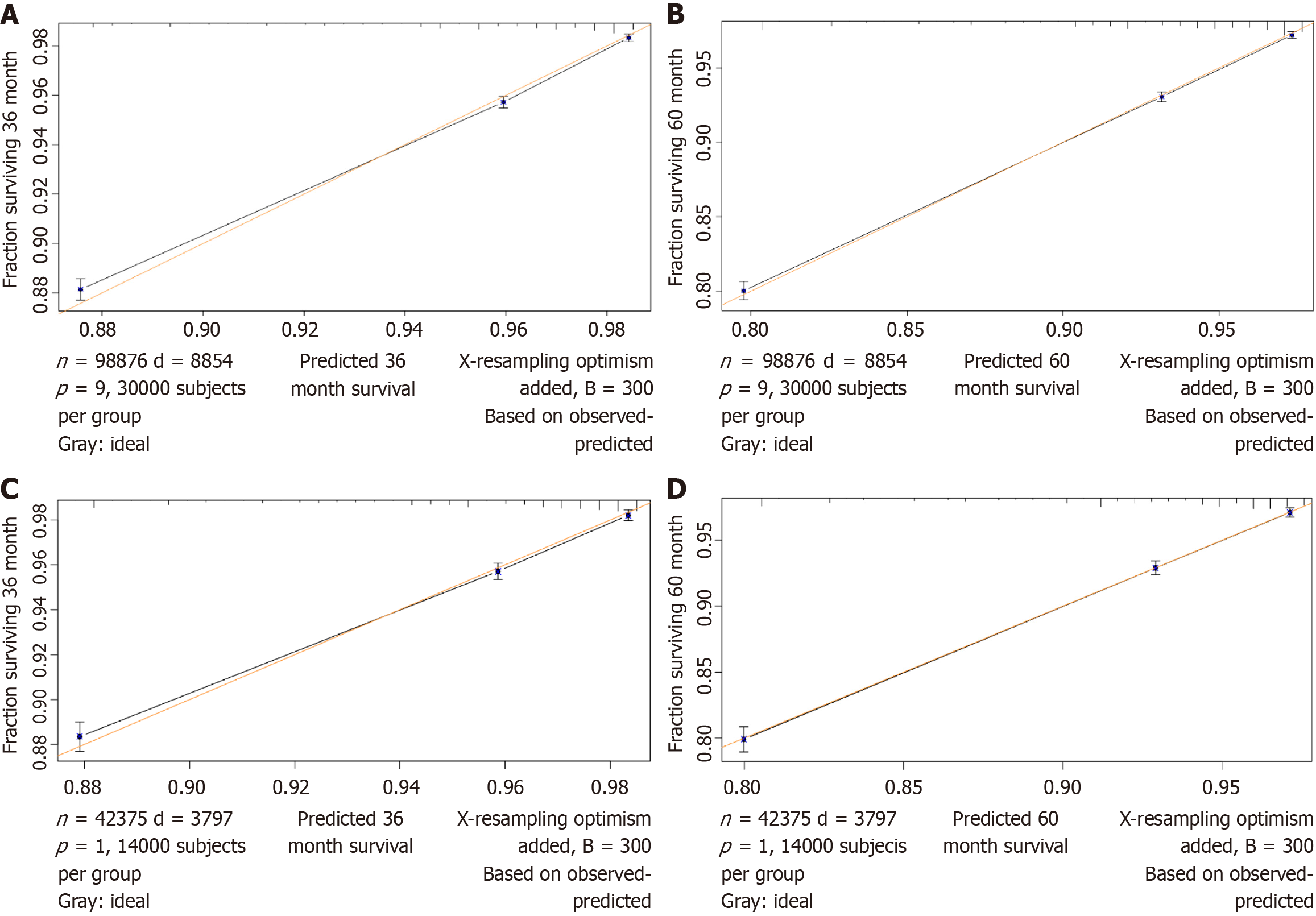
In the training cohort, the C-index of the nomogram for predicting CVD-specific mortality was 0.728 (95%CI: 0.722-0.734), indicating good discrimination. Figure 4A and B show a corrected graph of the prediction accuracy of the nomogram, which shows good consistency between the actual and predicted 3- and 5-year CVD-specific mortality, with a slope of nearly 45°.
Similarly, in the testing cohort, the C-index of the nomogram for CVD-specific mortality was 0.734 (95%CI: 0.725-0.743). Figure 4C and D show the corrected graph of the prediction accuracy of the nomogram, which showed good consistency between the actual and predicted 3- and 5-year cardiovascular mortality rates, with a slope of nearly 45°.
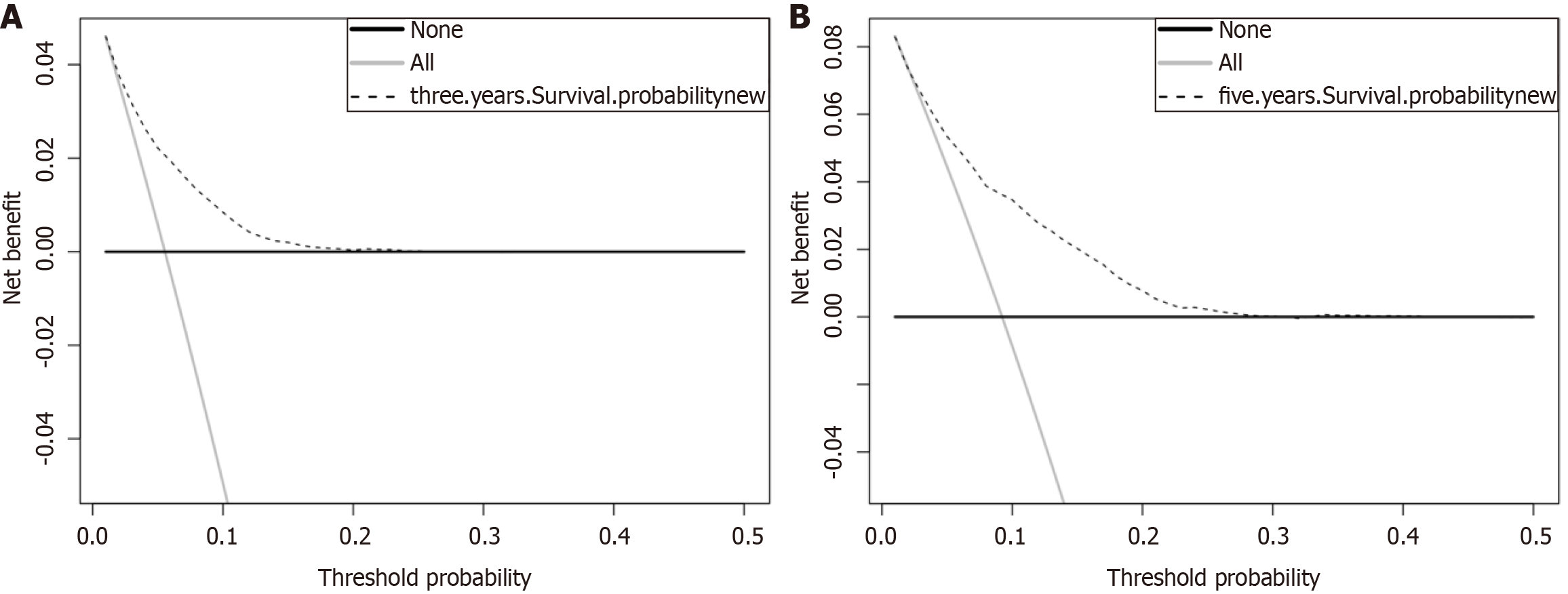
The DCA was plotted to evaluate how clinical benefits affected patients (Figure 5). According to the DCA, our nomogram had a positive net benefit in the training and testing cohorts, with a wide threshold probability range.
According to previous studies, older patients with CRC had a significantly higher risk of CVD morbidity and CVD-specific mortality than the general population[5]. Researchers have analyzed various causes of death in patients with different types of cancer and have emphasized that CVD was the most prevalent cause of non-cancer death in patients with cancer[13,14]. In recent years, a new international discipline called cardio-oncology has emerged that integrates cardiology and oncology organically and is receiving extensive attention. Cardio-oncology as a new discipline has now become a research hotspot. In a Canadian study, researchers found that CVD was the leading non-cancer cause of death among older patients with CRC[15]. Our findings were consistent with this Canadian study. In our study, we focused on the cardio-oncological health of older patients with CRC and found that the CVD-specific mortality was 8.96% among older patients with CRC, the second-leading cause of death, after CRC-specific mortality (29.35%). Therefore, it is very important to explore the prognostic factors of CVD-specific mortality in older patients with CRC.
To our best knowledge, the prognostic factors of CVD-specific mortality for older patients with CRC have not yet been completely elucidated, there is no constructed nomogram model for CVD-specific mortality in older patients with CRC. Therefore, our study focused on identifying the prognostic risk factors for CVD-specific mortality among older patients with CRC. In this study, we performed LASSO regression analysis to reduce the risk of over-fitting the model and suggested that the age at diagnosis, sex, marital status, year of diagnosis, surgery, and chemotherapy were the key prognostic factors associated with CVD-specific mortality among older patients with CRC. We synthesized a variety of analysis methods to construct a nomogram-based prognostic evaluation model with these six key prognostic factors, and validated the prognostic model with a testing cohort. By using comprehensive analysis and further verification, we found good predictive effect and high reliability. To our knowledge, this is the largest contemporary cohort of CVD-specific mortality and construction of nomogram-based prognostic evaluation model for older patients with CRC.
Our results indicated that the risk of CRC-specific mortality and CVD-specific mortality were both negatively associated with age at diagnosis. Previous studies have reported that patients with cancer perpetually have a higher risk of dying from CVD than the general population in the United States, and the incidence of any CVD increased with age[16,17]. Meanwhile, age was also identified as a risk factor for anthracycline-induced cardiotoxicity in CRC patients[17].
Our results suggested that male patients had a positive correlation with the risk of CVD-specific mortality. Because estrogen lowers a woman’s risk of CVD, they are typically protected by estrogen. Increased levels of oxidative stress may interfere with the mechanisms of DNA repair and was associated with higher rates of CRC and CVD[18]. Men are less resilient to oxidative stress than women and also have a higher risk of myocardial infarctions than women[19-21]. In addition, in our study, married status was also associated with reduced CVD-specific mortality in older patients with CRC. Married patients generally had better outcomes than single patients, which may be partly related to a favorable family environment[22].
An increasing number of older patients with CRC undergo surgery, chemotherapy, and/or radiotherapy. The improved anti-tumor systemic treatments resulted in longer survival time of patients with CRC[23]. However, the chemotherapeutic agents for CRC, such as oxaliplatin, 5-fluorouracil, cetuximab, and bavacizumab exhibit potential cardiotoxicity that may lead to a progressive increase in CVD deaths. Some studies have reported that a significant number of patients with CRC suffered CVD events following treatment with capecitabine, oxaliplatin, and bevacizumab[24-26]. Unfortunately, there are no specific chemotherapy regimens for patients with cancer in the SEER database, so we were unable to draw conclusions about the effects of specific chemotherapy regimens on CVD-specific mortality in this study. In the future, we aim to collect relevant data from other research centers for analysis to obtain more accurate results.
We developed a nomogram-based model for predicting the CVD-specific mortality in older patients with CRC based on six key prognostic variables, which is the first for CRC patients > 60 years old based on the SEER database. The model could be well applied in clinical work. This nomogram provides a visual and convenient assessment tool not only for the follow-up management of patients with cancer but also to ensure early intervention measures for such a high-risk population to improve the prognosis of patients and reduce the burden on healthcare resources. The nomogram-based model emphasized the contributions of age at diagnosis, sex, marital status, year of diagnosis, surgery, and chemo
Older patients with CRC have a better cancer-specific prognosis, but the risk of CVD-specific death is still higher. Anti-tumor therapy could directly lead to CVD or increase the risk of CVD, which had a serious impact on the quality of life and health of patients with cancer. There is growing evidence showing the shared pathophysiology and overlapping risk factors between CRC and CVD in the field of cardio-oncology[27-29]. Cardio-oncology has explored an optimal approach to manage these patients via the active collaboration between oncologists and cardiologists[30]. Patients with CRC maybe benefit from clinical intervention, which could reduce the CVD events. In addition, this study also emphasizes the need for continuous and active surveillance during patient survival. This finding supports the early intervention of car
This study has some limitations. First, the SEER database lacked many important variables such as blood lipid data, height, weight, medical history, and chemotherapy regimens. Second, the SEER database includes data from many medical centers, and the data were hence heterogeneous. We have adopted strict inclusion and exclusion of indicators to reduce heterogeneity. Third, our nomogram currently lacks an external cohort for predictive efficacy. In the future, we aim to incorporate external cohorts to validate our nomogram.
The age at diagnosis, sex, marital status, year of diagnosis, surgery, and chemotherapy were independent prognostic factors associated with CVD-specific mortality in older patients with CRC. The proposed nomogram-based model of the CVD-specific mortality could be used to predict the accurate prognosis for older patients with CRC and could be adopted to assist clinicians including oncologists and cardiologists to provide screening recommendations and choose an opti
The authors acknowledge each of them for the continuous support received during this study. The authors acknowledge the Surveillance, Epidemiology, and End Results (SEER) Program.
| 1. | Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2268] [Cited by in RCA: 3264] [Article Influence: 652.8] [Reference Citation Analysis (2)] |
| 2. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 11922] [Article Influence: 2980.5] [Reference Citation Analysis (4)] |
| 3. | Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3058] [Cited by in RCA: 3299] [Article Influence: 412.4] [Reference Citation Analysis (3)] |
| 4. | Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, van Ballegooijen M, Goede SL, Ries LA. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1504] [Article Influence: 100.3] [Reference Citation Analysis (1)] |
| 5. | Chen J, Zheng Y, Wang H, Zhang D, Zhao L, Yu D, Lin Z, Zhang T. Cause of death among patients with colorectal cancer: a population-based study in the United States. Aging (Albany NY). 2020;12:22927-22948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Dekker JW, Gooiker GA, Bastiaannet E, van den Broek CB, van der Geest LG, van de Velde CJ, Tollenaar RA, Liefers GJ; Steering Committee of the ‘Quality Information System Colorectal Cancer’ Project. Cause of death the first year after curative colorectal cancer surgery; a prolonged impact of the surgery in elderly colorectal cancer patients. Eur J Surg Oncol. 2014;40:1481-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Aquina CT, Mohile SG, Tejani MA, Becerra AZ, Xu Z, Hensley BJ, Arsalani-Zadeh R, Boscoe FP, Schymura MJ, Noyes K, Monson JR, Fleming FJ. The impact of age on complications, survival, and cause of death following colon cancer surgery. Br J Cancer. 2017;116:389-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 8. | van de Poll-Franse LV, Haak HR, Coebergh JW, Janssen-Heijnen ML, Lemmens VE. Disease-specific mortality among stage I-III colorectal cancer patients with diabetes: a large population-based analysis. Diabetologia. 2012;55:2163-2172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Baraghoshi D, Hawkins ML, Abdelaziz S, Park J, Wan Y, Fraser AM, Smith KR, Deshmukh V, Newman M, Rowe KG, Snyder J, Lloyd S, Samadder NJ, Hashibe M. Long-term risk of cardiovascular disease among colorectal cancer survivors in a population-based cohort study. J Clin Oncol. 2018;36:113-113. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Wang R, Han L, Dai W, Mo S, Xiang W, Li Q, Xu Y, Cai G. Cause of death for elders with colorectal cancer: a real-world data analysis. J Gastrointest Oncol. 2020;11:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Tibshirani R. Regression Shrinkage and Selection Via the Lasso. J R Stat Soc Ser B: Stat Methodol. 1996;58:267-288. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8558] [Cited by in RCA: 6201] [Article Influence: 885.9] [Reference Citation Analysis (0)] |
| 12. | Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med. 1997;16:385-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 61] [Reference Citation Analysis (0)] |
| 13. | Ye Y, Otahal P, Marwick TH, Wills KE, Neil AL, Venn AJ. Cardiovascular and other competing causes of death among patients with cancer from 2006 to 2015: An Australian population-based study. Cancer. 2019;125:442-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Zaorsky NG, Churilla TM, Egleston BL, Fisher SG, Ridge JA, Horwitz EM, Meyer JE. Causes of death among cancer patients. Ann Oncol. 2017;28:400-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 444] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 15. | Raycraft T, Cheung WY, Yin Y, Speers C, Ko JJ, Mariano C. Causes of mortality in older patients with stage 3 colon cancer. J Geriatr Oncol. 2019;10:138-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Savji N, Rockman CB, Skolnick AH. Association Between Advanced Age and Vascular Disease in Different Arterial Territories: A Population Database of Over 3.6 Million Subjects. J Vasc Surg. 2013;58:1719-1720. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Volkova M, Russell R 3rd. Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment. Curr Cardiol Rev. 2011;7:214-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 419] [Cited by in RCA: 593] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 18. | Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. 2007;121:2381-2386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 673] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 19. | D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4272] [Cited by in RCA: 5255] [Article Influence: 309.1] [Reference Citation Analysis (0)] |
| 20. | Kander MC, Cui Y, Liu Z. Gender difference in oxidative stress: a new look at the mechanisms for cardiovascular diseases. J Cell Mol Med. 2017;21:1024-1032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 347] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 21. | White RE, Gerrity R, Barman SA, Han G. Estrogen and oxidative stress: A novel mechanism that may increase the risk for cardiovascular disease in women. Steroids. 2010;75:788-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Manfredini R, De Giorgi A, Tiseo R, Boari B, Cappadona R, Salmi R, Gallerani M, Signani F, Manfredini F, Mikhailidis DP, Fabbian F. Marital Status, Cardiovascular Diseases, and Cardiovascular Risk Factors: A Review of the Evidence. J Womens Health (Larchmt). 2017;26:624-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 23. | van Steenbergen LN, Elferink MAG, Krijnen P, Lemmens VEPP, Siesling S, Rutten HJT, Richel DJ, Karim-Kos HE, Coebergh JWW; Working Group Output of The Netherlands Cancer Registry. Improved survival of colon cancer due to improved treatment and detection: a nationwide population-based study in The Netherlands 1989-2006. Ann Oncol. 2010;21:2206-2212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Chang RY, Lee MY, Kan CB, Hsu WP, Hsiao PC. Oxaliplatin-induced acquired long QT syndrome with torsades de pointes and myocardial injury in a patient with dilated cardiomyopathy and rectal cancer. J Chin Med Assoc. 2013;76:466-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Allegra CJ, Yothers G, O'Connell MJ, Sharif S, Colangelo LH, Lopa SH, Petrelli NJ, Goldberg RM, Atkins JN, Seay TE, Fehrenbacher L, O'Reilly S, Chu L, Azar CA, Wolmark N. Initial safety report of NSABP C-08: A randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J Clin Oncol. 2009;27:3385-3390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 207] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 26. | Letarte N, Bressler LR, Villano JL. Bevacizumab and central nervous system (CNS) hemorrhage. Cancer Chemother Pharmacol. 2013;71:1561-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Kenzik KM, Balentine C, Richman J, Kilgore M, Bhatia S, Williams GR. New-Onset Cardiovascular Morbidity in Older Adults With Stage I to III Colorectal Cancer. J Clin Oncol. 2018;36:609-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Keramida K, Charalampopoulos G, Filippiadis D, Tsougos E, Farmakis D. Cardiovascular complications of metastatic colorectal cancer treatment. J Gastrointest Oncol. 2019;10:797-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Hayek SS, Ganatra S, Lenneman C, Scherrer-Crosbie M, Leja M, Lenihan DJ, Yang E, Ryan TD, Liu J, Carver J, Mousavi N, O'Quinn R, Arnold A, Banchs J, Barac A, Ky B. Preparing the Cardiovascular Workforce to Care for Oncology Patients: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;73:2226-2235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 30. | Whelton SP, Berning P, Blumenthal RS, Marshall CH, Martin SS, Mortensen MB, Blaha MJ, Dzaye O. Multidisciplinary prevention and management strategies for colorectal cancer and cardiovascular disease. Eur J Intern Med. 2021;87:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |