Published online Feb 26, 2024. doi: 10.4330/wjc.v16.i2.58
Peer-review started: November 22, 2023
First decision: December 23, 2023
Revised: January 1, 2024
Accepted: January 18, 2024
Article in press: January 18, 2024
Published online: February 26, 2024
Processing time: 90 Days and 13.4 Hours
Myeloproliferative neoplasms (MPN) are a group of diseases characterized by the clonal proliferation of hematopoietic progenitor or stem cells. They are clinically classifiable into four main diseases: chronic myeloid leukemia, essential thrombocythemia, polycythemia vera, and primary myelofibrosis. These pathologies are closely related to cardio- and cerebrovascular diseases due to the increased risk of arterial thrombosis, the most common underlying cause of acute myocardial infarction. Recent evidence shows that the classical Virchow triad (hypercoagulability, blood stasis, endothelial injury) might offer an explanation for such association. Indeed, patients with MPN might have a higher number and more reactive circulating platelets and leukocytes, a tendency toward blood stasis because of a high number of circulating red blood cells, endothelial injury or overactivation as a consequence of sustained inflammation caused by the neo
Core Tip: Myeloproliferative neoplasms (MPNs) are a group of three diseases: essential thrombocythemia, polycythemia vera, and primary myelofibrosis. MPNs have a high risk of acute coronary syndromes due to a pro-thrombotic state. This state is induced by abnormal cancer cells that tend to proliferate and secrete several inflammatory cytokines, sustaining a pro-inflammatory state throughout the body. Clinically, MPN patients need to be carefully evaluated for cytoreductive treatments and cardiovascular protective strategies.
- Citation: Tirandi A, Schiavetta E, Maioli E, Montecucco F, Liberale L. Inflammation as a cause of acute myocardial infarction in patients with myeloproliferative neoplasm. World J Cardiol 2024; 16(2): 58-63
- URL: https://www.wjgnet.com/1949-8462/full/v16/i2/58.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i2.58
Myeloproliferative neoplasms (MPNs) are a group of diseases characterized by the clonal proliferation of hematopoietic progenitor or stem cells. MPNs are subdivided into four main diseases: Chronic myeloid leukemia, essential thrombocythemia (ET), polycythemia vera (PV), and primary myelofibrosis. Thrombosis is one of the most common complications of MPNs, which can occur in both arterial and venous vessels. As such, patients with MPN are at high risk of cardio- and cerebrovascular diseases such as myocardial infarction, deep venous thrombosis, and stroke[1,2]. Epidemiologically, up to 75% of patients with MPNs experience a major adverse cardiovascular event (MACE) as a complication of their clinical condition, and about a third after a first acute coronary syndrome (ACS) have another MACE[3]. Of interest, ACS might precede the development of a clinically overt MPNs[4]. Such higher cardiovascular risk is probably related to the hyper-viscosity and thrombocytosis that are found in these neoplastic conditions. The main key elements that contribute to this pro-thrombotic state are the augmented number of circulating platelets and their hyperactivation, the marked leukocytosis, the Janus kinase 2 (JAK2) mutation, and the inflammatory state that especially concern the endothelium. In addition, the concomitant presence of classic cardiovascular risk factors (such as smoking, dyslipidemia, hypertension, etc.) further contributes to the higher risk of possible cardiovascular acute diseases in these patients. In this editorial, we comment on a recent article by Manan et al[5] published in the World Journal of Cardiology entitled “Acute myocardial infarction in myeloproliferative neoplasms”. We provide the key insights of the paper, re-discussing the main topics focusing on the major mechanism underlying the relation of MPNs and ACS.
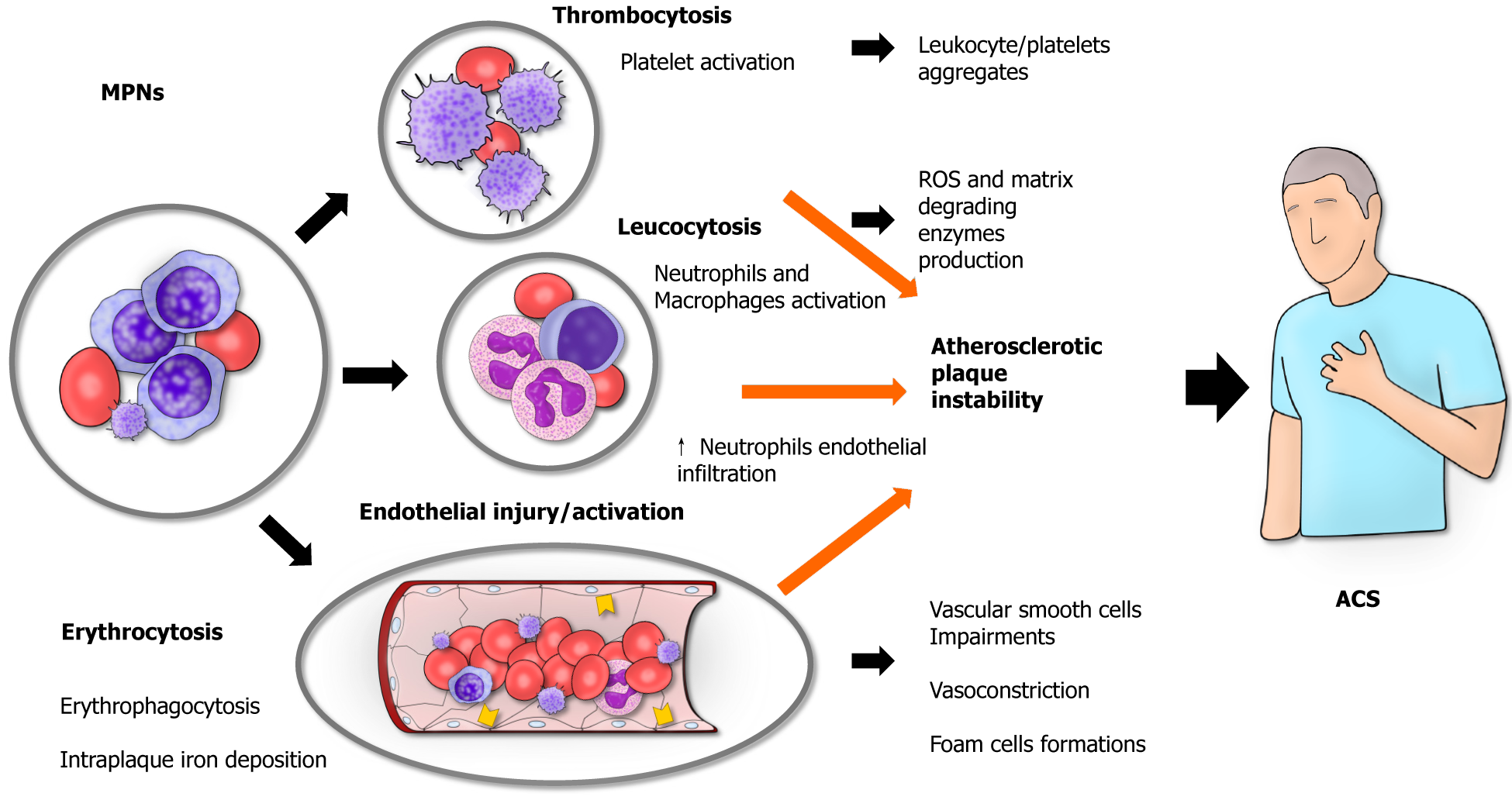
Inflammation plays a central role in the pathogenesis of cardiac diseases, particularly in the development of atherosclerotic disease[6]. In cases of MPN, the whole body undergoes a persistent inflammatory state, and patients typically suffer from inflammation-mediated symptoms such as fever, night sweats, weight loss, and fatigue[2]. Accordingly, in patients with ET and PV, the presence of high levels of C-reactive protein is associated with a higher risk of thrombosis[7]. Although more information is available concerning the role of inflammation in causing thrombosis, the underlying mechanisms through which MPNs contribute to the development of ACS are not completely understood. The concept is that thrombosis can affect both the arterial and venous vessels in MPN patients, and ACS are mainly caused by an arterial thrombosis of the coronary vessels[8]. The basic principle for the development of a thrombus remains the notorious Virchow triad (hypercoagulability, blood stasis, endothelial injury)[9] (Figure 1).
MPNs, especially ET, are associated with increased platelet count as well as their functionality impairment. ET is characterized by an overproduction of platelets from megakaryocytes as these cells become excessively sensitive to throm
Leukocytosis is known to be a non-specific marker of acute myocardial infarction (AMI)[14], where it is thought to reflect the inflammatory response toward myocardial necrosis in AMI patients. In MPNs patients, it can also be an expression of a more aggressive disease or an exaggerated inflammatory response[3]. As such, patients with AMI and marked leukocytosis are associated with a worse prognosis[15,16]. On the other hand, leukocytosis itself is a possible cause of AMI. For instance, acute leukemia patients with marked leukocytosis are known to possibly have acute myocardial infarction as a complication of their clinical condition[16]. In these patients, the presence of a pro-thrombophilic state and higher expression of adhesion molecules (e.g., CD56) are thought to favor the onset of ACS[17]. Similarly, patients with MPNs patients tend to have a pro-thrombotic state, and the presence of more circulating leukocytes can also reflect the presence of more reactive leukocytes with a tendency toward a dysregulated inflammatory response toward the onset of AMI. As such, leukocytosis in patients with PV can be considered as a possible hallmark of a higher card
JAK2 is a non-receptor tyrosine kinase in the Janus kinase family. JAK2 mutations are implicated in MPNs, including PV, ET, and myelofibrosis[19]. Furthermore, JAK2 mutation is also associated with a higher risk of ACS[3]. The most prevalent JAK2 mutation in MPNs is called JAK2V617F. This mutation consists of a substitution of a valine with phe
Blood stasis is typically found in MPNs patients. As all MPN relates with the expansion of a clone, and results in increased number of circulating cells a certain degree of blood stasis is expected in all patients with the disease[28]. The higher hematocrit found in PV patients is secondary to the higher number of circulating erythrocytes found in these patients. Such a higher number of circulating red blood cells is associated with blood stasis, blood flow disturbances, and hyper-viscosity[29], therefore favoring the development of thrombosis[30]. Similarly, in patients with ET cell count can hit high at over 1 million abnormal (see above) platelets/mL. Blood stasis together with the presence of over-reactive platelets can probably favor the development of thrombi because of platelet activation, as reported in studies on animal models that showed that platelet adhesion to the endothelium is directly related to the hematocrit levels[31]. Clinically, PV patients are more prone to have vascular complications, such ACS[18].
The endothelium is a pivotal player in the pathophysiology of arterial thrombosis. Indeed, under physiological conditions endothelial cells exert anti-thrombotic roles by producing several mediators including nitric oxide. The endothelium probably participates in the formation of thrombi as a consequence of the hyper-coagulability state rather than being the primary origin of thrombus formation[32]. However, under the pro-inflammatory pressure lead by the neoplastic clone, endothelial cells get dysfunctionally activated and secrete further pro-inflammatory cytokines propagating inflammation. Activated endothelial cells increase the expression of adhesion molecules including E-selectin on their surfaces in MPN patients[33]. Although E-selectin is not considered a marker of unstable coronary plaque[34], the endothelial overexpression of E-selectin can trigger an excessive leukocyte response in MPN patients. As such, even the smallest plaque tear might favor an exaggerated intracoronary activation of platelets, causing the clinical manifestation of ACS.
Furthermore, endothelial cells with mutated JAK2 have been found in patients carrying the JAK2 V617F mutation[35]. Of interest, such endothelial cells express a proadhesive phenotype with increased P-selectin expression that may be a further link with the increased thrombosis risk[36]. Indeed, therapeutic approaches aiming at P-selectin blockade have shown preclinical potential to reduce thrombosis. Increased atherosclerosis may not be the only link between AMI and MPNs, as almost 20% of AMI in MPNs occurs in patients without significant atherosclerotic occlusive disease[37]. Here, coronary vasoconstriction may play a role. Indeed, JAK2 V617F mice have shown increased arterial vasoconstriction due to their lower levels of nitric oxide, increased oxidative and inflammatory stress[38]. Specifically, erythrocytes-derived microvescicles have been deemed responsible for such phenotype and proteomic analysis of particles derived from JAK2V617F erythrocytes suggested MPO as the potential mediator[38].
MPNs are associated with cardiovascular diseases, especially those sustained by a thrombotic event. MPNs arise from clonal hematopoiesis of indeterminate potential (CHIP), whose investigation in the last years provided fundamental insight into the causal link between thrombosis and MPNs. CHIP is defined as the presence of a clonal mutation in a driver gene, occurring with a variant burden of ≥ 2% but without any clinical evidence of a hematologic neoplasm. Patients with CHIP show a 10-fold increased risk of developing any hematologic malignancy, including MPNs[39]. Of interest, the magnitude of risk enrichment due to CHIP is even higher than that of classical cardiovascular risk factors[39]. Experimental and clinical observations further point at inflammation as the culprit link between CHIP and cardiovascular disease[40,41]. Indeed, CHIP is nowadays seen as another characteristic of human aging, and it acco
Manan et al[5] reviewed the recent literature and provided insight into the pathogenesis and clinical consequences of the association between hematological and cardiovascular diseases. Further research is needed to establish cardiovascular preventive strategies for MPN patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen K, China S-Editor: Liu JH L-Editor: A P-Editor: Zhang YL
| 1. | Frederiksen H, Szépligeti S, Bak M, Ghanima W, Hasselbalch HC, Christiansen CF. Vascular Diseases In Patients With Chronic Myeloproliferative Neoplasms - Impact Of Comorbidity. Clin Epidemiol. 2019;11:955-967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Bhuria V, Baldauf CK, Schraven B, Fischer T. Thromboinflammation in Myeloproliferative Neoplasms (MPN)-A Puzzle Still to Be Solved. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Leiva O, Jenkins A, Rosovsky RP, Leaf RK, Goodarzi K, Hobbs G. Risk Factors for Death or Cardiovascular Events after Acute Coronary Syndrome in Patients with Myeloproliferative Neoplasms. Hematol Rep. 2023;15:398-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 4. | Gouri A, Yakhlef A, Dekaken A, Bentorki AA. Acute myocardial infarction revealing a polycythemia vera. Ann Biol Clin (Paris). 2012;70:489-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Manan MR, Kipkorir V, Nawaz I, Waithaka MW, Srichawla BS, Găman AM, Diaconu CC, Găman MA. Acute myocardial infarction in myeloproliferative neoplasms. World J Cardiol. 2023;15:571-581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 6. | Tirandi A, Montecucco F, Liberale L. Heart and vessels 'on fire'. Eur J Clin Invest. 2023;53:e14052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 7. | Barbui T, Carobbio A, Finazzi G, Vannucchi AM, Barosi G, Antonioli E, Guglielmelli P, Pancrazzi A, Salmoiraghi S, Zilio P, Ottomano C, Marchioli R, Cuccovillo I, Bottazzi B, Mantovani A, Rambaldi A; AGIMM and IIC Investigators. Inflammation and thrombosis in essential thrombocythemia and polycythemia vera: different role of C-reactive protein and pentraxin 3. Haematologica. 2011;96:315-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 8. | Nurkalem Z, Uslu N, Gorgulu S, Eren M. Left main coronary thrombosis with essential thrombocythemia. J Thromb Thrombolysis. 2006;22:165-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Chung I, Lip GY. Virchow's triad revisited: blood constituents. Pathophysiol Haemost Thromb. 2003;33:449-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Kawasaki H, Nakano T, Kohdera U, Kobayashi Y. Hypersensitivity of megakaryocyte progenitors to thrombopoietin in essential thrombocythemia. Am J Hematol. 2001;68:194-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Vannucchi AM, Barbui T. Thrombocytosis and thrombosis. Hematology Am Soc Hematol Educ Program. 2007:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Koupenova M, Kehrel BE, Corkrey HA, Freedman JE. Thrombosis and platelets: an update. Eur Heart J. 2017;38:785-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 13. | Ross DM, Liang HPH, Iqra Z, Whittaker S, Tan CW, Dale BJ, Chen VM. Platelets from patients with myeloproliferative neoplasms have increased numbers of mitochondria that are hypersensitive to depolarization by thrombin. Sci Rep. 2023;13:9172. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Green SM, Vowels J, Waterman B, Rothrock SG, Kuniyoshi G. Leukocytosis: a new look at an old marker for acute myocardial infarction. Acad Emerg Med. 1996;3:1034-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Kruk M, Przyłuski J, Kalińczuk L, Pregowski J, Kadziela J, Kaczmarska E, Petryka J, Kepka C, Klopotowski M, Chmielak Z, Ciszewski A, Demkow M, Karcz M, Witkowski A, Ruzyłło W. Hemoglobin, leukocytosis and clinical outcomes of ST-elevation myocardial infarction treated with primary angioplasty: ANIN Myocardial Infarction Registry. Circ J. 2009;73:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Pesaro AE, Nicolau JC, Serrano CV Jr, Truffa R, Gaz MV, Karbstein R, Giraldez RR, Kalil Filho R, Ramires JA. Influence of leukocytes and glycemia on the prognosis of patients with acute myocardial infarction. Arq Bras Cardiol. 2009;92:84-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Colović N, Bogdanović A, Virijević M, Vidović A, Tomin D. Acute Myocardial Infarction during Induction Chemotherapy for Acute MLL t(4;11) Leukemia with Lineage Switch and Extreme Leukocytosis. Srp Arh Celok Lek. 2015;143:734-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Landolfi R, Di Gennaro L, Barbui T, De Stefano V, Finazzi G, Marfisi R, Tognoni G, Marchioli R; European Collaboration on Low-Dose Aspirin in Polycythemia Vera (ECLAP). Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007;109:2446-2452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 286] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 19. | Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2647] [Cited by in RCA: 2747] [Article Influence: 137.4] [Reference Citation Analysis (0)] |
| 20. | Lussana F, Rambaldi A. Inflammation and myeloproliferative neoplasms. J Autoimmun. 2017;85:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 21. | Wolach O, Sellar RS, Martinod K, Cherpokova D, McConkey M, Chappell RJ, Silver AJ, Adams D, Castellano CA, Schneider RK, Padera RF, DeAngelo DJ, Wadleigh M, Steensma DP, Galinsky I, Stone RM, Genovese G, McCarroll SA, Iliadou B, Hultman C, Neuberg D, Mullally A, Wagner DD, Ebert BL. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 321] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 22. | Bonaventura A, Montecucco F, Dallegri F, Carbone F, Lüscher TF, Camici GG, Liberale L. Novel findings in neutrophil biology and their impact on cardiovascular disease. Cardiovasc Res. 2019;115:1266-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 23. | Bonaventura A, Liberale L, Carbone F, Vecchié A, Diaz-Cañestro C, Camici GG, Montecucco F, Dallegri F. The Pathophysiological Role of Neutrophil Extracellular Traps in Inflammatory Diseases. Thromb Haemost. 2018;118:6-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 24. | Liberale L, Holy EW, Akhmedov A, Bonetti NR, Nietlispach F, Matter CM, Mach F, Montecucco F, Beer JH, Paneni F, Ruschitzka F, Libby P, Lüscher TF, Camici GG. Interleukin-1β Mediates Arterial Thrombus Formation via NET-Associated Tissue Factor. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 25. | Scott LM. The JAK2 exon 12 mutations: a comprehensive review. Am J Hematol. 2011;86:668-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 26. | Vainchenker W, Kralovics R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood. 2017;129:667-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 444] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 27. | Shawky AM, Almalki FA, Abdalla AN, Abdelazeem AH, Gouda AM. A Comprehensive Overview of Globally Approved JAK Inhibitors. Pharmaceutics. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 178] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 28. | Hasselbalch HC, Elvers M, Schafer AI. The pathobiology of thrombosis, microvascular disease, and hemorrhage in the myeloproliferative neoplasms. Blood. 2021;137:2152-2160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 29. | Willerslev A, Hansen MM, Klefter ON, Bjerrum OW, Hasselbalch HC, Clemmensen SN, Larsen M, Munch IC. Non-invasive imaging of retinal blood flow in myeloproliferative neoplasms. Acta Ophthalmol. 2017;95:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Pearson TC, Wetherley-Mein G. Vascular occlusive episodes and venous haematocrit in primary proliferative polycythaemia. Lancet. 1978;2:1219-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 275] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 31. | Turitto VT, Weiss HJ. Red blood cells: their dual role in thrombus formation. Science. 1980;207:541-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 232] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 32. | Friedenberg WR, Roberts RC, David DE. Relationship of thrombohemorrhagic complications to endothelial cell function in patients with chronic myeloproliferative disorders. Am J Hematol. 1992;40:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Musolino C, Alonci A, Allegra A, Spatari G, Bellomo G, Tringali O, Quartarone C, Squadrito G, Quartarone M. Increased levels of the soluble adhesion molecule E-selectin in patients with chronic myeloproliferative disorders and thromboembolic complications. Am J Hematol. 1998;57:109-112. [PubMed] [DOI] [Full Text] |
| 34. | Galvani M, Ferrini D, Ottani F, Nanni C, Ramberti A, Amboni P, Iamele L, Vernocchi A, Nicolini FA. Soluble E-selectin is not a marker of unstable coronary plaque in serum of patients with ischemic heart disease. J Thromb Thrombolysis. 2000;9:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Guy A, Gourdou-Latyszenok V, Le Lay N, Peghaire C, Kilani B, Dias JV, Duplaa C, Renault MA, Denis C, Villeval JL, Boulaftali Y, Jandrot-Perrus M, Couffinhal T, James C. Vascular endothelial cell expression of JAK2(V617F) is sufficient to promote a pro-thrombotic state due to increased P-selectin expression. Haematologica. 2019;104:70-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 36. | Guadall A, Lesteven E, Letort G, Awan Toor S, Delord M, Pognant D, Brusson M, Verger E, Maslah N, Giraudier S, Larghero J, Vanneaux V, Chomienne C, El Nemer W, Cassinat B, Kiladjian JJ. Endothelial Cells Harbouring the JAK2V617F Mutation Display Pro-Adherent and Pro-Thrombotic Features. Thromb Haemost. 2018;118:1586-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 37. | Pósfai É, Marton I, Borbényi Z, Nemes A. Myocardial infarction as a thrombotic complication of essential thrombocythemia and polycythemia vera. Anatol J Cardiol. 2016;16:397-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Poisson J, Tanguy M, Davy H, Camara F, El Mdawar MB, Kheloufi M, Dagher T, Devue C, Lasselin J, Plessier A, Merchant S, Blanc-Brude O, Souyri M, Mougenot N, Dingli F, Loew D, Hatem SN, James C, Villeval JL, Boulanger CM, Rautou PE. Erythrocyte-derived microvesicles induce arterial spasms in JAK2V617F myeloproliferative neoplasm. J Clin Invest. 2020;130:2630-2643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 39. | Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D, Ebert BL. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488-2498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3384] [Cited by in RCA: 3494] [Article Influence: 317.6] [Reference Citation Analysis (0)] |
| 40. | Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, Vuong J, Jacob S, Muralidhar V, Robertson AA, Cooper MA, Andrés V, Hirschi KK, Martin KA, Walsh K. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 1094] [Article Influence: 136.8] [Reference Citation Analysis (0)] |
| 41. | Libby P, Ebert BL. CHIP (Clonal Hematopoiesis of Indeterminate Potential): Potent and Newly Recognized Contributor to Cardiovascular Risk. Circulation. 2018;138:666-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 42. | Liberale L, Montecucco F, Schwarz L, Lüscher TF, Camici GG. Inflammation and cardiovascular diseases: lessons from seminal clinical trials. Cardiovasc Res. 2021;117:411-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 43. | Liberale L, Montecucco F, Tardif JC, Libby P, Camici GG. Inflamm-ageing: the role of inflammation in age-dependent cardiovascular disease. Eur Heart J. 2020;41:2974-2982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 211] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 44. | Liberale L, Badimon L, Montecucco F, Lüscher TF, Libby P, Camici GG. Inflammation, Aging, and Cardiovascular Disease: JACC Review Topic of the Week. J Am Coll Cardiol. 2022;79:837-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 230] [Article Influence: 76.7] [Reference Citation Analysis (0)] |