Published online Aug 26, 2024. doi: 10.4330/wjc.v16.i8.448
Revised: July 17, 2024
Accepted: July 23, 2024
Published online: August 26, 2024
Processing time: 129 Days and 19.4 Hours
Sodium-dependent glucose transporter 2 inhibitors (SGLT2i) have shown efficacy in reducing heart failure (HF) burden in a very heterogeneous groups of patients, raising doubts about some contemporary assumptions of their mechanism of action. We previously published a prospective observational study that evaluated mechanisms of action of SGLT2i in patients with type 2 diabetes who were in HF stages A and B on dual hypoglycemic therapy. Two groups of patients were in
To evaluate the outcomes regarding natriuretic peptide, oxidative stress, inflammation, blood pressure, heart rate, cardiac function, and body weight.
The study outcomes were examined by dividing each treatment arm into two subgroups according to baseline parameters of global longitudinal strain (GLS), N-terminal pro-brain natriuretic peptide, myeloperoxidase (MPO), high-sensitivity C-reactive protein (hsCRP), and systolic and diastolic blood pressure. To evaluate the possible predictors of observed changes in the SGLT2i arm during follow-up, a rise in stroke volume index, body mass index (BMI) decrease, and lack of heart rate increase, linear regression analysis was performed.
There was a greater reduction of MPO, hsCRP, GLS, and blood pressure in the groups with higher baseline values of mentioned parameters irrespective of the therapeutic arm after 6 months of follow-up. Significant independent predictors of heart rate decrease were a reduction in early mitral inflow velocity to early diastolic mitral annular velocity at the interventricular septal annulus ratio and BMI, while the predictor of stroke volume index increase was SGLT2i therapy itself.
SGLT2i affect body composition, reduce cardiac load, improve diastolic/systolic function, and attenuate the sym
Core Tip: This study evaluated the outcomes regarding natriuretic peptide, oxidative stress, inflammation, blood pressure, heart rate, cardiac function, and body weight derived from our published prospective observational study, which assessed sodium-dependent glucose transporter 2 inhibitors (SGLT2i) mechanisms of action in patients with type 2 diabetes and heart failure (HF) stages A and B on dual oral antidiabetic therapy. Mechanisms underlying favorable SGLT2i effects on HF are related to changes in body composition, reduced cardiac load, better cardiac function, and attenuation of sympathetic response, depending on the HF stage and patients’ specific characteristics. Nevertheless, glycemic control itself could contribute to heart function improvement.
- Citation: Grubić Rotkvić P, Rotkvić L, Đuzel Čokljat A, Cigrovski Berković M. Sodium-dependent glucose transporter 2 inhibitors effects on myocardial function in patients with type 2 diabetes and asymptomatic heart failure. World J Cardiol 2024; 16(8): 448-457
- URL: https://www.wjgnet.com/1949-8462/full/v16/i8/448.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i8.448
Heart failure (HF), a clinical syndrome defined by its progressive nature, is becoming a growing health problem, carrying a 5-year mortality risk of around 75%[1]. People living with diabetes, one of the largest global public health problem as well, are especially prone to developing cardiovascular disease (or to be specific-macrovascular complication of diabetes) but also diabetic cardiomyopathy, a particular form of cardiomyopathy that develops independently of concomitant macro- and microvascular diabetic complications[2,3]. Indeed, the risk of developing HF in people with diabetes is two- to five-fold greater compared to those without[4]. Sodium-dependent glucose transporters inhibitor (SGLT2i), initially developed for treating hyperglycemia, have become a new panacea in HF therapy since they showed efficacy in reducing cardiovascular risk and HF burden irrespective of the initial HF type (preserved, reduced, or mildly reduced left ventricle ejection fraction) and the presence of established HF at baseline as well as independently of the diabetic status[5-9]. Despite the abundance of new information regarding SGLT2i mechanisms of action, the present literature focuses primarily on patients without diabetes and with advanced HF stages (C and D). Nevertheless, we must be aware that early treatment is important to prevent the progression of asymptomatic HF, especially in patients with type 2 diabetes (T2DM) and diabetic cardiomyopathy where timely treatment could favorably impact the prognosis independently of atherosclerotic cardiovascular disease[10].
Grubić Rotkvić et al[11] previously published the design and results from a prospective observational study that evaluated the possible mechanisms of action of SGLT2i in patients with T2DM who were in HF stages A and B and hence at risk of developing symptomatic HF. The study population consisted of two groups of patients with T2DM followed for 6 months: the ones that received SGLT2i as an add-on agent and others on dipeptidyl peptidase-4 inhibitors (DPP-4i) as an add-on to metformin. Here, we examined the study outcomes by further dividing each treatment arm into two subgroups according to the ranking of baseline resting parameters of interest that did not demonstrate significant changes between the treatment groups at the 6 months follow-up in the main analysis (global longitudinal strain [GLS] of the left ventricle, N-terminal pro-brain natriuretic peptide [NT-proBNP], myeloperoxidase [MPO], high sensitivity C-reactive protein [hsCRP], systolic and diastolic arterial pressure). Furthermore, we evaluated the possible clinical/echocardiographic predictors of the observed changes in the SGLT2i arm: rise in stroke volume index, body mass index (BMI) decrease, and a lack of heart rate increase at 6 months.
The study design and population with inclusion and exclusion criteria were previously published[11]. Briefly, it was a prospective, observational, non-randomized, two-center study aiming to verify whether SGLT2i impact biomarkers of myocardial stress, inflammation, and oxidative stress (NT-proBNP, hsCRP, MPO, respectively), diastolic heart function, myocardial contractility and structure assessed by echocardiography, in patients with T2DM and HF stages A and B that were already on metformin but due to suboptimal glycemic control needed treatment intensification with a second antidiabetic agent. The choice of therapeutic drug to add was based on patient-related and drug-specific factors and according to the valid international and Croatian guidelines on second-line diabetic therapy at the time the study was conducted[12]. The groups that received SGLT2i as an add-on agent, and for comparison, DPP-4i as an add-on to metformin (due to their presumed neutral effect on the heart except saxagliptin), were followed for 6months. SGLT2i available in our country at that time were: dapagliflozin and empagliflozin, while DPP-4i were the following: sitagliptin, linagliptin, alogliptin, vildagliptin, and saxagliptin (the latter was not used due to a potential increased risk of HF hospitalization). In our study, the patients were taking mostly vildagliptin (90% of patients in the DPP-4i group), and the rest were on sitagliptin, linagliptin, and alogliptin. Among SGLT2i, the most used agent was empagliflozin (81% of patients in the SGLT2i group), while the remaining patients were taking dapagliflozin. The study was conducted at two University hospitals in Zagreb, Croatia, between 2019 and 2022. Inclusion criteria were as follows: the need for an add-on agent (SGLT2i or DPP-4i, saxagliptin excluded) in addition to metformin due to inadequate glycemic control; age ≥ 18 years; willingness to participate in the study; preserved kidney and hepatic function; stable doses of cardioactive drugs such as hypolipemics and antihypertensives if they had them; and stable usual daily physical activities and/or habits. The following exclusion criteria were applied: patients unlikely to comply with study protocol; inability to give informed consent; known atherosclerotic cardiovascular disease; history or symptoms and signs of HF; breastfeeding or pregnancy; acute or chronic inflammatory or autoimmune disease; history of active neoplastic disease within the last 5 years; or use of any other antidiabetic agent besides metformin. Overall, 64 consecutive patients who met the criteria, 32 in each treatment group, underwent blood pressure, heart rate and anthropometric measurements, blood sample analysis, and echocardiography examination according to international recommendations, at baseline, and after 6 months of follow-up. Baseline characteristics of the study population are presented and adapted from Grubić Rotkvić et al[11] in Table 1.
| Characteristics | Group | Total | P value | ||||||
| Metformin + DPP-4i | Metformin + SGLT2i | n | (%) | ||||||
| n | (%) | n | (%) | ||||||
| Age in yr | ≤ 60 | 12 | 37.5 | 19 | 59.4 | 31 | 49.2 | 0.178 | |
| 61-70 | 13 | 40.6 | 7 | 21.9 | 20 | 31.7 | |||
| > 70 | 7 | 21.9 | 6 | 18.8 | 13 | 19.0 | |||
| Total | 32 | 100.0 | 32 | 100.0 | 64 | 100.0 | |||
| Sex | Males | 18 | 56.3 | 18 | 56.3 | 36 | 56.3 | 0.999 | |
| Females | 14 | 43.8 | 14 | 43.8 | 28 | 43.8 | |||
| Total | 32 | 100.0 | 32 | 100.0 | 64 | 100.0 | |||
| Body mass index in kg/m2 | 18.51-24.99 | 2 | 6.3 | 2 | 3.1 | 0.005a | |||
| 25.00-29.99 | 15 | 46.9 | 10 | 31.3 | 25 | 39.1 | |||
| 30.00-34.99 | 14 | 43.8 | 11 | 34.4 | 25 | 39.1 | |||
| ≥ 35.00 | 1 | 3.1 | 11 | 34.4 | 12 | 18.8 | |||
| Total | 32 | 100.0 | 32 | 100.0 | 64 | 100.0 | |||
| Comorbidities | Arterial hypertension | 28 | 87.5 | 22 | 68.8 | 50 | 78.1 | 0.066 | |
| Hyperlipidemia | 20 | 62.5 | 16 | 50.0 | 36 | 56.3 | 0.337 | ||
| Drugs | ACEi/ARB | 24 | 75.0 | 19 | 59.4 | 43 | 67.2 | 0.178 | |
| Beta-blockers | 8 | 25.0 | 8 | 25.0 | 16 | 25.0 | 0.999 | ||
| Calcium channel blockers | 17 | 53.1 | 13 | 40.6 | 30 | 46.9 | 0.340 | ||
| Diuretics | 15 | 46.9 | 12 | 37.5 | 27 | 42.2 | 0.470 | ||
| Moxonidine | 8 | 25.0 | 5 | 15.6 | 13 | 20.3 | 0.378 | ||
| Statins | 20 | 62.5 | 16 | 50.0 | 36 | 56.3 | 0.337 | ||
| Fibrates | 1 | 3.1 | 2 | 6.3 | 3 | 4.7 | 0.567 | ||
Data were statistically processed using SPSS software (version 24.0; IBM SPSS Statistics for Windows; Armonk, NY, United States) under two-sided test conditions with a 5% significance level. Values are presented as the mean ± standard deviation, or the median and interquartile range, for variables with normal and non-normal distribution, respectively. Differences between the two groups were tested using the independent samples t-test and a non-parametric substitute for independent samples (Mann-Whitney U test) where the conditions for calculating the t-test were missing. Differences between initial and follow-up measurements were checked with repeated measures analysis of variance (taking into account potential confounding factors such as the covariates hemoglobin A1c (HbA1c), sex, age, and BMI), with Bonferroni correction for multiple comparisons. A t-test for independent samples was used to test the significance of differences in absolute changes in the follow-up period. Additionally, the differences between the two time points were analyzed for certain clinically relevant variables that did not show significant differences in the main analysis (GLS, NT-proBNP, MPO, hsCRP, systolic, and diastolic arterial pressure) by dividing the treatment groups into subgroups according to their initial values. All variables were divided according to their initial median, and a series of two-way tests was performed (analysis of covariance [ANCOVA], covariates: age, sex, BMI, and HbA1c) to test for differences between treatment groups concerning the categorization of patients according to the initial value. Furthermore, whether certain biochemical and echocardiographic parameter of clinical importance predicts the observed changes during time was verified by linear regression analysis. The analysis was performed for a group of variables in which the changes in the main analysis were more pronounced and/or clinically significant such as stroke volume index, BMI, and heart rate. Variables assumed to be related to a particular criterion were entered as potential predictors, as well as HbA1c, BMI, age, and sex (as a dummy variable, binarized) and treatment group (also a dummy variable). Three multivariate regression models for three dependent criteria were developed. The selection of predictors was based on the initial screening of their relationship with the dependent variable and assumed clinical relevance. All variables were entered into the model at the same time (method enter-a procedure for variable selection in which all variables in a block are entered in a single step). A biomedical statistician performed the statistical review of the study.
The ethics committees of the participating institutions approved the protocol, and all patients gave written informed consent. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee.
Two-way ANCOVA did not find a statistically significant difference between the two subgroups of patients according to initial NT-proBNP value: subgroups with NT-proBNP more or less than 68 pg/mL (F-ratio (F) (1.52) = 0.422, P = 0.519). There was no significant difference between treatment groups (F (1.52) = 3.032, P = 0.088), nor was there a significant interaction of two factors (group x initial NT-proBNP group): F (1.52) = 0.469, P = 0.497.
A borderline difference in MPO change between the two subgroups according to the initial MPO value (F (1.59) = 3.176, P = 0.052) was found. There was a greater reduction in the subgroup with a higher baseline value ≥ 91, 0.8 ng/mL. There was no significant difference between the treatment groups (F (1.59) = 1.797, P = 0.186), nor was there a significant interaction of two factors (group x initial MPO group): F(1.59) = 1.036, P = 0.314.
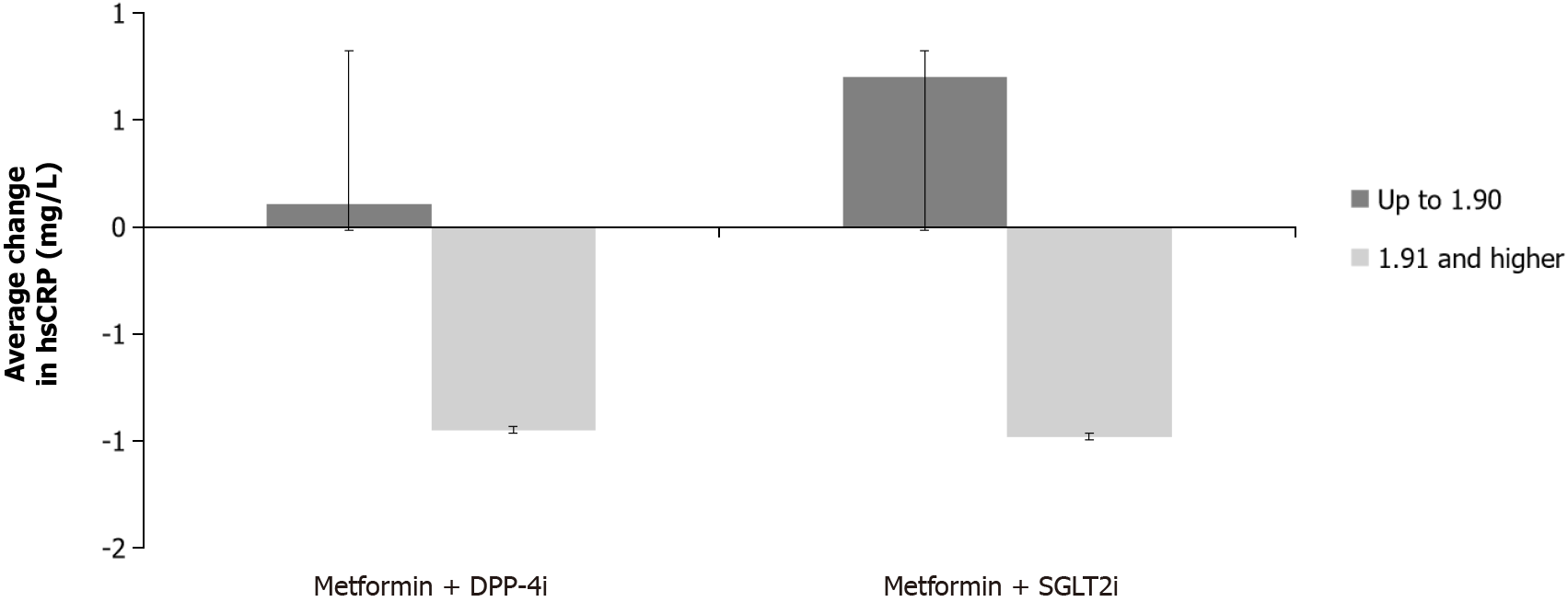
There was a significant difference in hsCRP change between the two subgroups according to the initial hsCRP value (F (1.59) = 6.838, P = 0.012), namely, a greater reduction in the average value was noticed in the subgroup that had an initial hsCRP ≥ 1.91 mg/L. There was no significant difference between the treatment groups (F (1.59) = 0.784, P = 0.380), nor was there a significant interaction between the two factors (group x initial hsCRP group): F (1.59) = 0.194, P = 0.662 (Figure 1).
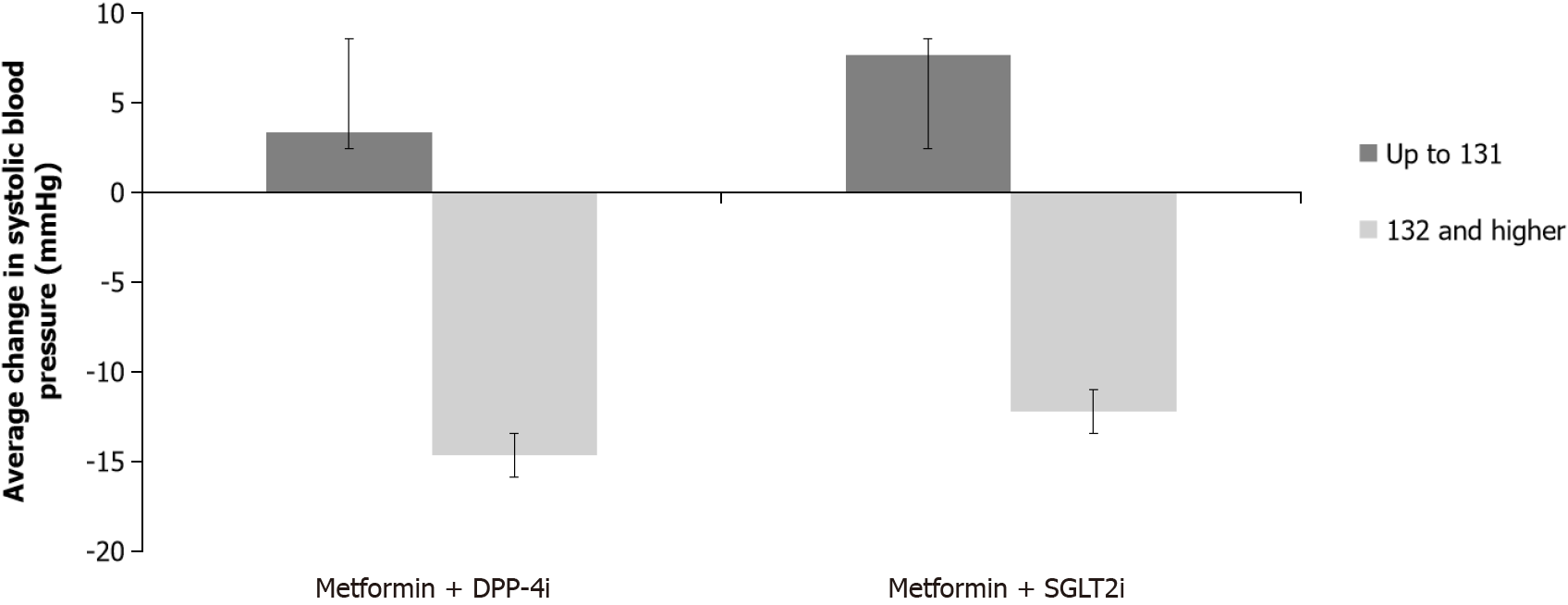
A significant difference in change in systolic pressure between the two subgroups according to the initial systolic pressure value (F (1.60) = 14.917, P < 0.001) was found. Systolic pressure decreased more in the subgroup that had an initial systolic pressure higher than 131 mmHg. No significant difference was found between treatment groups (F (1,60) = 0.281, P = 0.598); however there was a significant interaction of two factors (group x initial blood pressure group): F (1,60) = 7.867, P = 0.029, resulting from the fact that both DPP-4i and SGLT2i lowered systolic pressure in subgroups that had initial pressure > 131 mmHg, but in subgroups with initial pressure ≤ 131 mmHg, systolic pressure increased slightly higher in the SGLT2i group (Figure 2).
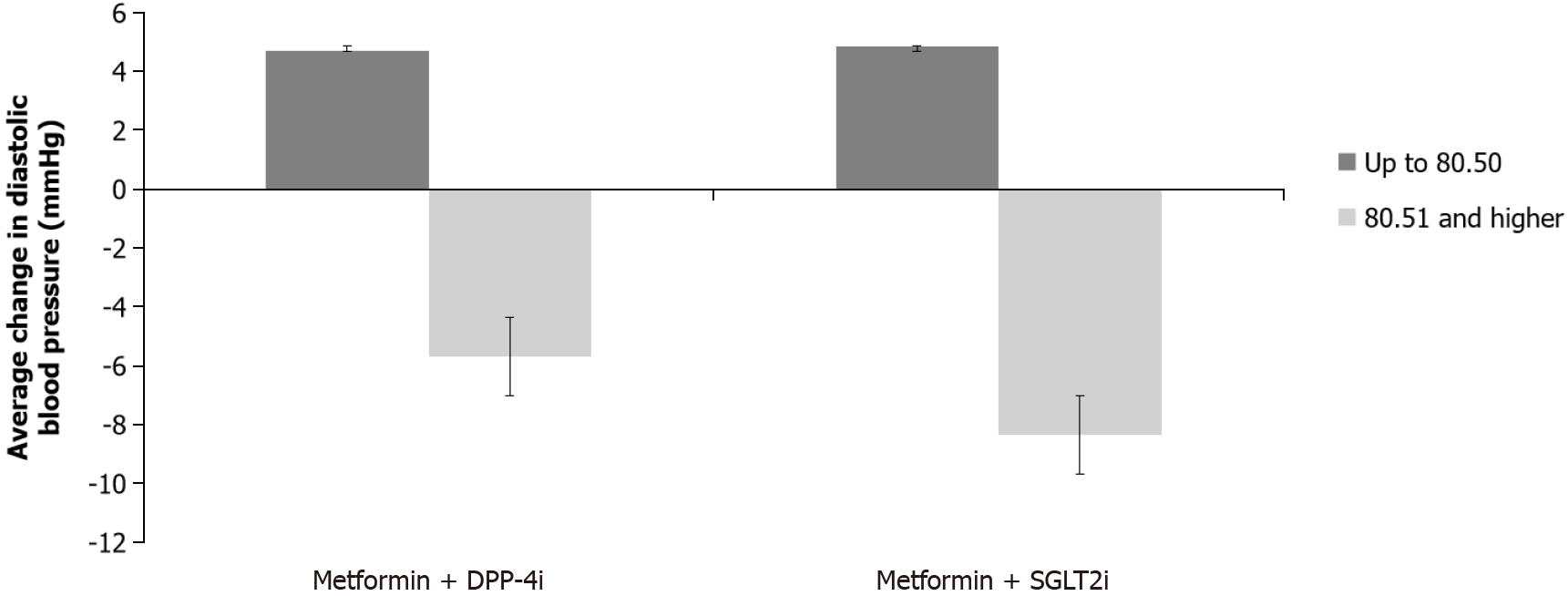
There was a statistically significant difference in the change in diastolic pressure between the two subgroups according to the initial diastolic pressure value (F (1.60) = 23.277, P < 0.0001). Diastolic pressure decreased more in the subgroup with initial pressure > 80.50 mmHg. No significant difference was found between treatment groups (F (1.60) = 0.423, P = 0.518), as well as in the interaction of two factors (group x initial blood pressure group): F (1.61) = 0.227, P = 0.636 (Figure 3).
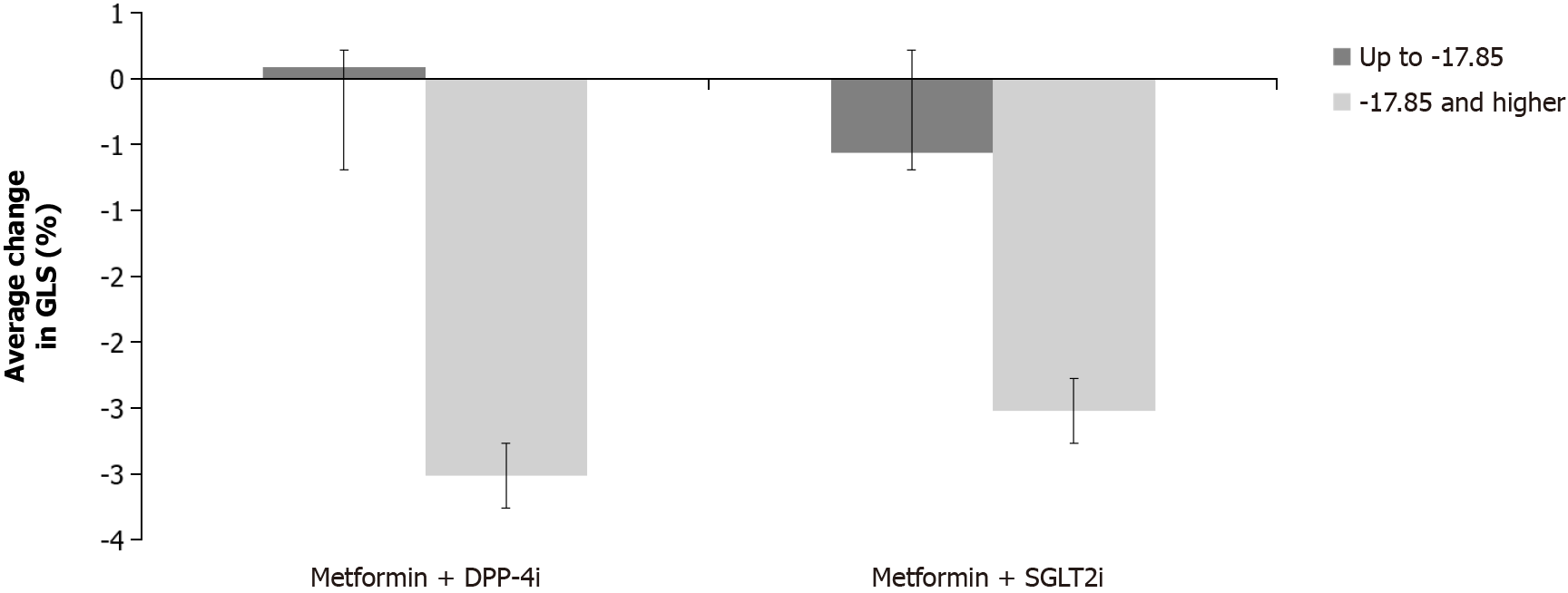
Two-way ANCOVA found a statistically significant difference between the two subgroups of patients according to the initial GLS (F (1.46) = 11.675, P < 0.001). In the subgroup where the initial GLS was > -17.84% the change was greater (resulting in more negative values hence better GLS). No significant difference was found between treatment groups (F (1.46) = 0.536, P = 0.468), nor was there a significant interaction of two factors (group x initial GLS group): F (1,46) = 0.270, P = 0.606 (Figure 4).
In the regression analysis model with an increase in stroke volume index as a criterion, the variables hemoglobin, erythrocytes, hematocrit, BMI, sex, age, HbA1c, and therapeutic group as potential predictors were entered. The model explained 23% of the variance (adjusted R2 = 0.231, F = 2.162, P = 0.071): SGLT2i therapy was the only significant independent predictor (beta-coefficient = 0.480, 95% confidence interval [CI]: 0.129-16.653, P = 0.047).
In the regression analysis model with BMI reduction as a criterion and potential predictors (relative wall thickness, low-density lipoprotein, triglycerides, high-density lipoprotein, HbA1c, age, sex, therapeutic group), the model explained 9% of the variance (adjusted R2 = 0.087, F = 1.349, P = 265) and there were no significant predictors.
Another regression analysis for the reduction of heart rate as a criterion was performed with the following possible predictors: early diastolic mitral annular velocity at the interventricular septal annulus (E’ septal), early mitral inflow velocity to early diastolic mitral annular velocity at the interventricular septal annulus ratio (E/E’), early mitral inflow velocity to atrial filling velocity ratio (E/A), early mitral inflow velocity (E-wave), GLS, left ventricular ejection fraction, tricuspid annular plane systolic excursion (TAPSE), as well as BMI, HbA1c, age, sex, and therapeutic group. For the reduction of heart rate, the model explains 16% of the variance (adjusted R2 = 0.165, F = 1.708, P = 0.113). Significant independent predictors of heart rate reduction were the E/E’ ratio (beta-coefficient = 0.728, 95%CI: 0.038-4.097, P = 0.047) and BMI (beta-coefficient = 3.450, 95%CI: 0.935-3.639, P = 0.002).
This subanalysis, where the patients were categorized according to the baseline measurements into those with higher and lower initial values (according to the obtained median), showed that there was no statistically significant difference between the two groups of patients concerning the initial NT-proBNP, which was consistent with our previous finding and could mean that volume homeostasis is not that significant in this particular patient population. Even in the literature, there have been inconsistent findings concerning the effect of SGLT2i on natriuretic peptides in different groups of patients regarding diabetes or HF status, ranging from slightly increasing and decreasing NT-proBNP to no effect at all[13-16]. In the EMPEROR-Reduced trial, SGLT2i empagliflozin showed a similar effect on reducing the risk of adverse cardiovascular outcomes across different NT-proBNP quartiles[17]. It could be hypothesized that SGLT2i enhances the beneficial physiological action of natriuretic peptides by the competition for the same target since there are reports from experimental studies that atrial natriuretic peptide inhibits renal SGLT2 activity, which could play a role in postponing the symptomatic phase of ventricular dysfunction in people with diabetes as in our specific group of patients with asymptomatic HF A and B stages[11,18].
Regarding MPO, there was a difference of marginal significance in the change of MPO values between the two subgroups according to baseline MPO. A greater reduction was noted in the group with a higher baseline value (≥ 91.08 ng/mL) without a significant difference between the treatment groups. There was also a significant difference in the change of hsCRP between the two subgroups categorized according to baseline hsCRP level. The average value decreased more in the group with initial hsCRP ≥ 1.91 mg/L. Again, no significant difference was found between therapeutic groups or the interaction of two factors. Previously, without categorizing the patients according to baseline MPO and hsCRP values, we did not find any differences during follow-up which could be interpreted in the time context since the effect on systemic inflammation and oxidative stress may only become apparent after a period longer than 6 months, especially in patients who have not yet developed chronic complications of diabetes[19]. However, it should not be ignored that in the categories of patients with higher hsCRP and MPO, the values were lowered during the follow-up period of 6 months in both treatment groups, stressing the potential effect of better glycemic control influencing oxidative stress and inflammation. Both oxidative stress and inflammation are closely related to the development of diabetic cardiomyopathy[2]. Comparing the previously mentioned average values of hsCRP and MPO from our study with literature data, borderline hsCRP values of low, average, and high cardiovascular risk were < 1.0 mg/L, 1.0-3.0 mg/L, and > 3.0 mg/L, respectively, whereas in the study by Nita et al[20], where the effect of fenofibrate was studied on basal MPO values in patients with T2DM on metformin monotherapy but with good glucoregulation, the median MPO was 55.0 (38.5-85.3) ng/mL[20,21]. Our population had a hsCRP median that would be classified as intermediate risk. The MPO was higher than in the study of Nita et al[20], perhaps due to inadequate glycemic control in our population when entering the study.
When the change in systolic pressure was analyzed concerning the therapeutic group and the division into subgroups according to the initial systolic pressure, there was a significant difference in the change in systolic pressure over time with a significant interaction of the two factors: The systolic pressure decreased with both DPP-4i and SGLT2i in subgroups that had initial pressure > 131 mmHg, while in subgroups with initial pressure ≤ 131 mmHg, systolic pressure increased, slightly more in the group on SGLT2i. There was also a statistically significant difference in the change of the diastolic pressure between the two subgroups, it decreased more in the group with initial pressure > 80.50 mmHg. Previous studies have mostly shown that SGLT2i lowers systolic blood pressure, and according to a recent meta-analysis, there was no reduction in diastolic blood pressure. However, the data are still not uniform, for example in the EMPEROR-Preserved and EMPEROR-Reduced randomized trials with empagliflozin in patients with reduced and preserved left ventricular ejection fraction, marginal changes were found between the groups treated with empagliflozin compared to placebo, so it was concluded that empagliflozin is effective in HF favorable outcomes independently of its antihypertensive effect. Moreover, similar to our study, there was an overall increase in systolic blood pressure at low baseline values (< 110 mmHg) as well as a small drop in systolic blood pressure at higher baseline values (> 130 mmHg) in those trials. The effect of empagliflozin was maintained at systolic blood pressure even < 110 mmHg, without causing any harm which is of clinical relevance since physicians often hesitate to initiate treatment due to the fear of over-lowering the blood pressure[7,22,23]. Reports of arterial pressure reduction also exist for DPP-4i[24]. Taken together, both drugs could account for a beneficial effect on blood pressure which could also be due to better glycemic control in this specific population.
Furthermore, when the patients were categorized depending on the initial GLS value, there was a statistically sig
In the regression analysis model with BMI reduction as a criterion and potential predictors (relative wall thickness, low-density lipoprotein, triglycerides, high-density lipoprotein, HbA1c, age, sex, therapeutic group), no significant predictors were determined.
A regression analysis was performed for the increase in stroke volume index as a criterion, and the biochemical variables hemoglobin, erythrocytes, hematocrit and BMI, sex, age, HbA1c, and therapeutic group were entered as potential predictors. Therapy with SGLT2i proved to be the only significant independent predictor of an increase in stroke volume index. The possible explanation for the rise in stroke volume index observed in our main analysis could be the decrease in systemic vascular resistance (afterload), which could be mediated through small increases in hematocrit (also linked to SGLT2i and confirmed in our study as well) that consequently increases blood viscosity and reduce vascular resistance (creating an increase in friction in the area of the microvasculature and stimulating vasoactive substances such as nitric oxide)[11,25]. Moreover, reducing afterload facilitates ventricular emptying and leads to an increase in stroke volume while diminishing myocardial oxygen demand which is more energy efficient.
Due to the clinical importance of possible predictors of heart rate reduction (or at least the absence of an increase which would be expected due to diuretic properties of SGLT2i), a regression analysis was performed with the following possible predictors: E’ septal, E/E’, E/A, E-wave (parameters of diastolic function) and GLS, left ventricular ejection fraction, TAPSE (systolic function parameters), as well as BMI, HbA1c, age, sex, and therapeutic group. The analysis showed that the lowering of BMI and E/E’ in our study contributes to heart rate reduction during SGLT2i therapy. It is known that both obesity and diastolic dysfunction, potential targets of SGLT2 inhibition, are associated with increased sympathetic activity[11,26,27].
As the results of these subanalyses showed effects in both therapeutic groups after stratification into more specific subgroups, it is certainly possible that the improvement of glycemia itself contributed to the improvement of heart function emphasizing that patients with T2DM encompass a different category of patients with potentially distinct pathophysiological mechanisms involved in the process of the failing heart. However, we also need to account for the limitations of this study being a subanalysis with a rather small sample size (hence possible non-trustable null findings that showed no significant differences between the two therapeutic groups in the sub-analyses) and potentially lasting for a too short period for some effects to be seen. Furthermore, there is a possibility of differences between individual agents within the same class as we included the DPP-4i and SGLT2i that were available in our country. Due to a relatively small sample size, we could not investigate extensive multivariable associations with outcomes. To adjust for the potential confounding effect of adiposity, the gold standard would be to measure abdominal visceral adipose tissue using magnetic resonance imaging and/or computed tomography instead of a BMI, but this is an expensive approach and is not always available in routine clinical practice[28]. In addition, there are also conflicting results concerning the possible DPP-4i antioxidant and anti-inflammatory effects, which in experimental studies suggested cardiovascular efficiency but were not consistently proven in human studies[29,30]. Nevertheless, it seems that our comparators are not completely neutral on cardiac function. However, mechanisms underlying the benefits of SGLT2i and their undoubtedly favorable effects in HF are multifactorial-connected to rapid changes in body composition, reduced cardiac load, improvement in diastolic and systolic parameters, and probably attenuation of sympathetic response, while depending on the stage of HF and patients’ specific characteristic as well as comorbidities. Additional studies are needed to enlighten the mechanisms underlying this complex cardiometabolic interplay.
| 1. | Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW, Fonarow GC. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J Am Coll Cardiol. 2017;70:2476-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 752] [Article Influence: 94.0] [Reference Citation Analysis (1)] |
| 2. | Huynh K, Bernardo BC, McMullen JR, Ritchie RH. Diabetic cardiomyopathy: mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol Ther. 2014;142:375-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 435] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 3. | Levelt E, Gulsin G, Neubauer S, McCann GP. MECHANISMS IN ENDOCRINOLOGY: Diabetic cardiomyopathy: pathophysiology and potential metabolic interventions state of the art review. Eur J Endocrinol. 2018;178:R127-R139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 4. | Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1406] [Cited by in RCA: 1455] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 5. | McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381:1995-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 4389] [Article Influence: 731.5] [Reference Citation Analysis (0)] |
| 6. | Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1634] [Cited by in RCA: 1907] [Article Influence: 317.8] [Reference Citation Analysis (0)] |
| 7. | Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383:1413-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1875] [Cited by in RCA: 3132] [Article Influence: 626.4] [Reference Citation Analysis (0)] |
| 8. | Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M; EMPEROR-Preserved Trial Investigators. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021;385:1451-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3009] [Cited by in RCA: 2724] [Article Influence: 681.0] [Reference Citation Analysis (0)] |
| 9. | Solomon SD, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Lindholm D, Wilderäng U, Öhrn F, Claggett B, Langkilde AM, Petersson M, McMurray JJV. Dapagliflozin in heart failure with preserved and mildly reduced ejection fraction: rationale and design of the DELIVER trial. Eur J Heart Fail. 2021;23:1217-1225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 210] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 10. | Writing Committee Members; ACC/AHA Joint Committee Members. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure. J Card Fail. 2022;28:e1-e167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 224] [Article Influence: 74.7] [Reference Citation Analysis (0)] |
| 11. | Grubić Rotkvić P, Ćelap I, Bralić Lang V, Jug J, Snagić A, Huljev Šipoš I, Cigrovski Berković M. Impact of SGLT2 inhibitors on the mechanisms of myocardial dysfunction in type 2 diabetes: A prospective non-randomized observational study in patients with type 2 diabetes mellitus without overt heart disease. J Diabetes Complications. 2023;37:108541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 12. | American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S111-S124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 682] [Article Influence: 170.5] [Reference Citation Analysis (0)] |
| 13. | Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853-862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 540] [Cited by in RCA: 651] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 14. | Eickhoff MK, Dekkers CCJ, Kramers BJ, Laverman GD, Frimodt-Møller M, Jørgensen NR, Faber J, Danser AHJ, Gansevoort RT, Rossing P, Persson F, Heerspink HJL. Effects of Dapagliflozin on Volume Status When Added to Renin-Angiotensin System Inhibitors. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 15. | Jensen J, Omar M, Kistorp C, Poulsen MK, Tuxen C, Gustafsson I, Køber L, Gustafsson F, Faber J, Fosbøl EL, Bruun NE, Brønd JC, Forman JL, Videbæk L, Møller JE, Schou M. Twelve weeks of treatment with empagliflozin in patients with heart failure and reduced ejection fraction: A double-blinded, randomized, and placebo-controlled trial. Am Heart J. 2020;228:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 16. | Nesti L, Pugliese NR, Sciuto P, Trico D, Dardano A, Baldi S, Pinnola S, Fabiani I, Di Bello V, Natali A. Effect of empagliflozin on left ventricular contractility and peak oxygen uptake in subjects with type 2 diabetes without heart disease: results of the EMPA-HEART trial. Cardiovasc Diabetol. 2022;21:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 17. | Januzzi JL Jr, Zannad F, Anker SD, Butler J, Filippatos G, Pocock SJ, Ferreira JP, Sattar N, Verma S, Vedin O, Schnee J, Iwata T, Cotton D, Packer M; EMPEROR-Reduced Trial Committees and Investigators. Prognostic Importance of NT-proBNP and Effect of Empagliflozin in the EMPEROR-Reduced Trial. J Am Coll Cardiol. 2021;78:1321-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 18. | Majowicz MP, Gonzalez Bosc LV, Albertoni Borghese MF, Delgado MF, Ortiz MC, Sterin Speziale N, Vidal NA. Atrial natriuretic peptide and endothelin-3 target renal sodium-glucose cotransporter. Peptides. 2003;24:1971-1976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Bendotti G, Montefusco L, Lunati ME, Usuelli V, Pastore I, Lazzaroni E, Assi E, Seelam AJ, El Essawy B, Jang J, Loretelli C, D'Addio F, Berra C, Ben Nasr M, Zuccotti G, Fiorina P. The anti-inflammatory and immunological properties of GLP-1 Receptor Agonists. Pharmacol Res. 2022;182:106320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 149] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 20. | Nita C, Bala C, Porojan M, Hancu N. Fenofibrate improves endothelial function and plasma myeloperoxidase in patients with type 2 diabetes mellitus: an open-label interventional study. Diabetol Metab Syndr. 2014;6:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC Jr, Taubert K, Tracy RP, Vinicor F; Centers for Disease Control and Prevention; American Heart Association. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4411] [Cited by in RCA: 4640] [Article Influence: 210.9] [Reference Citation Analysis (0)] |
| 22. | Li M, Yi T, Fan F, Qiu L, Wang Z, Weng H, Ma W, Zhang Y, Huo Y. Effect of sodium-glucose cotransporter-2 inhibitors on blood pressure in patients with heart failure: a systematic review and meta-analysis. Cardiovasc Diabetol. 2022;21:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 23. | Bhm M, Anker S, Mahfoud F, Lauder L, Filippatos G, Ferreira JP, Pocock SJ, Brueckmann M, Saloustros I, Schler E, Wanner C, Zannad F, Packer M, Butler J. Empagliflozin, irrespective of blood pressure, improves outcomes in heart failure with preserved ejection fraction: the EMPEROR-Preserved trial. Eur Heart J. 2023;44:396-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Zhang J, Chen Q, Zhong J, Liu C, Zheng B, Gong Q. DPP-4 Inhibitors as Potential Candidates for Antihypertensive Therapy: Improving Vascular Inflammation and Assisting the Action of Traditional Antihypertensive Drugs. Front Immunol. 2019;10:1050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Martini J, Tsai AG, Cabrales P, Johnson PC, Intaglietta M. Increased cardiac output and microvascular blood flow during mild hemoconcentration in hamster window model. Am J Physiol Heart Circ Physiol. 2006;291:H310-H317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Lambert EA, Esler MD, Schlaich MP, Dixon J, Eikelis N, Lambert GW. Obesity-Associated Organ Damage and Sympathetic Nervous Activity. Hypertension. 2019;73:1150-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Verloop WL, Beeftink MM, Santema BT, Bots ML, Blankestijn PJ, Cramer MJ, Doevendans PA, Voskuil M. A systematic review concerning the relation between the sympathetic nervous system and heart failure with preserved left ventricular ejection fraction. PLoS One. 2015;10:e0117332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Pescatori LC, Savarino E, Mauri G, Silvestri E, Cariati M, Sardanelli F, Sconfienza LM. Quantification of visceral adipose tissue by computed tomography and magnetic resonance imaging: reproducibility and accuracy. Radiol Bras. 2019;52:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA; on behalf of the American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46 (Suppl 1):S140-S157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 529] [Article Influence: 264.5] [Reference Citation Analysis (1)] |
| 30. | Bigagli E, Luceri C, Dicembrini I, Tatti L, Scavone F, Giovannelli L, Mannucci E, Lodovici M. Effect of Dipeptidyl-Peptidase 4 Inhibitors on Circulating Oxidative Stress Biomarkers in Patients with Type 2 Diabetes Mellitus. Antioxidants (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |