Published online Jun 26, 2024. doi: 10.4330/wjc.v16.i6.363
Revised: May 1, 2024
Accepted: May 22, 2024
Published online: June 26, 2024
Processing time: 117 Days and 18.4 Hours
Inferior wall left ventricular aneurysms are rare, they develop after transmural myocardial infarction (MI) and may be associated with poorer prognosis. We present a unique case of a large aneurysm of the inferior wall complicated by ventricular tachycardia (VT) and requiring surgical resection and mitral valve replacement.
A 59-year-old male was admitted for VT one month after he had a delayed presentation for an inferior ST-segment elevation MI and was discovered to have a large true inferior wall aneurysm on echocardiography and confirmed on coronary computed tomography (CT) angiography. Due to the sustained VT, concern for aneurysm expansion, and persistent heart failure symptoms, the patient was referred for surgical resection of the aneurysm with patch repair, mitral valve replacement, and automated implantable cardioverter defibrillator insertion with significant improvement in functional and clinical status.
Inferior wall aneurysms are rare and require close monitoring to identify electrical or contractile sequelae. Coronary CT angiography can outline anatomic details and guide surgical intervention to ameliorate life-threatening complications and improve performance status.
Core Tip: This case report is intended to assist clinicians anticipate and recognize complications arising from true inferior wall aneurysms in a bid to expedite timely pharmacologic and surgical interventions. It will also help outline the role of multidisciplinary care in managing inferior wall aneurysm complications to improve quality of care and help provide guidance in utilizing different imaging modalities to evaluate ventricular aneurysms and help guide therapy.
- Citation: Anuforo A, Charlamb J, Draytsel D, Charlamb M. Massive inferior wall aneurysm presenting with ventricular tachycardia and refractory cardiomyopathy requiring multiple interventions: A case report. World J Cardiol 2024; 16(6): 363-369
- URL: https://www.wjgnet.com/1949-8462/full/v16/i6/363.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i6.363
The prevalence of myocardial infarction (MI) in American adults aged 20 and older is 3.1 percent[1]. That equates to about 1.5 million patients in the United States and the overall incidence of left ventricular aneurysms is 30-35 percent of acute transmural MI[2]. The clinical implications of these aneurysms are profound, manifesting as wall motion abnormalities, reinfarction, ventricular tachyarrhythmias, and an increased risk of sudden cardiac death[3]. Ventricular aneurysms have two major risk factors. The first is total occlusion of the left anterior descending artery and the second is failure to obtain patency of the infarcted artery. This can lead to a true ventricular aneurysm or a ventricular pseudoaneurysm. A true aneurysm is a full-thickness outpouching of the ventricular wall. While a pseudo-aneurysm is a ventricular wall rupture that remains contained within the pericardium[2]. Differentiating between a true and pseudo-aneurysm can be difficult but is vital as pseudo-aneurysms have a propensity to rupture leading to cardiac tamponade, shock, and death. True aneurysms generally do not carry these same risk factors[4]. Nonetheless, they can lead to mural thrombus, arrhythmia, and heart failure[5]. This report details the case of a patient with a true aneurysm found after sustained ventricular tachycardia (VT) post-cardiac catheterization and placement of a drug-eluting stent (DES). The patient subsequently had surgical resection of the aneurysm, mitral valve replacement, and intracardiac cryoablation below the mitral valve to prevent future VT episodes.
We present a 59-year-old male with cardiovascular comorbidities. He presented to our emergency department (ED) with new onset palpitations that had lasted about 90 minutes, wide complex tachycardia tracing on his smartwatch with a heart rate of around 160/min, and concerns for VT.
Before presentation, the patient had no fever, nausea, vomiting, chest pain, syncope, or dyspnea. His clinical presentation was most consistent with scar-related VT arising from the aneurysm wall. Potential differentials include supraventricular tachycardia with aberrancy, pre-excited supraventricular tachycardia, and antidromic atrioventricular reciprocating tachycardia.
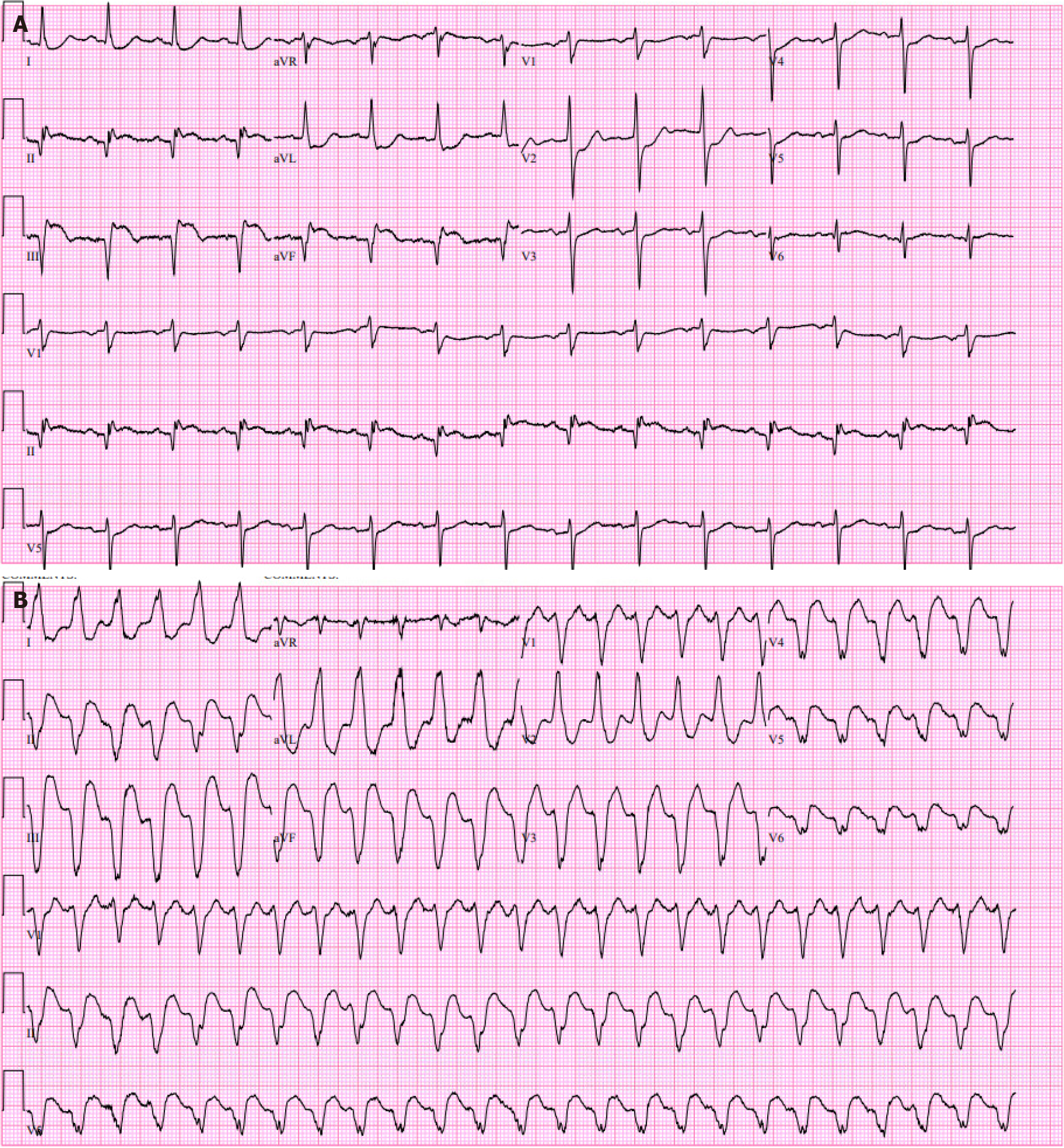
One month earlier the patient had a delayed presentation for an acute inferior wall ST segment elevation MI (STEMI) (Figure 1A) with admission high sensitivity cardiac Troponin (hs-cTn) elevated at 988 ng/L, peaked at 1225 ng/L and trended down to 954 ng/L. He underwent coronary angiography that revealed an occluded right coronary artery right coronary artery (RCA) and normal flow through the proximal, mid, and distal left anterior descending (LAD) and left circumflex artery. He then received percutaneous coronary intervention (PCI) with the placement of a DES to the occluded RCA and this hospital course was complicated by pericarditis managed with Aspirin and colchicine.
He had a history of essential hypertension, dyslipidemia, and type 2 diabetes mellitus and was on Amlodipine, Metoprolol, Atorvastatin, Metformin, and dual antiplatelet with Aspirin and Ticagrelor. Past surgical history was significant for left total shoulder and total knee replacements. The family history was significant for diabetes mellitus and heart disease in his father.
Vitals were most significant for tachycardia with a heart rate of 160/min, blood pressure of 122/98 mmHg, respiratory rate of 16/min, and SPO2 of 97% on ambient air. He was alert, oriented, and in no painful distress. He was well hydrated, with no neck vein distension. Chest auscultation was clear bilaterally and cardiac auscultation revealed an apical pan-systolic murmur without rubs or gallops. Abdominal exam was within normal limits and he was neurologically intact with warm and well-perfused extremities, with no lower extremity edema.
An electrocardiogram (EKG) in the ED confirmed monomorphic VT (Figure 1B). Lab testing was most significant for white blood count of 13.6 × 109/L and elevated hs-cTn at 117 ng/L, which increased to 140 ng/L. Other lab values were within normal limits.
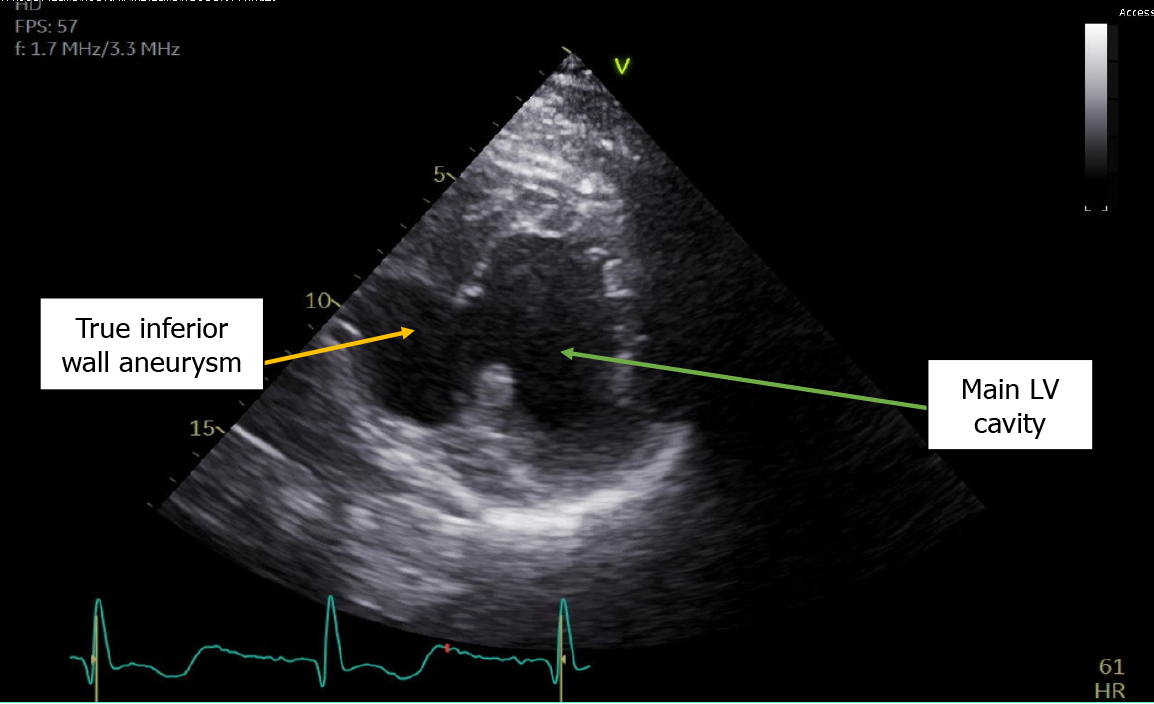
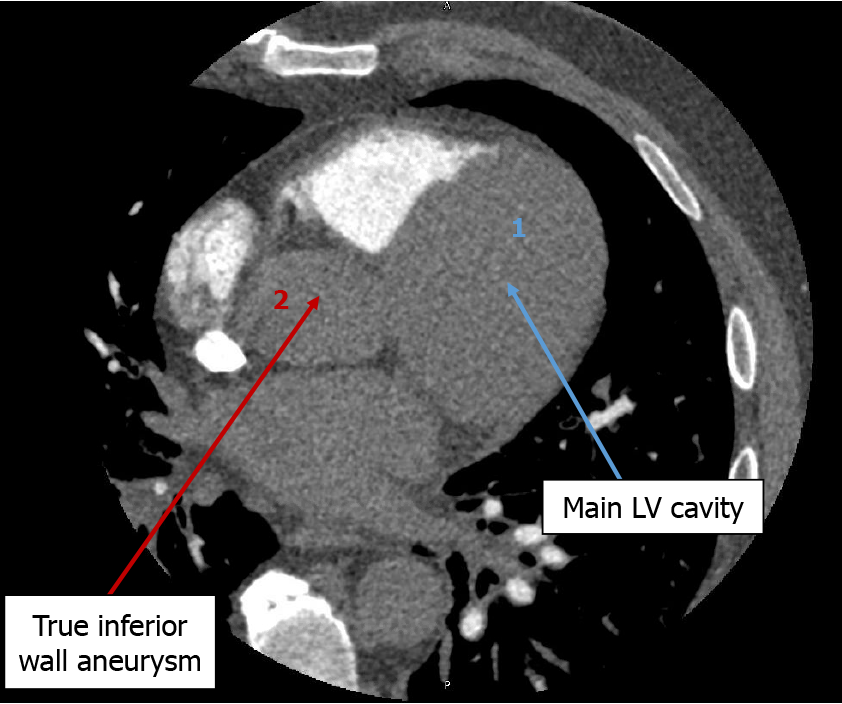
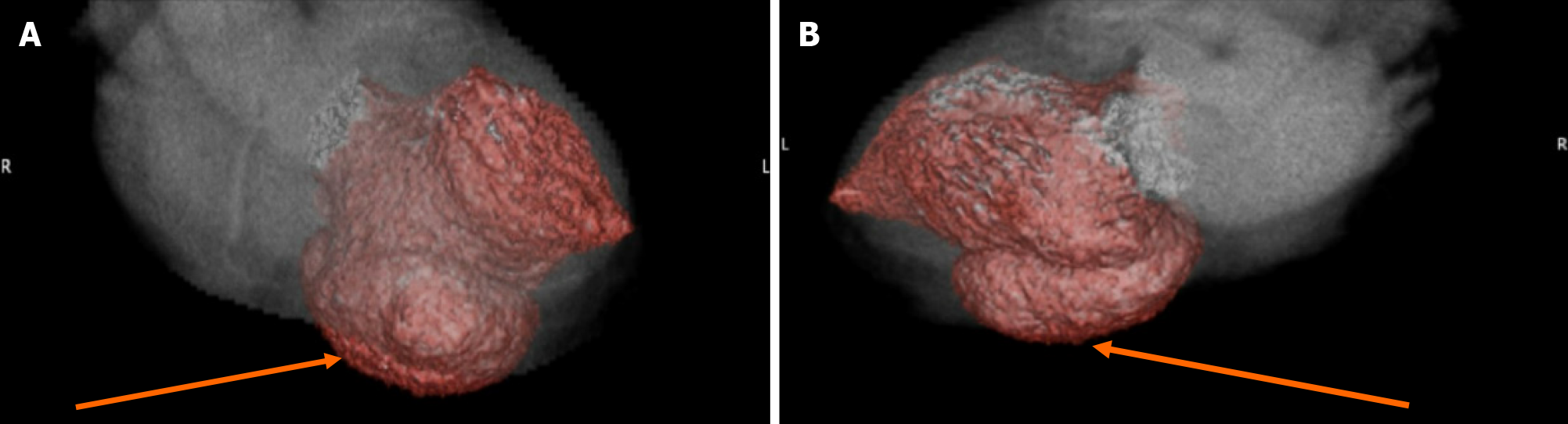
The echocardiogram showed hypokinetic and dyskinetic left ventricular walls, a large basal and mid-inferior-inferoseptal aneurysm with a wide neck, reduced systolic function, and a left ventricular ejection fraction (LVEF) 44% as well as moderate mitral insufficiency from a restricted posterior mitral valve leaflet and mild aortic insufficiency with a dilated ascending aorta of 4.4 cm (Figure 2). Prior echocardiogram during the STEMI had shown a dyskinetic segment involving the inferoseptal, basal, and mid-inferior wall which subsequently became aneurysmal. To further evaluate the aneu
Sustained scar-related VT secondary to an inferior wall left ventricular aneurysm post inferior STEMI.
He received a 150 mg Amiodarone bolus followed by an infusion at 1 mg/min and Esmolol at 50 mcg/kg/min in the ED. The esmolol dose was doubled and he received another 150 mg Amiodarone bolus for persistent VT and converted spontaneously to sinus rhythm just before anticipated electrical cardioversion. Repeat EKG showed loss of R wave progression in precordial leads and q waves with slight ST elevation in leads V3-V6. After the LAD stent placement, he was continued on dual antiplatelet therapy, high-intensity statin, guideline-directed medical therapy (GDMT) with Enalapril, Furosemide, Metoprolol succinate, Amiodarone, Dapagliflozin and a wearable cardioverter defibrillator (LifeVest).
Two months after the VT episode, due to concern for the aneurysm expansion, he was referred for LV aneurysm patch repair with polytetrafluoroethylene graft and bioprosthetic mitral valve replacement. He also received a surgical intracardiac cryoablation of the tissue between the mitral annulus and the scar of the inferior MI to prevent future recurrence of mitral annular VT. Surgery was complicated by prolonged mechanical ventilation and pneumosepsis. Due to the history of VT and persistently low pre-op LVEF of around 35%, the patient also had a dual-chamber automated implantable cardioverter defibrillator (AICD) placed and Amiodarone was discontinued due to bradycardia and prolonged QTc interval of 598 ms.
Three months postoperatively, he developed recurrent left-sided pleural effusions requiring two sessions of thoracentesis with drainage of 1.5 L and 0.7 L respectively. Due to postoperative persistent symptomatic LV dysfunction, GDMT was progressively optimized to include Entresto (replacing Enalapril), and Eplerenone in addition to previous medications with improvement in functional status and LVEF up to 50%.
This case outlines the complicated clinical course of a patient who developed a true inferior wall aneurysm complicated by sustained VT one month after presenting with an inferior wall STEMI despite reperfusion. It presents a unique intersection of ischemic complications combining ventricular arrhythmias post-STEMI with structural pathologies requiring combined AICD insertion and mitral valve replacement. In this case, due to unknown magnetic resonance imaging (MRI) compatibility of the patient’s orthopedic implants, it was agreed to obtain a cardiac gated coronary CT angiography scan as opposed to the gold standard for LV aneurysm diagnosis, which is cardiovascular MRI[6]. Most LV aneurysms (75%-80%) arise from the apical or anterior wall and are often associated with established risk factors like absence of collateralization in the setting of LAD coronary artery total occlusion, and incomplete or delayed reperfusion[7,8]. True inferior aneurysms are rare but have similar risk factors with the culprit vessel often being the RCA and persistent inferior ST elevation on EKG is usually consistent with the development of an aneurysm[9]. Aneurysm development arises from infarct expansion, which occurs in about 35%-45% of anterior MI and less frequently at other locations[10].
Following the advent of thrombolysis and PCI, large ventricular aneurysms have become uncommon complications of MIs[11,12]. A true aneurysm occurring a month after a STEMI raises questions about the underlying substrate and the potential role of delayed myocardial healing and remodeling. While pseudoaneurysms are at a higher risk of rupture, true aneurysms balloon out in systole causing a loss of kinetic energy required to maintain cardiac output, thus carrying a higher risk of heart failure, the stasis favors thrombus formation, and the scar tissue forms an arrhythmogenic substrate for ventricular arrhythmias[13,14]. Complications of aneurysm formation are responsible for a six-fold increase in the mortality of acute coronary syndrome, death is usually from sudden cardiac death[15].
This case highlights a greater need for arrhythmia risk stratification in post-infarction patients with regional myocardial dysfunction as well as the need for further exploration of potential underlying arrhythmogenic substrates, the possible role of scar-related reentry mechanisms and the impact of worsening myocardial dysfunction. Furthermore, this patient had a dual-chamber AICD placed for secondary prevention of recurrent VT managed with Amiodarone, and worsening LVEF of 35% after a trial of a LifeVest while on GDMT. Due to the patient’s elevated risk of sudden cardiac death on account of depressed LVEF, history or recurrent VT, and STEMI within the past 40 d, the patient had been placed on a LifeVest. More specific indications would include LVEF ≤ 35% within 90 d of coronary artery bypass graft, newly diagnosed but potentially reversible nonischemic cardiomyopathy, or severe heart failure awaiting transplantation[16]. However, some months after VT ablation and after a device check revealed no recurrent episodes of VT, it was decided to discontinue Amiodarone due to persistent bradycardia, markedly prolonged QTc interval of 598 ms, and the increased risk of Torsades.
Inferior aneurysms could be associated with mitral regurgitation (MR) from disruption of papillary muscle anatomy due to scar formation and subsequent mitral leaflet tethering[17]. This patient had moderate MR from dyskinetic walls and a restricted posterior mitral valve leaflet further complicating his heart failure symptoms, hence mitral valve replacement was required. The coexistence of mitral valve disease emphasizes the complexity of managing concurrent structural heart disease and arrhythmias.
Uncomplicated ventricular aneurysms are usually managed pharmacologically with GDMT and anticoagulation[17]. However, in the setting of persistent ventricular arrhythmia or refractory heart failure unresponsive to GDMT (in this case), surgical intervention is strongly indicated[13]. Surgical repair significantly reduces heart failure symptoms and VT episodes[11]. Of note, the perioperative risk of mortality for aneurysmal repair is high particularly when additional coronary artery bypass or valvular surgery is required especially when the LVEF is 35% or less[9,18]. This patient had an inferoseptal/inferobasal aneurysmectomy with patch repair, concomitant bioprosthetic mitral valve replacement, and AICD implantation with significant improvement at one-year follow-up. VT Cryoablation was guided by anatomical landmarks and performed from the scar area to the posterior mitral annulus (P2/P3 area) both from the LV endocardium side and epicardium side during the MV replacement procedure. This emphasizes the challenges of managing post-infarction patients, including the potential impact of ventricular mechanics and arrhythmia substrate.
Also noteworthy was the fact that the decompensated heart failure state postoperatively was complicated by symptomatic pleural effusions requiring thoracentesis procedures with drainage of 2.2 L. In this patient, further postoperative optimization of GDMT was associated with improvements in LVEF, pleural effusion, and functional status. This case underscores the evolving landscape of collaborative care involving interventional cardiology, cardiac surgery, and electrophysiology in addressing multifaceted cardiac pathologies.
This case provides valuable insights into the management of high-risk patients with complex post-infarction complications and underscores the importance of vigilant monitoring and risk assessment in the post-STEMI period in patients with inferior wall involvement. Additionally, it reveals the significant complications, perioperative challenges, and guideline-directed interventions for a true inferior wall left ventricular aneurysm and aims to contribute to the enhancement of treatment protocols within the realm of cardiology.
| 1. | Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:188-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 912] [Cited by in RCA: 950] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 2. | Sattar Y, Alraies MC. Ventricular Aneurysm. 2023 Apr 3. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 3. | Epstein JI, Hutchins GM. Subepicardial aneurysms: a rare complication of myocardial infarction. Am J Med. 1983;75:639-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Cho MN, Mehta SK, Matulevicius S, Weinstein D, Wait MA, McGuire DK. Differentiating true versus pseudo left ventricular aneurysm: a case report and review of diagnostic strategies. Cardiol Rev. 2006;14:e27-e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Meshram R, Vaibhav V, Agrawal S, Khorwal G, Sharma K. Myocardial Infarction With Ventricular Wall Aneurysm: A Case Report. Cureus. 2022;14:e29017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 6. | Fang CJ. Left ventricular aneurysm and pseudoaneurysm following acute myocardial infarction. 2024. Available from: https://www.uptodate.com/contents/left-ventricular-aneurysm-and-pseudoaneurysm-following-acute-myocardial-infarction. |
| 7. | Gorlin R, Klein MD, Sullivan JM. Prospective correlative study of ventricular aneurysm. Mechanistic concept and clinical recognition. Am J Med. 1967;42:512-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 246] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Dubnow MH, Burchell HB, Titus JL. Postinfarction ventricular aneurysm. A clinicomorphologic and electrocardiographic study of 80 cases. Am Heart J. 1965;70:753-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 199] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Aggarwal S, Clark DE, Muñoz D, Shah A, Armstrong C, Dendy JM, Hughes SG. Massive Left Ventricular Aneurysm After Inferior Infarction. AIM Clinical Cases. 2023;2. [DOI] [Full Text] |
| 10. | Weisman HF, Healy B. Myocardial infarct expansion, infarct extension, and reinfarction: pathophysiologic concepts. Prog Cardiovasc Dis. 1987;30:73-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 142] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Dor V, Sabatier M, Di Donato M, Montiglio F, Toso A, Maioli M. Efficacy of endoventricular patch plasty in large postinfarction akinetic scar and severe left ventricular dysfunction: comparison with a series of large dyskinetic scars. J Thorac Cardiovasc Surg. 1998;116:50-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 210] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Tikiz H, Balbay Y, Atak R, Terzi T, Genç Y, Kütük E. The effect of thrombolytic therapy on left ventricular aneurysm formation in acute myocardial infarction: relationship to successful reperfusion and vessel patency. Clin Cardiol. 2001;24:656-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Gong FF, Vaitenas I, Malaisrie SC, Maganti K. Mechanical Complications of Acute Myocardial Infarction: A Review. JAMA Cardiol. 2021;6:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 14. | Libby PP, Bonow R, Mann D, Zipes D. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 8th edition. https://www.scholars.northwestern.edu/en/publications/braunwalds-heart-disease-a-textbook-of-cardiovascular-medicine-8t-2. |
| 15. | Abildstrom SZ, Ottesen MM, Rask-Madsen C, Andersen PK, Rosthøj S, Torp-Pedersen C, Køber L. Sudden cardiovascular death following myocardial infarction: the importance of left ventricular systolic dysfunction and congestive heart failure. Int J Cardiol. 2005;104:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Granger CB, Hammill SC, Hlatky MA, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018;138:e272-e391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 305] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 17. | Yüksel M, Polat N, Vuruskan E, Ardıç İ. Inferior Wall Aneurysm of the Left Ventricle and Severe Mitral Regurgitation Following Acute Myocardial Infarction. Electron J Gen Med. 2013;10:50-52. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Michael H, John P, Walter J. Paulus-Cardiology. 3rd ed. E-Book Google Books. Available from: https://books.google.com/books/about/Cardiology_E_Book.html?id=DMPowl6M_6MC. |