Published online Mar 26, 2024. doi: 10.4330/wjc.v16.i3.149
Peer-review started: November 23, 2023
First decision: December 27, 2023
Revised: January 9, 2024
Accepted: February 6, 2024
Article in press: February 6, 2024
Published online: March 26, 2024
Processing time: 118 Days and 22.1 Hours
Obesity has become a serious public health issue, significantly elevating the risk of various complications. It is a well-established contributor to Heart failure with preserved ejection fraction (HFpEF). Evaluating HFpEF in obesity is crucial. Epicardial adipose tissue (EAT) has emerged as a valuable tool for validating prognostic biomarkers and guiding treatment targets. Hence, assessing EAT is of paramount importance. Cardiovascular magnetic resonance (CMR) imaging is acknowledged as the gold standard for analyzing cardiac function and mor
To assess the diagnostic utility of CMR for evaluating heart failure with preserved ejection fraction [HFpEF; left ventricular (LV) ejection fraction ≥ 50%] by measuring the epicardial adipose tissue (EAT) volumes and EAT mass in obese patients.
Sixty-two obese patients were divided into two groups for a case-control study based on whether or not they had heart failure with HFpEF. The two groups were defined as HFpEF+ and HFpEF-. LV geometry, global systolic function, EAT volumes and EAT mass of all subjects were obtained using cine magnetic resonance sequences.
Forty-five patients of HFpEF- group and seventeen patients of HFpEF+ group were included. LV mass index (g/m2) of HFpEF+ group was higher than HFpEF- group (P < 0.05). In HFpEF+ group, EAT volumes, EAT volume index, EAT mass, EAT mass index and the ratio of EAT/[left atrial (LA) left-right (LR) diameter] were higher compared to HFpEF- group (P < 0.05). In multivariate analysis, Higher EAT/LA LR diameter ratio was associated with higher odds ratio of HFpEF.
EAT/LA LR diameter ratio is highly associated with HFpEF in obese patients. It is plausible that there may be utility in CMR for assessing obese patients for HFpEF using EAT/LA LR diameter ratio as a diagnostic biomarker. Further prospective studies, are needed to validate these proof-of-concept findings.
Core Tip: The purpose of this research is to assess the diagnostic utility of cardiovascular magnetic resonance for evaluating heart failure with preserved ejection fraction (HFpEF) by measuring the epicardial adipose tissue (EAT) volumes in obesity. There is a strong correlation between increased EAT volumes and HFpEF in obesity. Moreover, EAT/Left atrial left-right (LA LR) diameter ratio is highly associated with HFpEF in obesity. Given the significant findings, there may be some diagnostic utility in cardiac magnetic resonance for assessing obesity for HFpEF.
- Citation: Shao JW, Chen BH, Abu-Shaban K, Baiyasi A, Wu LM, Ma J. Epicardial adipose tissue in obesity with heart failure with preserved ejection fraction: Cardiovascular magnetic resonance biomarker study. World J Cardiol 2024; 16(3): 149-160
- URL: https://www.wjgnet.com/1949-8462/full/v16/i3/149.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i3.149
Obesity has become a serious public health issue, significantly elevating the risk of various complications, including heart disease, type 2 diabetes, and hypertension[1]. It is a well-established contributor to heart failure (HF)[2]. Heart failure with preserved ejection fraction (HFpEF) is a prevalent and deadly clinical syndrome characterized by HF with a left ventricular ejection fraction (LVEF) ≥ 50%. Within the broader HFpEF population, the obesity-HFpEF phenotype has been identified as a distinct subset, potentially necessitating specific treatments[3]. Recently, there is growing recognition of the importance of anti-atherogenic and anti-inflammatory effects, known as 'meta-inflammatory' mechanisms, in the treatment of "obese HFpEF"[4]. Therefore, evaluating HFpEF in obesity is crucial.
Epicardial adipose tissue (EAT) refers to the fat surrounding the heart in the epicardium, also known as visceral fat[5]. Studies have linked EAT with HF, revealing higher EAT volume in HF patients with HFpEF[6,7]. Consequently, EAT has emerged as a valuable tool for validating prognostic biomarkers and guiding treatment targets[8,9]. Hence, assessing EAT is of paramount importance.
Accurate detection and quantification of EAT can be accomplished through 2-dimensional (2D) echocardiography, contrast-free computed tomography (CT), and magnetic resonance imaging (MRI)[10]. Echocardiography, a widely used cardiac imaging method for EAT measurement, does not expose patients to ionizing radiation[11,12]. However, it predominantly provides 2D cardiac images, measuring only the thickness, not the volume or mass of EAT[13]. Additionally, echocardiography-derived measurements may be more prone to inter-observer errors compared to cross-sectional modalities. Consequently, echocardiography is only accurate for measuring the maximum EAT thickness[14]. Nevertheless, the definitive EAT thickness threshold for use as a prognostic biomarker is yet to be determined[15]. Moreover, the applicability of EAT thickness is often constrained by suboptimal acoustic windows in obese patients.
More recently, the heightened EAT in patients exhibiting the HFpEF phenotype can be assessed through CT, potentially indicating adverse cardiac function[16]. Evaluation of cardiac function is feasible. However, CT is constrained by radiation exposure. Cardiovascular magnetic resonance (CMR) imaging is acknowledged as the gold standard for analyzing cardiac function and morphology[17]. Utilizing three-dimensional cine images, CMR enables accurate and reproducible quantification of EAT thickness, volume, and mass. Some recent CMR studies have compared EAT quantities in HFpEF groups with controls, emphasizing the need to focus on EAT beyond an individual's overall body fat concerning HFpEF[18,19]. However, it is conceivable that obesity could confound such findings due to the general increase in adipose tissue throughout the body. To our knowledge, no studies have investigated EAT metrics (including volume or mass) in obese patients using CMR to determine the association with HFpEF and whether EAT metrics could serve as a biomarker for predicting HFpEF in the obese population. Therefore, this study aims to employ CMR to examine EAT in the obese population with and without HFpEF, considering the association with co-morbidities, biomarkers, contractility parameters, and myocardial function assessed by CMR.
The study followed a case-control, prospective clinical design, enrolling 69 obese individuals from October 2019 to August 2020 at Shanghai Jiao Tong University School of Medicine Affiliated Renji Hospital. HFpEF patients meeting specific criteria were included: (1) Left ventricular (LV) ejection fraction ≥ 50%, assessed by echocardiography; (2) New York Heart Association class ≥ II, with either E/e′ > 13 and mean e′septal and lateral wall < 9 cm/s on echocardiography; (3) plasma brain natriuretic peptide (BNP) > 35 pg/mL[20]. Exclusion criteria were: (1) general contraindication to CMR; (2) poor imaging quality; (3) heart failure with mid-range ejection fraction (HFmrEF) and heart failure with reduced ejection fraction (HFrEF); (4) congenital heart disease; (5) acute ischemic cardiac injury; (6) hypertrophic cardiomyopathy; (7) greater than moderate valvular disease; (8) sarcoidosis; (9) amyloidosis; (10) thalassemia; and (11) hemochromatosis. The study complied with the 1964 Declaration of Helsinki and subsequent amendments.
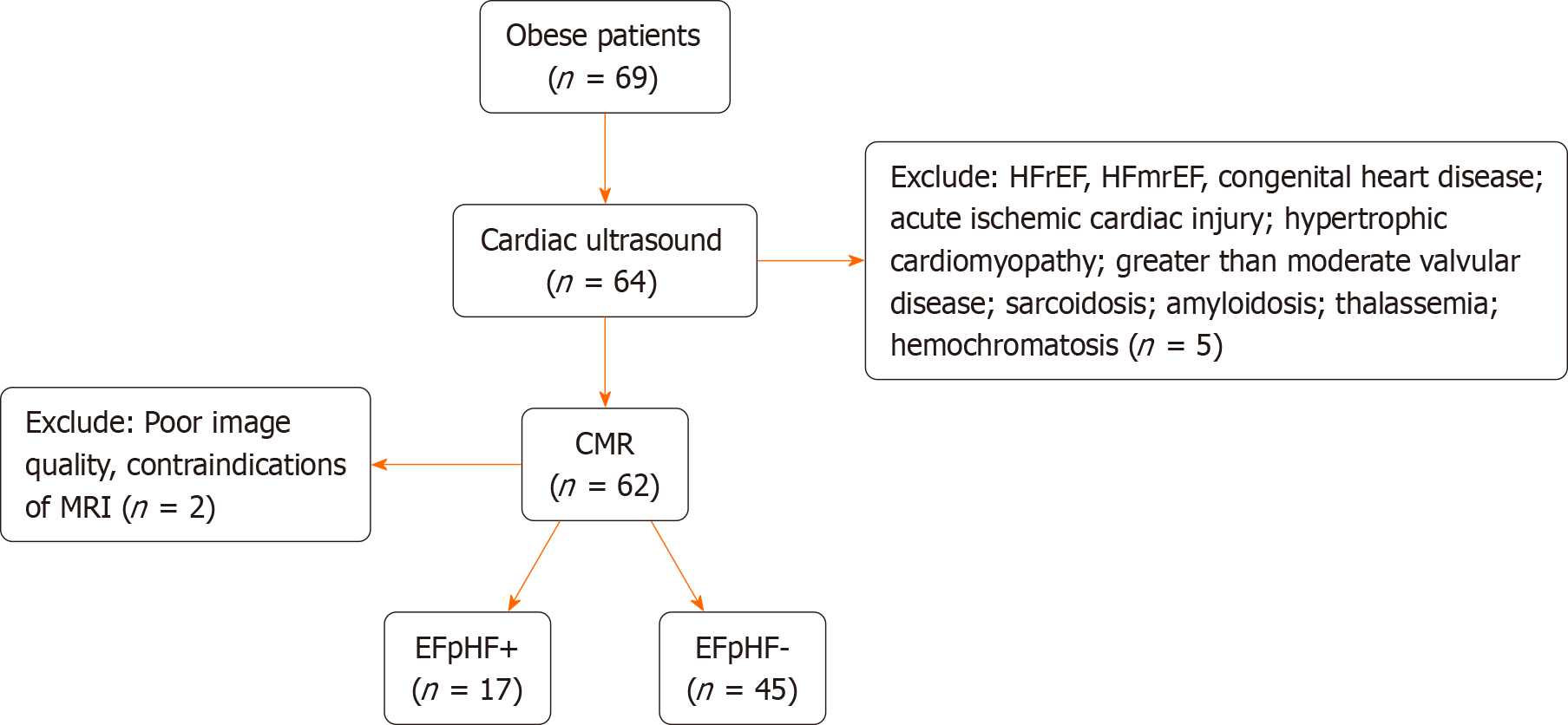
Five patients were excluded due to exclusion criteria, and 2 were excluded for poor image quality and MRI contraindications. Seventeen obese patients with HFpEF and 45 obese patients without HFpEF, meeting inclusion criteria with matched gender and age, were recruited. Obesity was defined as a body mass index (BMI) ≥ 30.0 kg/m2, following Asian-Pacific cutoff points[21]. All participants provided written, informed consent. BMI (kg/m2) was calculated, and measurements included blood pressure, serum cholesterol, serum triglycerides, serum high-density lipoprotein cholesterol, and serum low-density lipoprotein cholesterol. Fasting glucose and hemoglobin A1c levels were also assessed. The study flow diagram is depicted in Figure 1.
All examination data were obtained using a 3.0 Tesla magnetic resonance scanner (Prisma, Siemens, Erlangen, Germany) equipped with a 32-channel cardiac coil. Cine imaging was acquired through retrospective ECG gating with balanced steady-state free-precession during horizontal and vertical long-axis views, and in 16 short-axis slices covering the entire left ventricle to evaluate left ventricular function and cardiac mechanics. Data in the short-axis plane were collected at the mid-ventricular level. Imaging parameters comprised a repetition time of 326.6 ms, echo time of 1.09 ms, flip angle of 35°, field of view of 385 × 385 mm2, matrix of 156 × 192, slice thickness of 8 mm, slice gap of 4 mm, receiver bandwidth of 1085 Hz/px, GRAPPA acceleration factor 2, linear phase-encoding ordering, and 25 cardiac phases.
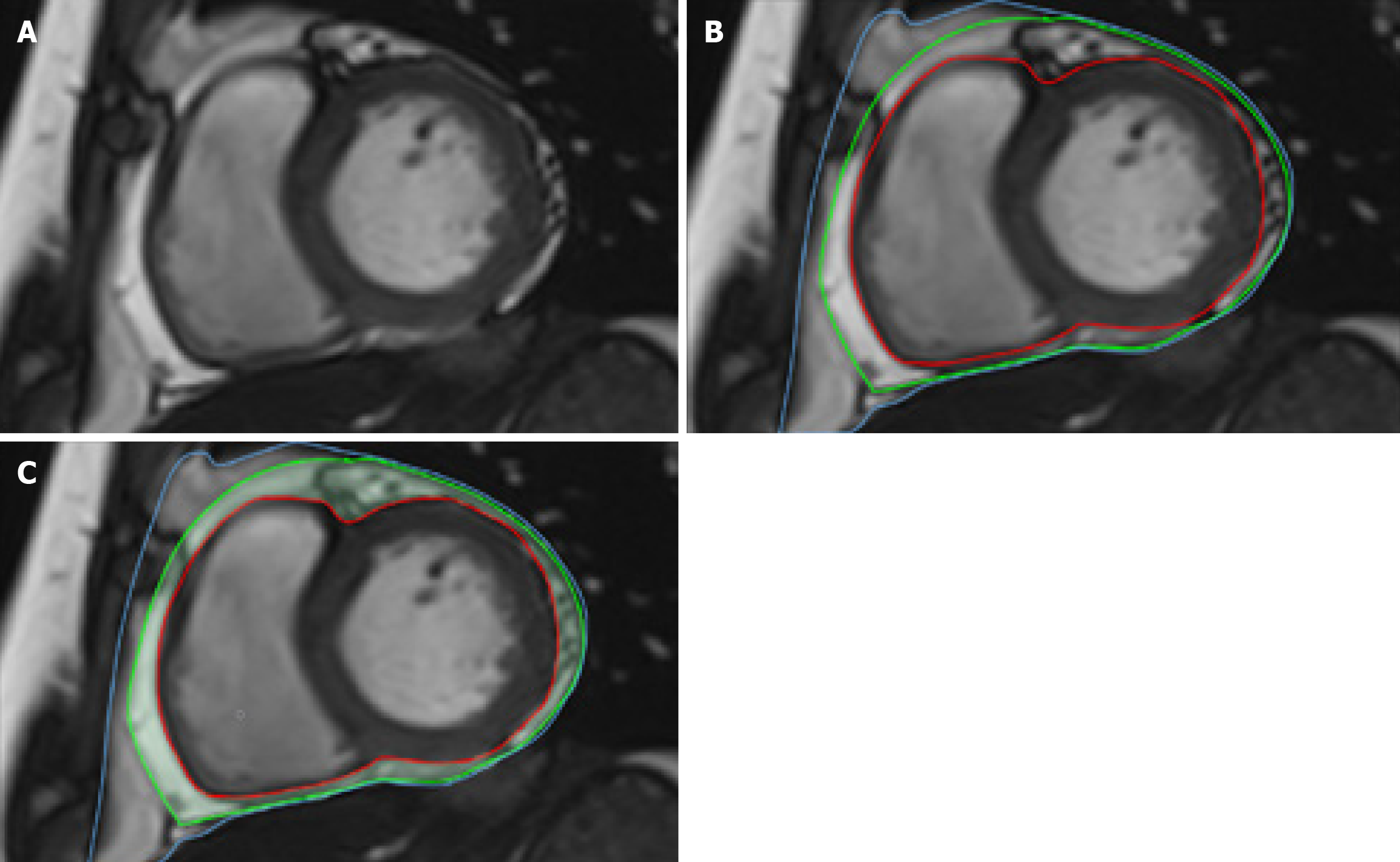
EAT was defined as the fat between the myocardium and the visceral pericardium. The borders of the EAT image were manually delineated on contiguous end-diastolic short-axis slices from the base to the apex using commercially available software (cvi42, Circle Cardiovascular Imaging Inc., Calgary, Canada) (Figure 2). Additionally, LV endocardial and epicardial borders were manually outlined slice by slice based on the initial contour set at end-diastole. EAT mass was estimated by multiplying the EAT volume by 0.92[22]. CMR image analyses were independently conducted by two experienced radiologists who were blinded to the study. LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), and LV mass were measured and normalized to body surface area. LV stroke volume (LVSV) was calculated by subtracting LVESV from LVEDV. LVEF was computed as LVSV/LVEDV × 100%. The measurement of left atrial anterior-posterior (LA AP) diameter and left atrial left-right (LA LR) diameter followed a previously reported method[23].
The normality of continuous samples was assessed using the Kolmogorov-Smirnov test for normal distribution. Group comparisons were conducted using Student’s t-test for continuous variables or Fisher’s exact test for categorical variables. Initial univariate analyses and stepwise multivariate linear regression analyses were executed to identify predictors of the odds of HFpEF in the obese population. Covariates with a univariate P value < 0.10 were included in the multivariate logistic regression analysis[24,25]. Pearson’s correlation coefficient was employed for correlation analyses. A P value < 0.05 was considered significant. Intra- and inter-observer repeatability of parameters derived from CMR were assessed using the intra-class correlation coefficient (ICC) in 30 randomly selected patients from the same cohort[26]. An ICC > 0.75 was considered indicative of good agreement[27]. Descriptive and comparative statistical analyses were carried out using SPSS version 23.0 (IBM Corp., Armonk, United States) and GraphPad Prism v. 8.0 (GraphPad Software, Inc., CA, United States).
Table 1 summarizes the baseline characteristics. The mean ages of the obese populations with HFpEF (HFpEF+) and without HFpEF (HFpEF-) were 42.94 ± 3.37 years and 36.60 ± 1.80 years, respectively (P > 0.05). In the HFpEF+ group, 17.6% were older than 60 years, compared to 0.02% in the HFpEF- group. Among HFpEF+ patients, 64.7% were males, while 55.6% of HFpEF- patients were males. No significant differences were observed in body surface area (BSA), BMI, BNP, and resting diastolic blood pressure, but there were significant differences between the two groups (P < 0.05). The prevalence of fatty liver was higher in the HFpEF- group (58.8%) compared to the HFpEF+ group (28.9%) (P = 0.0253), with no significant differences in other complications. Resting systolic blood pressure (SBP), regardless of medication control, was significantly higher in HFpEF+ patients than in the HFpEF- group (P = 0.0370).
| Parameter | HFpEF+ (n = 17) | HFpEF- (n = 45) | P value |
| Age (yr) | 42.94 ± 3.37 | 36.60 ± 1.80 | 0.0819 |
| > 60A | 3 (17.6) | 1 (0.02) | 0.0275a |
| Male gender | 11 (64.7) | 25 (55.6) | 0.5227 |
| BSA (m2) | 2.17 ± 0.07 | 2.13 ± 0.04 | 0.639 |
| Weight (kg) | 102.60 ± 5.49 | 103.00 ± 4.37 | 0.9667 |
| BMI (kg/m2) | 35.78 ± 1.28 | 33.5 ± 0.99 | 0.2093 |
| Systolic blood pressure (mmHg) | 139.80 ± 4.73 | 130.90 ± 1.84 | 0.0370a |
| Diastolic blood pressure (mmHg) | 86.59 ± 3.21 | 82.51 ± 1.61 | 0.2174 |
| Complications | |||
| Diabetes | 9 (20.0) | 4 (23.5) | 0.7653 |
| Hypertension | 6 (35.3) | 6 (13.3) | 0.052 |
| Hyperlipidemia | 8 (47.1) | 13 (28.9) | 0.1652 |
| Hyperuricemia | 4 (23.5) | 12 (26.7) | 0.8051 |
| Fatty liver | 10 (58.8) | 13 (28.9) | 0.0253a |
| Biomarkers | |||
| BNP (pg/mL) | 22.67 ± 5.21 | 21.63 ± 2.95 | 0.86 |
| Laboratory investigations | |||
| Serum cholesterol (mmol/L) | 5.01 ± 0.26 | 5.06 ± 0.16 | 0.8666 |
| Serum triglycerides (mmol/L) | 2.13 ± 0.21 | 1.85 ± 0.17 | 0.3609 |
| Serum HDL (mmol/L) | 1.06 ± 0.06 | 1.17 ± 0.03 | 0.0766 |
| Serum LDL (mmol/L) | 3.05 ± 0.20 | 3.12 ± 0.13 | 0.7818 |
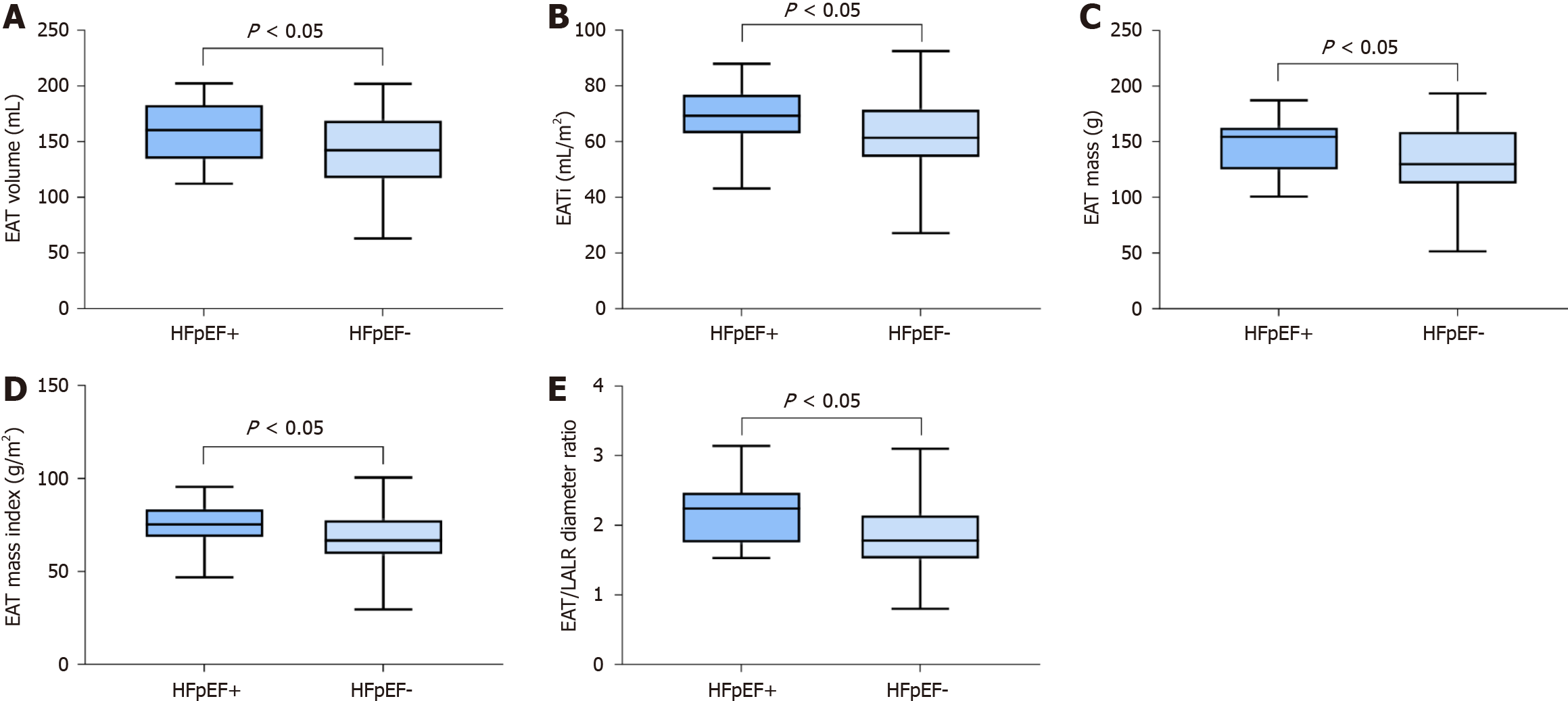
The measurements' results are detailed in Table 2. In terms of morphological characteristics, the HFpEF+ group displayed significant remodeling with a greater LV mass index compared to the HFpEF- group. No significant differences were observed in other morphological and functional parameters between the two groups. Regarding epicardial adipose tissue, both EAT volume and EAT mass were significantly larger in HFpEF+ individuals, and these differences persisted after adjustment for BSA (P = 0.04 for EAT volume/BSA and P = 0.04 for EAT mass/BSA). A significant difference in the EAT/LA LR diameter ratio was observed between the two groups (P = 0.02) (Figure 3).
| CMR parameters | HFpEF+ (n = 17) | HFpEF- (n = 45) | P value |
| Conventional parameters | |||
| LVEF (%) | 0.60 ± 0.03 | 0.64 ± 0.01 | 0.0797 |
| LV mass index (g/m2) | 61.38 ± 4.55 | 52.68 ± 1.40 | 0.0181a |
| LVEDD (mm) | 52.74 ± 1.80 | 50.20 ± 0.57 | 0.0833 |
| LVMWT (mm) | 10.32 ± 0.40 | 9.66 ± 0.25 | 0.1765 |
| LVEDVi (mL/m2) | 74.00 ± 5.52 | 69.16 ± 1.72 | 0.2716 |
| LVESVi (mL/m2) | 32.12 ± 5.87 | 24.84 ± 0.78 | 0.0592 |
| LA AP diameter (mm) | 41.37 ± 1.53 | 42.15 ± 0.87 | 0.6518 |
| LA LR diameter (mm) | 67.85 ± 2.55 | 70.63 ± 1.18 | 0.2686 |
| Epicardial adipose tissue | |||
| EAT volume (mL) | 160.00 ± 7.13 | 139.80 ± 5.39 | 0.0449a |
| EATi (mL/m2) | 74.20 ± 3.16 | 65.37 ± 2.23 | 0.0360a |
| EAT mass (g) | 147.20 ± 6.56 | 128.60 ± 4.98 | 0.0451a |
| EAT mass index (g/m2) | 68.26 ± 2.91 | 60.10 ± 2.06 | 0.0359a |
| EAT/LA AP diameter ratio | 3.56 ± 0.18 | 3.11 ± 0.14 | 0.0843 |
| EAT/LA LR diameter ratio | 2.19 ± 0.11 | 1.84 ± 0.08 | 0.0203a |
| EAT/LV mass ratio | 1.21 ± 0.09 | 1.17 ± 0.06 | 0.7737 |
| EAT/LV volume ratio | 0.99 ± 0.08 | 0.92 ± 0.04 | 0.4158 |
The correlation analysis results of all four epicardial adipose tissue parameters (EAT volume, EATi, EAT mass, EAT mass index) with eight CMR-measured LV morphological and functional parameters are presented in Table 3. No significant correlations were observed.
| Pearson’s correlation (r, P) | EAT volume (mL) | EATi (mL/m2) | EAT mass (g) | EAT mass index (g/m2) |
| LVEF (%) | -0.101, 0.698 | 0.245, 0.343 | -0.101, 0.698 | 0.245, 0.343 |
| LV mass index (g/m2) | 0.286, 0.265 | -0.039, 0.881 | 0.286, 0.265 | -0.039, 0.882 |
| LVEDD (mm) | 0.083, 0.751 | -0.102, 0.696 | 0.083, 0.751 | -0.102, 0.697 |
| LVMWT (mm) | 0.022, 0.932 | -0.314, 0.219 | 0.022, 0.932 | -0.314, 0.219 |
| LVEDVi (mL/m2) | -0.018, 0.946 | -0.213, 0.412 | -0.018, 0.946 | -0.213, 0.412 |
| LVESVi (mL/m2) | 0.028, 0.916 | -0.213, 0.411 | 0.028, 0.916 | -0.213, 0.411 |
| LA AP diameter (mm) | 0.354, 0.179 | 0.158, 0.558 | 0.354, 0.179 | 0.158, 0.558 |
| LA LR diameter (mm) | 0.231, 0.389 | 0.193, 0.474 | 0.231, 0.389 | 0.193, 0.474 |
In univariate logistic regression analysis, EAT mass index [odds ratio (OR) = 1.05, P = 0.04, 95%CI: 1.00-1.10], EATi (OR = 1.05, P = 0.04, 95%CI: 1.00-1.09), and EAT/LA LR diameter ratio (OR = 3.99, P = 0.03, 95%CI: 1.17-13.58) showed significant associations with HFpEF. EAT volume (OR = 1.02, P = 0.051, 95%CI: 1.00-1.04) trended toward an association with HFpEF. In multivariate analysis, the variable associated with HFpEF in the obese population was the EAT/LA LR diameter ratio (OR = 4.60, P = 0.02, 95%CI: 1.22-17.35) (Table 4).
| Variables | Lower/upper | |
| Univariate analysis (OR, 95%CI, P value) | Multivariate analysis (OR, 95%CI, P value) | |
| Age (yr) | 1.040 (0.994, 1.088) 0.087 | 1.046 (0.979, 1.117) 0.183 |
| BMI (kg/m2) | 1.057 (0.969, 1.154) 0.213 | |
| BMI > 35 kg/m2 | 2.812 (0.558, 14.179) 0.210 | |
| Diabetes | 1.231 (0.323, 4.689) 0.761 | |
| Hypertension | 3.545 (0.952, 13.201) 0.059 | 4.580 (1.008, 20.803) 0.049a |
| LVEF (%) | 0.002 (0.000, 7.482) 0.136 | |
| LVEDVi (mL/m2) | 1.020 (0.984, 1.058) 0.288 | |
| LVESVi (mL/m2) | 1.063 (0.958, 1.178) 0.249 | |
| LA AP diameter (mm) | 0.977 (0.884, 1.080) 0.646 | |
| LA LR diameter (mm) | 0.963 (0.900, 1.030) 0.267 | |
| EAT mass index (g/m2) | 1.049 (1.002, 1.098) 0.042a | 0.963 (1.054, 0.880) 0.416 |
| EAT mass (g) | 1.020 (1.000, 1.041) 0.051 | |
| EATi (mL/m2) | 1.045 (1.002, 1.090) 0.042a | |
| EAT volume (mL) | 1.019 (1.000, 1.038) 0.051 | |
| EAT/LA AP diameter ratio | 1.794 (0.915, 3.519) 0.089 | |
| EAT/LA LR diameter ratio | 3.989 (1.171, 13.584) 0.027a | 9.226 (1.070, 79.512) 0.043a |
| EAT/LV mass ratio | 1.236 (0.300, 5.098) 0.770 | |
| EAT/LV volume ratio | 2.299 (0.317, 16.658) 0.410 | |
Table 5 summarizes the ICC values for both intraobserver and interobserver reproducibility. The eight CMR-measured parameters demonstrated high reproducibility, ranging from 0.71 to 0.93 for intra-observer and 0.88 to 0.98 for inter-observer, respectively.
| Intra-observer | Inter-observer | |||||
| CV (%) | ICC | 95%CI | CV (%) | ICC | 95%CI | |
| EAT volume (mL) | 24.6 | 0.929 | (0.885, 0.939) | 18.4 | 0.900 | (0.746, 0.963) |
| EATi (mL/m2) | 23.0 | 0.913 | (0.846, 0.951) | 17.6 | 0.903 | (0.753, 0.964) |
| EAT mass (g) | 26.3 | 0.931 | (0.878, 0.962) | 17.9 | 0.900 | (0.746, 0.963) |
| EAT mass index (g/m2) | 23.7 | 0.913 | (0.846, 0.951) | 31.0 | 0.978 | (0.941, 0.992) |
| EAT/LA AP diameter ratio | 35.9 | 0.734 | (0.561, 0.846) | 18.5 | 0.882 | (0.696, 0.957) |
| EAT/LA LR diameter ratio | 28.4 | 0.710 | (0.526, 0.831) | 19.9 | 0.928 | (0.807, 0.974) |
| EAT/LV mass ratio | 34.7 | 0.870 | (0.774, 0.927) | 31.3 | 0.978 | (0.941, 0.992) |
| EAT/LV volume ratio | 29.4 | 0.929 | (0.874, 0.961) | 31.6 | 0.973 | (0.928, 0.963) |
In this study, we conducted a comprehensive comparison of EAT volume, mass, and functional characteristics, as determined by CMR, among individuals with obesity in the absence of HFpEF (HFpEF-) and HFpEF+ groups. The main findings of our study are as follows: (1) EAT volume and EAT mass were significantly increased in the obese HFpEF+ group compared to the obese HFpEF- group, and these differences persisted after adjustment for BSA; (2) in the obese population, the EAT/LA LR diameter ratio can serve as an alternative method to differentiate between HFpEF+ and HFpEF- groups; and (3) a higher EAT/LA LR diameter ratio was associated with a higher risk of HFpEF after adjusting for potential confounders.
The utilization of CMR for EAT measurement in our study provides a comprehensive assessment of cardiac structure and function in individuals with HFpEF[28]. Additionally, our study contributes to the existing literature by implementing and evaluating the quantification of EAT using MRI during diastole[29]. In a prior study, we demonstrated CMR's sensitivity and accuracy in detecting conventional atrial geometry in dialysis patients with HFpEF[30]. The present study, employing CMR to measure EAT, holds significant strengths over prior investigations examining the association between EAT and HFpEF in an obese population.
Our findings revealed that EAT volume and EAT mass, determined by CMR, were significantly higher in the HFpEF+ group compared to the HFpEF- group, with significant differences in EATi and EAT mass index as well. EAT, recognized as a risk factor for heart failure, particularly in the obese population[30,31], is implicated as an independent risk factor for HFpEF[32,33]. EAT's invasion into and around coronary arteries contributes to microvascular dysfunction, ventricular dilatation, and heart failure[12]. Adipocytes within EAT possess endocrine functions, synthesizing aldosterone and angiotensinogen[34]. Moreover, EAT serves as a marker for inflammatory factors[35]. Consistent with previous echocardiographic studies associating EAT thickness with HFpEF[36], our results further support this relationship.
In our study, there was a significant increase in LV mass index in the HFpEF+ group compared to the HFpEF- group. The space between the myocardial surface and the visceral pericardium may be filled with EAT, potentially covering the entire epicardium[37]. In the obese population, the excess EAT could impose an increased burden on both ventricles, ultimately leading to left ventricular hypertrophy[38]. These findings are consistent with prior investigations into obesity. A previous study utilizing CMR demonstrated that individuals with uncomplicated obesity and HFpEF exhibited extensive LV geometric remodeling, impaired ventricular function, and increased myocardial thickness[39].
Our research revealed that the EAT/LA LR diameter ratio was higher in the HFpEF+ group compared to the HFpEF- group, and this ratio was significantly associated with HFpEF. While no prior study has specifically investigated changes in the EAT/LA LR diameter ratio, it has been demonstrated to be impaired before left atrial enlargement in obese patients with HFpEF experiencing diastolic heart failure[40]. A recent study utilizing transthoracic echocardiography indicated that increased EAT thickness was linked to poorer left atrial function in HFpEF[41]. Additionally, another echocardiography-related study suggested that the presence of increased EAT is associated with a greater increase in cardiac filling pressures in patients with the obese phenotype of HFpEF[11]. Thus, the utilization of EAT/LA LR, assessed through CMR, could play a crucial role in the differentiation and diagnosis of obese HFpEF in clinical practice in the future. The EAT/LA LR diameter ratio may serve as a novel imaging biomarker.
Our study demonstrated no correlation between the four epicardial adipose tissue parameters (EAT volume, EATi, EAT mass, EAT mass index) and CMR-measured LV morphological and functional parameters. This finding aligns with some of the current studies[39]. It is plausible that our sample size is relatively small, and more conclusive results may emerge in the future with a larger sample size.
According to prior studies, age significantly contributes to EAT accumulation and may exert a substantial influence on its buildup[42]. In our study, there was no significant difference in age between the two groups, indicating that the effect of age on EAT was excluded. Despite the lack of statistical significance, there appears to be a trend towards older age in patients with HFpEF, supported by a higher proportion of subjects aged over 60 years in the HFpEF+ cohort. Additionally, resting SBP was significantly higher in HFpEF+ patients than in the HFpEF- group. Patients with HFpEF exhibit reduced aortic distensibility and increased systolic blood pressure[43]. Previous findings suggest that obesity has a detrimental impact on prehypertension and hypertension, irrespective of general obesity or abdominal obesity presence[44].
EAT/LA LR diameter ratio is highly associated with HFpEF in obese patients. It is plausible that there may be utility in CMR for assessing obese patients for HFpEF using EAT/LA LR diameter ratio as a diagnostic biomarker. Further prospective studies, are needed to validate these proof-of-concept findings.
Obesity has become a serious public health issue, significantly elevating the risk of various complications. It is a well-established contributor to Heart failure with preserved ejection fraction (HFpEF). Evaluating HFpEF in obesity is crucial. Epicardial adipose tissue (EAT) has emerged as a valuable tool for validating prognostic biomarkers and guiding treatment targets. Hence, assessing EAT is of paramount importance. Cardiovascular magnetic resonance (CMR) imaging is acknowledged as the gold standard for analyzing cardiac function and morphology. We hope to use CMR to assess EAT as a bioimaging marker to evaluate HFpEF in obese patients.
The aim of this study was to clarify the utility of using CMR-measured EAT as a diagnostic biomarker for assessing HFpEF in obese patients.
This study aims to employ CMR to examine EAT in the obese population with and without HFpEF, considering the association with co-morbidities, biomarkers, contractility parameters, and myocardial function assessed by CMR.
The study was designed as a case-control, prospective clinical study. Obese patients were divided into two groups for a case-control study based on whether or not they had heart failure with HFpEF. The two groups were defined as HFpEF+ and HFpEF-. LV geometry, global systolic function, EAT volumes and EAT mass of all subjects were obtained using cine magnetic resonance sequences. The novelty of this study is to investigate EAT metrics (including volume or mass) in obese patients using CMR to determine whether or not EAT metrics are associated with HFpEF and whether EAT metrics appear to be a biomarker for predicting HFpEF in the obese population.
Forty-five patients of HFpEF- group and seventeen patients of HFpEF+ group were included. LV mass index (g/m2) of HFpEF+ group was higher than HFpEF- group (P < 0.05). In HFpEF+ group, EAT volumes, EAT volume index, EAT mass, EAT mass index and EAT/ left atrial (LA) left-right (LR) diameter ratio were higher compared to HFpEF- group. In multivariate analysis, higher EAT/LA LR diameter ratio was independently associated with higher odds ratio (OR = 4.597) of HFpEF.
There was a strong correlation between increased EAT volumes and HFpEF in the obese. EAT/LA LR diameter ratio is highly associated with HFpEF in the obese.
Given the significant findings, there may be some diagnostic utility in CMR for assessing the obese for HFpEF.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Imaging science and photographic technology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bloomfield DA, United States S-Editor: Liu JH L-Editor: A P-Editor: Zheng XM
| 1. | Ren G, Kim T, Kim HS, Young ME, Muccio DD, Atigadda VR, Blum SI, Tse HM, Habegger KM, Bhatnagar S, Coric T, Bjornsti MA, Shalev A, Frank SJ, Kim JA. A Small Molecule, UAB126, Reverses Diet-Induced Obesity and its Associated Metabolic Disorders. Diabetes. 2020;69:2003-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Joyce E, Lala A, Stevens SR, Cooper LB, AbouEzzeddine OF, Groarke JD, Grodin JL, Braunwald E, Anstrom KJ, Redfield MM, Stevenson LW; Heart Failure Apprentice Network. Prevalence, Profile, and Prognosis of Severe Obesity in Contemporary Hospitalized Heart Failure Trial Populations. JACC Heart Fail. 2016;4:923-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | German CA, Brubaker PH, Nelson MB, Fanning J, Ye F, Kitzman DW. Relationships Between Objectively Measured Physical Activity, Exercise Capacity, and Quality of Life in Older Patients With Obese Heart Failure and Preserved Ejection Fraction. J Card Fail. 2021;27:635-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Clemenza F, Citarrella R, Patti A, Rizzo M. Obesity and HFpEF. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Sato T, Aizawa Y, Yuasa S, Kishi S, Fuse K, Fujita S, Ikeda Y, Kitazawa H, Takahashi M, Sato M, Okabe M. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc Diabetol. 2018;17:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 260] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 6. | Song Y, Song F, Wu C, Hong YX, Li G. The roles of epicardial adipose tissue in heart failure. Heart Fail Rev. 2022;27:369-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | van Woerden G, Gorter TM, Westenbrink BD, Willems TP, van Veldhuisen DJ, Rienstra M. Epicardial fat in heart failure patients with mid-range and preserved ejection fraction. Eur J Heart Fail. 2018;20:1559-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 202] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 8. | Rabkin SW, Campbell H. Comparison of reducing epicardial fat by exercise, diet or bariatric surgery weight loss strategies: a systematic review and meta-analysis. Obes Rev. 2015;16:406-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 9. | Monti CB, Schiaffino S, Galimberti Ortiz MDM, Capra D, Zanardo M, De Benedictis E, Luporini AG, Spagnolo P, Secchi F, Sardanelli F. Potential role of epicardial adipose tissue as a biomarker of anthracycline cardiotoxicity. Insights Imaging. 2021;12:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Nelson AJ, Worthley MI, Psaltis PJ, Carbone A, Dundon BK, Duncan RF, Piantadosi C, Lau DH, Sanders P, Wittert GA, Worthley SG. Validation of cardiovascular magnetic resonance assessment of pericardial adipose tissue volume. J Cardiovasc Magn Reson. 2009;11:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Koepp KE, Obokata M, Reddy YNV, Olson TP, Borlaug BA. Hemodynamic and Functional Impact of Epicardial Adipose Tissue in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2020;8:657-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 158] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 12. | Ayton SL, Gulsin GS, McCann GP, Moss AJ. Epicardial adipose tissue in obesity-related cardiac dysfunction. Heart. 2022;108:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Chuang ML, Danias PG, Riley MF, Hibberd MG, Manning WJ, Douglas PS. Effect of increased body mass index on accuracy of two-dimensional echocardiography for measurement of left ventricular volume, ejection fraction, and mass. Am J Cardiol. 2001;87:371-374, A10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | van Woerden G, van Veldhuisen DJ, Gorter TM, Ophuis B, Saucedo-Orozco H, van Empel VPM, Willems TP, Geelhoed B, Rienstra M, Westenbrink BD. The value of echocardiographic measurement of epicardial adipose tissue in heart failure patients. ESC Heart Fail. 2022;9:953-957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 15. | Monti CB, Codari M, De Cecco CN, Secchi F, Sardanelli F, Stillman AE. Novel imaging biomarkers: epicardial adipose tissue evaluation. Br J Radiol. 2020;93:20190770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Maimaituxun G, Kusunose K, Yamada H, Fukuda D, Yagi S, Torii Y, Yamada N, Soeki T, Masuzaki H, Sata M, Shimabukuro M. Deleterious Effects of Epicardial Adipose Tissue Volume on Global Longitudinal Strain in Patients With Preserved Left Ventricular Ejection Fraction. Front Cardiovasc Med. 2020;7:607825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Salem NA, Batouty NM, Tawfik AM, Sobh DM, Gadelhak B, Hendawy SR, Laimon W. Epicardial and Perihepatic Fat as Cardiometabolic Risk Predictors in Girls with Turner Syndrome: A Cardiac Magnetic Resonance Study. J Clin Res Pediatr Endocrinol. 2021;13:408-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Wu CK, Lee JK, Hsu JC, Su MM, Wu YF, Lin TT, Lan CW, Hwang JJ, Lin LY. Myocardial adipose deposition and the development of heart failure with preserved ejection fraction. Eur J Heart Fail. 2020;22:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 19. | Haykowsky MJ, Nicklas BJ, Brubaker PH, Hundley WG, Brinkley TE, Upadhya B, Becton JT, Nelson MD, Chen H, Kitzman DW. Regional Adipose Distribution and its Relationship to Exercise Intolerance in Older Obese Patients Who Have Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2018;6:640-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 20. | van der Meer P, Gaggin HK, Dec GW. ACC/AHA Versus ESC Guidelines on Heart Failure: JACC Guideline Comparison. J Am Coll Cardiol. 2019;73:2756-2768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 167] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 21. | World Health Organization. Regional Office for the Western Pacific The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia (2000). Available from: https://apps.who.int/iris/handle/10665/206936. |
| 22. | Zhuang B, Li S, Xu J, Zhou D, Yin G, Zhao S, Lu M. Age- and Sex-Specific Reference Values for Atrial and Ventricular Structures in the Validated Normal Chinese Population: A Comprehensive Measurement by Cardiac MRI. J Magn Reson Imaging. 2020;52:1031-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Davidovich D, Gastaldelli A, Sicari R. Imaging cardiac fat. Eur Heart J Cardiovasc Imaging. 2013;14:625-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4758] [Cited by in RCA: 5361] [Article Influence: 184.9] [Reference Citation Analysis (0)] |
| 25. | Siddiqui J, Bala F, Sciacca S, Falzon AM, Benger M, Matloob SA, Miller FNAC, Simister RJ, Chatterjee I, Sztriha LK, Davagnanam I, Booth TC. COVID-19 Stroke Apical Lung Examination Study: A Diagnostic and Prognostic Imaging Biomarker in Suspected Acute Stroke. AJNR Am J Neuroradiol. 2021;42:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med. 2016;15:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9979] [Cited by in RCA: 15713] [Article Influence: 1745.9] [Reference Citation Analysis (0)] |
| 27. | Zhu L, Pan Z, Ma Q, Yang W, Shi H, Fu C, Yan X, Du L, Yan F, Zhang H. Diffusion Kurtosis Imaging Study of Rectal Adenocarcinoma Associated with Histopathologic Prognostic Factors: Preliminary Findings. Radiology. 2017;284:66-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 28. | van Woerden G, van Veldhuisen DJ, Gorter TM, van Empel VPM, Hemels MEW, Hazebroek EJ, van Veldhuisen SL, Willems TP, Rienstra M, Westenbrink BD. Importance of epicardial adipose tissue localization using cardiac magnetic resonance imaging in patients with heart failure with mid-range and preserved ejection fraction. Clin Cardiol. 2021;44:987-993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol. 2015;11:363-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 428] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 30. | Zhou H, An DA, Ni Z, Xu J, Zhou Y, Fang W, Lu R, Ying L, Huang J, Yao Q, Li D, Hu J, Chen B, Shen J, Jin H, Wei Y, Ouchi E, Xu L, Wu LM, Mou S. Incremental diagnostic value of CMR-derived LA strain and strain rate in dialysis patients with HFpEF. Eur J Radiol. 2022;151:110285. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 31. | Wong C, Marwick TH. Obesity cardiomyopathy: pathogenesis and pathophysiology. Nat Clin Pract Cardiovasc Med. 2007;4:436-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 32. | Chrysant SG, Chrysant GS. Obesity-related heart failure with preserved ejection fraction: new treatment strategies. Hosp Pract (1995). 2019;47:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | van Woerden G, van Veldhuisen DJ, Westenbrink BD, de Boer RA, Rienstra M, Gorter TM. Connecting epicardial adipose tissue and heart failure with preserved ejection fraction: mechanisms, management and modern perspectives. Eur J Heart Fail. 2022;24:2238-2250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 34. | Savji N, Meijers WC, Bartz TM, Bhambhani V, Cushman M, Nayor M, Kizer JR, Sarma A, Blaha MJ, Gansevoort RT, Gardin JM, Hillege HL, Ji F, Kop WJ, Lau ES, Lee DS, Sadreyev R, van Gilst WH, Wang TJ, Zanni MV, Vasan RS, Allen NB, Psaty BM, van der Harst P, Levy D, Larson M, Shah SJ, de Boer RA, Gottdiener JS, Ho JE. The Association of Obesity and Cardiometabolic Traits With Incident HFpEF and HFrEF. JACC Heart Fail. 2018;6:701-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 313] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 35. | Tarsitano MG, Pandozzi C, Muscogiuri G, Sironi S, Pujia A, Lenzi A, Giannetta E. Epicardial Adipose Tissue: A Novel Potential Imaging Marker of Comorbidities Caused by Chronic Inflammation. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Lin JL, Sung KT, Lai YH, Yen CH, Yun CH, Su CH, Kuo JY, Liu CY, Chien CY, Cury RC, Bezerra HG, Hung CL. Epicardial Adiposity in Relation to Metabolic Abnormality, Circulating Adipocyte FABP, and Preserved Ejection Fraction Heart Failure. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Wu Y, Zhang A, Hamilton DJ, Deng T. Epicardial Fat in the Maintenance of Cardiovascular Health. Methodist Debakey Cardiovasc J. 2017;13:20-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 38. | Malavazos AE, Di Leo G, Secchi F, Lupo EN, Dogliotti G, Coman C, Morricone L, Corsi MM, Sardanelli F, Iacobellis G. Relation of echocardiographic epicardial fat thickness and myocardial fat. Am J Cardiol. 2010;105:1831-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 39. | Liu J, Li J, Pu H, He W, Zhou X, Tong N, Peng L. Cardiac remodeling and subclinical left ventricular dysfunction in adults with uncomplicated obesity: a cardiovascular magnetic resonance study. Quant Imaging Med Surg. 2022;12:2035-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 40. | Dzeshka MS, Lip GY, Snezhitskiy V, Shantsila E. Cardiac Fibrosis in Patients With Atrial Fibrillation: Mechanisms and Clinical Implications. J Am Coll Cardiol. 2015;66:943-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 385] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 41. | Jin X, Hung CL, Tay WT, Soon D, Sim D, Sung KT, Loh SY, Lee S, Jaufeerally F, Ling LH, Richards AM, van Melle JP, Voors AA, Lam CSP. Epicardial adipose tissue related to left atrial and ventricular function in heart failure with preserved versus reduced and mildly reduced ejection fraction. Eur J Heart Fail. 2022;24:1346-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 42. | Colom C, Viladés D, Pérez-Cuellar M, Leta R, Rivas-Urbina A, Carreras G, Ordóñez-Llanos J, Pérez A, Sánchez-Quesada JL. Associations between epicardial adipose tissue, subclinical atherosclerosis and high-density lipoprotein composition in type 1 diabetes. Cardiovasc Diabetol. 2018;17:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Reil JC, Hohl M, Reil GH, Granzier HL, Kratz MT, Kazakov A, Fries P, Müller A, Lenski M, Custodis F, Gräber S, Fröhlig G, Steendijk P, Neuberger HR, Böhm M. Heart rate reduction by If-inhibition improves vascular stiffness and left ventricular systolic and diastolic function in a mouse model of heart failure with preserved ejection fraction. Eur Heart J. 2013;34:2839-2849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 44. | Yuan Y, Sun W, Kong X. Relationship between metabolically healthy obesity and the development of hypertension: a nationwide population-based study. Diabetol Metab Syndr. 2022;14:150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |