Published online May 26, 2024. doi: 10.4330/wjc.v16.i5.293
Revised: April 24, 2024
Accepted: May 13, 2024
Published online: May 26, 2024
Processing time: 108 Days and 2.7 Hours
In severe cases of coronary artery disease, percutaneous coronary intervention provide promising results. The stent used could be a drug-eluting stent (DES) or a titanium-nitride-oxide coated stent (TiNOS).
To compare the 5-year effectiveness and safety of the two stent types.
The following systematic review and meta-analysis was conducted in accordance with the preferred reporting items for systematic reviews and meta-analysis guidelines, and PubMed/MEDLINE, Scopus, and Cochrane Central were searched from inception till August 2023. Primary outcomes were major adverse cardiac events (MACE), cardiac death, myocardial infarction (MI), cardiac death or MI, and ischemia-driven total lesion revascularization (ID-TLR).
Four randomized controlled trials (RCT), which analyzed a sum total of 3045 patients with acute coronary syndrome (ACS) after a median follow-up time of 5 years were included. Though statistically insignificant, an increase in the ID-TLR was observed in patients receiving TiNOSs vs DESs. In addition, MI, cardiac death and MI, and definite stent thrombosis (DST) were significantly decreased in the TiNOS arm. Baseline analysis revealed no significant results with meta-regression presenting non-ST elevated MI (NSTEMI) as a statistically significant covariate in the outcome of MACE.
TiNOS was found to be superior to DES in terms of MI, cardiac death or MI, and DST outcomes, however, the effect of the two stent types on ID-TLR and MACE was not significant. A greater number of studies are required to establish an accurate comparison of patient outcomes in TiNOS and DES.
Core Tip: Acute coronary syndrome (ACS) is characterized by reduced blood flow to the myocardium. While percutaneous coronary intervention with drug-eluting stents (DES) remains the standard management of ACS patients, titanium-nitride-oxide-coated stents (TiNOS) are a relatively newer intervention with relatively lower host immune reactions. In order to facilitate clinical practice guidelines in ACS patients requiring stent placement, it is imperative to compare and assess the safety and efficacy of DES and TiNOS. Therefore, this meta-analysis compared the two interventions in terms of major adverse cardiac events, cardiac death, myocardial infarction, and ischemia-driven total lesion revascularization outcomes.
- Citation: Fahim MAA, Salman A, Khan HA, Hasan SM, Bhojani MF, Aslam S, Haq AZU, Bejugam VR, Nasir BM, Gul W, Moeed A, Abdalla AS, Majid M, Asghar MS, Hasibuzzaman MA. Long-term outcomes of titanium-nitride-oxide coated stents and drug-eluting stents in acute coronary syndrome: A systematic review and meta-analysis. World J Cardiol 2024; 16(5): 293-305
- URL: https://www.wjgnet.com/1949-8462/full/v16/i5/293.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i5.293
Coronary artery disease (CAD) can be defined as a medical condition where the heart’s blood vessels become obstructed, resulting in ischemia and subsequent hypoxia to the cardiac tissue[1]. It is categorized into two groups: acute coronary syndrome (ACS) and chronic coronary syndrome (CCS) with the former differing from the latter mainly in the onset of symptoms[2].
ACS can manifest in a number of ways, namely ST-segment elevation myocardial infarction (STEMI), non-STEMI (NSTEMI), and unstable angina with subsequent arrhythmias. Clinically, a presentation with chest pain localized to the sternum, often described as a sensation of crushing or pressure can be seen. This discomfort may also radiate to the jaw and/or left arm[3].
Percutaneous coronary interventions (PCI) with drug-eluting stents (DES) have been established as the recommended treatment for ACS owing to their superior efficacy compared to bare metal stents (BMS). The introduction of immunosuppressant and anti-proliferative drugs such as sirolimus, everolimus, paclitaxel, and zotarolimus confers the benefit of lower rates of early cellular proliferation, inflammation, and therefore restenosis[4,5]. However, the comparatively newer titanium-nitride-oxide coated stents (TiNOS) have proven to be at par. The titanium-nitride coating affords the benefit of inducing lower levels of host immune reactions compared to other BMS such as non-coated stainless steel, better early vascular healing, lower rates of malapposition, and better stent coverage[6,7]. Additionally, TiNOS have been reported to require a lower duration of post-procedure anticoagulant therapy, owing to their anti-thrombotic nature[8].
In recent years, there have been several randomized controlled trials (RCT) comparing the clinical outcomes of TiNOSs and DESs in patients with ACS with the latest report being on the 5-year follow-up of patients in the TIDE-ACS trial a study that bears a considerable sample size. This study, and the most recent meta-analysis by Daoud et al[9] concluded that TiNOSs are a non-inferior and safe alternative to DES in ACS patients[9,10]. However, there is no meta-analysis to date, which has assessed the long-term efficacy and safety of TiNOSs and DESs with a sufficient sample size. Therefore, we aimed to compare the 5-year effectiveness and safety of the two stent types, using the latest data from the RCTs evaluating outcomes 5 years post-procedure.
The following systematic review and meta-analysis was conducted in accordance with the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines with a PRISMA checklist[11]. The research questionnaire was formulated utilizing the patient, intervention, control, and outcome (PICO) framework[12] and a search strategy based on the aforementioned questionnaire comprising of the Boolean operators “AND” and “OR” along with various MeSH terms was run on three separate databases: PubMed/MEDLINE, Scopus, and Cochrane Central. These databases were then systematically searched from inception till August 2023, without any restrictions or filters on the basis of language, year of publication, author names, country, institution of publication, or any other aspect applied, and all relevant RCTs and observational studies were selected. The terms utilized in the search strategy included ‘bioactive’, ‘Titanium’, ‘nitride’, ‘oxide’, ‘TiNO’, ‘TNO’, ‘BAS’, ‘stent’, ‘DES’, ‘drug’ and ‘eluting stent’. As this study sees publicly available data, study registry and institutional review board approval were not required. A more detailed overview of the search strategy used is given in Supplementary Table 1. Additionally, to identify grey literature ClinicalTrials.gov, Medrxiv.org, and Google Scholar were searched. This systematic review and meta-analysis is registered in the International Prospective Register of Systematic Reviews (PROSPERO ID: CRD42024534358).
Following the comprehensive literature search, all articles retrieved were exported to the EndNote Reference library (Version X7.5; Clarivate Analytics, Philadelphia, PA, United Sates), where duplicates were identified and removed accordingly. Two independent authors (Muhammad Ahmed Ali Fahim and Afia Salman) initially screened the remaining articles on the basis of Title and Abstract, after which full texts were evaluated to confirm relevance. Any disagreements between the two authors were resolved after discussion with a third author (Hira Anas Khan).
Studies complying with the following inclusion criteria were included in our analysis: Presented their findings in english literature; patients above 18 years of age; patients with CAD who received coronary PCI; comparative studies involving implantation of either a TiNOS or DES; outcomes reported at a 5-year follow-up; provided the outcomes as risk ratios (RR), odd ratios or raw data that could be utilized to calculate them; studies reporting one or more of our primary and or secondary outcomes. Our exclusion criteria included conference abstracts, letters, case reports, and studies containing inadequate original data for further analysis.
We defined our primary outcomes of interest as major adverse cardiac event [MACE, which we defined as a composite of cardiac death, myocardial infarction (MI) or ischemia-driven total lesion revascularization (ID-TLR)], cardiac death, MI, cardiac death or MI and ID-TLR. Our secondary outcomes of interest were defined as all-cause death and definite stent thrombosis (DST).
For this study, the program RevMan (version 5.4.1; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020) was used for all meta-analyses and subgroup analysis with the study population being divided into ACS or ACS and CCS subgroups. While Comprehensive Meta Analyst (version 3.7) was used for all meta-regression analyses. A random-effects model was used, for both, and RR along with their 95% Confidence Intervals (CI) were pooled. A P value of > 0.05 was considered statistically significant for all outcomes. Furthermore, heterogeneity was assessed with the Higgins I2 test. A value of I2 = 25%-50% was considered mild, 50%-75% moderate, and > 75% significant heterogeneity. Meta-regression results were reported as coefficients (Coeff) and P values.
The study, baseline patient, procedural, and angiographic characteristics were extracted onto an Excel Sheet and verified by two independent authors (Muhammad Ahmed Ali Fahim and Afia Salman). Any disagreements were resolved after consultation with a third author (Hira Anas Khan). Extracted data included, study name, year of publication, study design, study location, sample size, type of DES, study outcomes number of patients in each group, follow-up period, general patient characteristics (age and sex), comorbidities (diabetes mellitus, hypertension), risk factors (smoking, family history) prior cardiac events/procedures [MI, PCI, coronary artery bypass graft (CABG)], cause of PCI (NSTEMI, STEMI, unstable angina), reference vessel diameter, lesion length, total stents per lesion, direct stenting, stents per (culprit) Lesion, stent diameter, primary and secondary endpoints. For certain studies particular baseline and study characteristics were not accessible in the documents pertaining to the 5-year outcomes. As a result, the publication of the same trial for 1-year outcomes was utilized to comprehensively extract these characteristics for further analysis and regression[13-15].
The Revised Cochrane risk-of-bias tool for randomized trials (ROB 2)[16] tool was used by two independent authors (Muhammad Ahmed Ali Fahim and Afia Salman) to assess the quality of each RCT reported in this meta-analysis. The studies were analyzed according to their randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, and selection of the reported results. All inconsistencies were resolved with discussion and agreement. Additionally, the quality of evidence of each outcome was assessed utilizing GRADEpro[17].
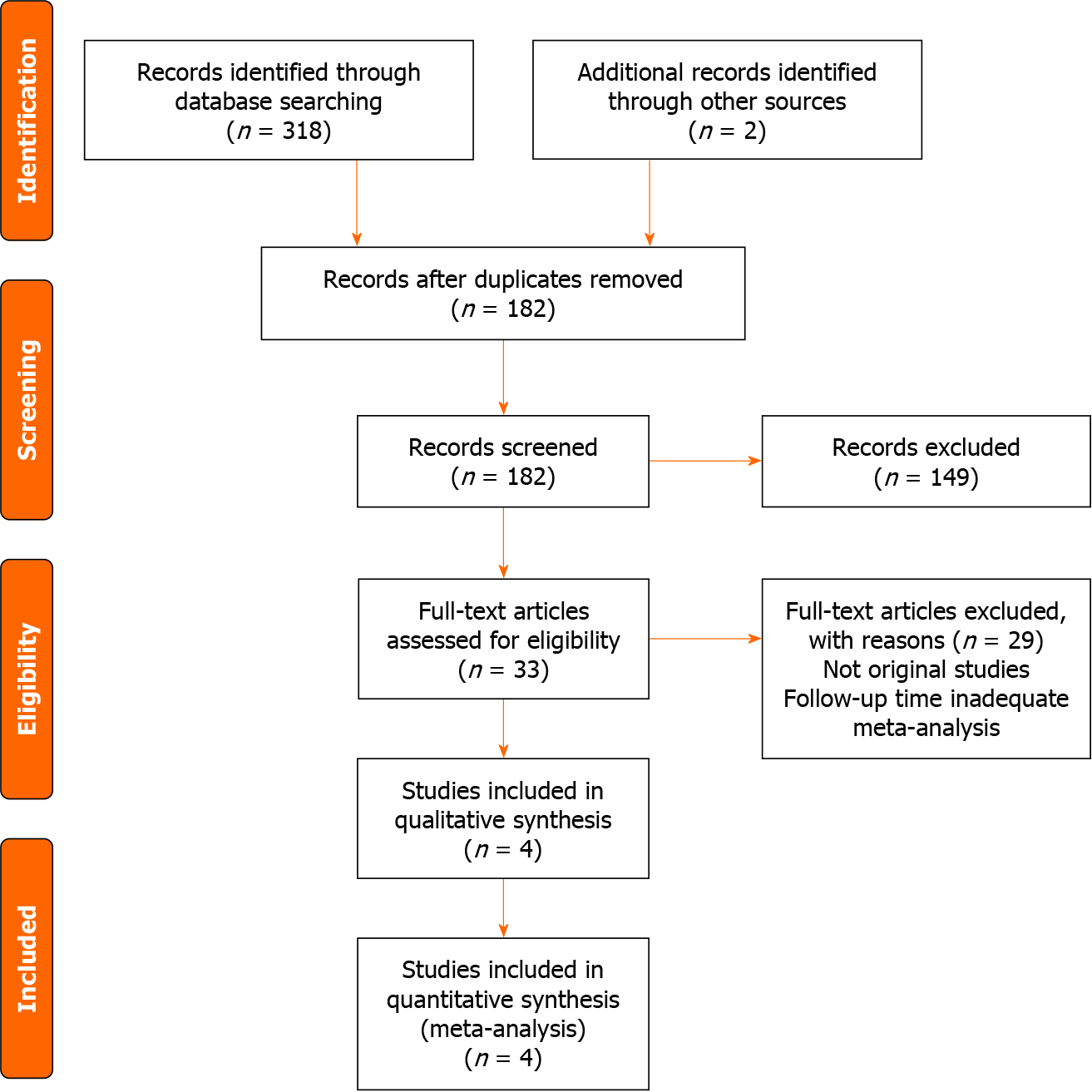
After retrieval of a total of 320 studies from the aforementioned resources, 4 studies[10,18-20], all being RCTs, were narrowed down and included in the meta-analysis. A more detailed explanation of the process is represented in the PRISMA flow chart as shown in Figure 1. The following outcomes of MACE, ID-TLR, cardiac death, MI, cardiac death or MI, DST, and all-cause death were extracted, pooled and analyzed for a sum total of 3045 patients with ACS after a median follow-up time of 5 years. The studies that presented patient data as pooled ACS and CCS were sub-grouped accordingly. Tables 1 and 2 identify the study characteristics and baseline patient, procedural, and angiographic characteristics of the included studies respectively.
| Study name | Year of publication | Study design | Study location | Sample size | Intervention | Control | Study outcomes | Follow-up duration |
| TIDE | 2011 | RCT | Switzerland | 302 | TiNO-coated stent | Zotarolimus-eluting stent | In-stent late lumen loss; MACE; death; cardiac death; MI; clinically-indicated TLR; clinically-indicated TVR; repeat vascularization; cardiac death or MI; stroke; cardiac death, MI, or clinically indicated TLR | 5 years |
| BASE ACS | 2016 | RCT | Finland | 827 | TiNO-coated stent | Everolimus-eluting stent | MACE; cardiac death; non-cardiac death; total death; non-fatal MI; cardiac death or MI; ischemia-driven TLR; DST | 5 years (median) |
| TITAX AMI | 2013 | RCT | Finland | 425 | TiNO-coated stent | Paclitaxel-eluting stent | MACE; cardiac death; recurrent MI; cardiac death or recurrent MI; ischemia-driven TLR; DST; all-cause death | 5 years |
| TIDES ACS | 2023 | RCT | Five European countries | 1491 | TiNO-coated stent | Everolimus-eluting stent | Cardiac death; MI; ischemia-driven TLR; major bleeding; cardiac death or MI; stent thrombosis; non-cardiac death; all-cause death | 5 years |
| TIDE (2011) | BASE ACS (2016) | TITAX AMI (2013) | TIDES ACS (2023) | ||
| Sample size n (TiNOS/DES) | 302 (152/150) | 827 (417/410) | 425 (214/211) | 1491 (989/502) | |
| Age (yr), mean ± SD | TiNOS | 65.9 ± 9.0 | 62.9 ± 12.0 | 64 ± 11 | 62.7 ± 10.9 |
| DES | 63.4 ± 10.5 | 63.0 ± 11.8 | 64 ± 11 | 62.6 ± 10.5 | |
| Male sex, n | TiNOS | 124 | 317 | 162 | 745 |
| DES | 118 | 312 | 157 | 383 | |
| Female sex, n | TiNOS | 28 | 100 | 52 | 244 |
| DES | 32 | 98 | 54 | 119 | |
| Diabetes mellitus | TiNOS | 30 (19.7) | 65 (15.6) | 48 (22) | 140 (14.2) |
| DES | 28 (18.7) | 75 (18.3) | 33 (16) | 63 (12.5) | |
| Hypertension | TiNOS | 105 (69.1) | 201 (48.2) | 122 (57) | 463 (46.8) |
| DES | 113 (75.3) | 212 (51.7) | 106 (50) | 219 (43.6) | |
| Current smoking/smoking | TiNOS | 53 (34.9) | 144 (34.5) | 113 (53) | 309 (31.2) |
| DES | 43 (28.7) | 134 (32.7) | 97 (46) | 180 (35.9) | |
| Family history of IHD/CAD | TiNOS | 45 (29.6) | 192 (46.0) | 103 (48) | 503 (50.9) |
| DES | 47 (31.3) | 185 (45.1) | 95 (45) | 247 (49.2) | |
| Prior MI | TiNOS | 42 (27.6) | 56 (13.4) | 33 (15) | 75 (7.6) |
| DES | 32 (21.3) | 40 (9.8) | 20 (9) | 45 (9.0) | |
| Prior PCI | TiNOS | 39 (25.7) | 40 (9.6) | 22 (10) | 69 (7.0) |
| DES | 38 (25.3) | 43 (10.5) | 10 (5) | 33 (6.6) | |
| Prior CABG | TiNOS | 12 (7.9) | 20 (4.8) | 16 (7) | 6 (6.0) |
| DES | 4 (2.7) | 17 (4.1) | 13 (6) | 6 (1.2) | |
| STEMI | TiNOS | 0 (0) | 162 (38.8) | 83 (39) | 444 (44.9) |
| DES | 0 (0) | 159 (38.8) | 97 (46) | 239 (47.6) | |
| Unstable angina | TiNOS | 14 (9.2) | 49 (11.8) | 0 (0) | 126 (12.7) |
| DES | 16 (10.7) | 64 (15.6) | 0 (0) | 61 (12.2) | |
| NSTEMI | TiNOS | 50 (32.9) | 206 (49.4) | 131 (61) | 458 (46.3) |
| DES | 63 (42.0) | 187 (45.6) | 114 (54) | 226 (45.0) | |
| Reference vessel diameter (mm), mean ± SD | TiNOS | 2.88 ± 0.47 | 3.13 ± 0.43 | 3.16 ± 0.45 | 3.20 ± 0.45 |
| DES | 2.90 ± 0.53 | 3.14 ± 0.43 | 3.11 ± 0.50 | 3.21 ± 0.45 | |
| Lesion length (mm), mean ± SD | TiNOS | 13.1 ± 8.1 | 14.4 ± 5.4 | 13.6 ± 5.6 | 14.9 ± 6.5 |
| DES | 14.2 ± 8.9 | 14.3 ± 6.5 | 13.2 ± 6.4 | 14.8 ± 5.9 | |
| Direct stenting | TiNOS | 76 (33.2) | 134 (32.1) | 26 (12) | 225 (22.8) |
| DES | 66 (29.7) | 126 (30.7) | 32 (15) | 145 (28.9) | |
| Total stent length per lesion (mm), mean ± SD | TiNOS | 19.3 ± 11.1 | 20.8 ± 9.4 | 18.5 ± 6.4 | 20.5 ± 7.8 |
| DES | 19.6 ± 10.0 | 20.6 ± 8.2 | 19.2 ± 7.2 | 20.6 ± 7.2 | |
| Stents per (culprit) lesion, mean ± SD | TiNOS | 1.28 ± 0.55 | 1.15 ± 0.38 | 1.1 ± 0.3 | 1.13 ± 0.38 |
| DES | 1.17 ± 0.45 | 1.14 ± 0.36 | 1.1 ± 0.4 | 1.14 ± 0.37 | |
| Stent diameter (mm), mean ± SD | TiNOS | 3.02 ± 0.46 | 3.15 ± 0.44 | 3.16 ± 0.42 | 3.22 ± 1.14 |
| DES | 3.01 ± 0.50 | 3.15 ± 0.45 | 3.11 ± 0.45 | 3.19 ± 0.43 | |
The risks of bias of the included RCTs are demonstrated in Supplementary Figure 1. The overall risk of bias across the RCTs is 75%. Two studies had a high risk of bias for deviation from the intended interventions (TIDE, TITAX-AMI). Additionally, one study represented a high risk of bias for the measurement of the outcomes (BASE-ACS). Furthermore, potential bias existed in one study for the measurement of outcomes (TIDES-ACS). The individual risk of bias summary for each RCT is given in Supplementary Figure 2.
An analysis was performed on the following compiled categorical and continuous baseline patient, procedural and angiographic characteristics of age, gender, diabetes mellitus, hypertension, smoking, family history for ischemic heart disease (IHD) or CAD, prior MI, prior PCI, prior CABG, reference vessel diameter, lesion length, stent per (culprit) lesion, stent diameter, total stent length per lesion, direct stenting, and NSTEMI the results of which are given in Supplementary Tables 2 and 3. Analysis results for all revealed a P value of greater than 0.05 indicating no significant differences in characteristics between the 2 arms patients were randomized in.
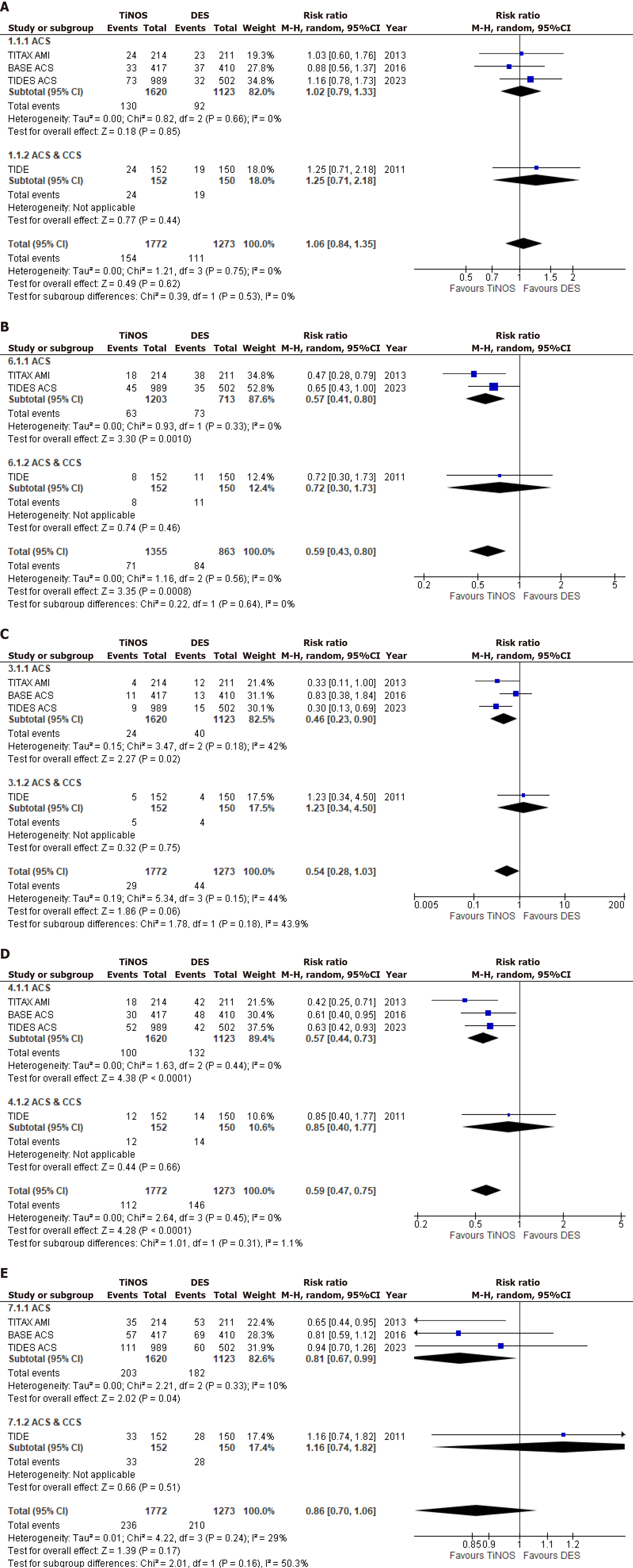
ID-TLR was an outcome of interest across all 4 studies. Upon pooling the results, a slightly increased occurrence of ID-TLR was observed in patients receiving TiNOSs as compared to those receiving DES. However, this disparity did not achieve statistical significance. (RR = 1.06, 95%CI = 0.84-1.35; P = 0.62; I² = 0%). Pooling data from three studies that evaluated the incidence of MI underscored a significant risk reduction associated with TiNOSs between the 2 groups (RR = 0.59, 95%CI = 0.43-0.80; P = 0.0008; I² = 0%). Additionally, all four studies assessed cardiac death and analysis demonstrated a lower incidence of the outcome in the TiNOS group when compared to the DES group; however, this divergence did not attain statistical significance (RR = 0.54, 95%CI = 0.28-1.03; P = 0.06; I² = 44%). The composite endpoint of cardiac death or MI was reported by all 4 studies and a statistically significant reduction in risk was apparent in patients treated with TiNOSs as opposed to those with DESs (RR = 0.59, 95%CI = 0.47-0.75; P < 0.0001; I² = 0%). In terms of MACE, a collective analysis of all studies exhibited a decrease in TiNOS in patients randomized between the two groups but no statistically significant divergence (RR = 0.86, 95%CI = 0.70-1.06; P = 0.17; I² = 29%). Forest plots for primary outcomes are represented in Figure 2.
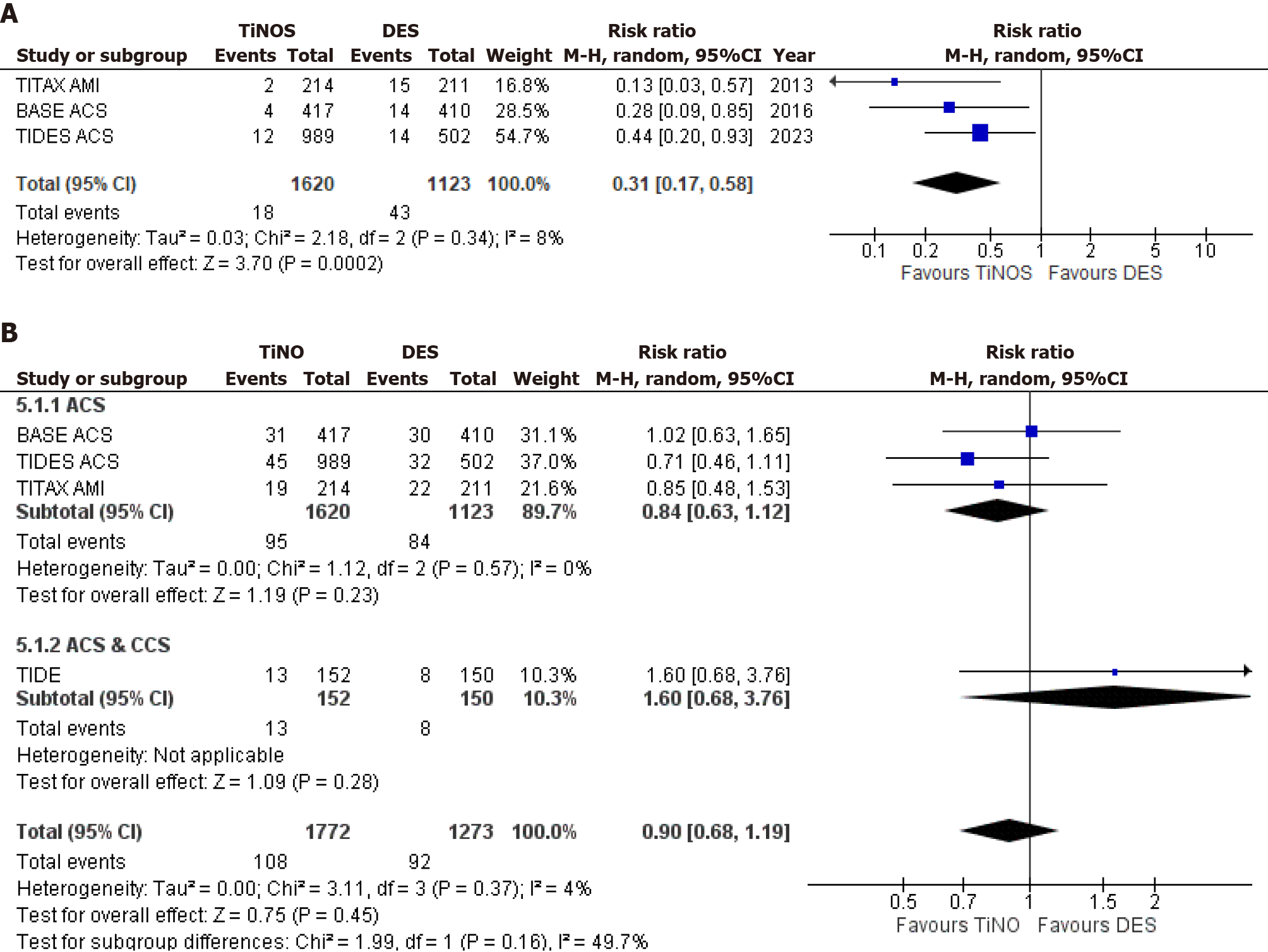
DST was evaluated across three out of four studies, revealing a statistically significant decrease in TiNOSs over DESs (RR = 0.31, 95%CI = 0.17-0.58; P = 0.0002; I² = 8%). Lastly, the analysis of all-cause death, inclusive of all studies, demonstrated a statistically non-significant decrease in TiNOS patients as compared to DES patients (RR = 0.90, 95%CI = 0.68-1.19; P = 0.45; I² = 4%). Forest plots for secondary outcomes are represented in Figure 3.
Studies were sub-grouped according to the representation of patients as ACS or ACS and CCS wherever possible. The outcome of ID-TLR had no heterogeneity overall and this trend continued when studies were sub-grouped. Similar to the overall effect both subgroups presented with the result of a non-significant increase in ID-TLR in TiNOS patients when compared with DES patients. [RR = 1.02, 95%CI = 0.79-1.33, I2 = 0% P = 0.85; RR = 1.25, 95%CI = 0.71-2.18, I2 = not available (NA), P = 0.44]. Furthermore, no subgroup differences were found (I2 = 0%). Similarly, for MI no heterogeneity was present overall or when studies sub-grouped, with both subgroups showing a decrease in MI in the TiNOS patients arm. However, this decrease was significant in the ACS subgroup while insignificant in the ACS and CCS one. (RR = 0.57, 95%CI = 0.41-0.80, I2 = 0%, P = 0.0010; RR = 0.72, 95%CI = 0.30-1.73, I2 = NA, P = 0.46). Additionally, no differences between both were shown (I2 = 0%). When assessing cardiac death mild heterogeneity was shown overall and when studies sub-grouped with the ACS subgroup it showed a slight decrease. The results of both subgroups differed greatly with the ACS subgroup showing a significant decrease in cardiac death while the ACS and CCS subgroup showed a non-significant increase in the aforementioned outcome when TiNOSs were compared with DESs. (RR = 0.46, 95%CI = 0.23-0.90, I2 = 42%, P = 0.02; RR = 1.23, 95%CI = 0.34-4.50, I2 = NA, P = 0.75). Subgroup differences having mild heterogeneity were seen (I2 = 43.9%). No heterogeneity was seen overall or in subgroups for the composite outcome of cardiac death or MI. Both subgroups showed a decrease in the outcome for the TiNOS arm however this decrease was significant in the ACS subgroup and insignificant in the ACS and CCS one. (RR = 0.57, 95%CI = 0.44-0.73, I2 = 0%, P < 0.0001; RR = 0.85, 95%CI = 0.40-1.77, I2 = NA, P = 0.66). Slight subgroup differences were shown (I2 = 1.1%). MACE showed moderate heterogeneity overall but when subgroup analysis was performed and the TIDE study was isolated from the ACS studies the heterogeneity of the ACS subgroup decreased greatly. The results of both subgroups differed with the ACS subgroup showing a significant decrease in MACE while the ACS and CCS subgroup showed a nonsignificant increase in the outcome in patients treated with TiNOSs as compared to DESs. (RR = 0.81, 95%CI = 0.67-0.99, I2 = 10%, P = 0.04; RR = 1.16, 95%CI = 0.74-1.82, I2 = NA, P = 0.51). Subgroup differences having moderate heterogeneity were seen (I2 = 50.3%). The outcome of All-Cause Death revealed slight heterogeneity overall and none in the ACS subgroup. The results of both subgroups were insignificant but differed in the sense that the ACS subgroup showed a decrease in all-cause death while the ACS and CCS subgroups showed an increase in the outcome for the TiNOS arm. (RR = 0.84, 95%CI = 0.63-1.12,
We assessed age, male gender, female gender, diabetes mellitus, hypertension, smoking, family history for IHD or CAD, prior MI, prior PCI, prior CABG, NSTEMI, reference vessel diameter, lesion length, direct stenting, total stent length per lesion, stent per (culprit) lesion, and stent diameter as covariates having an impact on specific outcomes. The outcomes assessed in the meta-regression included MACE, ID-TLR, cardiac death, cardiac death or MI, and all-cause death the results for which are represented in Supplementary Tables 4-8. All covariates for all outcomes revealed an insignificant 2-sided P value except for the covariate of NSTEMI% which had a significant result when assessed for the outcome of MACE (Coeff: -0.0369, P = 0.0416). Scatter plots are presented in the Supplementary Figures 3-87.
The quality of evidence was graded ‘High’ or ‘Moderate’ after assessment using GRADEpro with the results of ID TLR, Cardiac Death, All Cause Death and MACEs being rated as ‘Moderate’ quality after downgrading evidence in the field of ‘Imprecision’ due to individual studies having wide CIs and results being opposite. The remaining outcomes of MI, cardiac death or MI and DST being graded as ‘High’. A detailed explanation of each outcome is offered in Supplementary Table 9.
The findings of our meta-analysis and systematic review grossly favor TiNOSs over DESs. ID-TLR, the primary outcome being assessed, was found to be numerically higher with TiNOSs, whereas all other outcomes, namely cardiac death, MI, DST, MACE, and all-cause death were significantly lower in PCI with TiNOSs when compared to the occurrence of the same outcomes with DES. Meta-regression was performed for multiple variables such as age (years), male gender, female gender, diabetes mellitus, hypertension, smoking, family history for IHD or CAD, prior MI, prior PCI, prior CABG, reference vessel diameter (mm), lesion length (mm), NSTEMI, stent diameter (mm), total stent per lesion (mm) and stent per (culprit) lesion. However, none were found to be significant contributors to any outcomes being measured in our analysis except for NSTEMI for the outcome of MACE. Furthermore, a baseline analysis found no significant difference between the characteristics of the 2 arms proving that all statistically significant outcomes in our study were not due to any baseline angiographic or procedural discrepancies between the TiNOS or DES groups.
ID-TLR was found to be higher with TiNOSs (RR = 1.06, 95%CI = 0.84-1.35; P = 0.62; I² = 0%), although statistically insignificant, as compared to DESs which is in coherence with the findings of a previous meta-analysis by Daoud et al[9], as well as a comparative study by Limacher et al[21]. Although there is insufficient literature highlighting the exact cause of this occurrence, possible reasons for this could be the increased incidence of diabetes mellitus and peripheral arterial disease in patients undergoing PCI with TiNOSs in the TIDE ACS trial, a major contributor to our pooled data. Both factors have been identified as independent predictors of ID-TLR for between 2 and 4 years after PCI by a study conducted by Kurihara et al[22]. However, the validity of this hypothesis may be challenged as the mentioned study shows outcomes after treatment with DESs rather than TiNOSs. When assessed through meta-regression diabetes mellitus showed no significant association with this outcome. Early studies, such as one conducted by Varho et al[23] suggest that TiNOSs may cause greater early neointimal hyperplasia [Median (interquartile range) neointimal hyperplasia of 203 (106) µm vs 42.2 (41) µm] but similar coronary flow reserve, and fractional flow rate (FFR) compared to DES. As FFR has emerged as a valuable predictor of ID-TLR[24], this accentuates the statistical insignificance of increased ID-TLR with the TiNOS arm of this study.
TiNOSs showed statistically significant lower rates of DST vs DESs (RR = 0.31, 95%CI = 0.17-0.58; P = 0.0002; I² = 8%). These findings are consistent with those of Daoud et al[9]. According to a study, TiNOSs afford better endothelization than other BMS due to their biocompatibility which results in a less aggressive host response against the inserted stent, lower resultant inflammation, and hence, less likely thrombosis. Additionally, they have exhibited lower fibrinogen and platelet deposition, key modulators in the process[5,6]. Other important contributors to stent thrombosis are stent malapposition and insufficient stent coverage as they create a prothrombotic state due to low endothelial shear stress which causes the production of various chemical factors and according to a cohort study by Sia et al[7], Varho et al[23] and an RCT by Karjalainen et al[25], the incidence of malapposed and uncovered stents is lower with TiNOSs. The clinicians may reconsider their choice of stent types based on the different stent thrombosis outcomes. This also calls for further studies that investigate the optimal patient selection criteria based on the coagulation profile and the medical comorbidities.
These arguments can also be extended to explain the lower rates of MACE (RR = 0.86, 95%CI = 0.70-1.06; P = 0.17; I² = 29%), cardiac death (RR = 0.54, 95%CI = 0.28-1.03; P = 0.06; I² = 44%), MI (RR = 0.59, 95%CI = 0.43-0.80; P = 0.0008; I² = 0%), the composite endpoint of cardiac death or MI (RR = 0.59, 95%CI = 0.47-0.75; P < 0.0001; I² = 0%), along with the fact that while Varho et al[23] reports the opposite, as mentioned earlier, TiNOSs have the advantage of lower neointimal hyperplasia and resultant restenosis as reported by a more recent study conducted on rabbit iliac artery specimens[26]. The overall impact is satisfactory perfusion and therefore, lower probability of cardiac death. However, this is contradicted by Pilgrim et al[15] who showed an increased incidence of in-stent late loss, defined as loss in diameter in the lumen following PCI and in segment binary restenosis with TiNOSs compared to DES. However, it is important to note that this study reports outcomes 1-year post-procedure without any further follow-up reports, which were not assessed in our study.
Upon subgroup analysis, the ACS subgroup displayed a statistically significant difference in favor of TiNOSs for MACE (RR = 0.81, 95%CI = 0.67-0.99; P = 0.04) and cardiac death (RR = 0.46, 95%CI = 0.23-0.90; P = 0.02). The significance in ACS-only studies with regard to this result can be explained by the greater likelihood of early stent thrombosis in ACS compared to CCS, as concluded by Yamamoto et al[27] which can eventually result in cardiac death as already discussed. In this setting, TiNOSs anticoagulant properties in a thrombotic environment which is found in ACS aid the prognosis in patients. However, our meta-regression reported no significant association of Cardiac death or MI with any of our analyzed covariates. The argument is supported further by Karjalainen et al[28], who report a significantly lower incidence of MI and MACE after the use of BMS.
As stated previously, pooling data from three studies underscored a significant risk reduction associated with TiNOSs in MI (RR = 0.59, 95%CI = 0.43-0.80; P = 0.0008; I² = 0%). The I² = 0% suggests that there was no significant variability among the pool of subjects of all three studies, hence, strengthening the reliability of the pool data analysis. Previous studies such as the one conducted by Bouisset et al[10] also display that there is a significant decrease in the risk of MI occurring after using TiNOSs as compared to DESs. Daoud et al[9], further support this by highlighting that there is a lower risk of recurrent non-fatal MI occurring when TiNOSs are used.
The analysis of all-cause death suggests a broad measure of mortality. Our analysis of all-cause death, inclusive of all studies, demonstrated no statistically significant distinction between TiNOSs and DESs (RR = 0.90, 95%CI = 0.68-1.19; P = 0.45; I² = 4%). This infers that there is a possibility that the observed difference in mortality of the two stents could have occurred due to chance which leads it to being not significant with little variability among the studies. Upon subgroup analysis, as well as regression for the mentioned covariates, the non-significant trend persisted suggesting that the lack of statistical significance is not influenced by subgroup factors without any association between all-cause death and other factors. Similarly, as per the study conducted by Brener et al[29], there is no significant difference in cardiovascular vs non-cardiovascular mortality post-PCI regardless of the stent used.
While assessing NSTEMI as a covariate to possibly have an effect on the outcomes, we observed a statistically significant association with MACE. A multitude of previous research has presented an association of STEMI with MACE[30-33]. Ours as well as Fath-Ordoubadi et al[34] are among the few that have presented the contrary. This may possibly be due to the fact that long-term follow-up studies have smaller sample sizes and incomplete reporting of outcomes of interest[35].
It is important to note that this study may be limited in its extent to elucidate the comparison between TiNOSs and DESs accurately. Due to the limited number of studies, the data may not be representative. Furthermore, all pooled data has been derived from European countries without subgroup studies on participant ethnicities which limits its generalizability. Since the prognosis for ACS and subsequent PCI as a whole is multifactorial including race as a potential risk factor[36], this warrants a detailed study that focuses on the differences in outcomes in people of different races and ethnicities. The data used included patients with both ACS and CCS. However, subgroup studies were conducted to tackle this discrepancy. The trials included also displayed a difference in the type of DES used which could have impacted the results of each and hence, our analysis, even if only to a very limited extent. Another factor that limits the accuracy of our results is the bias due to the deviation from intended intervention specifically in the TIDE and TITAX-AMI studies. For example, in the TITAX-AMI trial, a greater percentage of patients were administered glycoprotein IIa/IIIb inhibitors in patients being treated with TiNOSs than DESs. Additionally, most trials are limited due to the lack of angiographic follow up which may have a possible contribution to the results favoring DESs with regard to ID-TLR or other outcomes favoring TiNOSs over DESs.
The findings of this systematic review and meta-analysis spark international collaborations and consensus efforts among researchers to share larger data sizes in order to optimal stent selection strategies in the management of ACS. Future studies could delve deeper into patient-reported outcomes and quality-of-life measures associated with different stent types. Moreover, the available evidence can be utilized to reinforce the refinement of TiNOSs and further development of novel stent strategies. Further studies can conduct a comparative analysis of healthcare costs associated with different ACS treatment approaches, which can lead to a lesser financial burden on the healthcare systems. Lastly, with the advent of newer technologies, the development of clinical practice guidelines is crucial to the management of ACS patients requiring stent placement.
Although DES was deemed to be an evolutionary move from BMS, resulting in their frequent use in PCI, TiNOSs have proved to be comparable if not superior to DESs with regard to efficacy and safety in the management of ACS. It has proven to elicit rapid healing and lower rates of long-term complications such as MI and ST even in patients with comorbidities such as diabetes. Nonetheless, it is important to conduct more studies to further evaluate these findings and fill in gaps in the literature to get better insight into the true potential of these stents.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country of origin: Bangladesh
Peer-review report’s classification
Scientific Quality: Grade D
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade C
P-Reviewer: Yu M, China S-Editor: Li L L-Editor: A P-Editor: Yuan YY
| 1. | Shahjehan RD, Bhutta BS. Coronary Artery Disease. 2023 Aug 17. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 2. | Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2791] [Cited by in RCA: 4530] [Article Influence: 906.0] [Reference Citation Analysis (0)] |
| 3. | Bhatt DL, Lopes RD, Harrington RA. Diagnosis and Treatment of Acute Coronary Syndromes: A Review. JAMA. 2022;327:662-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 392] [Article Influence: 130.7] [Reference Citation Analysis (1)] |
| 4. | Hong SJ, Hong MK. Drug-eluting stents for the treatment of coronary artery disease: A review of recent advances. Expert Opin Drug Deliv. 2022;19:269-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Windecker S, Mayer I, De Pasquale G, Maier W, Dirsch O, De Groot P, Wu YP, Noll G, Leskosek B, Meier B, Hess OM; Working Group on Novel Surface Coating of Biomedical Devices (SCOL). Stent coating with titanium-nitride-oxide for reduction of neointimal hyperplasia. Circulation. 2001;104:928-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 101] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Karjalainen PP, Nammas W. Titanium-nitride-oxide-coated coronary stents: insights from the available evidence. Ann Med. 2017;49:299-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Sia J, Nammas W, Collet C, De Bruyne B, Karjalainen PP. Comparative study of neointimal coverage between titanium-nitric oxide-coated and everolimus-eluting stents in acute coronary syndromes. Rev Esp Cardiol (Engl Ed). 2023;76:150-156. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Córdoba-Soriano JG, Gutiérrez-Díez A, Del Blanco BG, Núñez J, Amat-Santos IJ, Oteo JF, Romaguera R, Gallardo-López A, Lozano Ruíz-Poveda F, Baello P, Aguar P, Jerez-Valero M, Jiménez-Díaz VA, Serra B, Cascon JD, Morales-Ponce FJ, Portero-Portaz JJ, Melehi El Assali D, Cerrato-García P, Jiménez-Mazuecos J. Bioactive or Drug-Eluting Stents in 75 Years or Older Patients: The BIODES-75 Registry. Cardiovasc Revasc Med. 2022;42:114-120. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Daoud FC, Létinier L, Moore N, Coste P, Karjalainen PP. Efficacy and Safety of TiNO-Coated Stents versus Drug-Eluting Stents in Acute Coronary Syndrome: Systematic Literature Review and Meta-Analysis. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 10. | Bouisset F, Sia J, Mizukami T, Karjalainen PP, Tonino PAL, Pijls NHJ, Van der Heyden J, Romppanen H, Kervinen K, Airaksinen JKE, Lalmand J, Frambach P, Roza da Costa B, Collet C, De Bruyne B; TIDES-ACS Study Group. Titanium-Nitride-Oxide-Coated vs Everolimus-Eluting Stents in Acute Coronary Syndrome: 5-Year Clinical Outcomes of the TIDES-ACS Randomized Clinical Trial. JAMA Cardiol. 2023;8:703-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 11. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13343] [Article Influence: 833.9] [Reference Citation Analysis (0)] |
| 12. | Huang X, Lin J, Demner-Fushman D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA Annu Symp Proc. 2006;2006:359-363. [PubMed] |
| 13. | Karjalainen PP, Niemelä M, Airaksinen JK, Rivero-Crespo F, Romppanen H, Sia J, Lalmand J, de Bruyne B, Debelder A, Carlier M, Nammas W, Ylitalo A, Hess OM; BACE-ACS study investigators. A prospective randomised comparison of titanium-nitride-oxide-coated bioactive stents with everolimus-eluting stents in acute coronary syndrome: the BASE-ACS trial. EuroIntervention. 2012;8:306-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Tonino PAL, Pijls NHJ, Collet C, Nammas W, Van der Heyden J, Romppanen H, Kervinen K, Airaksinen JKE, Sia J, Lalmand J, Frambach P, Penaranda AS, De Bruyne B, Karjalainen PP; TIDES-ACS Study Group. Titanium-Nitride-Oxide-Coated Versus Everolimus-Eluting Stents in Acute Coronary Syndrome: The Randomized TIDES-ACS Trial. JACC Cardiovasc Interv. 2020;13:1697-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Pilgrim T, Räber L, Limacher A, Löffel L, Wenaweser P, Cook S, Stauffer JC, Togni M, Vogel R, Garachemani A, Moschovitis A, Khattab AA, Seiler C, Meier B, Jüni P, Windecker S. Comparison of titanium-nitride-oxide-coated stents with zotarolimus-eluting stents for coronary revascularization a randomized controlled trial. JACC Cardiovasc Interv. 2011;4:672-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 15191] [Article Influence: 2531.8] [Reference Citation Analysis (0)] |
| 17. | McMaster University, Evidence Prime Inc. GRADEpro GDT: GRADEpro guideline development tool [software]. 2024. [cited 3 May 2024]. Available from: https://www.gradepro.org/. |
| 18. | Karjalainen PP, Nammas W, Ylitalo A, de Bruyne B, Lalmand J, de Belder A, Rivero-Crespo F, Kervinen K, Airaksinen JKE. Long-term clinical outcome of titanium-nitride-oxide-coated stents versus everolimus-eluting stents in acute coronary syndrome: Final report of the BASE ACS trial. Int J Cardiol. 2016;222:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Pilgrim T, Räber L, Limacher A, Wenaweser P, Cook S, Stauffer JC, Garachemani A, Moschovitis A, Meier B, Jüni P, Windecker S. Five-year results of a randomised comparison of titanium-nitride-oxide-coated stents with zotarolimus-eluting stents for coronary revascularisation. EuroIntervention. 2015;10:1284-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Tuomainen PO, Ylitalo A, Niemelä M, Kervinen K, Pietilä M, Sia J, Nyman K, Nammas W, Airaksinen KE, Karjalainen PP. Five-year clinical outcome of titanium-nitride-oxide-coated bioactive stents versus paclitaxel-eluting stents in patients with acute myocardial infarction: long-term follow-up from the TITAX AMI trial. Int J Cardiol. 2013;168:1214-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Limacher A, Räber L, Laube E, Lauterburg A, Lötscher S, Hess N, Moschovitis A, Baldinger SH, Wenaweser P, Meier B, Hess OM, Jüni P. Clinical long-term outcome after implantation of titanium nitride-oxide coated stents compared with paclitaxel- or sirolimus-eluting stents: propensity-score matched analysis. EuroIntervention. 2012;7:1043-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Kurihara K, Ashikaga T, Sasaoka T, Yoshikawa S, Isobe M; Tokyo-MD PCI Study Investigators. Incidence and predictors of early and late target lesion revascularization after everolimus-eluting stent implantation in unselected patients in japan. Catheter Cardiovasc Interv. 2017;90:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Varho V, Kiviniemi TO, Nammas W, Sia J, Romppanen H, Pietilä M, Airaksinen JK, Mikkelsson J, Tuomainen P, Perälä A, Karjalainen PP. Early vascular healing after titanium-nitride-oxide-coated stent versus platinum-chromium everolimus-eluting stent implantation in patients with acute coronary syndrome. Int J Cardiovasc Imaging. 2016;32:1031-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Sato T, Goto S, Kishi S, Yamaguchi K, Warisawa T, Kozuki A, Toshihiro S, Tsuchida K, Yokoi H, Kazuya K, Akazawa K, Aizawa Y. Predictors and outcomes of ischemia-driven target lesion revascularization in deferred lesion based on fractional flow reserve: a multi-center retrospective cohort study. Cardiovasc Diagn Ther. 2022;12:485-494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 25. | Karjalainen P, Paana T, Ylitalo A, Sia J, Nammas W. Optical coherence tomography follow-up 18 months after titanium-nitride-oxide-coated versus everolimus-eluting stent implantation in patients with acute coronary syndrome. Acta Radiol. 2017;58:1077-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Cherian AM, Joseph J, Nair MB, Nair SV, Maniyal V, Menon D. Successful Reduction of Neointimal Hyperplasia on Stainless Steel Coronary Stents by Titania Nanotexturing. ACS Omega. 2020;5:17582-17591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Yamamoto F, Natsuaki M, Kuramitsu S, Ohya M, Otake H, Horie K, Yamanaka F, Shiomi H, Nakazawa G, Ando K, Kadota K, Saito S, Kimura T, Node K; REAL-ST Registry Investigators. Outcomes of Drug-Eluting Stent Thrombosis After Treatment for Acute Versus Chronic Coronary Syndrome. JACC Cardiovasc Interv. 2021;14:1082-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Karjalainen PP, Ylitalo A, Airaksinen JK, Nammas W. Five-year clinical outcome of titanium-nitride-oxide-coated bioactive stent implantation in a real-world population: a comparison with paclitaxel-eluting stents: the PORI registry. J Interv Cardiol. 2011;24:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Brener SJ, Tarantini G, Leon MB, Serruys PW, Smits PC, von Birgelen C, Crowley A, Ben-Yehuda O, Stone GW. Cardiovascular and Noncardiovascular Death After Percutaneous Coronary Intervention: Insights From 32 882 Patients Enrolled in 21 Randomized Trials. Circ Cardiovasc Interv. 2018;11:e006488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Hirsch A, Verouden NJ, Koch KT, Baan J Jr, Henriques JP, Piek JJ, Rohling WJ, van der Schaaf RJ, Tijssen JG, Vis MM, de Winter RJ. Comparison of long-term mortality after percutaneous coronary intervention in patients treated for acute ST-elevation myocardial infarction versus those with unstable and stable angina pectoris. Am J Cardiol. 2009;104:333-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Polonski L, Gasior M, Gierlotka M, Osadnik T, Kalarus Z, Trusz-Gluza M, Zembala M, Wilczek K, Lekston A, Zdrojewski T, Tendera M; PL-ACS Registry Pilot Group. A comparison of ST elevation versus non-ST elevation myocardial infarction outcomes in a large registry database: are non-ST myocardial infarctions associated with worse long-term prognoses? Int J Cardiol. 2011;152:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | García-García C, Subirana I, Sala J, Bruguera J, Sanz G, Valle V, Arós F, Fiol M, Molina L, Serra J, Marrugat J, Elosua R. Long-term prognosis of first myocardial infarction according to the electrocardiographic pattern (ST elevation myocardial infarction, non-ST elevation myocardial infarction and non-classified myocardial infarction) and revascularization procedures. Am J Cardiol. 2011;108:1061-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Chan MY, Sun JL, Newby LK, Shaw LK, Lin M, Peterson ED, Califf RM, Kong DF, Roe MT. Long-term mortality of patients undergoing cardiac catheterization for ST-elevation and non-ST-elevation myocardial infarction. Circulation. 2009;119:3110-3117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 191] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 34. | Fath-Ordoubadi F, Spaepen E, El-Omar M, Fraser DG, Khan MA, Neyses L, Danzi GB, Roguin A, Paunovic D, Mamas MA. Outcomes in patients with acute and stable coronary syndromes; insights from the prospective NOBORI-2 study. PLoS One. 2014;9:e88577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Gouda P, Savu A, Bainey KR, Kaul P, Welsh RC. Long-term risk of death and recurrent cardiovascular events following acute coronary syndromes. PLoS One. 2021;16:e0254008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Roumeliotis A, Claessen B, Sartori S, Cao D, Koh WJ, Qiu H, Nicolas J, Chandiramani R, Goel R, Chiarito M, Sweeny J, Barman N, Krishnan P, Kini A, Sharma SK, Dangas G, Mehran R. Impact of Race/Ethnicity on Long Term Outcomes After Percutaneous Coronary Intervention with Drug-Eluting Stents. Am J Cardiol. 2022;167:1-8. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |