Published online Jun 26, 2024. doi: 10.4330/wjc.v16.i6.329
Revised: April 22, 2024
Accepted: April 28, 2024
Published online: June 26, 2024
Processing time: 140 Days and 5.6 Hours
Lipoprotein(a) [Lp(a)] is a causal risk factor for atherosclerotic cardiovascular diseases; however, its role in acute coronary syndrome (ACS) remains unclear.
To investigate the hypothesis that the Lp(a) levels are altered by various con
From September 2009 to May 2016, 377 patients with ACS who underwent emergent coronary angiography, and 249 who completed ≥ 1000 d of follow-up were enrolled. Lp(a) levels were measured using an isoform-independent assay at each time point from before percutaneous coronary intervention (PCI) to 48 h after PCI. The primary endpoint was the occurrence of major adverse cardiac events (MACE; cardiac death, other vascular death, ACS, and non-cardiac vas
The mean circulating Lp(a) level decreased significantly from pre-PCI (0 h) to 12 h after (19.0 mg/dL to 17.8 mg/dL, P < 0.001), and then increased significantly up to 48 h after (19.3 mg/dL, P < 0.001). The changes from 0 to 12 h [Lp(a)Δ0-12] significantly correlated with the basal levels of creatinine [Spearman’s rank correlation coefficient (SRCC): -0.181, P < 0.01] and Lp(a) (SRCC: -0.306, P < 0.05). Among the tertiles classified according to Lp(a)Δ0-12, MACE was significantly more frequent in the lowest Lp(a)Δ0-12 group than in the remaining two tertile groups (66.2% vs 53.6%, P = 0.034). A multivariate analysis revealed that Lp(a)Δ0-12 [hazard ratio (HR): 0.96, 95% confidence interval (95%CI): 0.92-0.99] and basal creatinine (HR: 1.13, 95%CI: 1.05-1.22) were independent determinants of subsequent MACE.
Circulating Lp(a) levels in patients with ACS decreased significantly after emergent PCI, and a greater decrease was independently associated with a worse prognosis.
Core Tip: Two hundred and forty-nine patients with acute coronary syndrome were enrolled in the study. Lipoprotein(a) [Lp(a)] levels were measured before percutaneous coronary intervention (PCI) to 48 h after using an isoform-independent assay. Lp(a) levels decreased significantly from pre-PCI (0 h) to 12 h after, and then increased up to 48 h after. The changes from 0 to 12 h [Lp(a)Δ0-12] were significantly correlated with basal creatinine and Lp(a). Among the tertiles classified according to the changes from 0 to 12 h [Lp(a)Δ0-12], major adverse cardiac events (MACE) were significantly more frequent in the lowest Lp(a)Δ0-12 group than in the other two groups. Multivariate analysis revealed that Lp(a)Δ0-12 and basal creatinine were independent determinants of subsequent MACE.
- Citation: Saeki Y, Sawaguchi J, Akita S, Takamura TA, Fujibayashi K, Wakasa M, Akao H, Kitayama M, Kawai Y, Kajinami K. Initial decrease in the lipoprotein(a) level is a novel prognostic biomarker in patients with acute coronary syndrome. World J Cardiol 2024; 16(6): 329-338
- URL: https://www.wjgnet.com/1949-8462/full/v16/i6/329.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i6.329
Lipoprotein(a) [Lp(a)] is a well-known causal risk factor for myocardial infarction, aortic valve stenosis, and other atherosclerotic diseases[1,2]. In the Lp(a) particle, apolipoprotein B (apo-B) was covalently bound to an apolipoprotein(a) [apo(a)]. The mechanism enhancing atherosclerosis by Lp(a) is considered to involve apo-B-containing lipoprotein, similar to low-density lipoprotein particles, as a carrier particle for oxidized phospholipid promoting pro-inflammatory cascade, and as its potential association with fibrinolysis owing to its structural similarity with plasminogen[2]. However, the precise mechanism underlying the Lp(a)-mediated development of atherosclerotic cardiovascular disease remains largely unknown.
Apo(a) is encoded by the LPA gene, and variation at the LPA locus is the strongest genetic determinant of circulating Lp(a) levels[3]. In contrast, within-individual variability has also been described, including an increase in autoimmune inflammatory conditions[4] and a decrease after bariatric surgery[5]. Regarding acute coronary events, Lp(a) levels during hospitalization for myocardial infarction or unstable angina have been investigated only in a small or modest sample size with various time courses[6-8]. One study reported a small decrease from days 1 to 3 in 59 patients with acute myocardial infarction[6] and an increase from days 1 to 21 in 18 patients with unstable angina pectoris[7]. Based on these discrepancies in previous study results, we hypothesized that circulating Lp(a) levels may be altered by the pathophy
In the present study, we applied the Lp(a) assay method, which has been reported to be resistant to the apo(a) isoform-dependent influence on measurement results, postulated frequently in commercially-available kits, as a potential confounder during data acquisition and interpretation.
To evaluate Lp(a) levels in the early phase of acute coronary syndrome (ACS), we conducted a single-center observational cohort study. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of our institution (I-115). Written informed consent was obtained from all patients prior to enrollment.
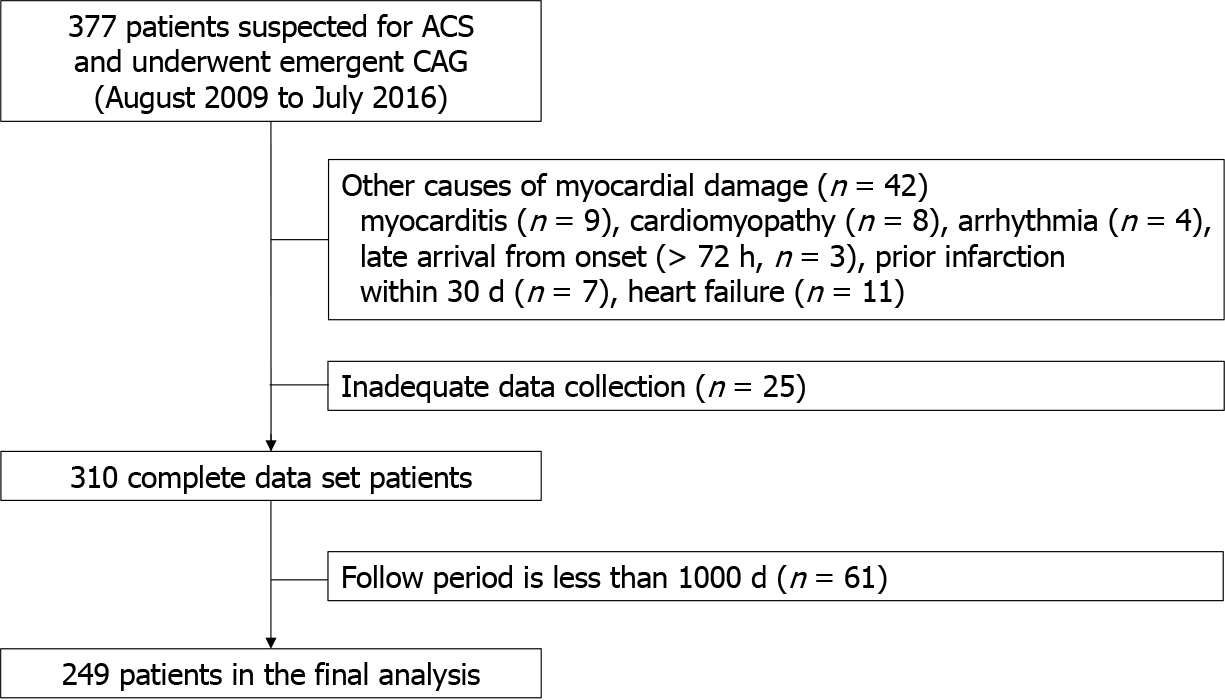
The inclusion criteria for the current study were patients with ACS who underwent emergent coronary angiography (CAG) between September 2009 and May 2016. The exclusion criteria were the absence of emergent CAG or admission for the treatment of conditions other than cardiac disease. Subjects presenting with myocardial damage due to concomitant cardiac diseases other than coronary stenosis, such as myocarditis (n = 9), cardiomyopathy (takotsubo n = 5, hypertrophic n = 3), ventricular tachyarrhythmia without coronary stenosis (n = 4), and heart failure (n = 11), were also excluded. In addition, patients whose myocardial infarction occurred ≥ 72 h before arrival (n = 3) or those with prior myocardial infarction within 30 days (n = 7) were excluded. A total of 377 patients who underwent emergent CAG suggesting ACS were enrolled, and 42 and 25 patients were excluded due to causes of myocardial damage and insufficient initial data collection, respectively. As a result, 310 individuals who successfully completed the data collection were selected as the study population. While a 1000-d follow-up was initially planned, 61 individuals dropped out during the course of the study, leaving a final cohort of 249 patients eligible for the analysis (Figure 1).
Myocardial infarction was diagnosed in cases with typical chest pain continuing ≥ 20 min, electrocardiogram (ECG) with ST elevation in at least two contiguous leads (≥ 2 mm in precordial or ≥ 1 mm in limb leads) or newly-identified left bundle branch block[9,10], associated with elevation of creatine kinase-myocardial isoform (CK-MB) or troponin T.
Peripheral blood samples to measure Lp(a), high-sensitivity C-reactive protein (hs-CRP), and other laboratory tests were drawn at following time-points; before CAG and at 3 h, 6 h, 12 h, 24 h, and 48 h after percutaneous coronary intervention (PCI). In patients with no coronary occlusion nor severe stenosis responsible for myocardial ischemia, peripheral blood samples were drawn at the same time points. Serum was separated after centrifugation (2500 × g) at 4 °C for 10 min and stored at -80 °C until use. Lp(a) levels were determined using an immunoturbidemetric assay kit (Denka Seiken., Tokyo, Japan), which is a well-validated assay with good reproducibility (coefficient of variation, 1.2%-2.2%) and is generally insensitive to apo(a) size heterogeneity[11]. Other laboratory measurements were performed by using standard methods at each timepoint.
All patients underwent emergent CAG, and PCI was immediately performed for lesions considered the culprit. In cases having ≥ 2 coronary occlusions, a lesion consistent with findings of ECG and echocardiogram was identified as the target. The detail of procedures depended on the interventional cardiology staff (T-AT, MK, and YK). A loading dose of antiplatelet agents (aspirin and P2Y12 inhibitors) was orally administered, and, before the procedure, 70 to 100 IU/kg of unfractionated heparin was given. The activated clotting time was maintained at ≥ 250 s with additional heparin administration during PCI. Primary PCI success was defined as TIMI flow grade ≥ 3 antegrade achievement with < 20% residual stenosis. Dual antiplatelet treatment was maintained during ≥ 6 months if bleeding complications requiring cessation of antiplatelet therapy did not occur, as previously described[12].
All patients were followed by an outpatient clinic at Kanazawa Medical University Hospital. Guideline-recommended medical treatments, including antiplatelet agents, statins, beta blockers, and an angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, were administered to lead the clinical and laboratory values reaching the targets for secondary prevention, if tolerable. Statins were given on hospital days 1 to 4, depending on the coronary care unit stay length, renal/liver function, and previous drug-related adverse events history; however, they were started by day 2 in most patients. Definition of major adverse cardiac events (MACE) was a composite of all-cause death, nonfatal myocardial infarction, stroke, and new angina pectoris. Newly-identified angina was defined as that in which the culprit lesion differed from the initial ACS event. Restenosis of the culprit lesion requiring revascularization was included in the MACEs category.
Continuous variables were described as the mean ± SD. Categorical variables were shown as numbers and percentages. The median with interquartile ranges was calculated for asymmetrically distributed data. Repeated measures of a one-way analysis of variance (with covariates if necessary) were applied in the assessment of serial changes in biomarker levels, and a paired t-test with Bonferroni correction for multiple tests was used to compare single time points. For variables with asymmetrical distributions, inter-group differences were compared using Kruskal-Wallis test and Scheffe’s multiple comparison test. To examine between-biomarker data, Pearson’s correlation coefficient (r) and Spearman’s rank correlation coefficient (SRCC) were applied to data with a normal distribution and non-parametric measures, respectively. In order to analyze the between-group characteristics, the chi-square test was applied. Univariate and multivariate analyses were performed using the Cox proportional hazards model.
The time to MACE was evaluated by means of the following two models: Model 1, variables demonstrated as risk factors for coronary heart disease in the Japanese Cohort, Suita Study[13]; age, sex, current smoking, presence of hypertension and diabetes, systolic blood pressure, pulse rate, HbA1c, low-density lipoprotein-cholesterol (LDL-C), baseline Lp(a), and the changes from 0 to 12 h [Lp(a)Δ0-12]. In model 2, well-established prognostic factors for ACS survivors (age, sex, peak CK-MB, creatinine, and Killip classification) and Lp(a)Δ0-12 were all included in the model. Outcomes were estimated by the Kaplan-Meier method and were compared with the log-rank test.
Bell Curve for Excel ver. 3.21 (Social Survey Research Information Co. Ltd., Tokyo, Japan) was used to statistical analyses, and all statistical tests were two sided. Statistical significance was set at P < 0.05.
Table 1 shows the baseline characteristics of the 249 patients with ACS included in the analysis. The majority of patients were male (79.0%), and more than half were smokers and were with a history of hypertension (52.6% and 59.8%, respectively). Statins were used in 26.9% of the patients; however, there was no significant difference in baseline Lp(a) levels between statin users and non-users. Therefore, we analyzed these patients together. The baseline LDL-C level was 122.4 mg/dL ± 36.6 mg/dL, and the creatinine level was 1.2 mg/dL ± 1.6 mg/dL.
| Variable | Unit | Value |
| Age | yr | 66.6 ± 12.6 |
| Sex (men/women) | % | 196/53 (79.0/21.0) |
| Medical history | ||
| Myocardial infarction | % | 11 (4.4) |
| Unstable angina pectoris | % | 5 (2.0) |
| Angina pectoris | % | 30 (12.0) |
| Atrial fibrillation | % | 14 (5.6) |
| Cerebral infarction | % | 17 (6.8) |
| Diabetes mellitus | % | 84 (33.7) |
| Hypertension | % | 149 (59.8) |
| Smoking | % | 131 (52.6) |
| Medication | ||
| Calcium channel blocker | % | 107 (43.0) |
| ACE-I/ARB | % | 81 (32.5) |
| Beta blocker | % | 22 (8.8) |
| Loop diuretics | % | 20 (8.0) |
| Nitrates | % | 37 (14.9) |
| Statin | % | 67 (26.9) |
| Fibrate | % | 5 (2.0) |
| Ezetimibe | % | 1 (0.4) |
| Insulin | % | 12 (4.8) |
| SGLT2 inhibitor | % | 2 (0.8) |
| Biguanide | % | 8 (3.2) |
| DPP4 Inhibitor | % | 18 (7.2) |
| BMI | kg/m2 | 23.8 ± 3.9 |
| Systolic blood pressure | mmHg | 134.0 ± 32.0 |
| Heart rate | bpm | 77.0 ± 22.0 |
| Laboratory data | ||
| Hemoglobin | mg/dL | 13.4 ± 2.0 |
| Glucose | mg/dL | 190.2 ± 91.3 |
| Hemoglobin A1c | % | 6.5 ± 1.3 |
| Total cholesterol | mg/dL | 189.0 ± 41.1 |
| Triglyceride | mg/dL | 118.81 ± 76.31 |
| LDL-cholesterol | mg/dL | 122.4 ± 36.6 |
| non-HDL cholesterol | mg/dL | 146.2 ± 40.0 |
| BNP | pg/mL | 278.9 ± 1288.0 |
| Creatinine | IU/L | 1.2 ± 1.6 |
| Alanine aminotransferase | IU/L | 58.5 ± 306.5 |
| peak CK | IU/L | 2973.0 ± 3257.0 |
| peak CK-MB | IU/L | 300.0 ± 668.0 |
According to the above-mentioned diagnostic criteria, the enrolled patients consisted of 169 patients with ST-elevation myocardial infarction (STEMI) patients, 47 patients with non-STEMI, and 33 patients with unstable angina pectoris. According to the Killip classification, 178 and 71 patients were classified as I + II and III + IV, respectively. Culprit lesions were in the left main coronary artery in five patients, left anterior descending artery in 113 patients, left circumflex artery in 31 patients, and right coronary artery in 50 patients. Immediate PCI was performed in 242 patients, including 51 with balloon dilation only and 191 with coronary stenting. Seven patients did not undergo PCI, five patients were eligible for thrombolytic therapy, and two did not undergo revascularization in the acute phase.
The changes in the levels of Lp(a), hs-CRP, CK, and CK-MB are shown in Table 2. Lp(a) initially decreased significantly to 24 h, and then increased to 48 h, which was not significantly different from that at 0 h. The mean hs-CRP levels gradually increased, and the CK and CK-MB levels both initially increased, peaked at 6 h, and then gradually decreased (these changes were both statistically significant). The change in Lp(a) from 0 h to 12 h [Lp(a)Δ0-12] was not associated with sex, age, or any associated condition. Regarding laboratory measurements, Lp(a)Δ0-12 showed significant and negative associations with basal levels of both creatinine (SRCC: -0.181, P < 0.01) and Lp(a) (SRCC: -0.306, P < 0.05). None of the remaining variables showed significant associations.
| Baseline | 3 h | 6 h | 12 h | 24 h | 48 h | ANOVA2 | |
| Lp(a), mg/dL | 19.0 ± 21.8 | 18.0 ± 20.6b | 18.0 ± 20.7b | 17.8 ± 20.1b | 18.2 ± 19.4a | 19.3 ± 19.2 | < 0.001 |
| hs-CRP1, ng/mL | 9584.4 (511.0, 4745.0) | 9784.1 (405.0, 5668.0) | 11295.9 (631.0, 7420.0) | 16680.2 (1730.0, 14700.0)b | 34628.8 (6720.0, 47200.0)b | 57233.6 (14400.0, 82600.0)b | < 0.001 |
| CK1, IU/L | 567.1 (112.0, 594.0) | 2410.3 (478.0, 3403.0)b | 2606.7 (579.0, 3893.0)b | 2228.0 (617.0, 3193.0)b | 1451.6 (454.0, 1918.0)b | 625.3 (200.0, 711.0) | < 0.001 |
| CK-MB1, IU/L | 53.1 (10.0, 57.0) | 221.1 (40.0, 309.0)b | 234.6 (54.0, 360.0)b | 176.8 (47.0, 261.0)b | 81.4 (26.0, 104.0) | 21.8 (10.0, 26.0)a | < 0.001 |
One hundred and forty-four patients developed MACE during the follow-up. Of the 17 patients who died, 13 died from heart disease, and 4 died from non-cardiac diseases (1 pneumonia, 2 cancer, and 1 unspecified cause). New angina pectoris was recorded in 111 patients, five of whom had unstable conditions. Nine patients had embolic events (seven with cerebral infarction and two with critical limb ischemia).
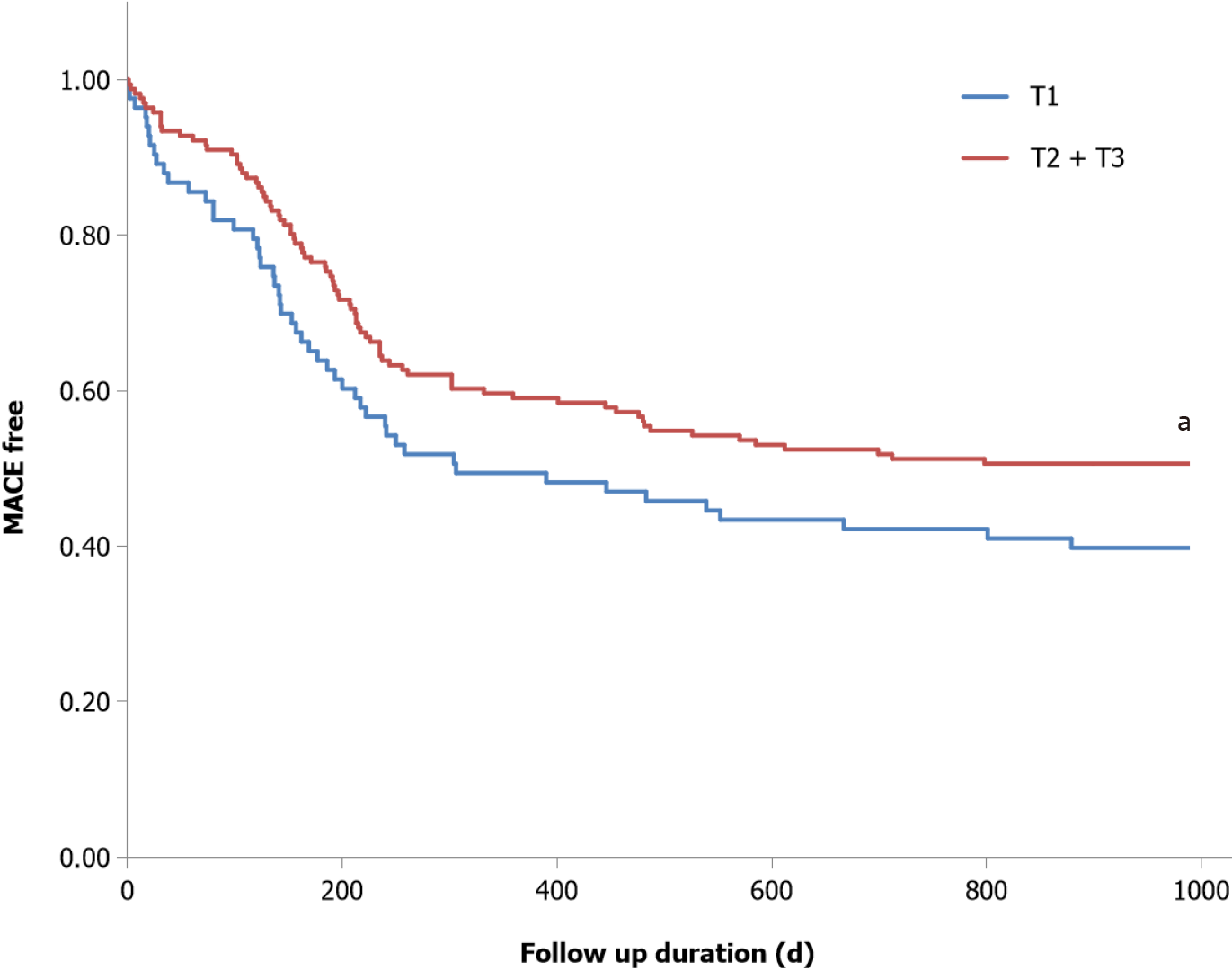
The prevalence of MACE was significantly greater in the tertile of Lp(a)Δ0-12; Q1 [Lp(a)Δ0-12: -31.6 to -1.8 mg/dL] than in Q2 + Q3 [Lp(a)Δ0-12: -1.8 to 8.3 mg/dL] (55 events vs 89 events, P < 0.05) (Figure 2). In univariate analysis, Lp(a)Δ0-12 and basal creatinine levels were significantly associated with the occurrence of MACE. In a multivariate analysis with the covariate as model-1, only Lp(a)Δ0-12 was identified as a significant and independent determinant [hazard ratio (HR): 0.95, P = 0.022]. In the analysis using model-2, Lp(a)Δ0-12 (HR: 0.96, P = 0.019) and basal creatinine (HR: 1.13, P < 0.01) were both significant and independent determinants (Table 3).
| Variable | Univariate cox regression | Multivariate cox regression (model-1) | Multivariate cox regression (model-2) | ||||||
| HR | 95%CI | P value | HR | 95%CI | P value | HR | 95%CI | P value | |
| Lp(a)Δ0-12 | 0.96 | 0.93 to 0.99 | 0.024a | 0.95 | 0.91 to 0.99 | 0.022a | 0.96 | 0.92 to 0.99 | 0.019a |
| Lp(a) 0 h (baseline) | 1.00 | 0.99 to 1.01 | 0.960 | 1.00 | 0.99 to 1.01 | 0.503 | 1.00 | 0.98 to 1.01 | 0.956 |
| Age | 0.99 | 0.98 to 1.01 | 0.481 | 1.00 | 0.98 to 1.01 | 0.833 | 1.00 | 0.98 to 1.01 | 0.721 |
| Sex | 1.50 | 0.97 to 2.33 | 0.068 | 1.46 | 0.89 to 2.37 | 0.131 | 1.50 | 0.94 to 2.40 | 0.091 |
| Smoking | 1.19 | 0.85 to 1.65 | 0.305 | 1.05 | 0.71 to 1.55 | 0.803 | |||
| Diabetes mellitus | 1.28 | 0.91 to 1.79 | 0.155 | 1.09 | 0.72 to 1.65 | 0.676 | |||
| Systolic blood pressure | 1.00 | 0.99 to 1.00 | 0.576 | 1.00 | 0.99 to 1.00 | 0.774 | |||
| Pulse rate | 1.07 | 0.97 to 1.19 | 0.192 | 1.06 | 0.93 to 1.21 | 0.388 | |||
| Hemoglobin A1c | 0.99 | 0.97 to 1.01 | 0.184 | 0.99 | 0.98 to 1.01 | 0.359 | |||
| HDL cholesterol | 1.00 | 0.99 to 1.00 | 0.561 | 1.00 | 0.99 to 1.00 | 0.795 | |||
| LDL cholesterol | 1.01 | 1.00 to 1.01 | 0.054 | ||||||
| Peak CK-MB | 1.00 | 0.99 to 1.00 | 0.056 | 1.00 | 1.00 to 1.00 | 0.447 | |||
| Creatinine | 1.16 | 1.09 to 1.24 | < 0.001b | 1.13 | 1.05 to 1.22 | < 0.01b | |||
| Killip classification | 1.50 | 1.06 to 2.13 | 0.021a | 1.39 | 0.96 to 2.02 | 0.078 | |||
In the present study, we showed that, during the acute phase of ACS, Lp(a) initially decreased and then returned to its basal level, and that a greater decrease, expressed as Lp(a)Δ0-12, was significantly associated with the occurrence of subsequent MACE, which was independent of other well-known prognostic factors of ACS.
It has already been established that elevated Lp(a) levels are an independent causal risk factor for atherosclerotic cardiovascular diseases and aortic valve disease. In addition, recent studies have indicated its role in heart failure as well[14]. Lp(a) levels are strongly determined by genetic variants of the LPA gene, particularly by size polymorphism in apo(a). Therefore, previous clinical studies investigating the potential role of Lp(a) in various disease conditions were conducted using a one-point evaluation under stable conditions.
Regarding ACS, only limited data are available for small groups of patients. An initial small decrease from day 1 (mean 6.3 mg/dL) to day 3 (mean 5.8 mg/dL) in 59 AMI patients[6], a transient initial increase from day 1 (mean 327 mg/L) to day 21 (mean 376 mg/L) in 18 patients with unstable angina[7], and an initial increase from day 1 (mean 64 nmol/L) to day 30 (mean 82 nmol/L) in 35 placebo-assigned AMI patients have been reported[8]. To our knowledge, the present study is the first to examine changes in circulating Lp(a) levels in the very early phase of ACS, using the largest sample size to date. A significant increase in Lp(a) from the acute phase of AMI to the six- month follow-up has recently been reported[15] as an extension of a previous study[8]. In these studies[8,15], initial measurements were performed within 24 h of the disease onset; therefore, the initial values might have been under-evaluated according to our current observations. In addition to the evaluation time point issue, the assay kits used in previous studies were highly variable, especially in terms of isoform influence. Therefore, the present study using the apo(a) isoform-resistant assay kit adds novel information to our knowledge about serial changes in circulating Lp(a) levels in patients with ACS.
The metabolism of Lp(a), synthesis, assembly, secretion, and clearance/catabolism have not yet been fully elucidated[16,17]. Similar to apolipoprotein B, apo(a) is exclusively synthesized in hepatocytes, suggesting the possibility that acute liver dysfunction may reduce apo(a) synthesis and Lp(a) assembly. None of the enrolled patients showed ALT elevation ≥ 100 IU/L or serum bilirubin elevation; therefore, the reduced production (also assembly and secretion) of Lp(a) resulting from liver dysfunction might play only a small role in the initial decrease in Lp(a) found in the present study. However, the precise mechanism underlying Lp(a) assembly remains unclear; therefore, an undetermined mechanism to reduce apo(a)-apolipoprotein B assembly may be involved.
The liver has also been established as the major site of Lp(a) clearance, followed to a much lesser extent by the kidneys and arterial wall[18,19]. Among the alternative pathways for Lp(a) clearance, toll-like receptor 2 (TLR2), which was proposed by a genome-wide association study[20], and the scavenger receptor BI (SR-BI), of which a rare genetic variant was reported in two human cohorts[21], are worth considering. Inflammatory processes play a pivotal role in the development of atherosclerotic vascular diseases, in which the activation of TLR2 in macrophages and other immune cells is crucial. Therefore, Lp(a) being catabolized by activated immune cells via TLR2 in patients with ACS after PCI, with a larger decrease indicating greater activation of this process, thereby leading to poor cardiovascular outcomes, seems to be an attractive hypothesis.
In the present study, baseline hs-CRP levels were not significantly associated with Lp(a)Δ0-12 (r = 0.0982, NS). This was also the case if the patient had unstable angina pectoris (r = -0.0994, NS), in which the influence of myocardial damage could be excluded. Further studies are needed to investigate the pathophysiology of this condition. Another potential pathway to consider is the SR-BI-mediated system, which is known to act as a high-density lipoprotein (HDL) receptor. Both human genetic studies[21] and cell biology experiments[22] have suggested that SR-BI plays a role in Lp(a) catabolism. In the present study, the fact that changes in HDL cholesterol from 0 h (44.1 mg/dL ± 10.6 mg/dL) to 12 h (41.8 mg/dL ± 9.2 mg/dL) showed a modest but significant association with Lp(a)Δ0-12 (r = 0.1948, P < 0.01) may support this possibility, which should be examined in further studies.
Our results are inconsistent with a previous report describing acute increases in both plasma Lp(a) and oxidized phospholipids after percutaneous coronary intervention in patients with stable coronary artery disease[23]. Differences in the clinical presentation of coronary artery disease (acute vs chronic coronary syndrome) would make it difficult to compare the study results directly; and thus, further studies are required to investigate this issue.
The present study clearly demonstrated that Lp(a) levels fluctuate during the acute phase of ACS; therefore, caution should be exercised when assessing Lp(a) levels. In addition, the determination of Lp(a) changes in the very acute phase of ACS may provide a better understanding of Lp(a) metabolism and pathophysiology in atherosclerotic vascular diseases. Another clinical implication of the present study is the modest but significant association between the initial decrease in both Lp(a) and HDL cholesterol levels. It could be hypothesized that a greater reduction in Lp(a) through the HDL-receptor might lead to worse cardiovascular effects through the atherogenic properties of Lp(a). Achieving of long-term reduction of Lp(a) levels by currently underdeveloping novel treatments could prevent this unfavorable effects in patients with high Lp(a) level.
Several limitations associated with the present study warrant mention. First, it was performed at a single center, indicating the potential for unrecognized bias. In addition, regarding the MACE criteria, we included new cases of angina pectoris because a small number of patients exhibited hard endpoints to sufficiently examine the prognostic value of Lp(a)Δ0-12. As shown in Table 4, the distribution of MACE types was compared according to the time course, but no specific trend was observed. A larger multicenter study would uncover the relationship between the Lp(a) change and the specific type of MACE. Second, this study enrolled all the type of patients diagnosed with ACS. We compared all variables derived from Lp(a) serial changes, including Lp(a)Δ0-12, but failed to find any significant differences across the ACS subgroups (data not shown). These findings suggest that the specific type of ACS manifestation did not play a major role in the current study. Theoretically, the influence of the amount of time from the ACS onset on the obtained results should be considered in the data interpretation. The time course of the Lp(a) levels found in this study can provide new ideas for future studies to investigate this issue. It is also possible that the severity of myocardial damages influences the occurrence of subsequent MACE. The lack of an association between well-known prognostic variables of ACS, such as peak CK levels or the Killip classification, suggests that Lp(a) itself or the pathophysiology responsible for Lp(a) changes might have an independent role. The introduction of a more sensitive assay for myocardial damage, such as troponin I assay, may also provide more detailed results. Third, most patients enrolled in this study were on statins the day after primary PCI without prior statin administration; thus, the possibility that statins played a role in subsequent Lp(a) changes could not be fully excluded. The beneficial effects of statin treatment after acute coronary events have been established; therefore, it is ethically difficult not to prescribe statin. Regarding the potential influence of prior medication on study results, statin are known to increase circulating Lp(a) levels[1,14,15]. In the present study, the mean baseline Lp(a) levels with prior statin treatment (22.2 mg/dL, n = 67) were slightly but statistically insignificantly higher than in those without such a history (17.8 mg/dL, n = 182). Lp(a) levels at 12 h with prior statin use (21.2 mg/dL, -4.5% from baseline) were comparable to those in patients without such a history (16.6 mg/dL, -6.5% from baseline). Further large-scale studies are needed to investigate this issue. Fourth, changes in apo-B-containing lipoprotein metabolism during the acute phase of ACS might influence circulating Lp(a) levels through altered apo-B production. However, neither baseline nor changes in lipid variables showed a significant relationship with Lp(a)Δ0-12.
| Event | Total | Within 1 wk | 1 wk to 1 month | 1-6 months | 6-12 months | After 12 months |
| Cardiac death | 13 | 2 | 4 | 4 | 1 | 2 |
| Other death | 4 | 2 | 0 | 1 | 0 | 1 |
| Nonfatal STEMI | 2 | 1 | 0 | 0 | 0 | 1 |
| Unstable angina | 5 | 0 | 0 | 3 | 0 | 2 |
| TLR or new PCI | 102 | 0 | 5 | 42 | 36 | 19 |
| New lesion with ischemia | 9 | 0 | 1 | 2 | 1 | 5 |
| Nonfatal stroke | 7 | 1 | 0 | 1 | 1 | 4 |
| EVT for PAD | 2 | 0 | 0 | 0 | 2 | 0 |
The initial decrease in Lp(a) levels after PCI for ACS, as expressed by Lp(a)Δ0-12, was associated with the occurrence of MACE within three years. The pathophysiology causing this alteration in Lp(a) metabolism appears to be a novel target to improve the outcomes after successful PCI in patients with ACS.
| 1. | Emerging Risk Factors Collaboration, Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, White IR, Marcovina SM, Collins R, Thompson SG, Danesh J. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1333] [Cited by in RCA: 1259] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 2. | Kronenberg F, Mora S, Stroes ESG, Ference BA, Arsenault BJ, Berglund L, Dweck MR, Koschinsky M, Lambert G, Mach F, McNeal CJ, Moriarty PM, Natarajan P, Nordestgaard BG, Parhofer KG, Virani SS, von Eckardstein A, Watts GF, Stock JK, Ray KK, Tokgözoğlu LS, Catapano AL. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement. Eur Heart J. 2022;43:3925-3946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 587] [Article Influence: 195.7] [Reference Citation Analysis (0)] |
| 3. | Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 980] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 4. | Missala I, Kassner U, Steinhagen-Thiessen E. A Systematic Literature Review of the Association of Lipoprotein(a) and Autoimmune Diseases and Atherosclerosis. Int J Rheumatol. 2012;2012:480784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Lin BX, Weiss MC, Parikh M, Berger JS, Fisher EA, Heffron SP. Changes in lipoprotein(a) following bariatric surgery. Am Heart J. 2018;197:175-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Andreassen AK, Berg K, Torsvik H. Changes in Lp(a) lipoprotein and other plasma proteins during acute myocardial infarction. Clin Genet. 1994;46:410-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Oshima S, Uchida K, Yasu T, Uno K, Nonogi H, Haze K. Transient increase of plasma lipoprotein(a) in patients with unstable angina pectoris. Does lipoprotein(a) alter fibrinolysis? Arterioscler Thromb. 1991;11:1772-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Vavuranakis MA, Jones SR, Ziogos E, Blaha MJ, Williams MS, Foran P, Schindler TH, Lai S, Schulman SP, Gerstenblith G, Leucker TM. The Trajectory of Lipoprotein(a) During the Peri- and Early Postinfarction Period and the Impact of Proprotein Convertase Subtilisin/Kexin Type 9 Inhibition. Am J Cardiol. 2022;171:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 9. | Japanese Society of Circulators, Japanese Society of Coronary Diseases, Japanese Society of Chest Surgery, Japanese Society of Concentrated Therapy Medicine, Japanese Society of Cardiovascular Interventional Therapy, Japanese Society of Cardiovascular Surgery, Japanese Society of Cardiology, the Japanese Society of Cardiovascular Rehabilitation, Japanese Society of Arrhythmias and Electrocardiogram. JCS 2018 Guideline on Diagnosis and Treatment of Acute Coronary Syndrome. December 12, 2022. [cited 1 March 2024]. Available from: https://www.j-circ.or.jp/cms/wp-content/uploads/2018/11/JCS2018_kimura.pdf. |
| 10. | Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, Filippatos G, Fox K, Huber K, Kastrati A, Rosengren A, Steg PG, Tubaro M, Verheugt F, Weidinger F, Weis M; ESC Committee for Practice Guidelines (CPG). Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008;29:2909-2945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1468] [Cited by in RCA: 1425] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 11. | Dati F, Tate JR, Marcovina SM, Steinmetz A; International Federation of Clinical Chemistry and Laboratory Medicine; IFCC Working Group for Lipoprotein(a) Assay Standardization. First WHO/IFCC International Reference Reagent for Lipoprotein(a) for Immunoassay--Lp(a) SRM 2B. Clin Chem Lab Med. 2004;42:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Sawaguchi J, Saeki Y, Oda M, Takamura TA, Fujibayashi K, Wakasa M, Akao H, Kitayama M, Kawai Y, Kajinami K. The circulating furin-cleaved/mature PCSK9 ratio has a potential prognostic significance in statin-naïve patients with acute ST elevation myocardial infarction. Atheroscler Plus. 2022;50:50-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Nakai M, Watanabe M, Kokubo Y, Nishimura K, Higashiyama A, Takegami M, Nakao YM, Okamura T, Miyamoto Y. Development of a Cardiovascular Disease Risk Prediction Model Using the Suita Study, a Population-Based Prospective Cohort Study in Japan. J Atheroscler Thromb. 2020;27:1160-1175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Steffen BT, Duprez D, Bertoni AG, Guan W, Tsai MY. Lp(a) [Lipoprotein(a)]-Related Risk of Heart Failure Is Evident in Whites but Not in Other Racial/Ethnic Groups. Arterioscler Thromb Vasc Biol. 2018;38:2498-2504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Ziogos E, Vavuranakis MA, Harb T, Foran PL, Blaha MJ, Jones SR, Lai S, Gerstenblith G, Leucker TM. Lipoprotein(a) concentrations in acute myocardial infarction patients are not indicative of levels at six month follow-up. Eur Heart J Open. 2023;3:oead035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 16. | Enkhmaa B, Berglund L. Non-genetic influences on lipoprotein(a) concentrations. Atherosclerosis. 2022;349:53-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 17. | Chemello K, Chan DC, Lambert G, Watts GF. Recent advances in demystifying the metabolism of lipoprotein(a). Atherosclerosis. 2022;349:82-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 18. | Hoover-Plow J, Huang M. Lipoprotein(a) metabolism: potential sites for therapeutic targets. Metabolism. 2013;62:479-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 19. | Kronenberg F. Causes and consequences of lipoprotein(a) abnormalities in kidney disease. Clin Exp Nephrol. 2014;18:234-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Mack S, Coassin S, Rueedi R, Yousri NA, Seppälä I, Gieger C, Schönherr S, Forer L, Erhart G, Marques-Vidal P, Ried JS, Waeber G, Bergmann S, Dähnhardt D, Stöckl A, Raitakari OT, Kähönen M, Peters A, Meitinger T, Strauch K, Kedenko L, Paulweber B, Lehtimäki T, Hunt SC, Vollenweider P, Lamina C, Kronenberg F. A genome-wide association meta-analysis on lipoprotein (a) concentrations adjusted for apolipoprotein (a) isoforms. J Lipid Res. 2017;58:1834-1844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 21. | Yang X, Sethi A, Yanek LR, Knapper C, Nordestgaard BG, Tybjærg-Hansen A, Becker DM, Mathias RA, Remaley AT, Becker LC. SCARB1 Gene Variants Are Associated With the Phenotype of Combined High High-Density Lipoprotein Cholesterol and High Lipoprotein (a). Circ Cardiovasc Genet. 2016;9:408-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Yang XP, Amar MJ, Vaisman B, Bocharov AV, Vishnyakova TG, Freeman LA, Kurlander RJ, Patterson AP, Becker LC, Remaley AT. Scavenger receptor-BI is a receptor for lipoprotein(a). J Lipid Res. 2013;54:2450-2457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Tsimikas S, Lau HK, Han KR, Shortal B, Miller ER, Segev A, Curtiss LK, Witztum JL, Strauss BH. Percutaneous coronary intervention results in acute increases in oxidized phospholipids and lipoprotein(a): short-term and long-term immunologic responses to oxidized low-density lipoprotein. Circulation. 2004;109:3164-3170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 205] [Article Influence: 9.8] [Reference Citation Analysis (0)] |