Published online May 26, 2024. doi: 10.4330/wjc.v16.i5.269
Revised: April 23, 2024
Accepted: May 14, 2024
Published online: May 26, 2024
Processing time: 91 Days and 16.1 Hours
Ibrutinib, a targeted therapy for B-cell malignancies, has shown remarkable efficacy in treating various hematologic cancers. However, its clinical use has raised concerns regarding cardiovascular complications, notably atrial fibrillation (AF). This comprehensive review critically evaluates the association between ibrutinib and AF by examining incidence, risk factors, mechanistic links, and management strategies. Through an extensive analysis of original research articles, this review elucidates the complex interplay between ibrutinib’s the
Core Tip: This review examines the association between ibrutinib, a Bruton’s tyrosine kinase inhibitor, and atrial fibrillation (AF). It explores the underlying mechanisms, clinical implications, and management strategies for AF in patients treated with ibru
- Citation: Mohyeldin M, Shrivastava S, Allu SVV. Ibrutinib and atrial fibrillation: An in-depth review of clinical implications and management strategies. World J Cardiol 2024; 16(5): 269-273
- URL: https://www.wjgnet.com/1949-8462/full/v16/i5/269.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i5.269
Ibrutinib, an oral Bruton’s tyrosine kinase (BTK) inhibitor, has emerged as a pivotal therapeutic agent for B-cell malignancies, particularly chronic lymphocytic leukemia and mantle cell lymphoma[1,2]. While ibrutinib has demon
Several studies have reported an increased incidence of AF in patients treated with ibrutinib[3-5,7,8]. In a pooled analysis of four randomized controlled trials, the incidence of AF was 6.5% in the ibrutinib group, whereas it was 1.6% in the comparator group (relative risk: 4.1; 95%CI: 2.2-7.5)[3]. The median time to AF onset was 2.8 months (range: 0.3-26.6 months)[3]. Risk factors associated with ibrutinib-related AF include older age, hypertension, prior history of AF, and the concomitant use of anticoagulants or antiplatelet agents[3,7]. Recent findings by Tang et al[9] further support the association between ibrutinib and cardiac side effects, including AF.
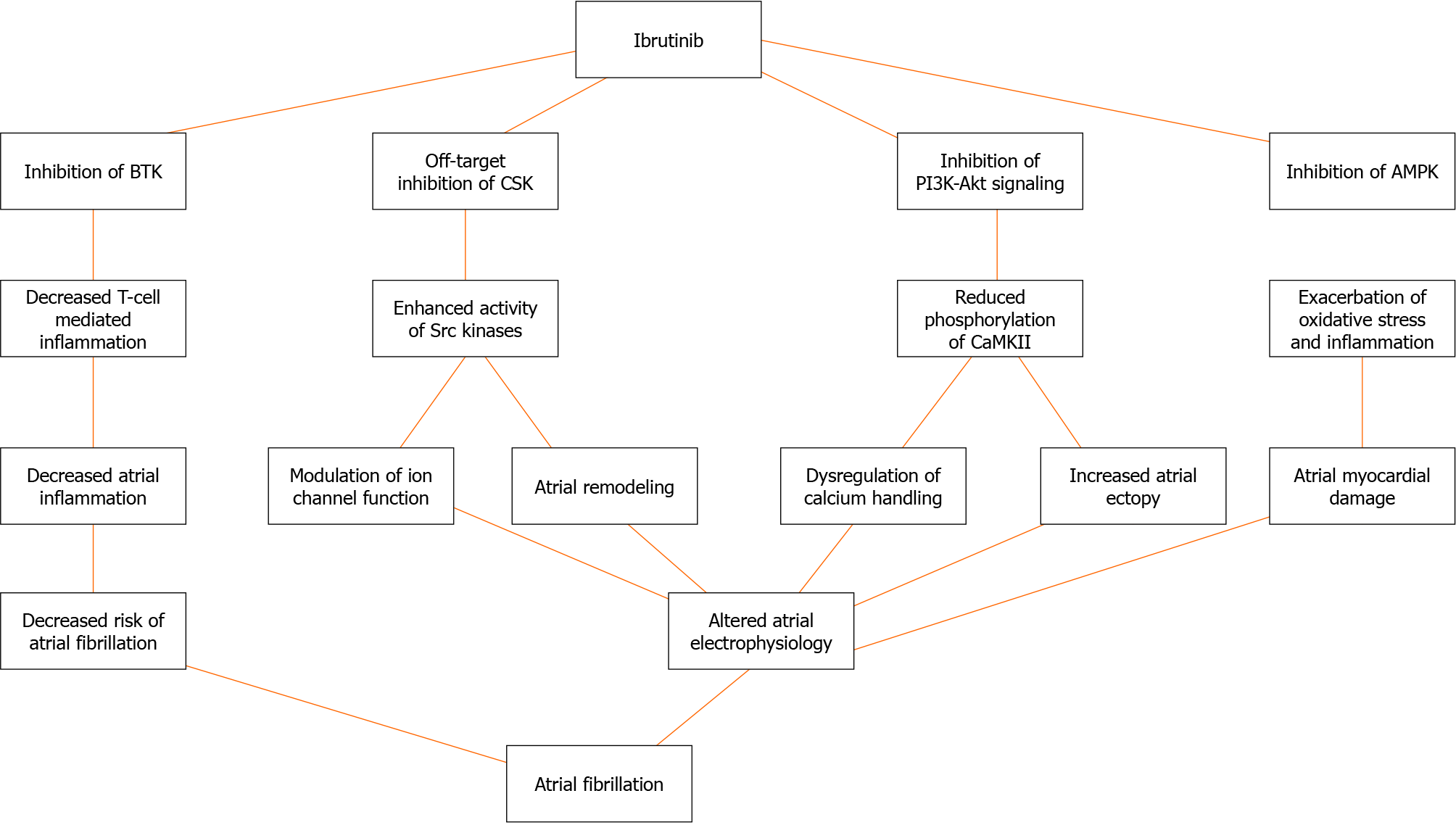
The mechanisms linking ibrutinib to AF are multifaceted and involve various pathways[5,10-12] (Figure 1). Ibrutinib has been shown to inhibit PI3K-Akt signaling, leading to reduced phosphorylation of the protein kinase Ca2+/calmodulin-dependent protein kinase II, which plays a crucial role in calcium handling and atrial electrophysiology[10-13]. The dysregulation of calcium homeostasis can trigger AF by promoting delayed afterdepolarizations and increasing atrial ectopy[11,12]. Additionally, ibrutinib inhibits the tyrosine kinase CSK, resulting in enhanced activity of Src kinases, which can modulate ion channel function and contribute to atrial remodeling[11,12,14]. Furthermore, ibrutinib has been associated with the inhibition of AMP-activated protein kinase, a key regulator of cellular energy homeostasis, potentially exacerbating oxidative stress and inflammation in the atrial myocardium[11,12]. McMullen et al[15] and Xiao et al[16] have provided evidence supporting the role of ibrutinib in increasing the risk of AF through inhibition of cardiac PI3K-Akt signaling and C-terminal Src kinase, respectively. Jiang et al[17] also highlighted ibrutinib’s promotion of AF via structural remodeling and calcium dysregulation in the atrium.
The association between ibrutinib and AF necessitates a multidisciplinary approach to patient care involving close collaboration among oncologists, cardiologists, and hematologists[10]. Baseline cardiovascular risk assessment, including electrocardiogram (ECG) and echocardiography, should be performed before initiating ibrutinib therapy[7]. Regular monitoring for signs and symptoms of AF, along with periodic ECG evaluations, is crucial for early detection and intervention[7].
Strategies for managing ibrutinib-associated AF involve a personalized approach tailored to individual patient characteristics and risk profiles[11,18]. In patients with a high risk of thromboembolism (CHA2DS2-VASc score ≥ 2), anticoagulation should be considered while weighing the benefits against the bleeding risk[12]. Novel oral anticoagulants have emerged as a promising option since their safety profile is more favorable than that of warfarin[12,19]. Rhythm control strategies, including pharmacological cardioversion and catheter ablation, may be considered in symptomatic patients or those with persistent AF[5].
Dose reduction or temporary interruption of ibrutinib may be necessary in cases of recurrent or symptomatic AF[7]. Alternative BTK inhibitors with potentially lower AF risk, such as acalabrutinib or zanubrutinib, can be considered in select patients[20,21]. Ongoing research aims to develop risk stratification tools and predictive models to identify patients with a higher risk of developing ibrutinib-related AF, enabling proactive management and personalized treatment decisions[7].
The association between ibrutinib and AF represents a significant challenge in the management of patients with B-cell malignancies. This comprehensive review highlights the incidence, risk factors, mechanistic links, clinical implications, and evolving management strategies related to this complex relationship. By integrating insights from original research articles, this review provides a robust evidence base to guide healthcare professionals in navigating the intricacies of ibrutinib therapy while prioritizing patient safety and cardiovascular well-being. Multidisciplinary collaboration, personalized risk assessment, and tailored management approaches are paramount in optimizing outcomes for patients receiving ibrutinib. Ongoing research efforts aimed at unraveling the underlying mechanisms, developing risk stratification tools, and exploring alternative therapeutic options could refine the approach to managing ibrutinib-associated AF. As the therapeutic landscape continues to evolve, this review serves as a valuable resource for healthcare professionals seeking to balance the remarkable efficacy of ibrutinib with the need for vigilant cardiovascular monitoring and proactive management strategies. By staying abreast of the latest evidence and adopting a patient-centric approach, clinicians can harness the transformative potential of ibrutinib while minimizing cardiovascular risks and ensuring the best possible outcomes for patients with B-cell malignancies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country of origin: Sudan
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade C
Creativity or Innovation: Grade C
Scientific Significance: Grade C
P-Reviewer: Du BB, China S-Editor: Li L L-Editor: A P-Editor: Yuan YY
| 1. | Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, Grant B, Sharman JP, Coleman M, Wierda WG, Jones JA, Zhao W, Heerema NA, Johnson AJ, Sukbuntherng J, Chang BY, Clow F, Hedrick E, Buggy JJ, James DF, O'Brien S. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1706] [Cited by in RCA: 1852] [Article Influence: 154.3] [Reference Citation Analysis (0)] |
| 2. | Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, Jurczak W, Advani RH, Romaguera JE, Williams ME, Barrientos JC, Chmielowska E, Radford J, Stilgenbauer S, Dreyling M, Jedrzejczak WW, Johnson P, Spurgeon SE, Li L, Zhang L, Newberry K, Ou Z, Cheng N, Fang B, McGreivy J, Clow F, Buggy JJ, Chang BY, Beaupre DM, Kunkel LA, Blum KA. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1178] [Cited by in RCA: 1316] [Article Influence: 109.7] [Reference Citation Analysis (0)] |
| 3. | Brown JR, Moslehi J, O'Brien S, Ghia P, Hillmen P, Cymbalista F, Shanafelt TD, Fraser G, Rule S, Kipps TJ, Coutre S, Dilhuydy MS, Cramer P, Tedeschi A, Jaeger U, Dreyling M, Byrd JC, Howes A, Todd M, Vermeulen J, James DF, Clow F, Styles L, Valentino R, Wildgust M, Mahler M, Burger JA. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017;102:1796-1805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 202] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 4. | Leong DP, Caron F, Hillis C, Duan A, Healey JS, Fraser G, Siegal D. The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood. 2016;128:138-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 187] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 5. | Hansen BJ, Zhao J, Li N, Zolotarev A, Zakharkin S, Wang Y, Atwal J, Kalyanasundaram A, Abudulwahed SH, Helfrich KM, Bratasz A, Powell KA, Whitson B, Mohler PJ, Janssen PML, Simonetti OP, Hummel JD, Fedorov VV. Human Atrial Fibrillation Drivers Resolved With Integrated Functional and Structural Imaging to Benefit Clinical Mapping. JACC Clin Electrophysiol. 2018;4:1501-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Wiczer TE, Levine LB, Brumbaugh J, Coggins J, Zhao Q, Ruppert AS, Rogers K, McCoy A, Mousa L, Guha A, Heerema NA, Maddocks K, Christian B, Andritsos LA, Jaglowski S, Devine S, Baiocchi R, Woyach J, Jones J, Grever M, Blum KA, Byrd JC, Awan FT. Cumulative incidence, risk factors, and management of atrial fibrillation in patients receiving ibrutinib. Blood Adv. 2017;1:1739-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 127] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 7. | Vick EJ, Patel K, Prouet P, Martin MG. Proliferation through activation: hemophagocytic lymphohistiocytosis in hematologic malignancy. Blood Adv. 2017;1:779-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Shanafelt TD, Parikh SA, Noseworthy PA, Goede V, Chaffee KG, Bahlo J, Call TG, Schwager SM, Ding W, Eichhorst B, Fischer K, Leis JF, Chanan-Khan AA, Hallek M, Slager SL, Kay NE. Atrial fibrillation in patients with chronic lymphocytic leukemia (CLL). Leuk Lymphoma. 2017;58:1630-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 9. | Tang CPS, McMullen J, Tam C. Cardiac side effects of bruton tyrosine kinase (BTK) inhibitors. Leuk Lymphoma. 2018;59:1554-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Kratzel A, Todt D, V'kovski P, Steiner S, Gultom M, Thao TTN, Ebert N, Holwerda M, Steinmann J, Niemeyer D, Dijkman R, Kampf G, Drosten C, Steinmann E, Thiel V, Pfaender S. Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 by WHO-Recommended Hand Rub Formulations and Alcohols. Emerg Infect Dis. 2020;26:1592-1595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 256] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 11. | Hopkins SR, Dominelli PB, Davis CK, Guenette JA, Luks AM, Molgat-Seon Y, Sá RC, Sheel AW, Swenson ER, Stickland MK. Face Masks and the Cardiorespiratory Response to Physical Activity in Health and Disease. Ann Am Thorac Soc. 2021;18:399-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 12. | Lambotte D, Kardol MJM, Schoenmakers B, Fret B, Smetcoren AS, De Roeck EE, Van der Elst M, De Donder L; D-SCOPE Consortium. Relational aspects of mastery for frail, older adults: The role of informal caregivers in the care process. Health Soc Care Community. 2019;27:632-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Tuomi JM, Xenocostas A, Jones DL. Increased Susceptibility for Atrial and Ventricular Cardiac Arrhythmias in Mice Treated With a Single High Dose of Ibrutinib. Can J Cardiol. 2018;34:337-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Ganatra S, Sharma A, Shah S, Chaudhry GM, Martin DT, Neilan TG, Mahmood SS, Barac A, Groarke JD, Hayek SS, Dani S, Venesy D, Patten R, Nohria A. Ibrutinib-Associated Atrial Fibrillation. JACC Clin Electrophysiol. 2018;4:1491-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 15. | McMullen JR, Boey EJ, Ooi JY, Seymour JF, Keating MJ, Tam CS. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 2014;124:3829-3830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 317] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 16. | Xiao L, Salem JE, Clauss S, Hanley A, Bapat A, Hulsmans M, Iwamoto Y, Wojtkiewicz G, Cetinbas M, Schloss MJ, Tedeschi J, Lebrun-Vignes B, Lundby A, Sadreyev RI, Moslehi J, Nahrendorf M, Ellinor PT, Milan DJ. Ibrutinib-Mediated Atrial Fibrillation Attributable to Inhibition of C-Terminal Src Kinase. Circulation. 2020;142:2443-2455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 17. | Jiang L, Li L, Ruan Y, Zuo S, Wu X, Zhao Q, Xing Y, Zhao X, Xia S, Bai R, Du X, Liu N, Ma CS. Ibrutinib promotes atrial fibrillation by inducing structural remodeling and calcium dysregulation in the atrium. Heart Rhythm. 2019;16:1374-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 18. | Pollak PT, Sun GR, Kim RB. Personalized Anticoagulation: Guided Apixaban Dose Adjustment to Compensate for Pharmacokinetic Abnormalities Related to Short-Bowel Syndrome. Can J Cardiol. 2018;34:342.e17-342.e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Khalid S, Yasar S, Khalid A, Spiro TP, Haddad A, Daw H. Management of Atrial Fibrillation in Patients on Ibrutinib: A Cleveland Clinic Experience. Cureus. 2018;10:e2701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Byrd JC, Harrington B, O'Brien S, Jones JA, Schuh A, Devereux S, Chaves J, Wierda WG, Awan FT, Brown JR, Hillmen P, Stephens DM, Ghia P, Barrientos JC, Pagel JM, Woyach J, Johnson D, Huang J, Wang X, Kaptein A, Lannutti BJ, Covey T, Fardis M, McGreivy J, Hamdy A, Rothbaum W, Izumi R, Diacovo TG, Johnson AJ, Furman RR. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med. 2016;374:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 670] [Cited by in RCA: 718] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 21. | Tam CS, Trotman J, Opat S, Burger JA, Cull G, Gottlieb D, Harrup R, Johnston PB, Marlton P, Munoz J, Seymour JF, Simpson D, Tedeschi A, Elstrom R, Yu Y, Tang Z, Han L, Huang J, Novotny W, Wang L, Roberts AW. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134:851-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 288] [Article Influence: 48.0] [Reference Citation Analysis (0)] |