Published online Nov 26, 2024. doi: 10.4330/wjc.v16.i11.632
Revised: August 27, 2024
Accepted: October 10, 2024
Published online: November 26, 2024
Processing time: 109 Days and 17.2 Hours
As a non-communicable disease, cardiovascular disorders have become the lea
Core Tip: Cardiovascular disease is the principal cause of non-communicable diseases with individuals succumbing to heart disease every thirty-three seconds and has a significant comorbidity with nonalcoholic fatty liver disease (NAFLD). These two disorders impact millions of individuals across the globe, yield significant disability and death to individuals, and have a common underlying cellular pathway with silent mating type information regulation 2 homolog 1 (Saccharomyces cerevi
- Citation: Maiese K. Cardiovascular and nonalcoholic fatty liver disease: Sharing common ground through SIRT1 pathways. World J Cardiol 2024; 16(11): 632-643
- URL: https://www.wjgnet.com/1949-8462/full/v16/i11/632.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i11.632
Non-communicable diseases (NCDs) lead to disability and death in a significant spectrum of individuals worldwide and encompass disorders that include cardiac disease, cancer, trauma, respiratory disease, stroke, Alzheimer’s disease, diabetes mellitus (DM), influenza and pneumonia, kidney disease, and suicide[1-3]. Cardiac and cardiovascular disorders are the most prominent of NCDs as the leading cause of death for both women and men and greater than one million individuals suffer a heart attack every year[4-7]. Several therapeutic pathways can lessen the severity of cardiovascular disease by addressing tobacco exposure, inadequate nutrition, hypertension, serum cholesterol values, obesity, infection, and the existence of DM[8-11].
In fact, DM not only is a risk factor for cardiovascular disease, but also for liver disease and the development of nonalcoholic fatty liver disease (NAFLD), a significant comorbidity of cardiovascular disorders[12-14]. NAFLD, also known as metabolic-dysfunction-associated steatotic liver disease, is increasing in presence throughout the world and this has occurred with concurrent the rise in DM globally[15-19]. NAFLD is a chronic disorder of the liver with excessive fat accumulation and is associated with at least one metabolic risk factor, such as obesity, DM, hypertension, elevated serum triglycerides, low serum high-density lipoprotein (HDL) cholesterol, and advanced age[13,20-23]. A recent paper by the authors Batta and Hatwal[24] brings to light the clinical link between cardiovascular disease and NAFLD and that in combination these disorders can lead to an increased risk of major impairment in cardiovascular function as well as cerebral function, such as stroke.
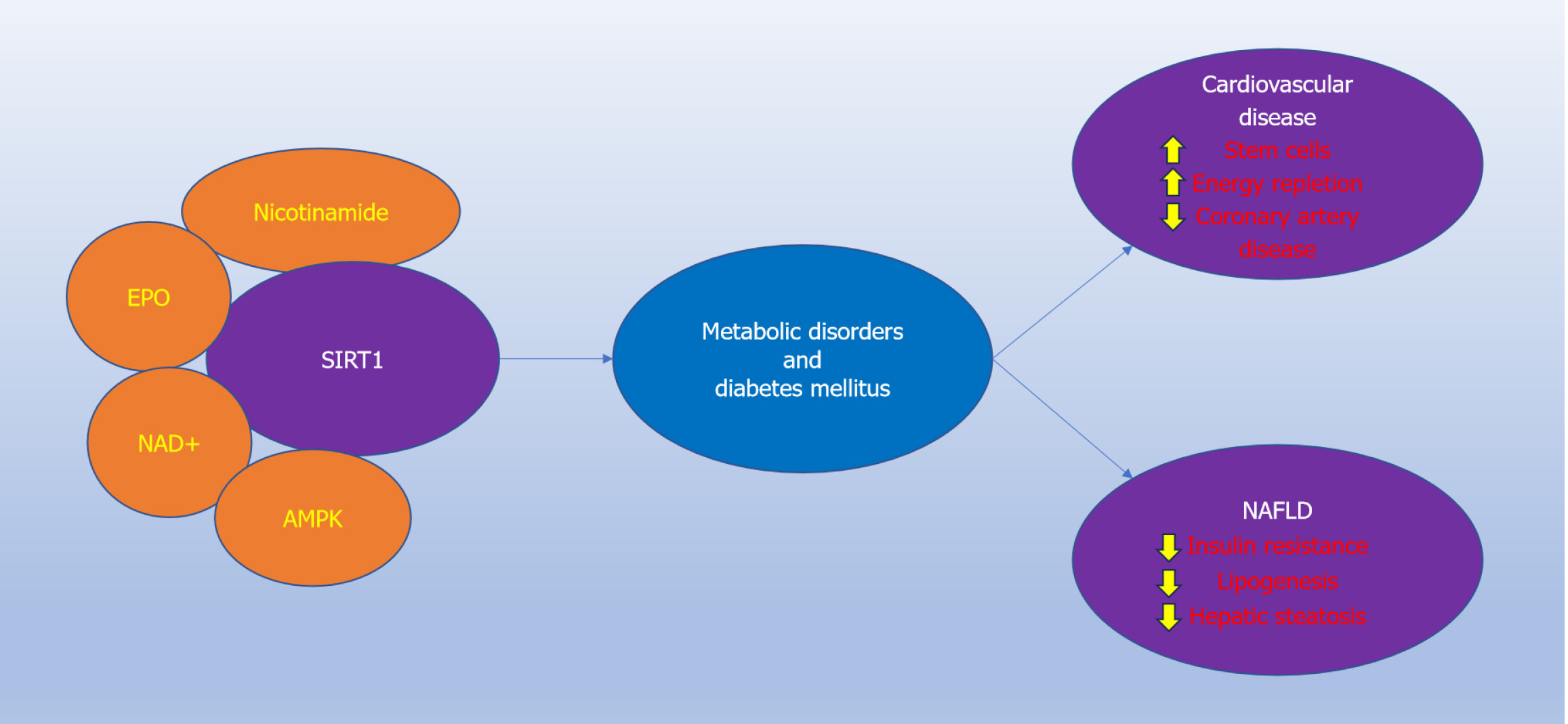
Given that metabolic disorders, such as DM, are common risk factors for the development of cardiovascular disease and NAFLD, it is significant to note that a common underlying cellular pathway that can oversee both of these disorders involves the silent mating type information regulation 2 homolog 1 (SIRT1; Saccharomyces cerevisiae)[25-27]. SIRT1 is present in the heart, skeletal muscle, pancreas, liver, brain, spleen, and adipose tissue[13,25,28-30]. SIRT1 is a member of the sirtuin family (sirtuin 1) and is a histone deacetylase that promotes transcription of DNA through the transfer of acetyl groups from e-N-acetyl lysine amino acids to DNA histones[31-33]. Nicotinamide adenine dinucleotide (NAD+), a coenzyme, functions as a SIRT1 substrate[22,28,34,35]. SIRT1 can control metabolic homeostasis[36,37] and functions closely with NAD+ and the vitamin nicotinamide[22,23,34,35]. As the amide form of the vitamin B3 (niacin), nicotinamide is the precursor for NAD+[31,38-40]. SIRT1 oversees nicotinamide phosphoribosyl-transferase that is required for NAD+ production and is tied to circadian clock gene rhythms[41]. It is important to note that sufficient levels of NAD+ are required to prevent vascular disease, dementia, and mitochondrial function[21,42,43]. Pools of cellular NAD+ are sus
In addition to maintaining metabolic homeostasis, SIRT1 also controls growth factor function through the activity of NAD+[50-53]. Erythropoietin (EPO) employs SIRT1 to preserve synaptic connections for memory function[54,55], enhance survival of cardiovascular cells[56,57], and protect against toxic events with liver cells[52,58]. At the cellular level, EPO oversees SIRT1 activity to prevent mitochondrial membrane depolarization, activation of BCL2 associated agonist of cell death (Bad), and caspase pathway activity[59-61].
SIRT1 offers a clinical target for both cardiovascular disease and hepatic disease that can lead to NAFLD (Figure 1). In regard to cardiovascular disease, SIRT1 can foster the function of stem cells[62-64] and enhance cardiac function and repair[65,66]. SIRT1 can improve the function of endothelial cells[42,67,68], reduce coronary artery disease[69,70], inhibit cardiac injury during DM and metabolic disorders[37,71-73], and assist with cellular energy repletion[74,75]. Through the prevention of cellular senescence[76] to allow progenitor cell differentiation with SIRT1, cardiovascular cells are afforded the ability for heightened resistance to injury[43,67,77,78]. In the broader cardiovascular systems, SIRT1 can oversee programmed cell death with apoptosis and autophagy, control cardiac remodeling through increased mitochondrial biogenesis, limit myocardial injury, reduce insulin resistance, and prevent cardiac hypertrophy[4,30,79-83].
Liver function is dependent upon both cellular metabolism and SIRT1 pathways[13,45,84,85]. Insulin sensitivity and the maintenance of mitochondrial function require SIRT1 activation[86-89]. Activation of SIRT1 can control hepatocyte processing of lipids and glucose level maintenance to lessen the risk of the onset of metabolic syndrome dysfunction[30,31,90]. If SIRT1 activity is limited in the pancreas and liver, insulin resistance can ensue especially during high fat consumption[13,91-93]. SIRT1 also can control de novo lipogenesis and resolve hepatic steatosis that may lead to NAFLD and require activation of related pathways of AMP-activated protein kinase (AMPK)[92,94-96]. The AMPK pathway is closely linked to SIRT1 and nicotinamide in overseeing cellular metabolic homeostasis[9,72,97-99]. In recent clinical studies, treatment with oleoylethanolamide, an endogenous peroxisome proliferator-activated receptor alpha agonist, in patients with NAFLD led to increased mRNA expression levels of SIRT1 with increases in HDL cholesterol and decreases in triglyceride levels, suggesting that SIRT1 is a therapeutic target for NAFLD[100]. In addition, increased exercise in patients with NAFLD may affect lipophagy, lipolytic pathways, and reduction in oxidative stress through SIRT1 activity[101]. Through SIRT1 pathways, exercise also affects cardiac fatty acid oxidation, tissue regeneration, improved metabolic status, dietary interventions for weight management, and reduction in age-related decline of cellular metabolic pathways[45,71,75].
Cardiovascular disorders and NAFLD impact a significant number of individuals throughout the globe and share common aspects of underlying disease pathology related to cellular metabolic dysfunction and the intricate pathways of SIRT1 involving NAD+, nicotinamide, trophic factors such as EPO, and AMPK. SIRT1 is an exciting clinical target for both cardiovascular disease and hepatic disorders, since SIRT1 activity can maintain cellular metabolic homeostasis, enhance stem cell function and differentiation, foster the survival of vascular endothelial cells, limit cardiac injury, control hepatocyte lipid production and insulin resistance, and limit hepatic steatosis that can result in NAFLD. Yet, the pathways of SIRT1 are complex and require intact cellular feedback pathways with nicotinamide, NAD+, growth factors, and AMPK since lack of close biological control can lead to unwanted clinical outcomes such as tumorigenesis[4,26,87,102,103]. In addition, multiple pathways intersect with SIRT1 that involve cellular metabolic disease[21,35,97,104-109], apoptosis and autophagy[26,110,111], oxidative stress, inflammation, and mitochondrial impairment[25,76,111-121], and clock genes with Wnt proteins impairment[105,111,117,118,122-124]. SIRT1 offers exciting possibilities for the advance
| 1. | IDF Diabetes Atlas. IDF Diabetes Atlas 9th edition. 2019. [cited 27 August 2024]. Available from: https://diabetesatlas.org/atlas/ninth-edition/. |
| 2. | Schell M, Wardelmann K, Kleinridders A. Untangling the effect of insulin action on brain mitochondria and metabolism. J Neuroendocrinol. 2021;33:e12932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Speer H, D'Cunha NM, Alexopoulos NI, McKune AJ, Naumovski N. Anthocyanins and Human Health-A Focus on Oxidative Stress, Inflammation and Disease. Antioxidants (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 4. | Maiese K. Innovative therapeutic strategies for cardiovascular disease. EXCLI J. 22:690-715. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Centers for Disease Control and Prevention. CDC WONDER. [cited 19 Ocober 2024]. Available from: https://wonder.cdc.gov/. |
| 6. | You H, Zhao Q, Dong M. The Key Genes Underlying Pathophysiology Correlation Between the Acute Myocardial Infarction and COVID-19. Int J Gen Med. 2022;15:2479-2490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Wang Z, Zhang G, Hu S, Fu M, Zhang P, Zhang K, Hao L, Chen S. Research progress on the protective effect of hormones and hormone drugs in myocardial ischemia-reperfusion injury. Biomed Pharmacother. 2024;176:116764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 8. | Centers for Disease Control and Prevention. National diabetes statistics report, 2020 : estimates of diabetes and its burden in the United States. Feb 14, 2020. [cited 19 Ocober 2024]. Available from: https://stacks.cdc.gov/view/cdc/85309. |
| 9. | Maiese K. Cornerstone Cellular Pathways for Metabolic Disorders and Diabetes Mellitus: Non-Coding RNAs, Wnt Signaling, and AMPK. Cells. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Bandelin-Franke L, Scheibenbogen C, Bobbert T. Post-COVID und Diabetes mellitus. Diabetologie. 2024;20:356-363. [DOI] [Full Text] |
| 11. | Ijaz K, Khan AU, Kamal Y, Irshad N. Effects of dapagliflozin against streptozotocin and isoproterenol-induced heart failure via investigating NLRP3 and PPAR-γ signaling. Pak J Pharm Sci. 2024;37:337-347. [PubMed] |
| 12. | Di Rosa M, Malaguarnera L. Chitotriosidase: A New Inflammatory Marker in Diabetic Complications. Pathobiology. 2016;83:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Sedik AA, Elgohary R, Khalifa E, Khalil WKB, I Shafey H, B Shalaby M, S O Gouida M, M Tag Y. Lauric acid attenuates hepato-metabolic complications and molecular alterations in high-fat diet-induced nonalcoholic fatty liver disease in rats. Toxicol Mech Methods. 2024;34:454-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 14. | Sun WD, Zhu XJ, Li JJ, Mei YZ, Li WS, Li JH. Nicotinamide N-methyltransferase (NNMT): a novel therapeutic target for metabolic syndrome. Front Pharmacol. 2024;15:1410479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 15. | Teng ML, Ng CH, Huang DQ, Chan KE, Tan DJ, Lim WH, Yang JD, Tan E, Muthiah MD. Global incidence and prevalence of nonalcoholic fatty liver disease. Clin Mol Hepatol. 2023;29:S32-S42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 325] [Article Influence: 162.5] [Reference Citation Analysis (0)] |
| 16. | Wang J, Chen S, Zhao X, Guo Q, Yang R, Zhang C, Huang Y, Ma L, Zhao S. Effect of PPARγ on oxidative stress in diabetes-related dry eye. Exp Eye Res. 2023;231:109498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 17. | Raut SK, Khullar M. Oxidative stress in metabolic diseases: current scenario and therapeutic relevance. Mol Cell Biochem. 2023;478:185-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 39] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 18. | Maiese K. Cellular Metabolism: A Fundamental Component of Degeneration in the Nervous System. Biomolecules. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Abo-Shady AM, Gheda SF, Ismail GA, Cotas J, Pereira L, Abdel-Karim OH. Antioxidant and Antidiabetic Activity of Algae. Life (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Anggreini P, Kuncoro H, Sumiwi SA, Levita J. Role of the AMPK/SIRT1 pathway in nonalcoholic fatty liver disease (Review). Mol Med Rep. 2023;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (1)] |
| 21. | Li JJ, Sun WD, Zhu XJ, Mei YZ, Li WS, Li JH. Nicotinamide N-Methyltransferase (NNMT): A New Hope for Treating Aging and Age-Related Conditions. Metabolites. 2024;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 22. | Liu Y, Cheng C, Gao H, Zhu XJ, He X, Zhou MX, Gao Y, Lu YW, Song XH, Xiao XH, Wang JB, Xu CJ, Ma ZT. Restoring energy metabolism by NAD(+) supplement prevents alcohol-induced liver injury and boosts liver regeneration. Food Sci Nutr. 2024;12:5100-5110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 23. | Maiese K. New Insights for nicotinamide: Metabolic disease, autophagy, and mTOR. Front Biosci (Landmark Ed). 2020;25:1925-1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Batta A, Hatwal J. Excess cardiovascular mortality in men with non-alcoholic fatty liver disease: A cause for concern! World J Cardiol. 2024;16:380-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (3)] |
| 25. | Farid HA, Sayed RH, El-Shamarka ME, Abdel-Salam OME, El Sayed NS. PI3K/AKT signaling activation by roflumilast ameliorates rotenone-induced Parkinson's disease in rats. Inflammopharmacology. 2024;32:1421-1437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Pandaram A, Paul J, Wankhar W, Thakur A, Verma S, Vasudevan K, Wankhar D, Kammala AK, Sharma P, Jaganathan R, Iyaswamy A, Rajan R. Aspartame Causes Developmental Defects and Teratogenicity in Zebra Fish Embryo: Role of Impaired SIRT1/FOXO3a Axis in Neuron Cells. Biomedicines. 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 27. | Yu X, Chen M, Wu J, Song R. Research progress of SIRTs activator resveratrol and its derivatives in autoimmune diseases. Front Immunol. 2024;15:1390907. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Maiese K. Cognitive Impairment in Multiple Sclerosis. Bioengineering (Basel). 2023;10. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Abo El-Magd NF, El-Kashef DH, El-Sherbiny M, Eraky SM. Hepatoprotective and cognitive-enhancing effects of hesperidin against thioacetamide-induced hepatic encephalopathy in rats. Life Sci. 2023;313:121280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 30. | Ramadhan AY, Soetikno V. Molecular Adaptation of Cardiac Remodeling in Metabolic Syndrome: Focus on AMPK, SIRT1 and PGC-1a. Mol Cell Biomed Sci. 2024;8:15. [DOI] [Full Text] |
| 31. | Maiese K. The impact of aging and oxidative stress in metabolic and nervous system disorders: programmed cell death and molecular signal transduction crosstalk. Front Immunol. 2023;14:1273570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 32. | Zhang RB, Ren L, Ding DP, Wang HD, Peng J, Zheng K. Protective Effect of the SIRT1-Mediated NF-κB Signaling Pathway against Necrotizing Enterocolitis in Neonatal Mice. Eur J Pediatr Surg. 2023;33:386-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (1)] |
| 33. | Dhillon VS, Shahid M, Deo P, Fenech M. Reduced SIRT1 and SIRT3 and Lower Antioxidant Capacity of Seminal Plasma Is Associated with Shorter Sperm Telomere Length in Oligospermic Men. Int J Mol Sci. 2024;25. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Verma P, Srivastava A, Prajapati P, Tandon P, Shimpi MR. Molecular Structure, Hydrogen Bonding Interactions and Docking Simulations of Nicotinamide (Monomeric and Trimeric Models) by Using Spectroscopy and Theoretical Approach. Polycycl Aromat Compd. 2024;44:1537-1555. [DOI] [Full Text] |
| 35. | Sorokoumova AA, Seryapina AA, Polityko YK, Yanshole LV, Tsentalovich YP, Gilinsky МА, Markel АL. Urine metabolic profile in rats with arterial hypertension of different genesis. Vavilovskii Zhurnal Genet Selektsii. 2024;28:299-307. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 36. | Yang J, Suo H, Song J. Protective role of mitoquinone against impaired mitochondrial homeostasis in metabolic syndrome. Crit Rev Food Sci Nutr. 2021;61:3857-3875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 37. | Jalgaonkar MP, Parmar UM, Kulkarni YA, Oza MJ. SIRT1-FOXOs activity regulates diabetic complications. Pharmacol Res. 2022;175:106014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 38. | Espinoza SE, Khosla S, Baur JA, de Cabo R, Musi N. Drugs Targeting Mechanisms of Aging to Delay Age-Related Disease and Promote Healthspan: Proceedings of a National Institute on Aging Workshop. J Gerontol A Biol Sci Med Sci. 2023;78:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 39. | Ramírez-Cruz A, Gómez-González B, Baiza-Gutman LA, Manuel-Apolinar L, Ángeles-Mejía S, López-Cervantes SP, Ortega-Camarillo C, Cruz-López M, Gómez-Olivares JL, Díaz-Flores M. Nicotinamide, an acetylcholinesterase uncompetitive inhibitor, protects the blood‒brain barrier and improves cognitive function in rats fed a hypercaloric diet. Eur J Pharmacol. 2023;959:176068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 40. | Tai SH, Chao LC, Huang SY, Lin HW, Lee AH, Chen YY, Lee EJ. Nicotinamide Deteriorates Post-Stroke Immunodepression Following Cerebral Ischemia-Reperfusion Injury in Mice. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 41. | Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 958] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 42. | Fangma Y, Wan H, Shao C, Jin L, He Y. Research Progress on the Role of Sirtuin 1 in Cerebral Ischemia. Cell Mol Neurobiol. 2023;43:1769-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Ministrini S, Puspitasari YM, Beer G, Liberale L, Montecucco F, Camici GG. Sirtuin 1 in Endothelial Dysfunction and Cardiovascular Aging. Front Physiol. 2021;12:733696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 44. | Watroba M, Szukiewicz D. Sirtuins at the Service of Healthy Longevity. Front Physiol. 2021;12:724506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 45. | Chong MC, Silva A, James PF, Wu SSX, Howitt J. Exercise increases the release of NAMPT in extracellular vesicles and alters NAD(+) activity in recipient cells. Aging Cell. 2022;21:e13647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 46. | Yamamoto H, Shimomura N, Oura K, Hasegawa Y. Nacre Extract from Pearl Oyster Shell Prevents D-Galactose-Induced Brain and Skin Aging. Mar Biotechnol (NY). 2023;25:503-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Trujillo-Rangel WÁ, Acuña-Vaca S, Padilla-Ponce DJ, García-Mercado FG, Torres-Mendoza BM, Pacheco-Moises FP, Escoto-Delgadillo M, García-Benavides L, Delgado-Lara DLC. Modulation of the Circadian Rhythm and Oxidative Stress as Molecular Targets to Improve Vascular Dementia: A Pharmacological Perspective. Int J Mol Sci. 2024;25. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 48. | Tabibzadeh S. Signaling pathways and effectors of aging. Front Biosci (Landmark Ed). 2021;26:50-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 49. | Ye M, Zhao Y, Wang Y, Xie R, Tong Y, Sauer JD, Gong S. NAD(H)-loaded nanoparticles for efficient sepsis therapy via modulating immune and vascular homeostasis. Nat Nanotechnol. 2022;17:880-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 50. | Maiese K. The Metabolic Basis for Nervous System Dysfunction in Alzheimer's Disease, Parkinson's Disease, and Huntington's Disease. Curr Neurovasc Res. 2023;20:314-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 51. | Sosa S, Bringas G, Urrutia N, Peñalver AI, López D, González E, Fernández A, Hernández ZM, Viña A, Peña Y, Batista JF, Valenzuela C, León K, Crombet T, Rodríguez T, Pérez L; ATHENEA Investigators. NeuroEPO plus (NeuralCIM(®)) in mild-to-moderate Alzheimer's clinical syndrome: the ATHENEA randomized clinical trial. Alzheimers Res Ther. 2023;15:215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 52. | Yang K, Zhang L, Chen W, Cheng J, Zhao X, Zhang Y, Li R, Zhou M, Yao Y, Li Y, Qiao Z. Expression of EPO and related factors in the liver and kidney of plain and Tibetan sheep. Histol Histopathol. 2023;38:1337-1347. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 53. | Zhu L, Yuan Q, Jing C, Sun L, Jiang L. Angiogenic responses are enhanced by recombinant human erythropoietin in a model of periventricular white matter damage of neonatal rats through EPOR-ERK1 signaling. J Neuropathol Exp Neurol. 2024;83:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 54. | Maiese K. Neurodegeneration, memory loss, and dementia: the impact of biological clocks and circadian rhythm. Front Biosci (Landmark Ed). 2021;26:614-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 55. | Jahan R, Yousaf M, Khan H, Shah SA, Khan AA, Bibi N, Javed F, Ijaz M, Ali A, Wei DQ. Zinc Ortho Methyl Carbonodithioate Improved Pre and Post-Synapse Memory Impairment via SIRT1/p-JNK Pathway against Scopolamine in Adult Mice. J Neuroimmune Pharmacol. 2023;18:183-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 56. | Cui L, Guo J, Zhang Q, Yin J, Li J, Zhou W, Zhang T, Yuan H, Zhao J, Zhang L, Carmichael PL, Peng S. Erythropoietin activates SIRT1 to protect human cardiomyocytes against doxorubicin-induced mitochondrial dysfunction and toxicity. Toxicol Lett. 2017;275:28-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 57. | Yuksel IO, Cagirci G, Koklu E, Yilmaz A, Kucukseymen S, Ellidag HY, Cay S, Yilmaz N, Arslan S. Erythropoietin stimulates the coronary collateral development in patients with coronary chronic total occlusion. Neth Heart J. 2016;24:609-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 58. | Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, Gerard RD, Garcia JA. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 390] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 59. | Kaur D, Behl T, Sehgal A, Singh S, Sharma N, Badavath VN, Ul Hassan SS, Hasan MM, Bhatia S, Al-Harassi A, Khan H, Bungau S. Unravelling the potential neuroprotective facets of erythropoietin for the treatment of Alzheimer's disease. Metab Brain Dis. 2022;37:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Memisoglu A, Kolgazi M, Yaman A, Bahadir E, Sirvanci S, Yeğen BÇ, Ozek E. Neuroprotective Effect of Erythropoietin on Phenylhydrazine-Induced Hemolytic Hyperbilirubinemia in Neonatal Rats. Neurochem Res. 2017;42:1026-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 61. | Rey F, Ottolenghi S, Giallongo T, Balsari A, Martinelli C, Rey R, Allevi R, Giulio AMD, Zuccotti GV, Mazzucchelli S, Foresti R, Samaja M, Carelli S. Mitochondrial Metabolism as Target of the Neuroprotective Role of Erythropoietin in Parkinson's Disease. Antioxidants (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 62. | Esmaeili M, Nasr-Esfahani MH, Shoaraye Nejati A, Safaeinejad Z, Atefi A, L Megraw T, Ghaedi K. PPARgamma dependent PEX11beta counteracts the suppressive role of SIRT1 on neural differentiation of HESCs. PLoS One. 2024;19:e0298274. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 63. | Zhao WJ, Liu X, Hu M, Zhang Y, Shi PZ, Wang JW, Lu XH, Cheng XF, Tao YP, Feng XM, Wang YX, Zhang L. Quercetin ameliorates oxidative stress-induced senescence in rat nucleus pulposus-derived mesenchymal stem cells via the miR-34a-5p/SIRT1 axis. World J Stem Cells. 2023;15:842-865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 64. | Zhou J, Chen H, Wang Q, Chen S, Wang R, Wang Z, Yang C, Chen A, Zhao J, Zhou Z, Mao Z, Zuo G, Miao D, Jin J. Sirt1 overexpression improves senescence-associated pulmonary fibrosis induced by vitamin D deficiency through downregulating IL-11 transcription. Aging Cell. 2022;21:e13680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 65. | Okada M, Kim HW, Matsu-ura K, Wang YG, Xu M, Ashraf M. Abrogation of Age-Induced MicroRNA-195 Rejuvenates the Senescent Mesenchymal Stem Cells by Reactivating Telomerase. Stem Cells. 2016;34:148-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 66. | Liu X, Chen H, Zhu W, Chen H, Hu X, Jiang Z, Xu Y, Zhou Y, Wang K, Wang L, Chen P, Hu H, Wang C, Zhang N, Ma Q, Huang M, Hu D, Zhang L, Wu R, Wang Y, Xu Q, Yu H, Wang J. Transplantation of SIRT1-engineered aged mesenchymal stem cells improves cardiac function in a rat myocardial infarction model. J Heart Lung Transplant. 2014;33:1083-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Begum MK, Konja D, Singh S, Chlopicki S, Wang Y. Endothelial SIRT1 as a Target for the Prevention of Arterial Aging: Promises and Challenges. J Cardiovasc Pharmacol. 2021;78:S63-S77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 68. | Zhang H, Yang X, Pang X, Zhao Z, Yu H, Zhou H. Genistein protects against ox-LDL-induced senescence through enhancing SIRT1/LKB1/AMPK-mediated autophagy flux in HUVECs. Mol Cell Biochem. 2019;455:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 69. | Saboori S, Koohdani F, Nematipour E, Yousefi Rad E, Saboor-Yaraghi AA, Javanbakht MH, Eshraghian MR, Ramezani A, Djalali M. Beneficial effects of omega-3 and vitamin E coadministration on gene expression of SIRT1 and PGC1α and serum antioxidant enzymes in patients with coronary artery disease. Nutr Metab Cardiovasc Dis. 2016;26:489-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 70. | Yuan L, Wang D, Wu C. Protective effect of liquiritin on coronary heart disease through regulating the proliferation of human vascular smooth muscle cells via upregulation of sirtuin1. Bioengineered. 2022;13:2840-2850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 71. | Wasserfurth P, Nebl J, Rühling MR, Shammas H, Bednarczyk J, Koehler K, Boßlau TK, Krüger K, Hahn A, Das AM. Impact of Dietary Modifications on Plasma Sirtuins 1, 3 and 5 in Older Overweight Individuals Undergoing 12-Weeks of Circuit Training. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 72. | Barcena ML, Tonini G, Haritonow N, Breiter P, Milting H, Baczko I, Müller-Werdan U, Ladilov Y, Regitz-Zagrosek V. Sex and age differences in AMPK phosphorylation, mitochondrial homeostasis, and inflammation in hearts from inflammatory cardiomyopathy patients. Aging Cell. 2023;22:e13894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 73. | Yu L, Liang H, Dong X, Zhao G, Jin Z, Zhai M, Yang Y, Chen W, Liu J, Yi W, Yang J, Yi D, Duan W, Yu S. Reduced silent information regulator 1 signaling exacerbates myocardial ischemia-reperfusion injury in type 2 diabetic rats and the protective effect of melatonin. J Pineal Res. 2015;59:376-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 74. | Planavila A, Iglesias R, Giralt M, Villarroya F. Sirt1 acts in association with PPARα to protect the heart from hypertrophy, metabolic dysregulation, and inflammation. Cardiovasc Res. 2011;90:276-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 216] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 75. | Kostić M, Korićanac G, Tepavčević S, Stanišić J, Romić S, Ćulafić T, Ivković T, Stojiljković M. Low-Intensity Exercise Affects Cardiac Fatty Acid Oxidation by Increasing the Nuclear Content of PPARα, FOXO1, and Lipin1 in Fructose-Fed Rats. Metab Syndr Relat Disord. 2023;21:122-131. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 76. | Younis RL, El-Gohary RM, Ghalwash AA, Hegab II, Ghabrial MM, Aboshanady AM, Mostafa RA, El-Azeem AHA, Farghal EE, Belal AAE, Khattab H. Luteolin Mitigates D-Galactose-Induced Brain Ageing in Rats: SIRT1-Mediated Neuroprotection. Neurochem Res. 2024;49:2803-2820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 77. | Desai SC, Macrin AD, Senthilvelan T, Panda RC. Identification of genes associated with accelerated biological ageing through computational analysis: a systematic review. Biotechnol Bioproc E. 2024;29:636-649. [DOI] [Full Text] |
| 78. | Maiese K. Prospects and Perspectives for WISP1 (CCN4) in Diabetes Mellitus. Curr Neurovasc Res. 2020;17:327-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 79. | Klionsky DJ, Abdel-Aziz AK, Abdelfatah S, Abdellatif M, Abdoli A, Abel S, Abeliovich H, Abildgaard MH, Abudu YP, Acevedo-Arozena A, Adamopoulos IE, Adeli K, Adolph TE, Adornetto A, Aflaki E, Agam G, Agarwal A, Aggarwal BB, Agnello M, Agostinis P, Agrewala JN, Agrotis A, Aguilar PV, Ahmad ST, Ahmed ZM, Ahumada-Castro U, Aits S, Aizawa S, Akkoc Y, Akoumianaki T, Akpinar HA, Al-Abd AM, Al-Akra L, Al-Gharaibeh A, Alaoui-Jamali MA, Alberti S, Alcocer-Gómez E, Alessandri C, Ali M, Alim Al-Bari MA, Aliwaini S, Alizadeh J, Almacellas E, Almasan A, Alonso A, Alonso GD, Altan-Bonnet N, Altieri DC, Álvarez ÉMC, Alves S, Alves da Costa C, Alzaharna MM, Amadio M, Amantini C, Amaral C, Ambrosio S, Amer AO, Ammanathan V, An Z, Andersen SU, Andrabi SA, Andrade-Silva M, Andres AM, Angelini S, Ann D, Anozie UC, Ansari MY, Antas P, Antebi A, Antón Z, Anwar T, Apetoh L, Apostolova N, Araki T, Araki Y, Arasaki K, Araújo WL, Araya J, Arden C, Arévalo MA, Arguelles S, Arias E, Arikkath J, Arimoto H, Ariosa AR, Armstrong-James D, Arnauné-Pelloquin L, Aroca A, Arroyo DS, Arsov I, Artero R, Asaro DML, Aschner M, Ashrafizadeh M, Ashur-Fabian O, Atanasov AG, Au AK, Auberger P, Auner HW, Aurelian L, Autelli R, Avagliano L, Ávalos Y, Aveic S, Aveleira CA, Avin-Wittenberg T, Aydin Y, Ayton S, Ayyadevara S, Azzopardi M, Baba M, Backer JM, Backues SK, Bae DH, Bae ON, Bae SH, Baehrecke EH, Baek A, Baek SH, Baek SH, Bagetta G, Bagniewska-Zadworna A, Bai H, Bai J, Bai X, Bai Y, Bairagi N, Baksi S, Balbi T, Baldari CT, Balduini W, Ballabio A, Ballester M, Balazadeh S, Balzan R, Bandopadhyay R, Banerjee S, Banerjee S, Bánréti Á, Bao Y, Baptista MS, Baracca A, Barbati C, Bargiela A, Barilà D, Barlow PG, Barmada SJ, Barreiro E, Barreto GE, Bartek J, Bartel B, Bartolome A, Barve GR, Basagoudanavar SH, Bassham DC, Bast RC Jr, Basu A, Batoko H, Batten I, Baulieu EE, Baumgarner BL, Bayry J, Beale R, Beau I, Beaumatin F, Bechara LRG, Beck GR Jr, Beers MF, Begun J, Behrends C, Behrens GMN, Bei R, Bejarano E, Bel S, Behl C, Belaid A, Belgareh-Touzé N, Bellarosa C, Belleudi F, Belló Pérez M, Bello-Morales R, Beltran JSO, Beltran S, Benbrook DM, Bendorius M, Benitez BA, Benito-Cuesta I, Bensalem J, Berchtold MW, Berezowska S, Bergamaschi D, Bergami M, Bergmann A, Berliocchi L, Berlioz-Torrent C, Bernard A, Berthoux L, Besirli CG, Besteiro S, Betin VM, Beyaert R, Bezbradica JS, Bhaskar K, Bhatia-Kissova I, Bhattacharya R, Bhattacharya S, Bhattacharyya S, Bhuiyan MS, Bhutia SK, Bi L, Bi X, Biden TJ, Bijian K, Billes VA, Binart N, Bincoletto C, Birgisdottir AB, Bjorkoy G, Blanco G, Blas-Garcia A, Blasiak J, Blomgran R, Blomgren K, Blum JS, Boada-Romero E, Boban M, Boesze-Battaglia K, Boeuf P, Boland B, Bomont P, Bonaldo P, Bonam SR, Bonfili L, Bonifacino JS, Boone BA, Bootman MD, Bordi M, Borner C, Bornhauser BC, Borthakur G, Bosch J, Bose S, Botana LM, Botas J, Boulanger CM, Boulton ME, Bourdenx M, Bourgeois B, Bourke NM, Bousquet G, Boya P, Bozhkov PV, Bozi LHM, Bozkurt TO, Brackney DE, Brandts CH, Braun RJ, Braus GH, Bravo-Sagua R, Bravo-San Pedro JM, Brest P, Bringer MA, Briones-Herrera A, Broaddus VC, Brodersen P, Brodsky JL, Brody SL, Bronson PG, Bronstein JM, Brown CN, Brown RE, Brum PC, Brumell JH, Brunetti-Pierri N, Bruno D, Bryson-Richardson RJ, Bucci C, Buchrieser C, Bueno M, Buitrago-Molina LE, Buraschi S, Buch S, Buchan JR, Buckingham EM, Budak H, Budini M, Bultynck G, Burada F, Burgoyne JR, Burón MI, Bustos V, Büttner S, Butturini E, Byrd A, Cabas I, Cabrera-Benitez S, Cadwell K, Cai J, Cai L, Cai Q, Cairó M, Calbet JA, Caldwell GA, Caldwell KA, Call JA, Calvani R, Calvo AC, Calvo-Rubio Barrera M, Camara NO, Camonis JH, Camougrand N, Campanella M, Campbell EM, Campbell-Valois FX, Campello S, Campesi I, Campos JC, Camuzard O, Cancino J, Candido de Almeida D, Canesi L, Caniggia I, Canonico B, Cantí C, Cao B, Caraglia M, Caramés B, Carchman EH, Cardenal-Muñoz E, Cardenas C, Cardenas L, Cardoso SM, Carew JS, Carle GF, Carleton G, Carloni S, Carmona-Gutierrez D, Carneiro LA, Carnevali O, Carosi JM, Carra S, Carrier A, Carrier L, Carroll B, Carter AB, Carvalho AN, Casanova M, Casas C, Casas J, Cassioli C, Castillo EF, Castillo K, Castillo-Lluva S, Castoldi F, Castori M, Castro AF, Castro-Caldas M, Castro-Hernandez J, Castro-Obregon S, Catz SD, Cavadas C, Cavaliere F, Cavallini G, Cavinato M, Cayuela ML, Cebollada Rica P, Cecarini V, Cecconi F, Cechowska-Pasko M, Cenci S, Ceperuelo-Mallafré V, Cerqueira JJ, Cerutti JM, Cervia D, Cetintas VB, Cetrullo S, Chae HJ, Chagin AS, Chai CY, Chakrabarti G, Chakrabarti O, Chakraborty T, Chakraborty T, Chami M, Chamilos G, Chan DW, Chan EYW, Chan ED, Chan HYE, Chan HH, Chan H, Chan MTV, Chan YS, Chandra PK, Chang CP, Chang C, Chang HC, Chang K, Chao J, Chapman T, Charlet-Berguerand N, Chatterjee S, Chaube SK, Chaudhary A, Chauhan S, Chaum E, Checler F, Cheetham ME, Chen CS, Chen GC, Chen JF, Chen LL, Chen L, Chen L, Chen M, Chen MK, Chen N, Chen Q, Chen RH, Chen S, Chen W, Chen W, Chen XM, Chen XW, Chen X, Chen Y, Chen YG, Chen Y, Chen Y, Chen YJ, Chen YQ, Chen ZS, Chen Z, Chen ZH, Chen ZJ, Chen Z, Cheng H, Cheng J, Cheng SY, Cheng W, Cheng X, Cheng XT, Cheng Y, Cheng Z, Chen Z, Cheong H, Cheong JK, Chernyak BV, Cherry S, Cheung CFR, Cheung CHA, Cheung KH, Chevet E, Chi RJ, Chiang AKS, Chiaradonna F, Chiarelli R, Chiariello M, Chica N, Chiocca S, Chiong M, Chiou SH, Chiramel AI, Chiurchiù V, Cho DH, Choe SK, Choi AMK, Choi ME, Choudhury KR, Chow NS, Chu CT, Chua JP, Chua JJE, Chung H, Chung KP, Chung S, Chung SH, Chung YL, Cianfanelli V, Ciechomska IA, Cifuentes M, Cinque L, Cirak S, Cirone M, Clague MJ, Clarke R, Clementi E, Coccia EM, Codogno P, Cohen E, Cohen MM, Colasanti T, Colasuonno F, Colbert RA, Colell A, Čolić M, Coll NS, Collins MO, Colombo MI, Colón-Ramos DA, Combaret L, Comincini S, Cominetti MR, Consiglio A, Conte A, Conti F, Contu VR, Cookson MR, Coombs KM, Coppens I, Corasaniti MT, Corkery DP, Cordes N, Cortese K, Costa MDC, Costantino S, Costelli P, Coto-Montes A, Crack PJ, Crespo JL, Criollo A, Crippa V, Cristofani R, Csizmadia T, Cuadrado A, Cui B, Cui J, Cui Y, Cui Y, Culetto E, Cumino AC, Cybulsky AV, Czaja MJ, Czuczwar SJ, D'Adamo S, D'Amelio M, D'Arcangelo D, D'Lugos AC, D'Orazi G, da Silva JA, Dafsari HS, Dagda RK, Dagdas Y, Daglia M, Dai X, Dai Y, Dai Y, Dal Col J, Dalhaimer P, Dalla Valle L, Dallenga T, Dalmasso G, Damme M, Dando I, Dantuma NP, Darling AL, Das H, Dasarathy S, Dasari SK, Dash S, Daumke O, Dauphinee AN, Davies JS, Dávila VA, Davis RJ, Davis T, Dayalan Naidu S, De Amicis F, De Bosscher K, De Felice F, De Franceschi L, De Leonibus C, de Mattos Barbosa MG, De Meyer GRY, De Milito A, De Nunzio C, De Palma C, De Santi M, De Virgilio C, De Zio D, Debnath J, DeBosch BJ, Decuypere JP, Deehan MA, Deflorian G, DeGregori J, Dehay B, Del Rio G, Delaney JR, Delbridge LMD, Delorme-Axford E, Delpino MV, Demarchi F, Dembitz V, Demers ND, Deng H, Deng Z, Dengjel J, Dent P, Denton D, DePamphilis ML, Der CJ, Deretic V, Descoteaux A, Devis L, Devkota S, Devuyst O, Dewson G, Dharmasivam M, Dhiman R, di Bernardo D, Di Cristina M, Di Domenico F, Di Fazio P, Di Fonzo A, Di Guardo G, Di Guglielmo GM, Di Leo L, Di Malta C, Di Nardo A, Di Rienzo M, Di Sano F, Diallinas G, Diao J, Diaz-Araya G, Díaz-Laviada I, Dickinson JM, Diederich M, Dieudé M, Dikic I, Ding S, Ding WX, Dini L, Dinić J, Dinic M, Dinkova-Kostova AT, Dionne MS, Distler JHW, Diwan A, Dixon IMC, Djavaheri-Mergny M, Dobrinski I, Dobrovinskaya O, Dobrowolski R, Dobson RCJ, Đokić J, Dokmeci Emre S, Donadelli M, Dong B, Dong X, Dong Z, Dorn Ii GW, Dotsch V, Dou H, Dou J, Dowaidar M, Dridi S, Drucker L, Du A, Du C, Du G, Du HN, Du LL, du Toit A, Duan SB, Duan X, Duarte SP, Dubrovska A, Dunlop EA, Dupont N, Durán RV, Dwarakanath BS, Dyshlovoy SA, Ebrahimi-Fakhari D, Eckhart L, Edelstein CL, Efferth T, Eftekharpour E, Eichinger L, Eid N, Eisenberg T, Eissa NT, Eissa S, Ejarque M, El Andaloussi A, El-Hage N, El-Naggar S, Eleuteri AM, El-Shafey ES, Elgendy M, Eliopoulos AG, Elizalde MM, Elks PM, Elsasser HP, Elsherbiny ES, Emerling BM, Emre NCT, Eng CH, Engedal N, Engelbrecht AM, Engelsen AST, Enserink JM, Escalante R, Esclatine A, Escobar-Henriques M, Eskelinen EL, Espert L, Eusebio MO, Fabrias G, Fabrizi C, Facchiano A, Facchiano F, Fadeel B, Fader C, Faesen AC, Fairlie WD, Falcó A, Falkenburger BH, Fan D, Fan J, Fan Y, Fang EF, Fang Y, Fang Y, Fanto M, Farfel-Becker T, Faure M, Fazeli G, Fedele AO, Feldman AM, Feng D, Feng J, Feng L, Feng Y, Feng Y, Feng W, Fenz Araujo T, Ferguson TA, Fernández ÁF, Fernandez-Checa JC, Fernández-Veledo S, Fernie AR, Ferrante AW Jr, Ferraresi A, Ferrari MF, Ferreira JCB, Ferro-Novick S, Figueras A, Filadi R, Filigheddu N, Filippi-Chiela E, Filomeni G, Fimia GM, Fineschi V, Finetti F, Finkbeiner S, Fisher EA, Fisher PB, Flamigni F, Fliesler SJ, Flo TH, Florance I, Florey O, Florio T, Fodor E, Follo C, Fon EA, Forlino A, Fornai F, Fortini P, Fracassi A, Fraldi A, Franco B, Franco R, Franconi F, Frankel LB, Friedman SL, Fröhlich LF, Frühbeck G, Fuentes JM, Fujiki Y, Fujita N, Fujiwara Y, Fukuda M, Fulda S, Furic L, Furuya N, Fusco C, Gack MU, Gaffke L, Galadari S, Galasso A, Galindo MF, Gallolu Kankanamalage S, Galluzzi L, Galy V, Gammoh N, Gan B, Ganley IG, Gao F, Gao H, Gao M, Gao P, Gao SJ, Gao W, Gao X, Garcera A, Garcia MN, Garcia VE, García-Del Portillo F, Garcia-Escudero V, Garcia-Garcia A, Garcia-Macia M, García-Moreno D, Garcia-Ruiz C, García-Sanz P, Garg AD, Gargini R, Garofalo T, Garry RF, Gassen NC, Gatica D, Ge L, Ge W, Geiss-Friedlander R, Gelfi C, Genschik P, Gentle IE, Gerbino V, Gerhardt C, Germain K, Germain M, Gewirtz DA, Ghasemipour Afshar E, Ghavami S, Ghigo A, Ghosh M, Giamas G, Giampietri C, Giatromanolaki A, Gibson GE, Gibson SB, Ginet V, Giniger E, Giorgi C, Girao H, Girardin SE, Giridharan M, Giuliano S, Giulivi C, Giuriato S, Giustiniani J, Gluschko A, Goder V, Goginashvili A, Golab J, Goldstone DC, Golebiewska A, Gomes LR, Gomez R, Gómez-Sánchez R, Gomez-Puerto MC, Gomez-Sintes R, Gong Q, Goni FM, González-Gallego J, Gonzalez-Hernandez T, Gonzalez-Polo RA, Gonzalez-Reyes JA, González-Rodríguez P, Goping IS, Gorbatyuk MS, Gorbunov NV, Görgülü K, Gorojod RM, Gorski SM, Goruppi S, Gotor C, Gottlieb RA, Gozes I, Gozuacik D, Graef M, Gräler MH, Granatiero V, Grasso D, Gray JP, Green DR, Greenhough A, Gregory SL, Griffin EF, Grinstaff MW, Gros F, Grose C, Gross AS, Gruber F, Grumati P, Grune T, Gu X, Guan JL, Guardia CM, Guda K, Guerra F, Guerri C, Guha P, Guillén C, Gujar S, Gukovskaya A, Gukovsky I, Gunst J, Günther A, Guntur AR, Guo C, Guo C, Guo H, Guo LW, Guo M, Gupta P, Gupta SK, Gupta S, Gupta VB, Gupta V, Gustafsson AB, Gutterman DD, H B R, Haapasalo A, Haber JE, Hać A, Hadano S, Hafrén AJ, Haidar M, Hall BS, Halldén G, Hamacher-Brady A, Hamann A, Hamasaki M, Han W, Hansen M, Hanson PI, Hao Z, Harada M, Harhaji-Trajkovic L, Hariharan N, Haroon N, Harris J, Hasegawa T, Hasima Nagoor N, Haspel JA, Haucke V, Hawkins WD, Hay BA, Haynes CM, Hayrabedyan SB, Hays TS, He C, He Q, He RR, He YW, He YY, Heakal Y, Heberle AM, Hejtmancik JF, Helgason GV, Henkel V, Herb M, Hergovich A, Herman-Antosiewicz A, Hernández A, Hernandez C, Hernandez-Diaz S, Hernandez-Gea V, Herpin A, Herreros J, Hervás JH, Hesselson D, Hetz C, Heussler VT, Higuchi Y, Hilfiker S, Hill JA, Hlavacek WS, Ho EA, Ho IHT, Ho PW, Ho SL, Ho WY, Hobbs GA, Hochstrasser M, Hoet PHM, Hofius D, Hofman P, Höhn A, Holmberg CI, Hombrebueno JR, Yi-Ren Hong CH, Hooper LV, Hoppe T, Horos R, Hoshida Y, Hsin IL, Hsu HY, Hu B, Hu D, Hu LF, Hu MC, Hu R, Hu W, Hu YC, Hu ZW, Hua F, Hua J, Hua Y, Huan C, Huang C, Huang C, Huang C, Huang C, Huang H, Huang K, Huang MLH, Huang R, Huang S, Huang T, Huang X, Huang YJ, Huber TB, Hubert V, Hubner CA, Hughes SM, Hughes WE, Humbert M, Hummer G, Hurley JH, Hussain S, Hussain S, Hussey PJ, Hutabarat M, Hwang HY, Hwang S, Ieni A, Ikeda F, Imagawa Y, Imai Y, Imbriano C, Imoto M, Inman DM, Inoki K, Iovanna J, Iozzo RV, Ippolito G, Irazoqui JE, Iribarren P, Ishaq M, Ishikawa M, Ishimwe N, Isidoro C, Ismail N, Issazadeh-Navikas S, Itakura E, Ito D, Ivankovic D, Ivanova S, Iyer AKV, Izquierdo JM, Izumi M, Jäättelä M, Jabir MS, Jackson WT, Jacobo-Herrera N, Jacomin AC, Jacquin E, Jadiya P, Jaeschke H, Jagannath C, Jakobi AJ, Jakobsson J, Janji B, Jansen-Dürr P, Jansson PJ, Jantsch J, Januszewski S, Jassey A, Jean S, Jeltsch-David H, Jendelova P, Jenny A, Jensen TE, Jessen N, Jewell JL, Ji J, Jia L, Jia R, Jiang L, Jiang Q, Jiang R, Jiang T, Jiang X, Jiang Y, Jimenez-Sanchez M, Jin EJ, Jin F, Jin H, Jin L, Jin L, Jin M, Jin S, Jo EK, Joffre C, Johansen T, Johnson GVW, Johnston SA, Jokitalo E, Jolly MK, Joosten LAB, Jordan J, Joseph B, Ju D, Ju JS, Ju J, Juárez E, Judith D, Juhász G, Jun Y, Jung CH, Jung SC, Jung YK, Jungbluth H, Jungverdorben J, Just S, Kaarniranta K, Kaasik A, Kabuta T, Kaganovich D, Kahana A, Kain R, Kajimura S, Kalamvoki M, Kalia M, Kalinowski DS, Kaludercic N, Kalvari I, Kaminska J, Kaminskyy VO, Kanamori H, Kanasaki K, Kang C, Kang R, Kang SS, Kaniyappan S, Kanki T, Kanneganti TD, Kanthasamy AG, Kanthasamy A, Kantorow M, Kapuy O, Karamouzis MV, Karim MR, Karmakar P, Katare RG, Kato M, Kaufmann SHE, Kauppinen A, Kaushal GP, Kaushik S, Kawasaki K, Kazan K, Ke PY, Keating DJ, Keber U, Kehrl JH, Keller KE, Keller CW, Kemper JK, Kenific CM, Kepp O, Kermorgant S, Kern A, Ketteler R, Keulers TG, Khalfin B, Khalil H, Khambu B, Khan SY, Khandelwal VKM, Khandia R, Kho W, Khobrekar NV, Khuansuwan S, Khundadze M, Killackey SA, Kim D, Kim DR, Kim DH, Kim DE, Kim EY, Kim EK, Kim HR, Kim HS, Hyung-Ryong Kim, Kim JH, Kim JK, Kim JH, Kim J, Kim JH, Kim KI, Kim PK, Kim SJ, Kimball SR, Kimchi A, Kimmelman AC, Kimura T, King MA, Kinghorn KJ, Kinsey CG, Kirkin V, Kirshenbaum LA, Kiselev SL, Kishi S, Kitamoto K, Kitaoka Y, Kitazato K, Kitsis RN, Kittler JT, Kjaerulff O, Klein PS, Klopstock T, Klucken J, Knævelsrud H, Knorr RL, Ko BCB, Ko F, Ko JL, Kobayashi H, Kobayashi S, Koch I, Koch JC, Koenig U, Kögel D, Koh YH, Koike M, Kohlwein SD, Kocaturk NM, Komatsu M, König J, Kono T, Kopp BT, Korcsmaros T, Korkmaz G, Korolchuk VI, Korsnes MS, Koskela A, Kota J, Kotake Y, Kotler ML, Kou Y, Koukourakis MI, Koustas E, Kovacs AL, Kovács T, Koya D, Kozako T, Kraft C, Krainc D, Krämer H, Krasnodembskaya AD, Kretz-Remy C, Kroemer G, Ktistakis NT, Kuchitsu K, Kuenen S, Kuerschner L, Kukar T, Kumar A, Kumar A, Kumar D, Kumar D, Kumar S, Kume S, Kumsta C, Kundu CN, Kundu M, Kunnumakkara AB, Kurgan L, Kutateladze TG, Kutlu O, Kwak S, Kwon HJ, Kwon TK, Kwon YT, Kyrmizi I, La Spada A, Labonté P, Ladoire S, Laface I, Lafont F, Lagace DC, Lahiri V, Lai Z, Laird AS, Lakkaraju A, Lamark T, Lan SH, Landajuela A, Lane DJR, Lane JD, Lang CH, Lange C, Langel Ü, Langer R, Lapaquette P, Laporte J, LaRusso NF, Lastres-Becker I, Lau WCY, Laurie GW, Lavandero S, Law BYK, Law HK, Layfield R, Le W, Le Stunff H, Leary AY, Lebrun JJ, Leck LYW, Leduc-Gaudet JP, Lee C, Lee CP, Lee DH, Lee EB, Lee EF, Lee GM, Lee HJ, Lee HK, Lee JM, Lee JS, Lee JA, Lee JY, Lee JH, Lee M, Lee MG, Lee MJ, Lee MS, Lee SY, Lee SJ, Lee SY, Lee SB, Lee WH, Lee YR, Lee YH, Lee Y, Lefebvre C, Legouis R, Lei YL, Lei Y, Leikin S, Leitinger G, Lemus L, Leng S, Lenoir O, Lenz G, Lenz HJ, Lenzi P, León Y, Leopoldino AM, Leschczyk C, Leskelä S, Letellier E, Leung CT, Leung PS, Leventhal JS, Levine B, Lewis PA, Ley K, Li B, Li DQ, Li J, Li J, Li J, Li K, Li L, Li M, Li M, Li M, Li M, Li M, Li PL, Li MQ, Li Q, Li S, Li T, Li W, Li W, Li X, Li YP, Li Y, Li Z, Li Z, Li Z, Lian J, Liang C, Liang Q, Liang W, Liang Y, Liang Y, Liao G, Liao L, Liao M, Liao YF, Librizzi M, Lie PPY, Lilly MA, Lim HJ, Lima TRR, Limana F, Lin C, Lin CW, Lin DS, Lin FC, Lin JD, Lin KM, Lin KH, Lin LT, Lin PH, Lin Q, Lin S, Lin SJ, Lin W, Lin X, Lin YX, Lin YS, Linden R, Lindner P, Ling SC, Lingor P, Linnemann AK, Liou YC, Lipinski MM, Lipovšek S, Lira VA, Lisiak N, Liton PB, Liu C, Liu CH, Liu CF, Liu CH, Liu F, Liu H, Liu HS, Liu HF, Liu H, Liu J, Liu J, Liu J, Liu L, Liu L, Liu M, Liu Q, Liu W, Liu W, Liu XH, Liu X, Liu X, Liu X, Liu X, Liu Y, Liu Y, Liu Y, Liu Y, Liu Y, Livingston JA, Lizard G, Lizcano JM, Ljubojevic-Holzer S, LLeonart ME, Llobet-Navàs D, Llorente A, Lo CH, Lobato-Márquez D, Long Q, Long YC, Loos B, Loos JA, López MG, López-Doménech G, López-Guerrero JA, López-Jiménez AT, López-Pérez Ó, López-Valero I, Lorenowicz MJ, Lorente M, Lorincz P, Lossi L, Lotersztajn S, Lovat PE, Lovell JF, Lovy A, Lőw P, Lu G, Lu H, Lu JH, Lu JJ, Lu M, Lu S, Luciani A, Lucocq JM, Ludovico P, Luftig MA, Luhr M, Luis-Ravelo D, Lum JJ, Luna-Dulcey L, Lund AH, Lund VK, Lünemann JD, Lüningschrör P, Luo H, Luo R, Luo S, Luo Z, Luparello C, Lüscher B, Luu L, Lyakhovich A, Lyamzaev KG, Lystad AH, Lytvynchuk L, Ma AC, Ma C, Ma M, Ma NF, Ma QH, Ma X, Ma Y, Ma Z, MacDougald OA, Macian F, MacIntosh GC, MacKeigan JP, Macleod KF, Maday S, Madeo F, Madesh M, Madl T, Madrigal-Matute J, Maeda A, Maejima Y, Magarinos M, Mahavadi P, Maiani E, Maiese K, Maiti P, Maiuri MC, Majello B, Major MB, Makareeva E, Malik F, Mallilankaraman K, Malorni W, Maloyan A, Mammadova N, Man GCW, Manai F, Mancias JD, Mandelkow EM, Mandell MA, Manfredi AA, Manjili MH, Manjithaya R, Manque P, Manshian BB, Manzano R, Manzoni C, Mao K, Marchese C, Marchetti S, Marconi AM, Marcucci F, Mardente S, Mareninova OA, Margeta M, Mari M, Marinelli S, Marinelli O, Mariño G, Mariotto S, Marshall RS, Marten MR, Martens S, Martin APJ, Martin KR, Martin S, Martin S, Martín-Segura A, Martín-Acebes MA, Martin-Burriel I, Martin-Rincon M, Martin-Sanz P, Martina JA, Martinet W, Martinez A, Martinez A, Martinez J, Martinez Velazquez M, Martinez-Lopez N, Martinez-Vicente M, Martins DO, Martins JO, Martins WK, Martins-Marques T, Marzetti E, Masaldan S, Masclaux-Daubresse C, Mashek DG, Massa V, Massieu L, Masson GR, Masuelli L, Masyuk AI, Masyuk TV, Matarrese P, Matheu A, Matoba S, Matsuzaki S, Mattar P, Matte A, Mattoscio D, Mauriz JL, Mauthe M, Mauvezin C, Maverakis E, Maycotte P, Mayer J, Mazzoccoli G, Mazzoni C, Mazzulli JR, McCarty N, McDonald C, McGill MR, McKenna SL, McLaughlin B, McLoughlin F, McNiven MA, McWilliams TG, Mechta-Grigoriou F, Medeiros TC, Medina DL, Megeney LA, Megyeri K, Mehrpour M, Mehta JL, Meijer AJ, Meijer AH, Mejlvang J, Meléndez A, Melk A, Memisoglu G, Mendes AF, Meng D, Meng F, Meng T, Menna-Barreto R, Menon MB, Mercer C, Mercier AE, Mergny JL, Merighi A, Merkley SD, Merla G, Meske V, Mestre AC, Metur SP, Meyer C, Meyer H, Mi W, Mialet-Perez J, Miao J, Micale L, Miki Y, Milan E, Milczarek M, Miller DL, Miller SI, Miller S, Millward SW, Milosevic I, Minina EA, Mirzaei H, Mirzaei HR, Mirzaei M, Mishra A, Mishra N, Mishra PK, Misirkic Marjanovic M, Misasi R, Misra A, Misso G, Mitchell C, Mitou G, Miura T, Miyamoto S, Miyazaki M, Miyazaki M, Miyazaki T, Miyazawa K, Mizushima N, Mogensen TH, Mograbi B, Mohammadinejad R, Mohamud Y, Mohanty A, Mohapatra S, Möhlmann T, Mohmmed A, Moles A, Moley KH, Molinari M, Mollace V, Møller AB, Mollereau B, Mollinedo F, Montagna C, Monteiro MJ, Montella A, Montes LR, Montico B, Mony VK, Monzio Compagnoni G, Moore MN, Moosavi MA, Mora AL, Mora M, Morales-Alamo D, Moratalla R, Moreira PI, Morelli E, Moreno S, Moreno-Blas D, Moresi V, Morga B, Morgan AH, Morin F, Morishita H, Moritz OL, Moriyama M, Moriyasu Y, Morleo M, Morselli E, Moruno-Manchon JF, Moscat J, Mostowy S, Motori E, Moura AF, Moustaid-Moussa N, Mrakovcic M, Muciño-Hernández G, Mukherjee A, Mukhopadhyay S, Mulcahy Levy JM, Mulero V, Muller S, Münch C, Munjal A, Munoz-Canoves P, Muñoz-Galdeano T, Münz C, Murakawa T, Muratori C, Murphy BM, Murphy JP, Murthy A, Myöhänen TT, Mysorekar IU, Mytych J, Nabavi SM, Nabissi M, Nagy P, Nah J, Nahimana A, Nakagawa I, Nakamura K, Nakatogawa H, Nandi SS, Nanjundan M, Nanni M, Napolitano G, Nardacci R, Narita M, Nassif M, Nathan I, Natsumeda M, Naude RJ, Naumann C, Naveiras O, Navid F, Nawrocki ST, Nazarko TY, Nazio F, Negoita F, Neill T, Neisch AL, Neri LM, Netea MG, Neubert P, Neufeld TP, Neumann D, Neutzner A, Newton PT, Ney PA, Nezis IP, Ng CCW, Ng TB, Nguyen HTT, Nguyen LT, Ni HM, Ní Cheallaigh C, Ni Z, Nicolao MC, Nicoli F, Nieto-Diaz M, Nilsson P, Ning S, Niranjan R, Nishimune H, Niso-Santano M, Nixon RA, Nobili A, Nobrega C, Noda T, Nogueira-Recalde U, Nolan TM, Nombela I, Novak I, Novoa B, Nozawa T, Nukina N, Nussbaum-Krammer C, Nylandsted J, O'Donovan TR, O'Leary SM, O'Rourke EJ, O'Sullivan MP, O'Sullivan TE, Oddo S, Oehme I, Ogawa M, Ogier-Denis E, Ogmundsdottir MH, Ogretmen B, Oh GT, Oh SH, Oh YJ, Ohama T, Ohashi Y, Ohmuraya M, Oikonomou V, Ojha R, Okamoto K, Okazawa H, Oku M, Oliván S, Oliveira JMA, Ollmann M, Olzmann JA, Omari S, Omary MB, Önal G, Ondrej M, Ong SB, Ong SG, Onnis A, Orellana JA, Orellana-Muñoz S, Ortega-Villaizan MDM, Ortiz-Gonzalez XR, Ortona E, Osiewacz HD, Osman AK, Osta R, Otegui MS, Otsu K, Ott C, Ottobrini L, Ou JJ, Outeiro TF, Oynebraten I, Ozturk M, Pagès G, Pahari S, Pajares M, Pajvani UB, Pal R, Paladino S, Pallet N, Palmieri M, Palmisano G, Palumbo C, Pampaloni F, Pan L, Pan Q, Pan W, Pan X, Panasyuk G, Pandey R, Pandey UB, Pandya V, Paneni F, Pang SY, Panzarini E, Papademetrio DL, Papaleo E, Papinski D, Papp D, Park EC, Park HT, Park JM, Park JI, Park JT, Park J, Park SC, Park SY, Parola AH, Parys JB, Pasquier A, Pasquier B, Passos JF, Pastore N, Patel HH, Patschan D, Pattingre S, Pedraza-Alva G, Pedraza-Chaverri J, Pedrozo Z, Pei G, Pei J, Peled-Zehavi H, Pellegrini JM, Pelletier J, Peñalva MA, Peng D, Peng Y, Penna F, Pennuto M, Pentimalli F, Pereira CM, Pereira GJS, Pereira LC, Pereira de Almeida L, Perera ND, Pérez-Lara Á, Perez-Oliva AB, Pérez-Pérez ME, Periyasamy P, Perl A, Perrotta C, Perrotta I, Pestell RG, Petersen M, Petrache I, Petrovski G, Pfirrmann T, Pfister AS, Philips JA, Pi H, Picca A, Pickrell AM, Picot S, Pierantoni GM, Pierdominici M, Pierre P, Pierrefite-Carle V, Pierzynowska K, Pietrocola F, Pietruczuk M, Pignata C, Pimentel-Muiños FX, Pinar M, Pinheiro RO, Pinkas-Kramarski R, Pinton P, Pircs K, Piya S, Pizzo P, Plantinga TS, Platta HW, Plaza-Zabala A, Plomann M, Plotnikov EY, Plun-Favreau H, Pluta R, Pocock R, Pöggeler S, Pohl C, Poirot M, Poletti A, Ponpuak M, Popelka H, Popova B, Porta H, Porte Alcon S, Portilla-Fernandez E, Post M, Potts MB, Poulton J, Powers T, Prahlad V, Prajsnar TK, Praticò D, Prencipe R, Priault M, Proikas-Cezanne T, Promponas VJ, Proud CG, Puertollano R, Puglielli L, Pulinilkunnil T, Puri D, Puri R, Puyal J, Qi X, Qi Y, Qian W, Qiang L, Qiu Y, Quadrilatero J, Quarleri J, Raben N, Rabinowich H, Ragona D, Ragusa MJ, Rahimi N, Rahmati M, Raia V, Raimundo N, Rajasekaran NS, Ramachandra Rao S, Rami A, Ramírez-Pardo I, Ramsden DB, Randow F, Rangarajan PN, Ranieri D, Rao H, Rao L, Rao R, Rathore S, Ratnayaka JA, Ratovitski EA, Ravanan P, Ravegnini G, Ray SK, Razani B, Rebecca V, Reggiori F, Régnier-Vigouroux A, Reichert AS, Reigada D, Reiling JH, Rein T, Reipert S, Rekha RS, Ren H, Ren J, Ren W, Renault T, Renga G, Reue K, Rewitz K, Ribeiro de Andrade Ramos B, Riazuddin SA, Ribeiro-Rodrigues TM, Ricci JE, Ricci R, Riccio V, Richardson DR, Rikihisa Y, Risbud MV, Risueño RM, Ritis K, Rizza S, Rizzuto R, Roberts HC, Roberts LD, Robinson KJ, Roccheri MC, Rocchi S, Rodney GG, Rodrigues T, Rodrigues Silva VR, Rodriguez A, Rodriguez-Barrueco R, Rodriguez-Henche N, Rodriguez-Rocha H, Roelofs J, Rogers RS, Rogov VV, Rojo AI, Rolka K, Romanello V, Romani L, Romano A, Romano PS, Romeo-Guitart D, Romero LC, Romero M, Roney JC, Rongo C, Roperto S, Rosenfeldt MT, Rosenstiel P, Rosenwald AG, Roth KA, Roth L, Roth S, Rouschop KMA, Roussel BD, Roux S, Rovere-Querini P, Roy A, Rozieres A, Ruano D, Rubinsztein DC, Rubtsova MP, Ruckdeschel K, Ruckenstuhl C, Rudolf E, Rudolf R, Ruggieri A, Ruparelia AA, Rusmini P, Russell RR, Russo GL, Russo M, Russo R, Ryabaya OO, Ryan KM, Ryu KY, Sabater-Arcis M, Sachdev U, Sacher M, Sachse C, Sadhu A, Sadoshima J, Safren N, Saftig P, Sagona AP, Sahay G, Sahebkar A, Sahin M, Sahin O, Sahni S, Saito N, Saito S, Saito T, Sakai R, Sakai Y, Sakamaki JI, Saksela K, Salazar G, Salazar-Degracia A, Salekdeh GH, Saluja AK, Sampaio-Marques B, Sanchez MC, Sanchez-Alcazar JA, Sanchez-Vera V, Sancho-Shimizu V, Sanderson JT, Sandri M, Santaguida S, Santambrogio L, Santana MM, Santoni G, Sanz A, Sanz P, Saran S, Sardiello M, Sargeant TJ, Sarin A, Sarkar C, Sarkar S, Sarrias MR, Sarkar S, Sarmah DT, Sarparanta J, Sathyanarayan A, Sathyanarayanan R, Scaglione KM, Scatozza F, Schaefer L, Schafer ZT, Schaible UE, Schapira AHV, Scharl M, Schatzl HM, Schein CH, Scheper W, Scheuring D, Schiaffino MV, Schiappacassi M, Schindl R, Schlattner U, Schmidt O, Schmitt R, Schmidt SD, Schmitz I, Schmukler E, Schneider A, Schneider BE, Schober R, Schoijet AC, Schott MB, Schramm M, Schröder B, Schuh K, Schüller C, Schulze RJ, Schürmanns L, Schwamborn JC, Schwarten M, Scialo F, Sciarretta S, Scott MJ, Scotto KW, Scovassi AI, Scrima A, Scrivo A, Sebastian D, Sebti S, Sedej S, Segatori L, Segev N, Seglen PO, Seiliez I, Seki E, Selleck SB, Sellke FW, Selsby JT, Sendtner M, Senturk S, Seranova E, Sergi C, Serra-Moreno R, Sesaki H, Settembre C, Setty SRG, Sgarbi G, Sha O, Shacka JJ, Shah JA, Shang D, Shao C, Shao F, Sharbati S, Sharkey LM, Sharma D, Sharma G, Sharma K, Sharma P, Sharma S, Shen HM, Shen H, Shen J, Shen M, Shen W, Shen Z, Sheng R, Sheng Z, Sheng ZH, Shi J, Shi X, Shi YH, Shiba-Fukushima K, Shieh JJ, Shimada Y, Shimizu S, Shimozawa M, Shintani T, Shoemaker CJ, Shojaei S, Shoji I, Shravage BV, Shridhar V, Shu CW, Shu HB, Shui K, Shukla AK, Shutt TE, Sica V, Siddiqui A, Sierra A, Sierra-Torre V, Signorelli S, Sil P, Silva BJA, Silva JD, Silva-Pavez E, Silvente-Poirot S, Simmonds RE, Simon AK, Simon HU, Simons M, Singh A, Singh LP, Singh R, Singh SV, Singh SK, Singh SB, Singh S, Singh SP, Sinha D, Sinha RA, Sinha S, Sirko A, Sirohi K, Sivridis EL, Skendros P, Skirycz A, Slaninová I, Smaili SS, Smertenko A, Smith MD, Soenen SJ, Sohn EJ, Sok SPM, Solaini G, Soldati T, Soleimanpour SA, Soler RM, Solovchenko A, Somarelli JA, Sonawane A, Song F, Song HK, Song JX, Song K, Song Z, Soria LR, Sorice M, Soukas AA, Soukup SF, Sousa D, Sousa N, Spagnuolo PA, Spector SA, Srinivas Bharath MM, St Clair D, Stagni V, Staiano L, Stalnecker CA, Stankov MV, Stathopulos PB, Stefan K, Stefan SM, Stefanis L, Steffan JS, Steinkasserer A, Stenmark H, Sterneckert J, Stevens C, Stoka V, Storch S, Stork B, Strappazzon F, Strohecker AM, Stupack DG, Su H, Su LY, Su L, Suarez-Fontes AM, Subauste CS, Subbian S, Subirada PV, Sudhandiran G, Sue CM, Sui X, Summers C, Sun G, Sun J, Sun K, Sun MX, Sun Q, Sun Y, Sun Z, Sunahara KKS, Sundberg E, Susztak K, Sutovsky P, Suzuki H, Sweeney G, Symons JD, Sze SCW, Szewczyk NJ, Tabęcka-Łonczynska A, Tabolacci C, Tacke F, Taegtmeyer H, Tafani M, Tagaya M, Tai H, Tait SWG, Takahashi Y, Takats S, Talwar P, Tam C, Tam SY, Tampellini D, Tamura A, Tan CT, Tan EK, Tan YQ, Tanaka M, Tanaka M, Tang D, Tang J, Tang TS, Tanida I, Tao Z, Taouis M, Tatenhorst L, Tavernarakis N, Taylor A, Taylor GA, Taylor JM, Tchetina E, Tee AR, Tegeder I, Teis D, Teixeira N, Teixeira-Clerc F, Tekirdag KA, Tencomnao T, Tenreiro S, Tepikin AV, Testillano PS, Tettamanti G, Tharaux PL, Thedieck K, Thekkinghat AA, Thellung S, Thinwa JW, Thirumalaikumar VP, Thomas SM, Thomes PG, Thorburn A, Thukral L, Thum T, Thumm M, Tian L, Tichy A, Till A, Timmerman V, Titorenko VI, Todi SV, Todorova K, Toivonen JM, Tomaipitinca L, Tomar D, Tomas-Zapico C, Tomić S, Tong BC, Tong C, Tong X, Tooze SA, Torgersen ML, Torii S, Torres-López L, Torriglia A, Towers CG, Towns R, Toyokuni S, Trajkovic V, Tramontano D, Tran QG, Travassos LH, Trelford CB, Tremel S, Trougakos IP, Tsao BP, Tschan MP, Tse HF, Tse TF, Tsugawa H, Tsvetkov AS, Tumbarello DA, Tumtas Y, Tuñón MJ, Turcotte S, Turk B, Turk V, Turner BJ, Tuxworth RI, Tyler JK, Tyutereva EV, Uchiyama Y, Ugun-Klusek A, Uhlig HH, Ułamek-Kozioł M, Ulasov IV, Umekawa M, Ungermann C, Unno R, Urbe S, Uribe-Carretero E, Üstün S, Uversky VN, Vaccari T, Vaccaro MI, Vahsen BF, Vakifahmetoglu-Norberg H, Valdor R, Valente MJ, Valko A, Vallee RB, Valverde AM, Van den Berghe G, van der Veen S, Van Kaer L, van Loosdregt J, van Wijk SJL, Vandenberghe W, Vanhorebeek I, Vannier-Santos MA, Vannini N, Vanrell MC, Vantaggiato C, Varano G, Varela-Nieto I, Varga M, Vasconcelos MH, Vats S, Vavvas DG, Vega-Naredo I, Vega-Rubin-de-Celis S, Velasco G, Velázquez AP, Vellai T, Vellenga E, Velotti F, Verdier M, Verginis P, Vergne I, Verkade P, Verma M, Verstreken P, Vervliet T, Vervoorts J, Vessoni AT, Victor VM, Vidal M, Vidoni C, Vieira OV, Vierstra RD, Viganó S, Vihinen H, Vijayan V, Vila M, Vilar M, Villalba JM, Villalobo A, Villarejo-Zori B, Villarroya F, Villarroya J, Vincent O, Vindis C, Viret C, Viscomi MT, Visnjic D, Vitale I, Vocadlo DJ, Voitsekhovskaja OV, Volonté C, Volta M, Vomero M, Von Haefen C, Vooijs MA, Voos W, Vucicevic L, Wade-Martins R, Waguri S, Waite KA, Wakatsuki S, Walker DW, Walker MJ, Walker SA, Walter J, Wandosell FG, Wang B, Wang CY, Wang C, Wang C, Wang C, Wang CY, Wang D, Wang F, Wang F, Wang F, Wang G, Wang H, Wang H, Wang H, Wang HG, Wang J, Wang J, Wang J, Wang J, Wang K, Wang L, Wang L, Wang MH, Wang M, Wang N, Wang P, Wang P, Wang P, Wang P, Wang QJ, Wang Q, Wang QK, Wang QA, Wang WT, Wang W, Wang X, Wang X, Wang Y, Wang Y, Wang Y, Wang YY, Wang Y, Wang Y, Wang Y, Wang Y, Wang Z, Wang Z, Wang Z, Warnes G, Warnsmann V, Watada H, Watanabe E, Watchon M, Wawrzyńska A, Weaver TE, Wegrzyn G, Wehman AM, Wei H, Wei L, Wei T, Wei Y, Weiergräber OH, Weihl CC, Weindl G, Weiskirchen R, Wells A, Wen RH, Wen X, Werner A, Weykopf B, Wheatley SP, Whitton JL, Whitworth AJ, Wiktorska K, Wildenberg ME, Wileman T, Wilkinson S, Willbold D, Williams B, Williams RSB, Williams RL, Williamson PR, Wilson RA, Winner B, Winsor NJ, Witkin SS, Wodrich H, Woehlbier U, Wollert T, Wong E, Wong JH, Wong RW, Wong VKW, Wong WW, Wu AG, Wu C, Wu J, Wu J, Wu KK, Wu M, Wu SY, Wu S, Wu SY, Wu S, Wu WKK, Wu X, Wu X, Wu YW, Wu Y, Xavier RJ, Xia H, Xia L, Xia Z, Xiang G, Xiang J, Xiang M, Xiang W, Xiao B, Xiao G, Xiao H, Xiao HT, Xiao J, Xiao L, Xiao S, Xiao Y, Xie B, Xie CM, Xie M, Xie Y, Xie Z, Xie Z, Xilouri M, Xu C, Xu E, Xu H, Xu J, Xu J, Xu L, Xu WW, Xu X, Xue Y, Yakhine-Diop SMS, Yamaguchi M, Yamaguchi O, Yamamoto A, Yamashina S, Yan S, Yan SJ, Yan Z, Yanagi Y, Yang C, Yang DS, Yang H, Yang HT, Yang H, Yang JM, Yang J, Yang J, Yang L, Yang L, Yang M, Yang PM, Yang Q, Yang S, Yang S, Yang SF, Yang W, Yang WY, Yang X, Yang X, Yang Y, Yang Y, Yao H, Yao S, Yao X, Yao YG, Yao YM, Yasui T, Yazdankhah M, Yen PM, Yi C, Yin XM, Yin Y, Yin Z, Yin Z, Ying M, Ying Z, Yip CK, Yiu SPT, Yoo YH, Yoshida K, Yoshii SR, Yoshimori T, Yousefi B, Yu B, Yu H, Yu J, Yu J, Yu L, Yu ML, Yu SW, Yu VC, Yu WH, Yu Z, Yu Z, Yuan J, Yuan LQ, Yuan S, Yuan SF, Yuan Y, Yuan Z, Yue J, Yue Z, Yun J, Yung RL, Zacks DN, Zaffagnini G, Zambelli VO, Zanella I, Zang QS, Zanivan S, Zappavigna S, Zaragoza P, Zarbalis KS, Zarebkohan A, Zarrouk A, Zeitlin SO, Zeng J, Zeng JD, Žerovnik E, Zhan L, Zhang B, Zhang DD, Zhang H, Zhang H, Zhang H, Zhang H, Zhang H, Zhang H, Zhang H, Zhang HL, Zhang J, Zhang J, Zhang JP, Zhang KYB, Zhang LW, Zhang L, Zhang L, Zhang L, Zhang L, Zhang M, Zhang P, Zhang S, Zhang W, Zhang X, Zhang XW, Zhang X, Zhang X, Zhang X, Zhang X, Zhang XD, Zhang Y, Zhang Y, Zhang Y, Zhang YD, Zhang Y, Zhang YY, Zhang Y, Zhang Z, Zhang Z, Zhang Z, Zhang Z, Zhang Z, Zhang Z, Zhao H, Zhao L, Zhao S, Zhao T, Zhao XF, Zhao Y, Zhao Y, Zhao Y, Zhao Y, Zheng G, Zheng K, Zheng L, Zheng S, Zheng XL, Zheng Y, Zheng ZG, Zhivotovsky B, Zhong Q, Zhou A, Zhou B, Zhou C, Zhou G, Zhou H, Zhou H, Zhou H, Zhou J, Zhou J, Zhou J, Zhou J, Zhou K, Zhou R, Zhou XJ, Zhou Y, Zhou Y, Zhou Y, Zhou ZY, Zhou Z, Zhu B, Zhu C, Zhu GQ, Zhu H, Zhu H, Zhu H, Zhu WG, Zhu Y, Zhu Y, Zhuang H, Zhuang X, Zientara-Rytter K, Zimmermann CM, Ziviani E, Zoladek T, Zong WX, Zorov DB, Zorzano A, Zou W, Zou Z, Zou Z, Zuryn S, Zwerschke W, Brand-Saberi B, Dong XC, Kenchappa CS, Li Z, Lin Y, Oshima S, Rong Y, Sluimer JC, Stallings CL, Tong CK. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)(1). Autophagy. 2021;17:1-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 887] [Cited by in RCA: 856] [Article Influence: 214.0] [Reference Citation Analysis (0)] |
| 80. | Xue P, Zhao J, Zheng A, Li L, Chen H, Tu W, Zhang N, Yu Z, Wang Q, Gu M. Chrysophanol alleviates myocardial injury in diabetic db/db mice by regulating the SIRT1/HMGB1/NF-κB signaling pathway. Exp Ther Med. 2019;18:4406-4412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 81. | Golatkar V, Bhatt LK. Artesunate attenuates isoprenaline induced cardiac hypertrophy in rats via SIRT1 inhibiting NF-κB activation. Eur J Pharmacol. 2024;977:176709. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 82. | Maiese K. Artificial Intelligence and Disease Signature Pathways: Driving Innovation to Elucidate Underlying Pathogenic Mechanisms. Curr Neurovasc Res. 2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 83. | Maiese K. Biological Gases, Oxidative Stress, Artificial Intelligence, and Machine Learning for Neurodegeneration and Metabolic Disorders. Med Gas Res. 2025;15:145-147. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 84. | BinMowyna MN, AlFaris NA. Kaempferol suppresses acetaminophen-induced liver damage by upregulation/activation of SIRT1. Pharm Biol. 2021;59:146-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 85. | Maiese K. Cognitive Impairment and Dementia: Gaining Insight through Circadian Clock Gene Pathways. Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 86. | Ju DT, Huang RS, Tsai BC, Su YC, Chiu PL, Chang YM, Padma VV, Ho TJ, Yao CH, Kuo WW, Huang CY. Folic Acid and Folinic Acid Protect Hearts of Aging Triple-transgenic Alzheimer's Disease mice via IGF1R/PI3K/AKT and SIRT1/AMPK Pathways. Neurotox Res. 2023;41:648-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 87. | Jobst M, Kiss E, Gerner C, Marko D, Del Favero G. Activation of autophagy triggers mitochondrial loss and changes acetylation profile relevant for mechanotransduction in bladder cancer cells. Arch Toxicol. 2023;97:217-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 88. | Chen L, Xu W, Zhang Y, Chen H, Han Y. Gandouling alleviates nerve injury through PI3K/Akt/FoxO1 and Sirt1/FoxO1 signaling pathway to inhibit autophagy in the rats model of Wilson's disease. Brain Behav. 2023;13:e3325. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 89. | Xiao X, Feng H, Liao Y, Tang H, Li L, Li K, Hu F. Identification of key circadian rhythm genes in skin aging based on bioinformatics and machine learning. Aging (Albany NY). 2023;15:11672-11689. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 90. | Caron AZ, He X, Mottawea W, Seifert EL, Jardine K, Dewar-Darch D, Cron GO, Harper ME, Stintzi A, McBurney MW. The SIRT1 deacetylase protects mice against the symptoms of metabolic syndrome. FASEB J. 2014;28:1306-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 91. | Ghiasi R, Naderi R, Sheervalilou R, Alipour MR. Swimming training by affecting the pancreatic Sirtuin1 (SIRT1) and oxidative stress, improves insulin sensitivity in diabetic male rats. Horm Mol Biol Clin Investig. 2019;40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 92. | Castaño D, Larequi E, Belza I, Astudillo AM, Martínez-Ansó E, Balsinde J, Argemi J, Aragon T, Moreno-Aliaga MJ, Muntane J, Prieto J, Bustos M. Cardiotrophin-1 eliminates hepatic steatosis in obese mice by mechanisms involving AMPK activation. J Hepatol. 2014;60:1017-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 93. | Chen YR, Fang SR, Fu YC, Zhou XH, Xu MY, Xu WC. Calorie restriction on insulin resistance and expression of SIRT1 and SIRT4 in rats. Biochem Cell Biol. 2010;88:715-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 94. | Geng C, Xu H, Zhang Y, Gao Y, Li M, Liu X, Gao M, Wang X, Liu X, Fang F, Chang Y. Retinoic acid ameliorates high-fat diet-induced liver steatosis through sirt1. Sci China Life Sci. 2017;60:1234-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 95. | Li Y, Xu S, Giles A, Nakamura K, Lee JW, Hou X, Donmez G, Li J, Luo Z, Walsh K, Guarente L, Zang M. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J. 2011;25:1664-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 248] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 96. | Maiese K. Dysregulation of metabolic flexibility: The impact of mTOR on autophagy in neurodegenerative disease. Int Rev Neurobiol. 2020;155:1-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 97. | Yang Z, Zhang L, Liu J, Li D. Litchi Pericarp Extract Treats Type 2 Diabetes Mellitus by Regulating Oxidative Stress, Inflammatory Response, and Energy Metabolism. Antioxidants (Basel). 2024;13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 98. | Alves HR, Lomba GSB, Gonçalves-de-Albuquerque CF, Burth P. Irisin, Exercise, and COVID-19. Front Endocrinol (Lausanne). 2022;13:879066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 99. | Gong Q, Wang H, Yu P, Qian T, Xu X. Protective or Harmful: The Dual Roles of Autophagy in Diabetic Retinopathy. Front Med (Lausanne). 2021;8:644121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 100. | Tutunchi H, Ebrahimi-Mameghani M, Hosseinzadeh-Attar MJ, Roshanravan N, Mobasseri M, Najafipour F, Naeini F, Naghshi S, Asghari S, Akbarzadeh M, Soleimanzadeh H, Ostadrahimi A. Effects of oleoylethanolamide supplementation on the expression of lipid metabolism-related genes and serum NRG4 levels in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Clin Nutr ESPEN. 2023;58:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 101. | Su P, Chen JG, Tang DH. Exercise against nonalcoholic fatty liver disease: Possible role and mechanism of lipophagy. Life Sci. 2023;327:121837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 102. | Sadria M, Seo D, Layton AT. The mixed blessing of AMPK signaling in Cancer treatments. BMC Cancer. 2022;22:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 103. | Lin L, Zheng X, Qiu C, Dongol S, Lv Q, Jiang J, Kong B, Wang C. SIRT1 promotes endometrial tumor growth by targeting SREBP1 and lipogenesis. Oncol Rep. 2014;32:2831-2835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 104. | Novoselova EG, Glushkova OV, Khrenov MO, Lunin SM, Novoselova TV, Sharapov MG, Parfenyuk SB. Peroxyredoxin 6 Protects RIN-M5F Pancreatic Beta Cells Against Streptozotocin-Induced Senescence. Cell Physiol Biochem. 2024;58:527-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 105. | Rajan PK, Udoh US, Finley R, Pierre SV, Sanabria J. The Biological Clock of Liver Metabolism in Metabolic Dysfunction-Associated Steatohepatitis Progression to Hepatocellular Carcinoma. Biomedicines. 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 106. | Rezaeimanesh N, Abbasi Kasbi N, Saeedi R, Sahraian MA, Razeghi Jahromi S, Naser Moghadasi A. Investigating the Correlation Between Cognitive Function and Fasting Blood Sugar, Fasting Insulin Level and Insulin Sensitivity in Patients With Multiple Sclerosis. Endocrinol Diabetes Metab. 2024;7:e70006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 107. | Tian Y, Ai R, Xiao X, Liu W, Cheng S, Zhu X. Mechanism of the effect of TREM2 on cognitive function in autistic mice. Cell Mol Biol (Noisy-le-grand). 2024;70:66-72. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 108. | Babighian S, Gattazzo I, Zanella MS, Galan A, D'Esposito F, Musa M, Gagliano C, Lapenna L, Zeppieri M. Nicotinamide: Bright Potential in Glaucoma Management. Biomedicines. 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 109. | Ziklo N, Bibi M, Sinai L, Salama P. Niacinamide Antimicrobial Efficacy and Its Mode of Action via Microbial Cell Cycle Arrest. Microorganisms. 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 110. | Qin P, Li Q, Zu Q, Dong R, Qi Y. Natural products targeting autophagy and apoptosis in NSCLC: a novel therapeutic strategy. Front Oncol. 2024;14:1379698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 111. | Singh K, Oladipupo SS. An overview of CCN4 (WISP1) role in human diseases. J Transl Med. 2024;22:601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 112. | Liu QQ, Wu GH, Wang XC, Xiong XW, Rui-Wang, Yao BL. The role of Foxo3a in neuron-mediated cognitive impairment. Front Mol Neurosci. 2024;17:1424561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 113. | Olivares-Costa M, Fabio MC, De la Fuente-Ortega E, Haeger PA, Pautassi R. New therapeutics for the prevention or amelioration of fetal alcohol spectrum disorders: a narrative review of the preclinical literature. Am J Drug Alcohol Abuse. 2024;1-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 114. | Parab S, Parekh N, Apte K, Singh D, Kumawat V, Bagwe-Parab S, et al. Unraveling the Mechanisms of Hydrophilic Vitamins in Alzheimer's and Parkinson's: Preclinical and Clinical Evidence. In: Shah AK, Tappia PS, Dhalla NS, editors. Hydrophilic Vitamins in Health and Disease. Cham: Springer: 2024. [DOI] [Full Text] |
| 115. | Wang R, Zhu Y, Qin LF, Xu ZG, Gao XR, Liu CB, Xu GT, Chen YZ. Comprehensive Bibliometric Analysis of Stem Cell Research in Alzheimer's Disease from 2004 to 2022. Dement Geriatr Cogn Disord. 2023;52:47-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 116. | Ibrahim WW, Sayed RH, Abdelhameed MF, Omara EA, Nassar MI, Abdelkader NF, Farag MA, Elshamy AI, Afifi SM. Neuroprotective potential of Erigeron bonariensis ethanolic extract against ovariectomized/D-galactose-induced memory impairments in female rats in relation to its metabolite fingerprint as revealed using UPLC/MS. Inflammopharmacology. 2024;32:1091-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 117. | Christopoulou ME, Aletras AJ, Papakonstantinou E, Stolz D, Skandalis SS. WISP1 and Macrophage Migration Inhibitory Factor in Respiratory Inflammation: Novel Insights and Therapeutic Potentials for Asthma and COPD. Int J Mol Sci. 2024;25. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 118. | González D, Campos G, Pütter L, Friebel A, Holland CH, Holländer L, Ghallab A, Hobloss Z, Myllys M, Hoehme S, Meindl-Beinker NM, Dooley S, Marchan R, Weiss TS, Hengstler JG, Godoy P. Role of WISP1 in Stellate Cell Migration and Liver Fibrosis. Cells. 2024;13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 119. | Li P, Chen L, Liu J. Network pharmacology and molecular docking approach to elucidate the mechanisms of safflower, phellodendron, scutellaria baicalensis, coptis, and gardenia in hand-foot syndrome. Front Med (Lausanne). 2024;11:1454776. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 120. | Mosharaf MP, Alam K, Gow J, Mahumud RA, Mollah MNH. Common molecular and pathophysiological underpinnings of delirium and Alzheimer's disease: molecular signatures and therapeutic indications. BMC Geriatr. 2024;24:716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 121. | Shafiek MS, Mekky RY, Nassar NN, El-Yamany MF, Rabie MA. Vortioxetine ameliorates experimental autoimmune encephalomyelitis model of multiple sclerosis in mice via activation of PI3K/Akt/CREB/BDNF cascade and modulation of serotonergic pathway signaling. Eur J Pharmacol. 2024;982:176929. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 122. | da Silveira EJD, Barros CCDS, Bottino MC, Castilho RM, Squarize C. The rhythms of histones in regeneration: The epigenetic modifications determined by clock genes. Exp Dermatol. 2024;33:e15005. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |