Published online Mar 26, 2024. doi: 10.4330/wjc.v16.i3.126
Peer-review started: December 28, 2023
First decision: January 17, 2024
Revised: January 17, 2024
Accepted: February 26, 2024
Article in press: February 26, 2024
Published online: March 26, 2024
Processing time: 83 Days and 20.4 Hours
The post-resuscitation period is recognized as the main predictor of cardiopulmonary resuscitation (CPR) outcomes. The first description of post-resuscitation syndrome and stony heart was published over 50 years ago. Major manifestations may include but are not limited to, persistent precipitating pathology, systemic ischemia/reperfusion response, post-cardiac arrest brain injury, and finally, post-cardiac arrest myocardial dysfunction (PAMD) after successful resuscitation. Why do some patients initially survive successful resuscitation, and others do not? Also, why does the myocardium response vary after resuscitation? These ques
Core Tip: Despite the advances in emergency and critical care management, the outcomes post-cardiac arrest (in-hospital or out-of-hospital) remain challenging. Post-cardiac arrest myocardial dysfunction and circulatory failure are the main predictors of cardiopulmonary resuscitation outcomes. The pattern, management, and outcome of these predictors differ between subjects based on many factors. A better understanding of the pathophysiology of these two predictors is of utmost importance to achieve better post-cardiac arrest outcomes. Although restoring spontaneous circulation is a vital sign, it should not be the end of the game or lone primary outcome; it calls for aggressive multi-disciplinary interventions and care.
- Citation: El-Menyar A, Wahlen BM. Cardiac arrest, stony heart, and cardiopulmonary resuscitation: An updated revisit. World J Cardiol 2024; 16(3): 126-136
- URL: https://www.wjgnet.com/1949-8462/full/v16/i3/126.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i3.126
Cardiac arrest and cardiopulmonary resuscitation: An estimated 17.7 million people died due to cardiovascular disease (CVD), and this number represents about 31% of the global deaths[1]. Sudden cardiac arrest (SCD) occurs in more than 800000 patients per year[2]. More than 100000 SCD have been noticed among the American female population, indicating this is a significant issue in health care[3]. It has been shown that outcomes after cardiac arrest improve significantly when cardiopulmonary resuscitation (CPR) is performed promptly at a high-quality level[4]. Interestingly, remarkable regional and interindividual differences exist in the survival rates of cardiac arrest incidence and outcomes[5,6]. However, the incidence of SCD depends on its definition[7]. The quality of CPR has changed over time, and accordingly, the likelihood of restoration of spontaneous circulation (ROSC) and survival after cardiac arrest is expected to be improved[8]. Despite advances in CPR, poor survival rates remain challenging, even with the ROSC. Almost one-tenth and one-quarter of the out-of-hospital (OHCA) and in-hospital (IHCA) cardiac arrests survive hospital discharge[9]. The survival rate after IHCA is approximately twice that of OHCA, as the earlier ROSC is achieved in almost 50% of the IHCA[10,11]. The post-resuscitation period is the main predictor of CPR outcomes, as during this period, a multi-systemic insult phenomenon called post-cardiac arrest syndrome (PCAS) occurs, including four elements of variable degrees and intensity[12,13]. This phenomenon may happen in five phases post-ROSC in terms of immediate (20 min), early (within 12 h), intermediate (within 72 h), recovery (after three days), and rehabilitation phase[14,15]. These elements include hypoxic brain injury, systemic ischemia–reperfusion injury (IRI), myocardial dysfunction, and the persistent underlying cause of cardiac arrest[12,13]. This phenomenon results from initial systemic ischemia and no-flow local circulations followed by reperfusion injury during resuscitation and the ROSC. During cardiac arrest, the brain and cardiac injuries occur and play a critical role in the patient's survival and quality of life. Secondary brain injury could lead to late death in approximately two-thirds and one-quarter of patients who sustained OHCA and IHCA, respectively. Whereas early death, which may occur within the first three days, is mainly related to post-cardiac arrest myocardial dysfunction (PAMD)[16]. PAMD is a commonly reversible sort of myocardial stunning that often responds to small doses of inotropes. Therefore, if detected and treated early, PAMD could reach its base level eight hours following ROSC, potentially improving on the first day and normalizing by the third day. Otherwise, in addition to the systemic IRI and vasodilation, multiorgan failure takes place and leads to death. Such IRI results in oxidative stress that causes cardiac injury and ventricular dysfunction, which peaks at 8-24 h after cardiac arrest. The systematic inflammatory response of IRI leads to several detrimental sequences such as vasoplegia, microcirculatory dysfunction, hypercoagulability, relative adrenal and Vasopressin insufficiency, immunosuppression, hyperglycemia, transient bacteremia, and eventually multiorgan failure[13]. The persistence of tissue hypoxia during cardiac arrest leads to the activation of immune, complement, and coagulation pathways. It ends up with systemic inflammatory response syndrome (SIRS) within three hours post-resuscitation[9]. Furthermore, the body organs' metabolism switches to anaerobic status due to the minimal cellular reserve and insufficient tissue oxygen delivery. The latter condition, in addition to the ongoing myocardial stunning (or PAMD), activated dysfunctional vascular endothelium, and microcirculatory failure, exaggerates the hemodynamic instability and organ failure[9].
The diagnosis of PAMD requires the presence of low cardiac index (CI), decreased left ventricular systolic and diastolic function, and right ventricular dysfunction after cardiac arrest and ROSC. Studies in patients with post-cardiac arrest provided evidence of reduced left ventricular ejection fraction (LVEF) within the first day after ROSC in two-thirds or more of cases[17-19]. Vasopressor dependence or the presence of shock is not an indicator of PAMD after cardiac arrest, as each or both may arise from vascular dysfunction in the absence of myocardial involvement[20]. On the other hand, the presence of myocardial dysfunction after cardiac arrest is not a forecast for the necessity of vasopressors or worse outcomes, at least when adjusted for the severity of cardiac arrest, shock, and vasopressor use. Nevertheless, experts still assume that the likelihood of PAMD is a significant cause of death after successful CPR[21]. Why do some patients initially survive successful resuscitation, and others do not? Also, why does the myocardium response vary after resuscitation? These questions have kept scientists busy for several decades. By modifying the conventional modalities of resuscitation together with new promising agents, rescuers will be able to salvage the jeopardized post-resuscitation myocardium and prevent its progression to a dismal, stony heart, which is the extreme form of PAMD[22]. The actual incidence of PAMD is still unclear in the literature because of the use of different definitions, small studies population, and diversity of cardiac function assessment[17]. Community awareness and staff education are crucial for improving and shortening the resuscitation time and attaining optimal short- and long-term outcomes. Awareness of PCAS components before and early after the restoration of circulation will improve the outcomes of CPR[23]. Restoration of adequate circulation and favorable long-term outcomes should be the main aim of resuscitation[24]. Several factors are associated with the development and impact of PAMD. These factors help healthcare providers anticipate which person would need early diagnostic evaluation, such as serial electrocardiogram, echocardiography, and specific treatments. Yao et al[25] have shown that almost 50% of OHCA is followed by myocardial dysfunction and that early myocardial dysfunction is not always associated with neurologically intact survival. The reversibility of PAMD reflects an aggressive on-time treatment strategy, and such dysfunction and the hemodynamic status should not affect the decision to discontinue treatment as both are usually reversible. After the initial phases of the ROSC, the neurologic status determines the patient's resuscitation outcome[26].
Furthermore, post-resuscitation shock, which is a complex pathophysiological condition occurring in 50%-70% of patients who experienced a cardiac arrest, is an early and transient complication of the post-resuscitation phase[27]. The optimal mean arterial pressure target during post-resuscitation shock needs further elaboration, and mechanical circulatory support could be required in selected cases whenever the neurological prognosis is expected to be favorable[27]. PAMD plays a role in early re-arrest after post-ROSC; it was reported in six percent of transported post-ROSC survivors[17,28]. Patients who develop re-arrest or another critical event (23%) are less likely to survive[28]. Of note, as a part of PAMD, the diastolic dysfunction measured by the isovolumetric relaxation time on echocardiography was found to be an independent predictor of mortality regardless of the patient's age, initial rhythm, duration of CPR, and doses of epinephrine[29].
Although defibrillation of a shockable rhythm as early as possible is the most critical factor in this sensitive period, the times before and after defibrillation and thoracic compressions (peri-shock) should be as short as possible[30,31]. A study showed that electrical shock of prolonged VF had an unfavorable outcome if a non-perfusing rhythm followed it compared to a primary asystole; this difference was attributed to the myocardial electrical injury[32]. Myocardial stunning and PAMD could also arise due to the use of defibrillators during resuscitation depending on the shock timing, frequency, amount of delivered energy, and waveform[33,34]. The electrical shock causes a decrease in the CI and contractility in addition to an increase in the ventricular end-diastolic pressure[17,34]. A prior study showed that the survival rate to hospital discharge was higher in patients presenting with pulseless electrical activity (PEA)/asystole without subsequent VT/VF than in patients with PEA/asystole with subsequent VT/VF[35]. Therefore, early defibrillation with concurrent high-quality CPR is critical for VF/pulseless VT, whereas epinephrine use with high-quality CPR is essential for better outcomes for non-shockable rhythms[36]. A meta-analysis showed that shockable rhythm conversion from asystole was associated with pre-hospital ROSC and survival to hospital discharge compared to PEA[37]. Also, earlier shockable rhythm conversion was associated with higher favorable neurological outcomes in OHCA patients within one month compared to late conversion.
More than a decade ago, Laurent et al[20] evaluated 165 survivors of OHCA and found that the higher the dosages of epinephrine and the number of defibrillations during CPR, the higher the likelihood of cardio-circulatory instability and the need for more vasopressor support, which was necessary for more than half of the patients. The mean LVEF is lower in patients with hemodynamic instability. Patients who presented with a low CI on the first day after a cardiac arrest are more likely to die in the hospital due to multiorgan failure. In patients who survived cardiac arrest, efforts and multi-disciplinary care could regain normal hemodynamic parameters within the first three days. Of note, the hemodynamic status did not influence the neurologic outcomes in some cases[20]. However, the autoregulation of the cerebral circulation is disrupted after ROSC, and therefore, up to three-thirds of cases require inotropic support cases due to microvascular impairment and PAMD-induced hypotension[17,38].
An interesting study on more than 600 patients in whom echocardiogram findings were recorded within three months before the occurrence of IHCA[21]. The authors found a 25% reduction of the LVEF from its baseline values within 72 h post-IHCA in 14% of the cohort. The likelihood of survival was lower in patients whose LVEF before their cardiac arrest was less than 45%.
In the targeted temperature management (TTM) study, the LVEF on the first-day post-OHCA was severely reduced in 28% of patients and moderately reduced in 48% of patients[19]. The LVEF patterns did not distinguish patients with higher and lower vasopressor requirements or those with different target temperatures (36 oC vs 33 oC), which precluded the association between PAMD and systemic hemodynamics.
Hypotension and shock in terms of SBP less than 90-100 mmHg or mean arterial pressure of less than 60-65 mmHg and the need for vasopressor use were reported in more than half of patients with ROSC in different studies. However, these factors were associated with adverse neurological outcomes and recurrence of cardiac arrest[17,39]. Furthermore, the mean arterial pressure and survival rate were inversely correlated after ROSC.
The pathophysiological processes after ROSC were described as initial low CI followed by vasodilation and subnormal systemic vascular resistance. The preliminary end stage is a capillary leak from the SIRS, which is responsible for drawing parallels to the septic shock-like states[20,40]. This period, with extreme vasoplegia, necessitates a continuous and rising need for vasopressors and peaks after one day, including an initial 6-hour period of apparent stabilization[20,40]. Following ROSC, endocrine dysfunctions occur in terms of pituitary-adrenal axis activation and functional adrenal abnormality with low cortisol secretion, which was more evident in non-survivor patients in some studies[41,42]. Also, relatively low vasopressin levels after cardiac arrest could contribute to the vasoplegia condition[43]. This was supported by an experimental study demonstrating that Vasopressin may prevent, to some extent, the cellular toxicity that could happen from excessive beta-adrenergic stimulation[17]. An RCT on IHCA showed that administration of vasopressin and epinephrine, methylprednisolone (during CPR), and a stress dose of hydrocortisone (during a shock stage of ROSC requiring vasopressors) is associated with better outcomes in terms of survival to discharge and favorable neurological status than epinephrine without Vasopressin[44]. A more recent study showed improvement occurred only in the ROSC in the vasopressin-methylprednisolone group rather than in the placebo group[45]. However, animal studies indicated that any vasopressin and/or epinephrine during resuscitation of OHCA is associated with reduced microcirculatory cerebral blood flow (CBF)[46,47]. During cardiac arrest, CBF is already losing 60% during chest compression only, and it needs at least 3 min of ROSC to be normalized[48]. The current Western guidelines do not recommend using Vasopressin and glucocorticoids in IHCA and OHCA[45,49]. However, few studies attributed the improvement of PAMD and peri-arrest cerebral ischemia to the impact of Vasopressin on the early improved post-arrest mean arterial pressure and central venous oxygen saturation, the use of less dosages of epinephrine, the shorter duration of CPR, and the use of methylprednisolone during resuscitation[36,44,50].
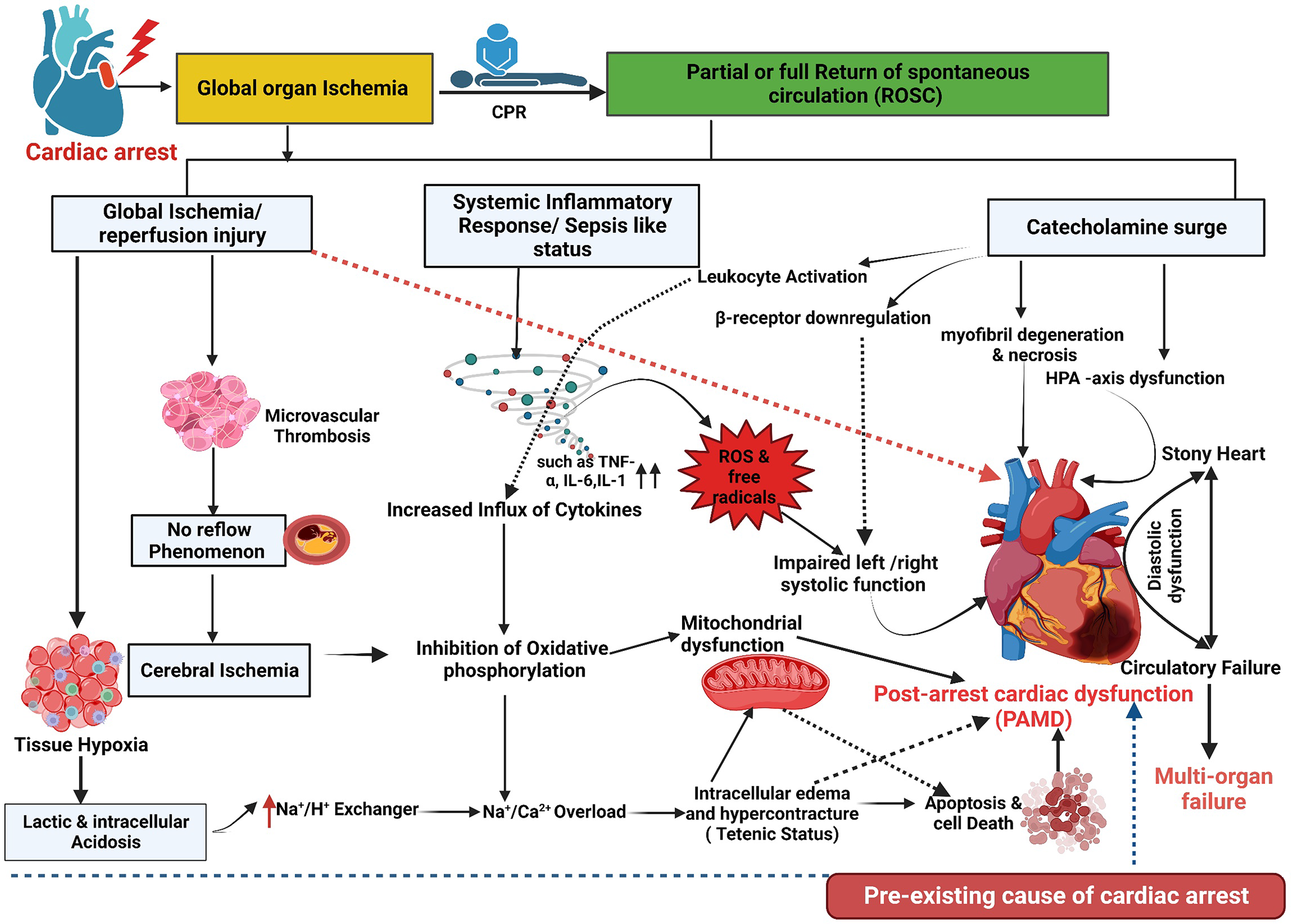
The main pathways that could explain the development of PAMD include the IRI, catecholamine-induced myocardial injury, cytokine-mediated cardiac dysfunction, microvascular dysfunction, adrenal insufficiency, mitochondrial dysfunction, cardiac stunning related to the harmful effect of direct-current countershock, and iatrogenic interventions like therapeutic hypothermia (TH), propofol, remifentanil, and vasopressors in some instances[17,51,52]. The cardiovascular IRI represents the primary chain between cardiac arrest and the development of PAMD, shock, and multiorgan failure; it initiates the release of pro-inflammatory cytokines, SIRS, and sepsis-like status[19,53,54]. The intensity of this inflammatory response is a determinant of the mortality post-ROSC. The intense cytokine activity directly depresses the myocardium and induces mitochondrial dysfunction with further lactic acidosis[55]. Excess catecholamines during CPR may lead to myocardial dysfunction by several mechanisms, including calcium overload, beta-receptor downregulation and desensitization, and overproduction of toxic reactive oxygen species (ROS); the latter may end up with ischemic myocardial contracture and stony heart[17,22,56]. Figure 1 shows the mechanism and pathophysiology of post-cardiac arrest myocardial dysfunction and stony heart.
IRI-induced coagulofibrinolytic changes mimic sepsis-like constellations but are less distinctive and accused of worse outcomes post-cardiac arrest as the coagulation and fibrinolysis are not adequately balanced during resuscitation[54,57]. The imbalance occurs during the early phase of cardiac resuscitation, as the resultant hyperfibrinolysis (t-PA release) is tracked by less endogenous fibrinolysis and fibrinolytic shutdown[57]. Augmented coagulation, followed by disseminated intravascular coagulation, leads to disturbances at the microcirculatory level, like the "no-reflow" phenomenon in the brain, and, finally, a multiorgan dysfunction[54].
The extension of the no-reflow phenomenon is multifactorial and mainly depends on the ischemic/hypoxia time and the coagulation system response[57,58].
As a result of the systemic IRI, the damage-associated molecular patterns (DAMPs) are substantially produced from the stressed cells and enhance the pro-inflammatory cytokines released from the immune and endothelial cells[59]. These DAMPs exaggerate the tissue factor-dependent coagulation and factor XII- and factor XI-dependent activation, and they inhibit the fibrinolysis process through the effects of the cell-free DNA, which is a form of the DAMP. Fibrinolytic shutdown occurs post-cardiac arrest secondary to the marked increases in PAI-1 after the first 24 h and DAMPs release. Studies showed that, during the post-arrest period, patients had higher plasma levels of DAMPs, including cell-free DNA, which were associated with higher hospital mortality as well[60].
It is not surprising that the severity of hyperfibrinolysis differs according to the underlying cause of arrest[61]. Previous studies indicated that the time from the onset of cardiac arrest to the first CPR and the duration of CPR are primary causes of hyperfibrinolysis[62].
Studies have shown that IL-6 Levels could predict the need for vasopressors, multiorgan failure, and mortality post-cardiac arrest[63,64]. A significant mediator of cytokine-induced cardiac dysfunction is the TNF-α; it directly affects myocardial inotropy, responsiveness to beta-adrenergic stimulation, and mitochondria function[65,66]. Therefore, trials aim to remove inevitably appearing cytokines after cardiac arrest that could impact the outcome after ROSC. For instance, Infliximab administered during the peri-arrest period showed some improvement in animal studies, whereas etanercept failed[67]. Unfortunately, there are limited animal and human studies, predominantly on cyclosporine and corticosteroids after cardiac arrest[67,68].
There is also a shadow when there is light. ROSC usually goes hand in hand with a flood of toxic ROS, leading to a second wave of injury. Energy depletion caused by cardiac arrest leads to muscle contraction like tetanic stimulation, consecutively to the thickening of the wall and a reduction in the cavity volume. Depending on its extent, this phenomenon can result in irreversible stony heart[56]. An initially promising NHE inhibitor (e.g., Cariporide) has shown, in animal studies, a reduction in PAMD, dysrhythmias, and mortality through its potential preventing effect on the cellular injury during the IRI[69,70].
The effects of TH and TTM on the neurological outcomes post-cardiac arrest have been demonstrated in various studies[71]. Mild TH affects hemodynamics by improving the inotropic property of the myocardium, preserving diastolic relaxation, reducing heart rate, and increasing the systemic vascular resistance (SVR), induction of ‘cold diuresis,' stabilization of MAP, and reducing the vasopressor dosages[72-74]. A prior study showed that TH displayed in the first 12 h a lower CI, lower heart rate, and higher SVR with no effect on the MAP and stroke volume[75]. However, Annborn et al[76] demonstrated no benefit on the survival or shock status after OHCA in patients treated with TTM at 33 °C compared to 36 °C. Nevertheless, after rewarming, a more extended period of vasopressor support is still needed in patients with OHCA[77]. The 2022 International Consensus on Cardiopulmonary Resuscitation recommended not to routinely use pre-hospital cooling with a rapid infusion of large volumes of cold intravenous fluid immediately after ROSC and suggested active fever prevention for at least 72 h for patients who remain comatose after ROSC[49]. Also, patients who remained in a coma and had mild hypothermia after ROSC should not be actively rewarmed to attain normal body temperature[49].
Low cardiac output requires volume replacement, possibly due to systemic capillary leakage from systemic IRI and cytokine release after ROSC. Therefore, administering at least 1 Liter of isotonic fluid should be standard in patients with low blood pressure after successful CPR to keep central venous pressure between 8 and 12 mmHg[17,78]. For close monitoring, invasive blood pressure monitoring is of utmost importance in hypotensive patients requiring vasopressors and or inotropes. Among various vasopressors that can be used to restore SBP ≥ 90 mmHg and MAP ≥ 70 mmHg in the first 72 h, norepinephrine is commonly used with a lower risk of arrhythmia. In contrast, vasopressors like dopamine increases the risk of arrhythmia and may increase mortality[78-80]. Epinephrine and Vasopressin with or without low-dose hydrocortisone are other choices to overcome refractory vasoplegia[17,44,81].
Inotropic support (i.e., dobutamine) benefits patients with pending end-organ perfusion impairment in terms of low urine output after fluid resuscitation, low cardiac output, low central venous oxygen saturation, refractory acidosis, and warranting PAC insertion[72,82]. There were two sides of the inotropic support after cardiac arrest. On the one hand, protocols that utilize goal-directed therapy suggest inotropic agents improve cardiac output and tissue oxygen delivery. On the other hand, it is known that inotropes cause dysrhythmias, and the optimal cardiac output may vary from patient to patient. The efficacy and use of vasopressors and inotropic agents are based on the relative receptor potency. There is diversity in their potency, reflecting the variation in the circulatory effects and the potential side effects. Thus, there is not enough evidence to point out which vasopressor or inotrope is superior to another in terms of survival and neurological outcome[82-86]. Therefore, the decision to use it should be taken carefully, and it is better to be used only for patients with a combination of low cardiac output plus evidence of inadequate tissue perfusion.
The PPOO is crucial after ROSC, particularly in IHCA patients, as most of the deaths in this group are related to refractory shock, recurrent arrest, and multiorgan failure, in contrast to neurological injury in addition to shock in OHCA patients[87,88]. However, the optimal MAP and mixed venous oxygen saturation values ensure acceptable cerebral perfusion without burdening other tissues like the myocardium, which remains unchanged. In this regard, Ameloot et al[89] proposed a range of 80 mmHg and 70% of these two parameters, respectively, to keep cerebral perfusion at 65%. To attain better organ perfusion, the global body ischemia post-cardiac arrest reflecting the mitochondrial dysfunction and oxidative phosphorylation impairment[90] needs further elaboration in both IHCA and OHCA in large sample-sized research.
In selected patients, mechanical circulatory support can restore hemodynamic stability and end-organ perfusion; it acts as a bridge to definitive therapy in patients with refractory shock to maximal medical treatment. This can be achieved using an intra-aortic balloon pump, Impella, left ventricular assist device, and venoarterial extracorporeal membrane oxygenator[91-93].
The decision to do and timing of coronary intervention after cardiac arrest due to myocardial infarction is an ongoing discussion. A recent meta-analysis showed that early intervention (within the first 24 h) was associated with significantly better survival and neurologic outcomes. However, it was graded as low-quality[94]. Moreover, this beneficial outcome was observed in patients without ST-segment elevation myocardial infarction, in contrast to non-statistically significant results in patients with ST-segment elevation myocardial infarction.
In a multicenter study including 2075 admissions with IHCA and OHCA, the IHCA patients had significantly higher comorbidities, lower lactate, greater utilization of invasive hemodynamics and mechanical circulatory support, lesser TTM and lesser in-hospital mortality (36.1% vs 44.1%) than IHCA patients[95]. Another study on 779 post-cardiac arrest patients[96] revealed that IHCA patients were older, less frequently male, and less frequently without comorbidity. The initial cardiac rhythm was more often non-shockable, all delay-times such as ROSC and no-flow, and time to advanced life support were shorter in IHCA. Cardiac cause of the arrest was less common, long-term neurological outcome was better, and the mortality at 30 d was lower in the IHCA than OHCA patients.
The pattern, management, and outcome of PAMD and post-cardiac arrest shock are different based on many factors, including IHCA vs OHCA, witnessed vs unwitnessed cardiac arrest, the underlying cause of arrest, and the duration of and protocol used for CPR. Although ROSC is a vital sign, it should not be the end of the game or lone primary outcome; it calls for aggressive multi-disciplinary interventions and care. The development of stony heart post-CPR and OHCA remain the main challenges in emergency and critical care medicine. A better understanding of the pathophysiology of PAMD and circulatory failure after ROSC is of utmost importance to achieve better post-cardiac arrest outcomes.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Qatar
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jha AK, United States S-Editor: Gong ZM L-Editor: A P-Editor: Guo X
| 1. | World Health Organization. Cardiovascular diseases (CVDs). 2017. Available from: http://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). |
| 2. | Berdowski J, Berg RA, Tijssen JG, Koster RW. Global incidences of out-of-hospital cardiac arrest and survival rates: Systematic review of 67 prospective studies. Resuscitation. 2010;81:1479-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1588] [Cited by in RCA: 1465] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 3. | Myat A, Song KJ, Rea T. Out-of-hospital cardiac arrest: current concepts. Lancet. 2018;391:970-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 349] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 4. | Meaney PA, Bobrow BJ, Mancini ME, Christenson J, de Caen AR, Bhanji F, Abella BS, Kleinman ME, Edelson DP, Berg RA, Aufderheide TP, Menon V, Leary M; CPR Quality Summit Investigators, the American Heart Association Emergency Cardiovascular Care Committee, and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Cardiopulmonary resuscitation quality: [corrected] improving cardiac resuscitation outcomes both inside and outside the hospital: a consensus statement from the American Heart Association. Circulation. 2013;128:417-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 703] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 5. | Girotra S, Cram P, Spertus JA, Nallamothu BK, Li Y, Jones PG, Chan PS; American Heart Association's Get With the Guidelines®‐Resuscitation Investigators. Hospital variation in survival trends for in-hospital cardiac arrest. J Am Heart Assoc. 2014;3:e000871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Perkins GD, Cooke MW. Variability in cardiac arrest survival: the NHS Ambulance Service Quality Indicators. Emerg Med J. 2012;29:3-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Kong MH, Fonarow GC, Peterson ED, Curtis AB, Hernandez AF, Sanders GD, Thomas KL, Hayes DL, Al-Khatib SM. Systematic review of the incidence of sudden cardiac death in the United States. J Am Coll Cardiol. 2011;57:794-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 246] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 8. | Wissenberg M, Lippert FK, Folke F, Weeke P, Hansen CM, Christensen EF, Jans H, Hansen PA, Lang-Jensen T, Olesen JB, Lindhardsen J, Fosbol EL, Nielsen SL, Gislason GH, Kober L, Torp-Pedersen C. Association of national initiatives to improve cardiac arrest management with rates of bystander intervention and patient survival after out-of-hospital cardiac arrest. JAMA. 2013;310:1377-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 880] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 9. | Randhawa VK, Grunau BE, Debicki DB, Zhou J, Hegazy AF, McPherson T, Nagpal AD. Cardiac Intensive Care Unit Management of Patients After Cardiac Arrest: Now the Real Work Begins. Can J Cardiol. 2018;34:156-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Jentzer JC, Clements CM, Murphy JG, Scott Wright R. Recent developments in the management of patients resuscitated from cardiac arrest. J Crit Care. 2017;39:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Girotra S, Nallamothu BK, Spertus JA, Li Y, Krumholz HM, Chan PS; American Heart Association Get with the Guidelines–Resuscitation Investigators. Trends in survival after in-hospital cardiac arrest. N Engl J Med. 2012;367:1912-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 668] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 12. | Rittenberger JC, Tisherman SA, Holm MB, Guyette FX, Callaway CW. An early, novel illness severity score to predict outcome after cardiac arrest. Resuscitation. 2011;82:1399-1404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 13. | Madder RD, Reynolds JC. Multidisciplinary Management of the Post-Cardiac Arrest Patient. Cardiol Clin. 2018;36:85-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Kang Y. Management of post-cardiac arrest syndrome. Acute Crit Care. 2019;34:173-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Nolan JP, Neumar RW, Adrie C, Aibiki M, Berg RA, Böttiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, Kern KB, Laurent I, Longstreth WT, Merchant RM, Morley P, Morrison LJ, Nadkarni V, Peberdy MA, Rivers EP, Rodriguez-Nunez A, Sellke FW, Spaulding C, Sunde K, Hoek TV. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation. 2008;79:350-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 739] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 16. | Pellis T, Sanfilippo F, Ristagno G. The optimal hemodynamics management of post-cardiac arrest shock. Best Pract Res Clin Anaesthesiol. 2015;29:485-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Jentzer JC, Chonde MD, Dezfulian C. Myocardial Dysfunction and Shock after Cardiac Arrest. Biomed Res Int. 2015;2015:314796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 18. | Ruiz-Bailén M, Aguayo de Hoyos E, Ruiz-Navarro S, Díaz-Castellanos MA, Rucabado-Aguilar L, Gómez-Jiménez FJ, Martínez-Escobar S, Moreno RM, Fierro-Rosón J. Reversible myocardial dysfunction after cardiopulmonary resuscitation. Resuscitation. 2005;66:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Bro-Jeppesen J, Annborn M, Hassager C, Wise MP, Pelosi P, Nielsen N, Erlinge D, Wanscher M, Friberg H, Kjaergaard J; TTM Investigators. Hemodynamics and vasopressor support during targeted temperature management at 33°C Versus 36°C after out-of-hospital cardiac arrest: a post hoc study of the target temperature management trial*. Crit Care Med. 2015;43:318-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 20. | Laurent I, Monchi M, Chiche JD, Joly LM, Spaulding C, Bourgeois B, Cariou A, Rozenberg A, Carli P, Weber S, Dhainaut JF. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol. 2002;40:2110-2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 485] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 21. | Gonzalez MM, Berg RA, Nadkarni VM, Vianna CB, Kern KB, Timerman S, Ramires JA. Left ventricular systolic function and outcome after in-hospital cardiac arrest. Circulation. 2008;117:1864-1872. [PubMed] [DOI] [Full Text] |
| 22. | El-Menyar AA. The resuscitation outcome: revisit the story of the stony heart. Chest. 2005;128:2835-2846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | El-Menyar AA. Pathophysiology and hemodynamic of postresuscitation syndrome. Saudi Med J. 2006;27:441-445. [PubMed] |
| 24. | El-Menyar AA. Postresuscitation myocardial stunning and its outcome: new approaches. Crit Pathw Cardiol. 2004;3:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Yao Y, Johnson NJ, Perman SM, Ramjee V, Grossestreuer AV, Gaieski DF. Myocardial dysfunction after out-of-hospital cardiac arrest: predictors and prognostic implications. Intern Emerg Med. 2018;13:765-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Bougouin W, Cariou A. Management of postcardiac arrest myocardial dysfunction. Curr Opin Crit Care. 2013;19:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Jozwiak M, Bougouin W, Geri G, Grimaldi D, Cariou A. Post-resuscitation shock: recent advances in pathophysiology and treatment. Ann Intensive Care. 2020;10:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 28. | Hartke A, Mumma BE, Rittenberger JC, Callaway CW, Guyette FX. Incidence of re-arrest and critical events during prolonged transport of post-cardiac arrest patients. Resuscitation. 2010;81:938-942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Deakin CD, Koster RW. Chest compression pauses during defibrillation attempts. Curr Opin Crit Care. 2016;22:206-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Cheskes S, Schmicker RH, Christenson J, Salcido DD, Rea T, Powell J, Edelson DP, Sell R, May S, Menegazzi JJ, Van Ottingham L, Olsufka M, Pennington S, Simonini J, Berg RA, Stiell I, Idris A, Bigham B, Morrison L; Resuscitation Outcomes Consortium (ROC) Investigators. Perishock pause: an independent predictor of survival from out-of-hospital shockable cardiac arrest. Circulation. 2011;124:58-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 305] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 31. | Niemann JT, Stratton SJ, Cruz B, Lewis RJ. Outcome of out-of-hospital postcountershock asystole and pulseless electrical activity versus primary asystole and pulseless electrical activity. Crit Care Med. 2001;29:2366-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Gazmuri RJ. Effects of repetitive electrical shocks on postresuscitation myocardial function. Crit Care Med. 2000;28:N228-N232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Toh N, Nishii N, Nakamura K, Tada T, Oe H, Nagase S, Kohno K, Morita H, Kusano KF, Ito H. Cardiac dysfunction and prolonged hemodynamic deterioration after implantable cardioverter-defibrillator shock in patients with systolic heart failure. Circ Arrhythm Electrophysiol. 2012;5:898-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Meaney PA, Nadkarni VM, Kern KB, Indik JH, Halperin HR, Berg RA. Rhythms and outcomes of adult in-hospital cardiac arrest. Crit Care Med. 2010;38:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 310] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 35. | Panchal AR, Bartos JA, Cabañas JG, Donnino MW, Drennan IR, Hirsch KG, Kudenchuk PJ, Kurz MC, Lavonas EJ, Morley PT, O'Neil BJ, Peberdy MA, Rittenberger JC, Rodriguez AJ, Sawyer KN, Berg KM; Adult Basic and Advanced Life Support Writing Group. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142:S366-S468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 1045] [Article Influence: 209.0] [Reference Citation Analysis (0)] |
| 36. | Chang WT, Ma MH, Chien KL, Huang CH, Tsai MS, Shih FY, Yuan A, Tsai KC, Lin FY, Lee YT, Chen WJ. Postresuscitation myocardial dysfunction: correlated factors and prognostic implications. Intensive Care Med. 2007;33:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 37. | Luo S, Zhang Y, Zhang W, Zheng R, Tao J, Xiong Y. Prognostic significance of spontaneous shockable rhythm conversion in adult out-of-hospital cardiac arrest patients with initial non-shockable heart rhythms: A systematic review and meta-analysis. Resuscitation. 2017;121:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 38. | Sundgreen C, Larsen FS, Herzog TM, Knudsen GM, Boesgaard S, Aldershvile J. Autoregulation of cerebral blood flow in patients resuscitated from cardiac arrest. Stroke. 2001;32:128-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 302] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 39. | Roberts BW, Kilgannon JH, Chansky ME, Mittal N, Wooden J, Parrillo JE, Trzeciak S. Multiple organ dysfunction after return of spontaneous circulation in postcardiac arrest syndrome. Crit Care Med. 2013;41:1492-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 40. | Oksanen T, Skrifvars M, Wilkman E, Tierala I, Pettilä V, Varpula T. Postresuscitation hemodynamics during therapeutic hypothermia after out-of-hospital cardiac arrest with ventricular fibrillation: a retrospective study. Resuscitation. 2014;85:1018-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | de Jong MF, Beishuizen A, de Jong MJ, Girbes AR, Groeneveld AB. The pituitary-adrenal axis is activated more in non-survivors than in survivors of cardiac arrest, irrespective of therapeutic hypothermia. Resuscitation. 2008;78:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Ito T, Saitoh D, Takasu A, Kiyozumi T, Sakamoto T, Okada Y. Serum cortisol as a predictive marker of the outcome in patients resuscitated after cardiopulmonary arrest. Resuscitation. 2004;62:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Omar S, Zedan A, Nugent K. Cardiac vasoplegia syndrome: pathophysiology, risk factors and treatment. Am J Med Sci. 2015;349:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 44. | Mentzelopoulos SD, Malachias S, Chamos C, Konstantopoulos D, Ntaidou T, Papastylianou A, Kolliantzaki I, Theodoridi M, Ischaki H, Makris D, Zakynthinos E, Zintzaras E, Sourlas S, Aloizos S, Zakynthinos SG. Vasopressin, steroids, and epinephrine and neurologically favorable survival after in-hospital cardiac arrest: a randomized clinical trial. JAMA. 2013;310:270-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 224] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 45. | Andersen LW, Isbye D, Kjærgaard J, Kristensen CM, Darling S, Zwisler ST, Fisker S, Schmidt JC, Kirkegaard H, Grejs AM, Rossau JRG, Larsen JM, Rasmussen BS, Riddersholm S, Iversen K, Schultz M, Nielsen JL, Løfgren B, Lauridsen KG, Sølling C, Pælestik K, Kjærgaard AG, Due-Rasmussen D, Folke F, Charlot MG, Jepsen RMHG, Wiberg S, Donnino M, Kurth T, Høybye M, Sindberg B, Holmberg MJ, Granfeldt A. Effect of Vasopressin and Methylprednisolone vs Placebo on Return of Spontaneous Circulation in Patients With In-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA. 2021;326:1586-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 46. | Voelckel WG, Lurie KG, McKnite S, Zielinski T, Lindstrom P, Peterson C, Wenzel V, Lindner KH, Benditt D. Effects of epinephrine and vasopressin in a piglet model of prolonged ventricular fibrillation and cardiopulmonary resuscitation. Crit Care Med. 2002;30:957-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Ristagno G, Sun S, Tang W, Castillo C, Weil MH. Effects of epinephrine and vasopressin on cerebral microcirculatory flows during and after cardiopulmonary resuscitation. Crit Care Med. 2007;35:2145-2149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 48. | Ristagno G, Tang W, Sun S, Weil MH. Cerebral cortical microvascular flow during and following cardiopulmonary resuscitation after short duration of cardiac arrest. Resuscitation. 2008;77:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 49. | Wyckoff MH, Greif R, Morley PT, Ng KC, Olasveengen TM, Singletary EM, Soar J, Cheng A, Drennan IR, Liley HG, Scholefield BR, Smyth MA, Welsford M, Zideman DA, Acworth J, Aickin R, Andersen LW, Atkins D, Berry DC, Bhanji F, Bierens J, Borra V, Böttiger BW, Bradley RN, Bray JE, Breckwoldt J, Callaway CW, Carlson JN, Cassan P, Castrén M, Chang WT, Charlton NP, Chung SP, Considine J, Costa-Nobre DT, Couper K, Couto TB, Dainty KN, Davis PG, de Almeida MF, de Caen AR, Deakin CD, Djärv T, Donnino MW, Douma MJ, Duff JP, Dunne CL, Eastwood K, El-Naggar W, Fabres JG, Fawke J, Finn J, Foglia EE, Folke F, Gilfoyle E, Goolsby CA, Granfeldt A, Guerguerian AM, Guinsburg R, Hirsch KG, Holmberg MJ, Hosono S, Hsieh MJ, Hsu CH, Ikeyama T, Isayama T, Johnson NJ, Kapadia VS, Kawakami MD, Kim HS, Kleinman M, Kloeck DA, Kudenchuk PJ, Lagina AT, Lauridsen KG, Lavonas EJ, Lee HC, Lin YJ, Lockey AS, Maconochie IK, Madar RJ, Malta Hansen C, Masterson S, Matsuyama T, McKinlay CJD, Meyran D, Morgan P, Morrison LJ, Nadkarni V, Nakwa FL, Nation KJ, Nehme Z, Nemeth M, Neumar RW, Nicholson T, Nikolaou N, Nishiyama C, Norii T, Nuthall GA, O'Neill BJ, Ong YG, Orkin AM, Paiva EF, Parr MJ, Patocka C, Pellegrino JL, Perkins GD, Perlman JM, Rabi Y, Reis AG, Reynolds JC, Ristagno G, Rodriguez-Nunez A, Roehr CC, Rüdiger M, Sakamoto T, Sandroni C, Sawyer TL, Schexnayder SM, Schmölzer GM, Schnaubelt S, Semeraro F, Skrifvars MB, Smith CM, Sugiura T, Tijssen JA, Trevisanuto D, Van de Voorde P, Wang TL, Weiner GM, Wyllie JP, Yang CW, Yeung J, Nolan JP, Berg KM; Collaborators. 2022 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations: Summary From the Basic Life Support; Advanced Life Support; Pediatric Life Support; Neonatal Life Support; Education, Implementation, and Teams; and First Aid Task Forces. Circulation. 2022;146:e483-e557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 89] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 50. | Skyschally A, Haude M, Dörge H, Thielmann M, Duschin A, van de Sand A, Konietzka I, Büchert A, Aker S, Massoudy P, Schulz R, Erbel R, Heusch G. Glucocorticoid treatment prevents progressive myocardial dysfunction resulting from experimental coronary microembolization. Circulation. 2004;109:2337-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 51. | Chalkias A, Xanthos T. Pathophysiology and pathogenesis of post-resuscitation myocardial stunning. Heart Fail Rev. 2012;17:117-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 52. | Bjelland TW, Dale O, Kaisen K, Haugen BO, Lydersen S, Strand K, Klepstad P. Propofol and remifentanil versus midazolam and fentanyl for sedation during therapeutic hypothermia after cardiac arrest: a randomised trial. Intensive Care Med. 2012;38:959-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 53. | Antonucci E, Fiaccadori E, Donadello K, Taccone FS, Franchi F, Scolletta S. Myocardial depression in sepsis: from pathogenesis to clinical manifestations and treatment. J Crit Care. 2014;29:500-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 205] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 54. | Adrie C, Adib-Conquy M, Laurent I, Monchi M, Vinsonneau C, Fitting C, Fraisse F, Dinh-Xuan AT, Carli P, Spaulding C, Dhainaut JF, Cavaillon JM. Successful cardiopulmonary resuscitation after cardiac arrest as a "sepsis-like" syndrome. Circulation. 2002;106:562-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 733] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 55. | Han F, Da T, Riobo NA, Becker LB. Early mitochondrial dysfunction in electron transfer activity and reactive oxygen species generation after cardiac arrest. Crit Care Med. 2008;36:S447-S453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 56. | Klouche K, Weil MH, Sun S, Tang W, Povoas HP, Kamohara T, Bisera J. Evolution of the stone heart after prolonged cardiac arrest. Chest. 2002;122:1006-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 57. | Wada T. Coagulofibrinolytic Changes in Patients with Post-cardiac Arrest Syndrome. Front Med (Lausanne). 2017;4:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 58. | Adrie C, Monchi M, Laurent I, Um S, Yan SB, Thuong M, Cariou A, Charpentier J, Dhainaut JF. Coagulopathy after successful cardiopulmonary resuscitation following cardiac arrest: implication of the protein C anticoagulant pathway. J Am Coll Cardiol. 2005;46:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 153] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 59. | Gando S, Otomo Y. Local hemostasis, immunothrombosis, and systemic disseminated intravascular coagulation in trauma and traumatic shock. Crit Care. 2015;19:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 60. | Gornik I, Wagner J, Gašparović V, Miličić D, Degoricija V, Skorić B, Gornik O, Lauc G. Prognostic value of cell-free DNA in plasma of out-of-hospital cardiac arrest survivors at ICU admission and 24h post-admission. Resuscitation. 2014;85:233-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 61. | Wada T, Gando S, Mizugaki A, Kodate A, Sadamoto Y, Murakami H, Maekawa K, Katabami K, Ono Y, Hayakawa M, Sawamura A, Jesmin S, Ieko M. Differences in coagulofibrinolytic changes between post-cardiac arrest syndrome of cardiac causes and hypoxic insults: a pilot study. Acute Med Surg. 2017;4:371-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 62. | Kim J, Kim K, Lee JH, Jo YH, Kim T, Rhee JE, Kang KW. Prognostic implication of initial coagulopathy in out-of-hospital cardiac arrest. Resuscitation. 2013;84:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 63. | Bro-Jeppesen J, Kjaergaard J, Wanscher M, Nielsen N, Friberg H, Bjerre M, Hassager C. Systemic Inflammatory Response and Potential Prognostic Implications After Out-of-Hospital Cardiac Arrest: A Substudy of the Target Temperature Management Trial. Crit Care Med. 2015;43:1223-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 64. | Vaahersalo J, Skrifvars MB, Pulkki K, Stridsberg M, Røsjø H, Hovilehto S, Tiainen M, Varpula T, Pettilä V, Ruokonen E; FINNRESUSCI Laboratory Study Group. Admission interleukin-6 is associated with post resuscitation organ dysfunction and predicts long-term neurological outcome after out-of-hospital ventricular fibrillation. Resuscitation. 2014;85:1573-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 65. | Moe GW, Marin-Garcia J, Konig A, Goldenthal M, Lu X, Feng Q. In vivo TNF-alpha inhibition ameliorates cardiac mitochondrial dysfunction, oxidative stress, and apoptosis in experimental heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1813-H1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 66. | Niemann JT, Rosborough JP, Youngquist S, Shah AP, Lewis RJ, Phan QT, Filler SG. Cardiac function and the proinflammatory cytokine response after recovery from cardiac arrest in swine. J Interferon Cytokine Res. 2009;29:749-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 67. | Niemann JT, Youngquist ST, Shah AP, Thomas JL, Rosborough JP. TNF-α blockade improves early post-resuscitation survival and hemodynamics in a swine model of ischemic ventricular fibrillation. Resuscitation. 2013;84:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 68. | Gill RS, Lee TF, Manouchehri N, Liu JQ, Lopaschuk G, Bigam DL, Cheung PY. Postresuscitation cyclosporine treatment attenuates myocardial and cardiac mitochondrial injury in newborn piglets with asphyxia-reoxygenation. Crit Care Med. 2013;41:1069-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Ayoub IM, Kolarova J, Gazmuri RJ. Cariporide given during resuscitation promotes return of electrically stable and mechanically competent cardiac activity. Resuscitation. 2010;81:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 70. | Cunningham CA, Coppler PJ, Skolnik AB. The immunology of the post-cardiac arrest syndrome. Resuscitation. 2022;179:116-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 71. | Arrich J, Holzer M, Herkner H, Müllner M. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2009;CD004128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 72. | Giraud R, Siegenthaler N, Bendjelid K. Cardiac index during therapeutic hypothermia: which target value is optimal? Crit Care. 2013;17:214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 73. | Jacobshagen C, Pelster T, Pax A, Horn W, Schmidt-Schweda S, Unsöld BW, Seidler T, Wagner S, Hasenfuss G, Maier LS. Effects of mild hypothermia on hemodynamics in cardiac arrest survivors and isolated failing human myocardium. Clin Res Cardiol. 2010;99:267-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 74. | Polderman KH, Tjong Tjin Joe R, Peerdeman SM, Vandertop WP, Girbes AR. Effects of therapeutic hypothermia on intracranial pressure and outcome in patients with severe head injury. Intensive Care Med. 2002;28:1563-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 179] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 75. | Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4038] [Cited by in RCA: 3809] [Article Influence: 165.6] [Reference Citation Analysis (0)] |
| 76. | Annborn M, Bro-Jeppesen J, Nielsen N, Ullén S, Kjaergaard J, Hassager C, Wanscher M, Hovdenes J, Pellis T, Pelosi P, Wise MP, Cronberg T, Erlinge D, Friberg H; TTM-trial investigators. The association of targeted temperature management at 33 and 36 °C with outcome in patients with moderate shock on admission after out-of-hospital cardiac arrest: a post hoc analysis of the Target Temperature Management trial. Intensive Care Med. 2014;40:1210-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 77. | Bro-Jeppesen J, Kjaergaard J, Søholm H, Wanscher M, Lippert FK, Møller JE, Køber L, Hassager C. Hemodynamics and vasopressor support in therapeutic hypothermia after cardiac arrest: prognostic implications. Resuscitation. 2014;85:664-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 78. | Peberdy MA, Callaway CW, Neumar RW, Geocadin RG, Zimmerman JL, Donnino M, Gabrielli A, Silvers SM, Zaritsky AL, Merchant R, Vanden Hoek TL, Kronick SL; American Heart Association. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S768-S786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 987] [Cited by in RCA: 959] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 79. | De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, Brasseur A, Defrance P, Gottignies P, Vincent JL; SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1194] [Cited by in RCA: 1153] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 80. | Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Böttiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, Kern KB, Laurent I, Longstreth WT Jr, Merchant RM, Morley P, Morrison LJ, Nadkarni V, Peberdy MA, Rivers EP, Rodriguez-Nunez A, Sellke FW, Spaulding C, Sunde K, Vanden Hoek T. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118:2452-2483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1061] [Cited by in RCA: 1116] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 81. | Mayr V, Luckner G, Jochberger S, Wenzel V, Ulmer H, Pajk W, Knotzer H, Friesenecker B, Lindner K, Hasibeder W, Dünser M. Arginine vasopressin in advanced cardiovascular failure during the post-resuscitation phase after cardiac arrest. Resuscitation. 2007;72:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 82. | Kakavas S, Chalkias A, Xanthos T. Vasoactive support in the optimization of post-cardiac arrest hemodynamic status: from pharmacology to clinical practice. Eur J Pharmacol. 2011;667:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 83. | Overgaard CB, Dzavík V. Inotropes and vasopressors: review of physiology and clinical use in cardiovascular disease. Circulation. 2008;118:1047-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 318] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 84. | Levy B, Buzon J, Kimmoun A. Inotropes and vasopressors use in cardiogenic shock: when, which and how much? Curr Opin Crit Care. 2019;25:384-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 85. | Scheeren TWL, Bakker J, Kaufmann T, Annane D, Asfar P, Boerma EC, Cecconi M, Chew MS, Cholley B, Cronhjort M, De Backer D, Dubin A, Dünser MW, Duranteau J, Gordon AC, Hajjar LA, Hamzaoui O, Hernandez G, Kanoore Edul V, Koster G, Landoni G, Leone M, Levy B, Martin C, Mebazaa A, Monnet X, Morelli A, Payen D, Pearse RM, Pinsky MR, Radermacher P, Reuter DA, Sakr Y, Sander M, Saugel B, Singer M, Squara P, Vieillard-Baron A, Vignon P, Vincent JL, van der Horst ICC, Vistisen ST, Teboul JL. Current use of inotropes in circulatory shock. Ann Intensive Care. 2021;11:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 86. | Bangash MN, Kong ML, Pearse RM. Use of inotropes and vasopressor agents in critically ill patients. Br J Pharmacol. 2012;165:2015-2033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 87. | Lemiale V, Dumas F, Mongardon N, Giovanetti O, Charpentier J, Chiche JD, Carli P, Mira JP, Nolan J, Cariou A. Intensive care unit mortality after cardiac arrest: the relative contribution of shock and brain injury in a large cohort. Intensive Care Med. 2013;39:1972-1980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 469] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 88. | Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30:2126-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 737] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 89. | Ameloot K, Meex I, Genbrugge C, Jans F, Boer W, Verhaert D, Mullens W, Ferdinande B, Dupont M, De Deyne C, Dens J. Hemodynamic targets during therapeutic hypothermia after cardiac arrest: A prospective observational study. Resuscitation. 2015;91:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 90. | Wiberg S, Stride N, Bro-Jeppesen J, Holmberg MJ, Kjærgaard J, Larsen S, Donnino MW, Hassager C, Dela F. Mitochondrial dysfunction in adults after out-of-hospital cardiac arrest. Eur Heart J Acute Cardiovasc Care. 2020;9:S138-S144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 91. | Manzo-Silberman S, Fichet J, Mathonnet A, Varenne O, Ricome S, Chaib A, Zuber B, Spaulding C, Cariou A. Percutaneous left ventricular assistance in post cardiac arrest shock: comparison of intra aortic blood pump and IMPELLA Recover LP2.5. Resuscitation. 2013;84:609-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 92. | Ben-Hamouda N, Ltaief Z, Kirsch M, Novy J, Liaudet L, Oddo M, Rossetti AO. Neuroprognostication Under ECMO After Cardiac Arrest: Are Classical Tools Still Performant? Neurocrit Care. 2022;37:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 93. | Jeung KW, Jung YH, Gumucio JA, Salcido DD, Menegazzi JJ. Benefits, key protocol components, and considerations for successful implementation of extracorporeal cardiopulmonary resuscitation: a review of the recent literature. Clin Exp Emerg Med. 2023;10:265-279. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 94. | Welsford M, Bossard M, Shortt C, Pritchard J, Natarajan MK, Belley-Côté EP. Does Early Coronary Angiography Improve Survival After out-of-Hospital Cardiac Arrest? A Systematic Review With Meta-Analysis. Can J Cardiol. 2018;34:180-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 95. | Carnicelli AP, Keane R, Brown KM, Loriaux DB, Kendsersky P, Alviar CL, Arps K, Berg DD, Bohula EA, Burke JA, Dixson JA, Gerber DA, Goldfarb M, Granger CB, Guo J, Harrison RW, Kontos M, Lawler PR, Miller PE, Nativi-Nicolau J, Newby LK, Racharla L, Roswell RO, Shah KS, Sinha SS, Solomon MA, Teuteberg J, Wong G, van Diepen S, Katz JN, Morrow DA. Characteristics, therapies, and outcomes of In-Hospital vs Out-of-Hospital cardiac arrest in patients presenting to cardiac intensive care units: From the critical care Cardiology trials network (CCCTN). Resuscitation. 2023;183:109664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 96. | Andersson A, Arctaedius I, Cronberg T, Levin H, Nielsen N, Friberg H, Lybeck A. In-hospital versus out-of-hospital cardiac arrest: Characteristics and outcomes in patients admitted to intensive care after return of spontaneous circulation. Resuscitation. 2022;176:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |