INTRODUCTION
Electrical storm (ES), also known as sympathetic storm and ventricular electric storm, refers to a state of unstable ventricular electrical activity. It is characterized by three or more episodes of ventricular tachycardia (VT) or ventricular fibrillation (VF) within a 24-hour period. This condition is dangerous and associated with a high mortality rate. The clinical occurrence of ES is on the rise with the increasing incidence of coronary atherosclerotic heart disease, heart failure, and other related conditions. Typically, it leads to evident hemodynamic disturbances. which is difficult to manage with medication alone, necessitating the use of electroconversion or defibrillation for termination. Clinically, the mortality rate of ES is high[1,2]. The most common cause of ES is malignant, arrhythmia triggered by acute myocardial ischemia. Excessive excitation of the sympathetic nervous system is an important factor that triggers ES. Nicorandil is a unique clinical drug with dual effects[3]. The first effect of nicorandil is to open adenosine triphosphate (ATP)-sensitive potassium ion channels. Another effect of nicorandil, similar to nitrate esters, is to open to the K+-ATP channels on the myocardial fiber cell membrane. This action leads to the dilation of coronary arteries, increased blood flow, reduced occurrence of angina, inhibition of coronary microvascular spasm, decreased endothelial cell damage, improvement in microcirculation, reduced myocardial fibrosis, and reduced ventricular arrhythmia[4]. Nicorandil can also reduce Ca2+ influx by opening the K+-ATP channel of myocardial cell mitochondria, prevent Ca2+ overload in mitochondria, and protect or restore mitochondrial function, simulating myocardial ischemic preconditioning to protect myocardial cells[5]. Hirose et al[6] also found that long-term use of nicorandil in gaq transgenic mice can shorten the QT interval and reduce the occurrence of ventricular premature contractions. The patient in this case used nicorandil to reduce the occurrence of ESs.
CASE PRESENTATION
Chief complaints
A 75-year-old female patient presented with intermittent chest tightness, shortness of breath for 10 days, and fainting once for 7 days.
History of present illness
Ten days ago, the patient had no obvious causes or triggers, and intermittent chest tightness and shortness of breath, which were obviously related to activities. Oral administration of cold medicine and Huoxiang Zhengqi water did not relieve the symptoms. Seven days ago, the patient suddenly had amaurosis during activity and developed syncope. A few minutes later, she woke up on her own, without incontinence, limb twitching, occasional nausea, vomiting, and sweating. She immediately went to Tongliao District Hospital by 120 ambulances, and was admitted to the Department of Cardiology with suspected coronary atherosclerotic heart disease.
History of past illness
Paroxysmal atrial fibrillation for > 6 years without treatment, hypertension for > 10 years (highest blood pressure 180/100 mmHg), intermittent oral administration of amlodipine besylate 5 mg/day, unmonitored blood pressure, and no history of infectious diseases, vaccination, food or drug allergies, or trauma. Fifteen years after lumbar spine fracture surgery and right lower limb fracture surgery; 20 years after hysterectomy for uterine fibroids, with no history of blood transfusion.
Personal and family history
No history of smoking and alcohol consumption, as well as no family genetic history.
Physical examination
Vital signs: Temperature 36.5 °C, blood pressure 145/80 mmHg, pulse rate 124 beats/minute, respiratory rate 20 breaths/minute.
Physical examination: Double lung smell and dry, wet rale, small heart boundary, heart rate 164 beats/minute, irregular, different first heart sound, short pulse, no murmur, soft abdomen, no tenderness and rebound pain, no edema in either limb, no obvious positive signs after residual body examination.
Laboratory examinations
In June 28, 2023, complete blood count (CBC): White blood cell (WBC) count (10.7 × 109/L); neutrophil (NEU) percentage (88.7%); NEU count (9.54 × 109/L); lymphocyte (LYM) percentage (7.1%); monocyte (MON) count (0.41 × 109/L); MON percentage (3.9%); eosinophil percentage (EOS%) (0.2%); platelet count (297 × 109/L); hemoglobin (HGB) (121 g/L); C-reactive protein (6.57 mg/L); N-terminal prohormone of brain natriuretic peptide (NT-ProBNP) (2677 pg/mL); high sensitivity troponin I (47.9 pg/mL); K+ (3.3 mmol/L); creatine kinase (CK) (7 U/L); CK-type M and B (CK-MB) (2.03 U/L); lactate dehydrogenase (LDH) (244 U/L); hydroxybutyrate dehydrogenase (HBDH) (146.2 U/L); D-dimer (DD) (1.27 mg/L); fibrinogen (FIB) (3.93 g/L). In July 5, 2023, WBC count (17.6 × 109/L); NEU percentage (83.9%); NEU count (14.76 × 109/L); LYM percentage (7.4%); MON count (1.43 × 109/L); MON percentage (8.2%); EOS% 0.4%; platelet count (352 × 109/L); HGB (137 g/L); FR-C-reactive protein (86.3 mg/L); CK (29 U/L); CK-MB-M (1.19 U/L); LDH (290 U/L); HBDH (177.2 U/L); NT-ProBNP (1940.5 pg/mL). In July 6, 2023, K+ (4.3 mmol/L); CK (27 U/L); CK-MB-M (13.4 U/L); LDH (315 U/L); HBDH (234 U/L); CBC: WBC count (16.01 × 109/L); NEU percentage (83.3%); NEU count (13.33 × 109/L); LYM percentage (6.6%); LYM count (1.06 × 109/L); MON count (1.55 × 109/L); MON percentage (9.7%); EOS% (0.2%); platelet count (354 × 109/L); HGB (134 g/L); FIB (6.03 g/L); DD (3.4 mg/L); NT-ProBNP (2669 pg/mL); high sensitivity troponin I (22 pg/mL); myoglobin < 21 ng/mL; alanine transaminase (18.8 U/L); g-glutamyl transpeptidase (106 U/L); aspartate transaminase (23.4 U/L); total protein (55.8 g/L); albumin (32.4 g/L); alkaline phosphatase (146 U/L); cystatin-C (1.05 mg/L). In July 7, 2023, CK (26.3 U/L); CK-MB-M (11.1 U/L); LDH (320.2 U/L); HBDH (235.7 U/L); urine analysis no abnormality seen; blood fat no abnormality seen. In July 8, 2023, WBC (11.38 × 109/L); NEU percentage (76.1%); NEU count (8.65 × 109/L); LYM percentage (13.7%); MON count (0.97 × 109/L); stool for routine occult blood (+); NT-ProBNP (2767 pg/mL); HGB A1c (5.3%). In July 9, 2023, CBC: WBC count (9.48 × 109/L); NEU percentage (68.5%); NEU count (8.65 × 109/L); LYM percentage (19.4%); stool for routine: Occult blood (-); arterial blood gas: pH = 7.44; K+ (4.43 mmol/L). In July 10, 2023, ST: Occult blood (-); arterial blood gas: pH = 7.44; K+ (4.8 mmol/L); NT-ProBNP (609.7 pg/mL). In July 13, 2023, WBC count (9.91 × 109/L); NEU percentage (71.8%); NEU count (7.12 × 109/L); LYM percentage (17.3%); platelet count (447 × 109/L); HGB (131 g/L); NT-ProBNP (129.2 pg/mL). In July 14, 2023, K+ (4.46 mmol/L); CBC: WBC count (9.14 × 109/L); NEU percentage (70.7%); NEU count (6.47 × 109/L); LYM percentage (18.2%); platelet count (420 × 109/L); HGB (130 g/L); FIB (4.17 g/L); DD (1.48 mg/L). In July 17, 2023, K+ (4.46 mmol/L); CBC: WBC count (8.03 × 109/L); NEU percentage (63.3%); NEU count (5.08 × 109/L); LYM percentage (24%); platelet count (364 × 109/L); HGB (129 g/L); NT-ProBNP (30.8 pg/mL).
Imaging examinations
In June 28, 2023, chest computed tomography, diagnostic opinions: (1) Double pneumonia changes, except for pulmonary edema; (2) Calcification in the upper lobe of the right lung; (3) Bilateral pleural effusion; (4) Heart enlargement; and (5) Multiple liver cysts. In June 30, 2023, cardiac color ultrasound showed: Double atrial enlargement, left ventricular wall movement abnormality, decreased left heart function, aortic valve calcification, posterior mitral annular calcification and regurgitation (mild), tricuspid regurgitation (mild), ejection fraction (EF) 33%. In July 6, 2023, bedside heart color ultrasound: Mitral regurgitation (small), aortic regurgitation (small), reduced cardiac function, EF 46%. Pleural color ultrasound showed bilateral pleural effusion, with a possible investigation range of 3 cm. In July 11, 2023, thoracic color ultrasound showed bilateral pleural effusion, with the right depth of 5.8 cm and left depth of 4.4 cm. In July 13, 2023, chest color ultrasound showed bilateral pleural effusion with a depth of 7.6 cm on the right and 3.9 cm on the left.
FINAL DIAGNOSIS
Coronary artery atherosclerotic heart disease, unstable angina arrhythmia, frequent ventricular premature beat, short array VT, VF, heart function grade III.
TREATMENT
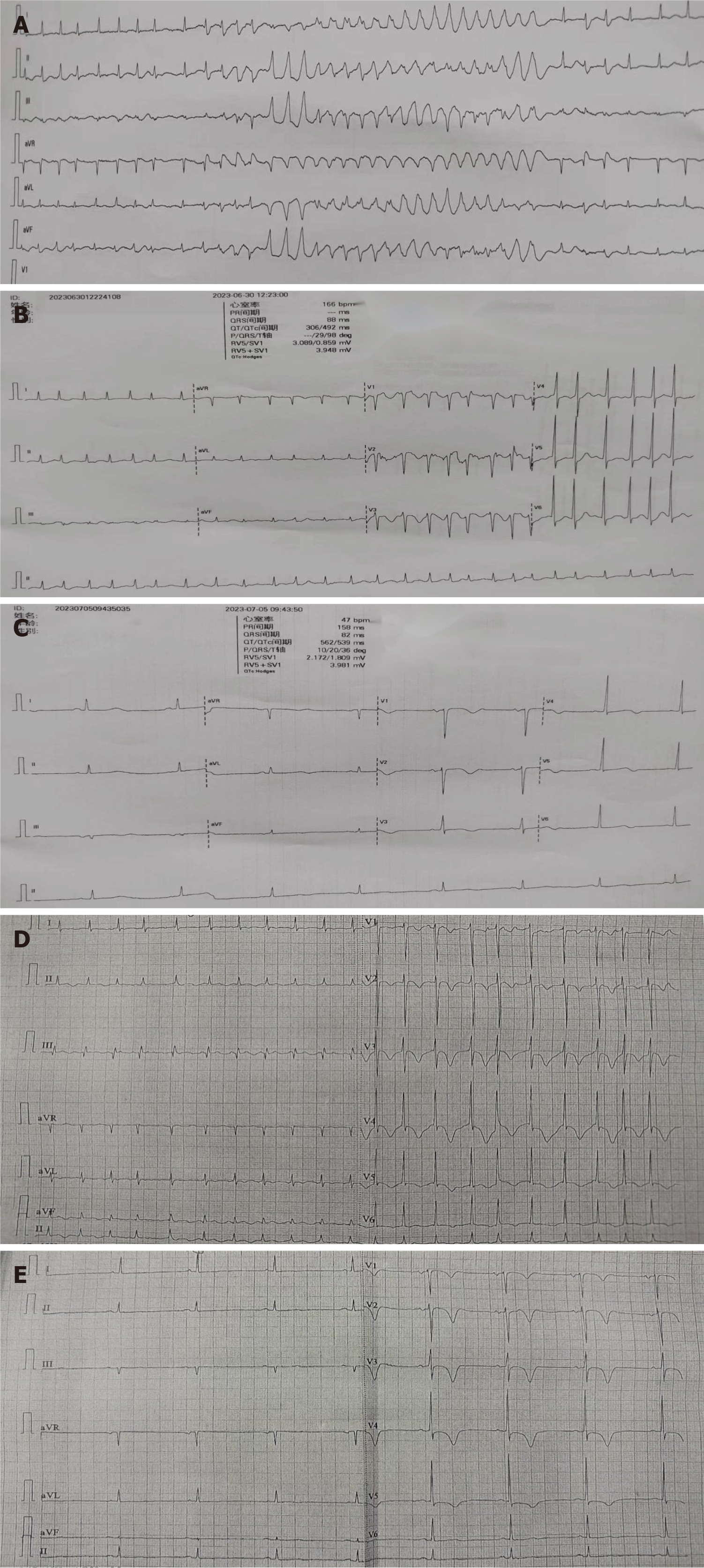
The patient was treated with oxygen inhalation, electrocardiography (ECG) monitoring, and oral drugs (aspirin, pravastatin, and metoprolol tartrate), hypodermic injection of low molecular weight heparin, drug infusion (cefoperazone sodium sulbactam sodium, ginkgo damol, tolasemide, furosemide, spironolactone diuresis and potassium supplementation). At 00:18 am the next day, under night monitoring, VF and loss of consciousness were detected. Cardiopulmonary resuscitation was performed, and sinus rhythm was restored after asynchronous 200 joules defibrillation. The aforementioned medication continued. However, there were still intermittent episodes of VT. On June 30, 2023, at 00:01 am, intravenous injection of lidocaine and infusion did not improve the symptoms. At 00:19 am, heart rhythm developed short array VT and VF after atrial fibrillation (Figure 1A). Given a 200 joules cardioversion immediately, sinus rhythm was still not restored. Immediately after intravenous injection of amiodarone, 150 mg was added to 150 mL glucose for continuous pumping. At 00:23 am, the ECG showed that atrial fibrillation, with a heart rate of 166 beats/minute, QTc 493 ms (Figure 1B). However, the patient still had intermittent seizure VT. For further treatment, she was transferred to the intensive care unit. The electrocardiogram of sinus bradycardia was performed at 09:43 am, heart rate was 47 beats/minute, QTc 539 ms, and amiodarone was stopped (Figure 1C). At 05:41 pm on July 5, 2023, ECG monitoring showed ventricular arrhythmia, and after electrical defibrillation, the patient still had intermittent attack, and received lidocaine intravenous injection and infusion at 04:59 am on July 6. The heart rhythm was continuous VT and VF after addition of esmolol; therefore, she was transferred to our department.
Figure 1 Patient outcomes.
A: The heart rhythm of patient developed short array ventricular tachycardia and ventricular fibrillation after atrial fibrillation; B: Atrial fibrillation, with a heart rate of 166 beats/minute, QTc 493 ms; C: Sinus rhythm, with a heart rate of 47 beats/minute, QTc 539 ms; D: Heart rhythm of patient was atrial fibrillation; E: Heart rhythm was converted to sinus rhythm with rate 49 beats/minute, QTc 433 ms.
When she was entered to the Department of Cardiology at our hospital, ECG monitoring showed sudden onset of short-term VT. After a few seconds, the heart rhythm spontaneously turned into sinus, which was followed by intravenous injection of 5 mg lidocaine. After sustained intravenous infusion of lidocaine, ventricular premature beats occurred intermittently. Potassium magnesium solution and nicorandil infusion were given. Ultrasonic cardiogram showed an EF of 45%, NT-ProBNP (2669 pg/mL), and chest ultrasound showed bilateral pleural effusion of approximately 3 cm. A definite diagnosis of heart failure was made, and recombinant human brain natriuretic peptide treatment was given. Emergency blood routine examination showed elevated WBCs, and symptomatic treatment with antibiotics was initiated. However, the patient still had intermittent premature ventricular beats and nicorandil was given with intravenous fluids. The heart rhythm was atrial fibrillation at 09:00 am (Figure 1D). After cedilanid intravenous injection, ECG still showed atrial fibrillation, and metoprolol tartrate tablets controlled heart rate. At 06:01 pm on July 8, 2023, heart rhythm was converted to sinus rhythm of 49 beats/minute, QTc 433 ms (Figure 1E). Metoprolol tartrate tablets were stopped but rivaroxaban, atorvastatin, sacubitril valsartan sodium tablets, torsemide and spironolactone were continued. No further ventricular premature beats occurred thereafter. On July 13, re-examination with chest ultrasound showed that the right-sided pleural effusion was 6.1 cm and the left side was 3.9 cm. The patient requested surgical treatment and underwent elective coronary angiography, which showed an irregular anterior descending branch with lesions from the opening to the proximal segment; the most severe stenosis was 70%-85%, and the anterior blood flow was thrombolysis in myocardial infarction (TIMI) grade 3. The circumflex artery was irregular, with 80%-90% stenosis in the distal segment and TIMI grade 3 forward blood flow. The right coronary artery was irregular, with diffuse long lesions in the proximal to distal segments; the most severe stenosis was 50%-60% in the proximal segment, 50%-60% in the middle segment, and 70%-80% in the distal segment. The anterior blood flow was TIMI grade 3. The patient had coronary heart disease. After consultation with her family, we decided to treat the descending branch lesion and chose a suitable time to treat the circumflex branch lesion. A Gureater 3.5 mm × 24 mm drug-eluting stent was implanted in the anterior descending artery lesion. After surgery, antiplatelet aggregation, stable plaque, crown expansion and diuresis were continued, and the patient was discharged after her condition improved. After discharge, treatment continued with oral aspirin, clopidogrel, rivaroxaban, atorvastatin, nicorandil, sacubitril valsartan sodium tablets, torsemide, and spironolactone.
OUTCOME AND FOLLOW-UP
The ventricular arrhythmia stopped and the patient’s condition improved and she was discharged. After discharge, she had no further attack of ventricular arrhythmia and her condition was stable.
DISCUSSION
ES is a prevalent form of ventricular arrhythmia in clinical cardiology, characterized by three or more occurrences within a 24-hour period. It arises from disrupted ventricular heart rhythm and presents with symptoms such as palpitations, dyspnea, chest discomfort, reduced exercise tolerance, and often triggered by emotional stress. In severe cases, it can lead to heart failure, posing a significant risk to life. Therefore, it is crucial to identify and correct the mechanisms of ventricular arrhythmias[7]. The pathogenesis of ventricular arrhythmia is multifaceted. The involvement of myocardial injury, neurohumoral changes, and abnormalities in calcium channels are the main causes of ES[8-10]. The initiation and perpetuation of ES rely on overexcitation of the sympathetic nervous system. This overexcitation triggers a swift surge in the secretion of endogenous catecholamines, prompting the influx of sodium and Ca2+ into the myocardial cell membrane, and the efflux of potassium ions (such as myocardial infarction, major trauma, surgery, emotional excitement, etc.). Consequently, the action potential of myocardial cells is prolonged, ultimately leading to the development of malignant arrhythmias. Previous studies have reported that long-term myocardial ischemia and hypoxia in patients with coronary heart disease may lead to myocardial fibrosis, and myocardial fibrosis is one of the causes of arrhythmia[6]. Patients with coronary heart disease often have arrhythmia, and most of them have ventricular phase contraction. Nicorandil inhibits myocardial fibrosis and reduces ventricular arrhythmia by improving the microcirculation. Defibrillation during the treatment of patients experiencing ESs can exacerbate the excessive excitation of the sympathetic nervous system.
In the case of patients exhibiting stable hemodynamics and nonorganic heart disease, treatment may not be necessary when symptoms are mild or absent. when symptoms become apparent, lidocaine can be administered as the preferred medication[11]. Originally developed as a local anesthetic, lidocaine possesses antiarrhythmic properties. It is used in various medical procedures such as epidural anesthesia, infiltration and surface anesthesia, as well as nerve conduction block[12]. Additionally, lidocaine can be used in cases where ventricular bradycardia or premature contractions are induced by acute myocardial infarction[13]. It is classified as a class 1B drug in the treatment of arrhythmia. These medications possess the ability to moderately impede sodium channels, thereby hindering the depolarization process during phase 4 of the action potential. Consequently, they diminish the rate of depolarization, widen the threshold, and alleviate abnormal autonomous activity. However, they do not impede the depolarization rate or conductivity during phase 0 of the action potential of myocardial cells[14]. Consequently, these drugs are suitable for treating VT in patients with stable hemodynamics. Nonetheless, they may elicit adverse reactions such as dizziness and consciousness disorders. Additionally, they may occasionally lead to sinus node suppression and atrioventricular block[15-17].
Amiodarone is a frequently used class III medication in the management of anti-arrhythmia. Its mechanisms of action include diminishing the autonomy of the sinoatrial node, impeding atrial conduction fibers, and obstructing sodium, potassium, and Ca2+ channels. It also prolongs the QT interval. Additionally, it influences α and beta adrenal hormones, exerting a noncompetitive inhibitory effect that prolongs the non-responsiveness of the sinoatrial node and ventricular muscles. This medication decelerates the conduction between the sinoatrial node and the atrioventricular node, thereby ameliorating arrhythmia[18-23]. In this particular case, the ECG showed ventricular arrhythmia. Despite continuous treatment involving lidocaine, amiodarone, and electrical defibrillation, VT did not show any improvement. Consequently, no ES occurred after the application of nicorandil.
Nicorandil, a potassium channel opener, is prominently characterized by shortening the action potential time course in the ventricle and Purkinje fibers. In the latest antiarrhythmic drug classification, it was designated as a class IIIb metabolism-dependent potassium channel opening agent, shortening the action potential duration and ECG QT interval in all cardiomyocytes except sinuatrial node cells[24]. Hirose et al[6] treated gaq transgenic mice with nicorandil over an extended period and revealed that the drug prevented the occurrence of arrhythmia by shortening the duration of action potentials and QT interval. K+-ATP channels are expressed by various types of cells in cardiac tissue, such as cardiomyocytes, vascular smooth muscle cells, and autonomic neurons. K+-ATP channels are also present in the sarcolemma and mitochondria of these cells. K+-ATP channels are nearly closed under normoxic conditions[25-27]. However, under ischemic conditions, depletion of intracellular ATP concentration and accumulation of ischemia-related metabolites, such as adenosine diphosphate and lactate, open the channels. Nicorandil is a mixed compound of ATP channel openers and nitrate and is commonly used as a coronary vasodilator for treatment of angina pectoris. In patients with acute myocardial infarction, nicorandil before coronary intervention may reduce infarct size and ischemia-reperfusion-induced arrhythmia by reducing the absence of rebleeding phenomenon[28-30]. Studies have shown that K+-ATP channel opening may have important biological actions that prevent cardiac fibrosis. Nicorandil attenuated myocardial-infarction-induced cardiac fibrosis in rats, and its beneficial actions on differentiations of fibroblast were blocked by adding glibenclamide which is a blocker of K+-ATP channels[31]. Nicorandil thus improves the microcirculation and reduces the ventricular arrhythmia by inhibiting myocardial fibrosis. Therefore, it can be concluded that nicorandil has the potential to prevent the development of malignant arrhythmias[31,32].
How does nicorandil contribute to the prevention of arrhythmias? After the patient experienced electrical defibrillation treatment, lidocaine treatment was tried. Lidocaine, a local anesthetic that also has antiarrhythmic properties, reduces the occurrence of ventricular arrhythmia by affecting stage 4 of action potential depolarization. However, in the present case, ventricular arrhythmia was not controlled by lidocaine. Subsequently amiodarone treatment was tried, but caused prolonged QTc and repolarization, which induced ventricular arrhythmia, aggravating the situation. Therefore, lidocaine and amiodarone were both discontinued. After these treatments failed to effectively control ventricular arrhythmias, we finally treated them with nicorandil. Subsequently, the patient was treated with a combination of lidocaine and nicorandil, resulting in the absence of VT. Because the outward or inward current can prolong the action potential, this current change is likely to induce ventricular arrhythmia. It is suggested that nicorandil prevents phase 2 early after depolarization[33], enabling shortening of the QT interval, as it can prolong the plateau phase by inhibiting a large inward current in this phase, thereby reactivating the L-type calcium channel and forming early and late depolarization of myocardial cells. In theory, the duration of the action potential can be regulated by the interplay between depolarization and repolarization currents, without necessitating automatic calcium release from the sarcoplasmic reticulum or activation of sodium and calcium inward currents. Any augmentation of inward current and reduction of outward current can lead to premature and delayed depolarization. In addition, under myocardial ischemia, it opens K+-ATP channels, and then nicorandil inhibited myocardial fibrosis, dilated coronary arteries, improved microcirculation, and reduced arrhythmia.
CONCLUSION
Nicorandil is a drug with dual effects. First, it is a metabolism-dependent K+ channel opening agent that can open ATP-sensitive potassium channels. Secondly, nicorandil has a similar effect as nitrate ester, and opens the K+-ATP channel on cardiac muscle fiber cell membranes, leading to coronary artery expansion, increased blood flow, reduced occurrence of angina, inhibition of coronary microvascular spasm, reduced endothelial cell damage, improved microcirculation, and reduced myocardial fibrosis and ventricular arrhythmia. Nicorandil can also reduce the influx of Ca2+ by opening K+-ATP channels in mitochondria, prevent calcium overload in mitochondria, and protect or restore mitochondrial function, mimicking myocardial ischemic preconditioning to protect cardiomyocytes. In the latest classification of antiarrhythmic drugs, nicorandil belongs to class B drugs. K+-ATP channels open when intracellular ATP decreases, K+ outflow increases, action potential plateau shortens, voltage-dependent Ca2+ channel activity decreases, Ca2+ influx decreases, shortening myocardial tissue action potential recovery time, refractory period and QT interval. In the present case, the patient had frequent ventricular arrhythmia due to myocardial ischemia and reperfusion, so the ventricular arrhythmia was controlled immediately by nicorandil. We conclude that nicorandil is a new treatment option for ventricular arrhythmia, especially with myocardial ischemia and hypoxia in coronary heart disease.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C, Grade C
Novelty: Grade B, Grade C
Creativity or Innovation: Grade B, Grade C
Scientific Significance: Grade C, Grade C
P-Reviewer: Nag DS S-Editor: Bai Y L-Editor: A P-Editor: Zhang XD