Published online Oct 26, 2024. doi: 10.4330/wjc.v16.i10.580
Revised: August 28, 2024
Accepted: September 19, 2024
Published online: October 26, 2024
Processing time: 204 Days and 3.6 Hours
Metabolic dysfunction-associated steatotic liver disease (MASLD), particularly in the presence of liver fibrosis, increases the risk of cardiovascular morbidity and mortality, but the nature of the cardio-hepatic interaction in the context type 2 diabetes mellitus (T2DM) is not fully understood.
To evaluate the changes in cardiac morphology and function in patients with T2DM and MASLD-associated liver fibrosis.
T2DM patients with MASLD underwent a medical evaluation that included an assessment of lifestyle, anthropometric measurements, vital signs, an extensive laboratory panel, and a standard echocardiography. Liver fibrosis was evaluated using two scores [Fibrosis-4 (FIB4) and Non-alcoholic fatty liver disease-Fibrosis Score (NFS)], and subjects were classified as having advanced fibrosis, no fibrosis, or an indeterminate risk. The correlations between structural and functional cardiac parameters and markers of liver fibrosis were evaluated through bivariate and multiple regression analyses. Statistical significance was set at P < 0.05.
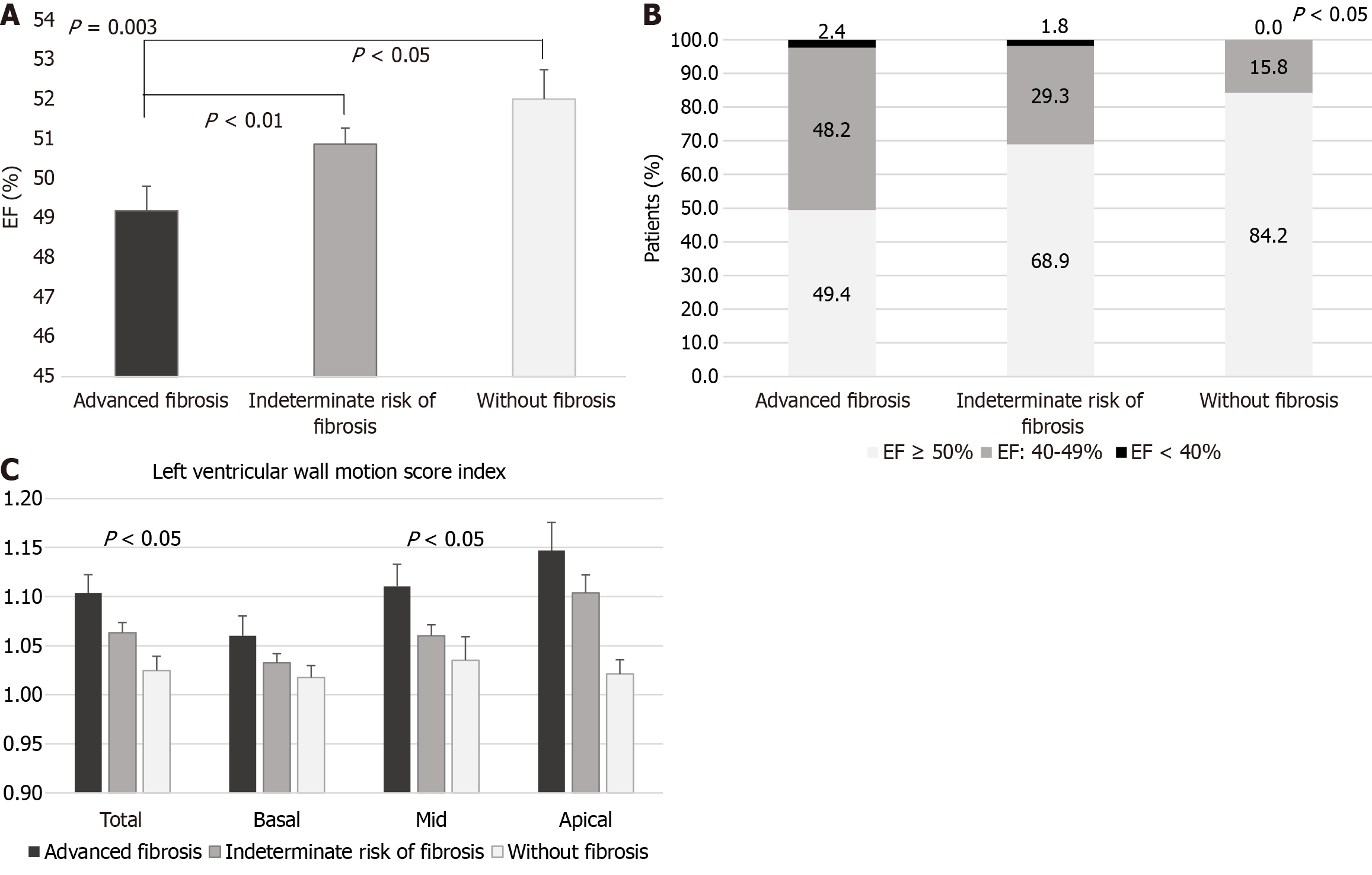
Data from 267 T2DM-MASLD subjects with complete assessment was analyzed. Patients with scores indicating advanced fibrosis exhibited higher interventricular septum and left ventricular (LV) posterior wall thickness, atrial diameters, LV end-systolic volume, LV mass index (LVMi), and epicardial adipose tissue thickness (EATT). Their mean ejection fraction (EF) was significantly lower (49.19% ± 5.62% vs 50.87% ± 5.14% vs 52.00% ± 3.25%; P = 0.003), and a smaller proportion had an EF ≥ 50% (49.40% vs 68.90% vs 84.21%; P = 0.0017). Their total and mid LV wall motion score indexes were higher (P < 0.05). Additionally, they had markers of diastolic dysfunction, with a higher E/e’ ratio [9.64 ± 4.10 vs 8.44 (2.43-26.33) vs 7.35 ± 2.62; P = 0.026], and over 70% had lateral e’ values < 10 cm/second, though without significant differences between groups. In multiple regression analyses, FIB4 correlated with left atrium diameter (LAD; β = 0.044; P < 0.05), and NFS with both LAD (β = 0.039; P < 0.05) and right atrium diameter (β = 0.041; P < 0.01), Moreover, LVMi correlated positively with age and EATT (β = 1.997; P = 0.0008), and negatively with serum sex-hormone binding protein (SHBP) concentrations (β = -0.280; P = 0.004). SHBP also correlated negatively with LAD (β = -0.036; P < 0.05).
T2DM patients with markers of MASLD-related liver fibrosis exhibit lower EF and present indicators of diastolic dysfunction and cardiac hypertrophy. Additionally, LVMi and LAD correlated negatively with serum SHBP concentrations.
Core Tip: Metabolic dysfunction-associated steatotic liver disease (MASLD) is frequently associated with type 2 diabetes mellitus (T2DM), and both conditions are important risk factors for cardiovascular disease. However, the nature of the cardio-liver interaction, particularly in patients with T2DM, is not completely elucidated. In this study we found that T2DM patients with MASLD-associated fibrosis, quantified by accessible scores (Fibrosis-4 and Non-alcoholic fatty liver disease-Fibrosis Score), present markers of systolic and diastolic dysfunction, as well as cardiac hypertrophy, particularly increased left atrial diameter. The left ventricular mass index and left atrial dimension also correlated negatively with serum concentrations of sex-hormone binding protein, which may serve as a valuable prognostic biomarker. Mechanistic studies that explain the correlations between liver fibrosis and cardiac remodeling in MASLD patients, both with and without T2DM, are greatly needed.
- Citation: Cernea S, Onișor D, Roiban AL, Benedek T, Rat N. Metabolic dysfunction-associated steatotic liver disease-associated fibrosis and cardiac dysfunction in patients with type 2 diabetes. World J Cardiol 2024; 16(10): 580-594
- URL: https://www.wjgnet.com/1949-8462/full/v16/i10/580.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i10.580
Metabolic dysfunction-associated steatotic liver disease (MASLD), previously termed non-alcoholic fatty liver disease (NAFLD), is defined by the presence of hepatic steatosis in combination with at least one cardiometabolic risk factor, without other causes of steatotic liver disease[1]. It encompasses a spectrum of conditions from simple hepatic steatosis to steatohepatitis (which may involve varying degrees of fibrosis, from mild to severe/cirrhosis), and to hepatocellular carcinoma[1,2].
A core pathogenetic mechanism of MASLD is insulin resistance, which implies a cross-talk between the liver and peripheral tissues, favoring the accumulation of lipids in the liver[3,4]. In fact, MASLD is considered part of a multi-systemic disease, alongside other components of metabolic syndrome [i.e., type 2 diabetes mellitus (T2DM), obesity, dyslipidemia, hypertension], for which insulin resistance is a key pathogenetic factor[5,6]. The relationship between T2DM and MASLD is bidirectional[6]. Literature data indicates that MASLD doubles the risk of T2DM, and the severity of hepatic fibrosis is independently correlated with a higher risk of incident diabetes[7,8]. On the other hand, T2DM increases the risk of MASLD by approximately two-fold and worsens the course of the disease toward more advanced stages (i.e., advanced fibrosis/cirrhosis, hepatocellular carcinoma, liver-related hospitalizations, and deaths)[6,9-11].
Moreover, MASLD significantly increases the risk of cardiovascular disease (CVD) and mortality in both individuals with and without T2DM, independent of other risk factors[12-15]. In patients with T2DM, the presence of MASLD nearly doubles the risk of CVD, suggesting potential synergistic effects of these two conditions on cardiovascular risk[13,16]. The presence of MASLD in hospitalized patients with CVD significantly raises the risk of all-cause mortality [hazard ratio (HR): 2.08; 95% confidence interval (95%CI): 1.56-2.59, P < 0.001][17]. Furthermore, the severity of MASLD fibrosis is associated with a higher risk of overall mortality [unadjusted relative risk for stage F0 vs F4: 3.42 (95%CI: 2.63-4.46); adjusted HR for stage F0-2 vs F3-4: 2.24 (95%CI: 1.48-3.39)][18]. Emerging evidence also suggests that the severity of liver fibrosis has a significant impact on the risk of fatal or non-fatal CVD events, independent of other cardiometabolic risk factors [pooled random-effects HR: 2.50 (95%CI: 1.68-3.72)][15].
Nevertheless, the nature of the relationship between liver fibrosis and CVD, particularly in the context of T2DM, is not entirely clear, as the coexistence of other cardiovascular risk factors complicates the deciphering of the independent contributions of each condition to the incidence and progression of the other. Some authors even question the causal link between MASLD and CVD[19]. On the other hand, some data suggest that markers of CVD (such as carotid intima-media thickness) may predict liver fibrosis in MASLD patients with T2DM[20]. Therefore, understanding the nature of these associations is important, as early screening and intervention for one disease may potentially ameliorate the progression of the other. However, few studies have investigated the relationship between liver fibrosis and cardiac morphology and function in patients with T2DM. One study showed that liver fibrosis was independently associated with diastolic dysfunction [odds ratio (OR): 1.58 (95%CI: 1.07-2.34, P = 0.022), while another reported an association with subclinical myocardial remodeling in T2DM subjects[21,22].
The aim of this study was to evaluate cardiac morphology and function in relation to markers of liver fibrosis in T2DM patients with MASLD.
The study enrolled patients with T2DM and NAFLD in the Outpatient Unit of the Emergency County Clinical Hospital of Târgu Mureș, Romania between July 2022 and July 2023. Patients were recruited from the Diabetes, Nutrition and Metabolic Diseases Outpatient Unit, and the Gastroenterology Department of the County Clinical Hospital, Târgu Mureș. Inclusion criteria were as follows: Adult subjects aged 30 years or older, with a previous diagnosis of T2DM and NAFLD (based on patient history and liver ultrasound). NAFLD was defined by the presence of hepatic steatosis/steatohepatitis in the absence of other secondary causes of liver disease (including viral or autoimmune hepatitis, excessive alcohol intake of ≥ 30 g/day for men and ≥ 20 g/day for women, specific drugs, toxins, hemochromatosis, Wilson’s disease or other known specific liver diseases). In July 2023, the term MASLD was proposed to replace NAFLD to better describe and classify the steatotic liver disease, potentially reducing stigma[23]. This new term was largely adopted thereafter, and emerging evidence indicates that the term MASLD can be used interchangeably with NAFLD[24]. Since our patients met the diagnostic criteria for MASLD (i.e., had liver steatosis and at least one cardiometabolic risk factor, T2DM), we adopted the new term to describe their liver condition. Exclusion criteria for this study included other types of diabetes, other chronic liver diseases (including liver transplant), malignant diseases in the last 5 years, severe autoimmune diseases, severe valvulopathy, and significant pericardial collections. The study was approved by the Ethics Committees of the Emergency County Clinical Hospital of Târgu Mureș (nr. 8120/05.04.2022), the County Clinical Hospital of Târgu Mureș (nr. 4873/24.05.2022), and the George Emil Palade University of Medicine, Pharmacy, Science and Technology of Târgu Mureș (nr. 1806/22.06.2022). All subjects signed an informed consent before being enrolled in the study.
The following data were collected: Demographic data, medical history (including therapy), lifestyle data (diet, coffee, tea and alcohol intake, physical exercise, sleep, smoking, stress) through general or specific questionnaires. Alcohol consumption was assessed using both a general questionnaire with secondary interview, and the AUDIT-C test. Anthropometric parameters (weight, height, waist circumference, hip circumference), heart rate, and blood pressure were measured using standard methods. The body mass index (BMI) was calculated as follows: Weight/height2 (kg/m2). The pO2 was measured under standard conditions using a pulse oximeter.
On the same day, fasting blood samples were collected between 7: 45 AM and 8: 15 AM, and serum aliquots were stored at -80 °C for subsequent analysis of the following parameters: Blood glucose, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, C-peptide, uric acid, creatinine, sex-hormone binding protein (SHBP), aspartate aminotransferase (ASAT), alanine aminotransferase (ALAT), gamma-glutamyl transpeptidase (GGT), direct bilirubin, albumin, ferritin, and haptoglobin. Blood for HbA1c measurement was drawn on the same occasion and stored at -80 °C for up to three months for later measurement. The biochemical tests were analyzed using a Cobas Integra 400plus (Roche Diagnostics, Germany). Albumin, haptoglobin, and HbA1c were measured using an immunoturbidimetric method, while uric acid, ASAT, ALAT, direct bilirubin, GGT, creatinine, glucose, and lipids were measured using a spectrophotometric method. C-peptide, ferritin and SHBP were analyzed on the Immulite 2000 XPI system (Siemens) using a solid-phase, two-site chemiluminescent immunometric assay. The complete blood count was analyzed shortly after the blood was drawn using a 5-differential hematology Mindray BC6200 analyzer. The Homeo
The hepatic fibrosis was estimated using two well-known and validated indices. The fibrosis-4 (FIB4) score was calculated using the formula: Age (years) × ASAT (U/L)/[platelet (109/L) × ALT1/2 (U/L)]. A FIB4 score < 1.3 rules out advanced fibrosis, a score > 2.67 indicates advanced fibrosis (F ≥ 2), while FIB4 values between 1.3 and 2.67 are considered to be indeterminate risk[27]. The NAFLD-Fibrosis Score (NFS) was calculated with the following formula:
The echocardiographic evaluation was performed on a subsequent day (within a 2-3 weeks interval) by an experienced cardiologist who was blinded to all other aspects of the study. The ultrasonographic assessment was conducted using a VIVID9 XDClear equipment (GE HealthCare). The quantification of cardiac chamber sizes and function was carried out in accordance with the recommendations of the ASE/EAC Guidelines[29].
The left ventricular (LV) ejection fraction (EF) was evaluated using the modified Simpson’s rule calculated by dividing the stroke volume by the end-diastolic LV volume. An EF was considered normal if the values were ≥ 50%[30]. The dimensions of the ventricles and atria, as well as the epicardial adipose tissue thickness (EATT), were measured in the parasternal long-axis view. The LV end-diastolic and end-systolic volumes were measured using 2D echocardiography in the apical 4-chamber view and 2-chamber view at the end of diastole and systole.
The LV mass (LVM) was calculated using the following formula: LVM (g) = 0.80 × [1.04 × (PWd (cm)+ IVSd (cm) + LVDd (cm))3 - (LVDd (cm))3] + 0.6, where 1.04 is the density of heart muscle (g/cm3), PWd is the LV posterior wall thickness at end-diastole, IVSd is the interventricular (IV) septum thickness at end-diastole, and LVDd is the LV end-diastolic dimension[29,31]. The LVM was indexed to the body surface area [LVM index (LVMi)] calculated using the DuBois formula[29,32]. The upper limits for normal values of LVMi were considered to be 95 g/m2 in women and 115 g/m2 in men[29].
The LV outflow tract (LVOT) velocity time integral was determined in the apical 5-chamber view using the pulsed-wave Doppler technique, with the pulse wave Doppler gate positioned at the LVOT level. Using the same technique in the apical 4-chamber view, the following parameters were determined on the Doppler curve: Maximum velocities of the E wave, A wave, e’ septal, e’ lateral, a’ septal, a’ lateral, and deceleration time (DcT). The E/A ratio e’/a’ septal, and e’/a’ lateral ratios were calculated. The average E/e’ ratio was calculated as the ratio of E to the average e’ (mean of e’ septal and e’ lateral).
Regional LV function was assessed in a 17-segment model. The basal and midventricular segments included anterior, anterolateral, anteroseptal, inferior, inferolateral, and inferoseptal segments, while the apical segments included anterior, septal, inferior, lateral, and the ‘‘apical cap (apex)’’ (myocardium beyond the end of the LV cavity)[29]. Total and segmental kinetics scores were calculated by assigning points according to the following grading: 1 point for normal kinetics; 2 points for hypokinesia, 3 points for akinesia. Total and segmental wall motion score indexes were calculated by dividing the wall motion scores by the number of segments.
Descriptive statistics were performed for all variables, and the normality of the data was assessed using the Kolmogorov-Smirnov test. Normally distributed continuous data are presented as the mean ± SD, while non-normally distributed variables are presented as median (min-max). Categorical variables are presented as frequency (%). Comparisons between groups were conducted using one-way ANOVA with Tukey post-test for normally distributed variables or Kruskal-Wallis test with Dunn post-test for non-normally distributed variables. The χ2 test was employed to analyze categorical variables. The relationship between two variables of interest was investigated using Spearman’s test, with data presented as correlation coefficients r (95%CI). Multiple regression analyses for more than two variables were employed to test the independent associations between liver fibrosis scores and cardiac parameters, as well as to evaluate the impact of independent variables on various echocardiographic parameters of interest. Statistical analyses were performed using GraphPad InStat 3 (GraphPad Software, United States). All tests were two-tailed, and statistical significance was assumed at P < 0.05.
In this study 278 T2DM patients with MASLD were enrolled. Of these, seven patients met the exclusion criteria, and four did not return for the cardiac ultrasound evaluation. Ultimately, data from 267 T2DM-MASLD patients were analyzed. The median age of the participants was 66 (36-82) years, and the median duration of diabetes was 10 (0-33) years. Of the total cohort, 45.32% were men, 76.40% lived in urban areas, 22.84% were employed, and 75.28% were retired. Their relevant medical history, lifestyle and other clinical characteristics are presented in Table 1.
| Patients’ characteristics | Value |
| Systolic/diastolic BP (mmHg) | 135.0 (95.0-190.0)/80.0 (51.0-107.5) |
| Heart rate (beats/min) | 74.0 (50.0-112.0) |
| pO2 (%) | 97.0 (90.0-99.0) |
| Lifestyle | |
| Smoking status | |
| Current smoker | 28 (10.49) |
| Former smoker | 107 (40.07) |
| Never smoked | 132 (49.44) |
| Coffee intake (cups/day) | 1.25 ± 0.80 |
| Alcohol intake (g/day) | 2.85± 5.37 |
| Anthropometric parameters | |
| BMI (kg/m2) | 34.11 ± 5.30 |
| Waist circumference (cm) | 109.16 ± 11.72 (F) |
| 114.92 ± 10.83 (M) | |
| Hip circumference (cm) | 110.21 ± 10.55 (F) |
| 108.86 ± 9.98 (M) | |
| Comorbidities | |
| Hypertension | 251 (94.00) |
| Dyslipidemia | 247 (92.51) |
| Coronary artery disease | 137 (51.31) |
| Heart failure | 99 (37.01) |
| Atrial fibrillation | 17 (6.37) |
| Peripheral arterial disease | 18 (6.74) |
| Stroke | 19 (7.11) |
| Diabetic neuropathy | 106 (39.70) |
| Diabetic retinopathy | 36 (13.48) |
| Chronic kidney disease | 49 (18.35) |
| Hyperuricemia | 58 (21.72) |
| Antihyperglycemic therapy | |
| Metformin | 262 (98.13) |
| GLP-1 RA | 85 (31.83) |
| SGLT2 inhibitors | 62 (23.22) |
| DPP-4 inhibitors | 19 (7.12) |
| Sulphonylureas | 27 (10.11) |
| Insulin | 66 (24.72) |
The median FIB4 value was 1.35 (0.4-17.3), and median NFS was 0.159 (-2.783 to 6.212). One FIB4 value was a significant outlier (134.15) and was excluded from further analysis. Among the study population, 11.2% had a FIB4 score > 2.67 suggesting advanced liver fibrosis, 44.2% had a FIB4 score between 1.3-2.67 (indicating an indeterminate risk of advanced fibrosis) and 44.6% had a FIB4 score < 1.3 (which rules out significant fibrosis). Regarding the NFS, 31.8% of subjects had a score > 0.676 indicating advanced liver fibrosis, 61% had a score between 0.676 and -1.455 (undetermined risk of liver fibrosis), while 7.1% had NFS values < -1.455, which excludes fibrosis.
To ensure a proper selection of patients in the advanced fibrosis and no fibrosis categories, we divided the study population into three groups according to the two liver fibrosis scores: Group 1 consisted of patients with both FIB4 > 2.67 and NFS > -1.455 or both NFS > 0.676 and FIB4 > 1.3 [suggestive of significant (or advanced) fibrosis], Group 3 included patients with both FIB4 < 1.3 and NFS < -1.455 (indicating no significant fibrosis, F0-1), and Group 2, included the remaining subjects (with indeterminate risk of advanced fibrosis). The laboratory data are shown according to the liver fibrosis categories in Table 2. Subjects with more advanced hepatic fibrosis exhibited higher markers of liver injury, as well as increased insulin resistance, uric acid and triglyceride levels, and lower LDL cholesterol and eGFR values. There was no significant difference among the three groups in terms of the proportion of patients receiving therapy with glucagon-like peptide-1 receptor agonists (GLP-1 RA) and/or sodium-glucose co-transporter 2 (SGLT2) inhibitors (47.6% vs 48.8% vs 52.6%, P = 0.9244).
| Parameter | Group 1 (advanced fibrosis), n = 84 | Group 2 (indeterminate risk of fibrosis), n = 164 | Group 3 (without fibrosis), n = 19 | P value |
| Uric acid (mg/dL) | 6.13 ± 1.44 | 5.86 ± 1.48 | 5.29 (3.59-8.17) | 0.0484 |
| Albumin (g/dL) | 4.59 ± 0.22a | 4.68 ± 0.23a | 4.70 ± 0.28 | 0.0118 |
| ALAT (U/L) | 17.22 (2.32-80.94) | 18.02 (4.18-92.79) | 25.36 ± 12.56 | 0.0921 |
| ASAT (U/L) | 22.54 (11.48-130.85)b | 19.91 (9.75-49.95)b | 19.30 (13.16-39.16) | 0.0034 |
| Direct bilirubin (mg/dL) | 0.21 (0.07-0.59)a | 0.20 (0.07-0.90) | 0.17 ± 0.06a | 0.0335 |
| GGT (U/L) | 32.78 (4.97-338.18)a | 27.51 (4.02-313.66)a | 27.13 (9.17-173.63) | 0.0360 |
| Total cholesterol (mg/dL) | 147.20 (91.38-279.48) | 155.45 (96.75-376.17) | 175.16 ± 51.20 | 0.1347 |
| HDL cholesterol (mg/dL) | 43.98 (27.86-65.09) | 43.75 (22.48-75.75) | 48.04 ± 11.77 | 0.3977 |
| LDL cholesterol (mg/dL) | 73.71 (36.57-186.09) | 84.73 (31.2-270.6) | 104.01 ± 39.94 | 0.0488 |
| Triglycerides (mg/dL) | 156.02 (70.06-573.36)a | 155.36 (62.37-609.08)a | 117.83 (68.15-382.84)a | 0.0305 |
| Blood glucose (mg/dL) | 137.59 (92.09-261.68) | 136.58 (87.34-326.2) | 142.75 ± 18.60 | 0.7290 |
| HbA1c (%) | 6.80 (4.6-10.01) | 6.80 (5.7-10.20) | 7.02 ± 0.72 | 0.5780 |
| C-peptide (ng/mL) | 3.68 (0.72-10.5)a,b | 3.00 (0.28-8.83)a | 2.62 ± 1.43b | 0.0039 |
| HOMA-IR | 3.04 (0.66-8.4)a | 2.61 (0.45-7.52) | 2.23 ± 1.21a | 0.0067 |
| eGFR (mL/min/1.73m²) | 82.53 (26.70-117.25)b,c | 91.38 (40.36-114.59)a,b | 97.17 ± 14.60a,c | < 0.0001 |
| Haptoglobin (g/L) | 1.59 ± 0.57 | 1.73 ± 0.61 | 1.73 ± 0.63 | 0.1997 |
| Ferritin (ng/mL) | 112.00 (8.79-781.00) | 92.30 (6.41-811.00) | 53.90 (9.72-543.0) | 0.6226 |
| SHBP (nmol/L) | 37.41 ± 15.12 | 32.80 (7.62-118.00) | 35.38 ± 15.68 | 0.3811 |
We first analyzed the ultrasound cardiac parameters evaluating the heart function and structure according to the liver fibrosis category (Table 3). The E, A and DcT were not measured in patients with atrial fibrillation, as this condition alters atrial filling. T2DM patients with MASLD and advanced liver fibrosis had significantly higher LVMi, left and right atrial diameters, IV septum thickness, and LV posterior wall thickness, LV end-systolic volume, and EATT. For other mea
| Echocardiographic parameters | Group 1 (advanced fibrosis), n = 84 | Group 2 (indeterminate risk of fibrosis), n = 164 | Group 3 (without fibrosis), n = 19 | P value |
| Morphologic parameters | ||||
| LV diastolic diameter (mm) | 51.37 ± 6.04 | 50.00 (38.00-71.00) | 48.53 ± 4.50 | 0.1482 |
| LV systolic diameter (mm) | 37.00 (24.00-57.00) | 36.00 (24.00-60.00) | 34.89 ± 6.44 | 0.4832 |
| IV septum thickness (mm) | 12.00 (8.00-16.00)b | 11.00 (7.00-16.00) | 10.00 (9.00-13.00)b | 0.0029 |
| LV posterior wall thickness (mm) | 12.00 (9.00-16.00)a | 11.00 (7.00-17.00)a | 11.00 (10.00-14.00) | 0.0277 |
| Left atrium diameter (mm) | 39.07 ± 5.15c | 37.00 (26.00-52.00)a | 34.31 ± 4.26a,c | 0.0008 |
| Right atrium diameter (mm) | 36.96 ± 6.03a | 35.47 ± 5.28 | 33.47 ± 4.68a | 0.0224 |
| Right ventricle diameter (mm) | 36.34 ± 5.50 | 35.00 (12.00-50.00) | 37.00 ± 5.18 | 0.5209 |
| Aortic annular diameter (mm) | 32.00 (20.00-42.00) | 32.00 (23.00-40.00) | 31.11 ± 3.05 | 0.2201 |
| Descending aorta diameter (mm) | 18.00 (13.00-33.00)b | 19.00 (10.00-30.00)b | 18.11 ± 1.60 | 0.0066 |
| LV end-diastolic volume (mm3) | 121.64 ± 31.18 | 109.00 (63.00-380.00) | 108.74 ± 17.85 | 0.1388 |
| LV end-systolic volume (mm3) | 66.70 ± 22.25a | 57.00 (21.00-290.00)a | 54.35 ± 14.16 | 0.0101 |
| Stroke volume (mm3) | 55.42 ± 16.60 | 54.00 (24.00-112.00) | 52.81 ± 13.48 | 0.7994 |
| EATT (mm) | 8.00 (3.00-17.00)b | 7.00 (3.00-14.00)b | 7.26 ± 2.51 | 0.0027 |
| LVMi (g/m2) | 119.16 ± 27.36 | 113.23 ± 24.82 | 104.39 ± 22.32 | 0.0474 |
| Increased LVMi, n (%) | 55 (66.27%) | 99 (60.37%) | 10 (52.63%) | 0.4687 |
| Functional parameters | ||||
| EF, n (%) | 49.19 ± 5.62 | 50.87 ± 5.14 | 52.00 ± 3.25a | 0.0030 |
| 49.00 (27.00-65.00)a,b | 50.00 (23.00-61.00)a,b | 52.00 (45.00-60.00) | ||
| EF-normal range, n (%) | 41 (49.40) | 113 (68.90) | 16 (84.21) | 0.0017 |
| E wave velocity (cm/second) | 72.59 ± 19.24 | 70.00 (32.00-158.00) | 71.83 ± 18.07 | 0.9292 |
| A wave velocity (cm/second) | 84.31 ± 21.25 | 86.10 ± 22.65 | 81.61 ± 27.54 | 0.6626 |
| Mitral valve E/A | 0.79 (0.38-1.52) | 0.80 (0.44-2.07) | 0.94 ± 0.28 | 0.5683 |
| e’ septal (cm/second) | 8.00 (3.00-16.00)b | 8.00 (4.00-15.00) | 10.50 ± 2.94b | 0.0068 |
| e’/a’ septal | 0.70 (0.43-2.0) | 0.78 (0.36-3.00) | 0.84 ± 0.24 | 0.3637 |
| E/e’ septal | 9.64 ± 4.10a | 8.44 (2.43-26.33) | 7.35 ± 2.62a | 0.0260 |
| e’ lateral (cm/second) | 8.00 (4.00-19.00) | 8.00 (4.00-18.00) | 8.00 (4.00-13.00) | 0.9551 |
| e’/a’ lateral | 0.73(0.40-2.00) | 0.71 (0.40-2.00) | 0.79 ± 0.28 | 0.7655 |
| E/e’ lateral | 9.15 ± 3.76 | 8.27 (2.83-25.0) | 8.15 (3.54-20.75) | 0.8768 |
| Average E/e’ ratio | 9.17 ± 3.58 | 8.39 (2.62-22.57) | 7.77 ± 2.34 | 0.3208 |
| DcT (msec) | 200.73 ± 59.07 | 194.59 ± 51.00 | 192.17 ± 43.19 | 0.6725 |
| LVOT VTI (cm) | 30.00 (11.20-78.00) | 30.00 (11.90-76.00) | 28.98 ± 7.27 | 0.5857 |
| LV segmental kinetics | ||||
| Total wall motion score index | 1.10 ± 0.17 | 1.06 ± 0.13 | 1.02 ± 0.06 | 0.0352 |
| Basal wall motion score index | 1.06 ± 0.18 | 1.03 ± 0.12 | 1.02 ± 0.05 | 0.6358 |
| Mid wall motion score index | 1.11 ± 0.21 | 1.06 ± 0.15 | 1.03 ± 0.1 | 0.0240 |
| Apical wall motion score index | 1.15 ± 0.26 | 1.10 ± 0.24 | 1.02 ± 0.06 | 0.0548 |
Moreover, patients with markers of advanced liver fibrosis had significantly lower EF (Figure 1A), and higher E/e’ septal, as well as higher total a LV wall motion score index (mainly due to higher mid-ventricular wall motion scores), indicative of cardiac dysfunction and dyskinesia (Table 3 and Figure 1B). A higher proportion of T2DM patients with more advanced liver fibrosis had decreased EF (50.6% vs 31.1% vs 15.8%; Ptrend = 0.0004; Figure 1C and Table 3).
Additionally, over half of the patients in each liver fibrosis category had increased LVMi values, with a slightly higher percentage in the advanced fibrosis group; however, the difference between the three groups was not significant (overall prevalence: 61.65%; Table 3). Moreover, a large proportion of patients in all three groups presented lateral e’ values < 10 cm/second, indicative of LV diastolic dysfunction, but the differences between groups were not significant (65.79% in group 1 vs 72.84% in group 2 vs 70.59% in group 3; P = 0.5384). Higher percentages of patients in the advanced liver fibrosis and indeterminate risk of fibrosis groups presented e’ septal values < 7 cm/second compared to the no liver fibrosis group, but the differences were not statistically significant (19.74% in group 1 vs 19.75% in group 2 vs 5.56% in group 3; P = 0.3308).
We further investigated the correlation between markers of liver fibrosis (using both FIB4 and NFS) and cardiac parameters by performing bivariate correlation and multiple regression analyses. The bivariate analyses indicated an association between markers of liver fibrosis and cardiac hypertrophy (mainly LVMi and atrial diameters), and dys
| FIB4, r (95%CI) | NFS, r (95%CI) | |
| LV diastolic diameter | 0.09 (-0.03; 0.22) | 0.14 (0.02; 0.26)a |
| IV septum thickness | 0.19 (0.07; 0.31)b | 0.23 (0.11; 0.35)c |
| LV posterior wall thickness | 0.12 (-0.004; 0.24) | 0.18 (0.06; 0.30)b |
| Left atrium diameter | 0.19 (0.07; 0.31)b | 0.17 (0.04; 0.29)b |
| Right atrium diameter | 0.17 (0.05; 0.29)b | 0.21 (0.09; 0.32)d |
| Aortic annular diameter | 0.13 (0.001; 0.25)a | 0.09 (-0.04; 0.21) |
| EATT | 0.12 (-0.004; 0.24) | 0.13 (0.01; 0.25)a |
| LV end-systolic volume | 0.08 (-0.05; 0.20) | 0.14 (0.01; 0.26)a |
| LVMi | 0.15 (0.02; 0.26)a | 0.19 (0.06; 0.30)b |
| EF (%) | -0.13 (-0.25; -0.01)a | -0.21 (-0.33; -0.09)d |
| e’ septal | -0.24 (-0.36; -0.12)e | -0.19 (-0.31; -0.07)b |
| a’ septal | -0.13 (-0.25; -0.004)a | -0.10 (-0.22; 0.03) |
| e’/a’ septal | -0.14 (-0.27; -0.02)a | -0.12 (-0.24; 0.01) |
| E/e’ septal | 0.19 (0.06; 0.31)b | 0.19 (0.07; 0.31)b |
| Total LV segmental kinetics score | 0.14 (0.01; 0.26)a | 0.16 (0.04; 0.28)b |
| β (95%CI); t ratio | β (95%CI); t ratio | |
| Model 1 | ||
| Left atrium diameter | 0.044 (0.007; 0.082); 2.307a | 0.039 (0.004; 0.074); 2.180a |
| Right atrium diameter | 0.019 (-0.014; 0.052); 1.107 | 0.041 (0.010; 0.072); 2.628b |
| Sex | 0.137 (-0.273; 0.546); 0.654 | -0.560 (-0.941; -0.179); 2.881b |
| Serum creatinine | 0.748 (-0.025; 1.521); 1.897 | 1.467 (0.747; 2.186); 3.995c |
| Model 2 | ||
| Left atrium diameter | 0.037 (0.0005; 0.073); 1.990a | 0.030 (-0.005; 0.065); 1.695 |
| C-peptide | 0.175 (0.071; 0.280); 3.282b | 0.099 (-0.002; 1.200); 1.920 |
| Right atrium diameter | 0.013 (-0.017; 0.043); 0.851 | 0.030 (0.001; 0.059); 2.021a |
| Serum creatinine | 0.410 (-0.328; 1.148); 1.089 | 0.843 (0.133; 1.533); 2.326a |
In the multiple regression analyses, atrial dimensions were independently associated with liver fibrosis markers. In model 1, which adjusted for several independent variables (sex, smoking status, systolic blood pressure, duration of diabetes, alcohol intake, presence of atrial fibrillation, serum creatinine), both FIB4 and NFS were independently associated with left atrium diameter (LAD; FIB4: R2 = 7.77%, P = 0.0221; NFS: R2 = 15.03%; P < 0.0001; Table 5). In model 2, which included cardiac parameters, as well as serum creatinine, C-peptide and LDL cholesterol values (correlated with both FIB4 and NFS in the bivariate analyses) as independent variables, liver fibrosis markers remained correlated with LAD, and C-peptide values (for FIB4; R2 = 11.19%, P < 0.0001) and right atrium diameter and creatinine values (for NFS; R2 = 12.56%, P < 0.0001), respectively (Table 5). The remaining parameters (not mentioned in Table 5) did not correlate with either of the fibrosis scores. In the multiple regression analyses, the LVMi was not associated significantly with either liver fibrosis scores.
In a separate bivariate analysis, LVMi correlated positively with uric acid values [r = 0.13 (0.010; 0.253), P = 0.0295], age [r = 0.16 (95%CI: 0.038; 0.279), P = 0.0083], and EATT [r = 0.15 (0.030; 0.272), P = 0.0122], while the negative correlation with SHBP concentration was not quite significant [r = -0.12 (-0.238; 0.005), P = 0.0538]. No other laboratory parameters or therapies with GLP-1 RA and/or SGLT2 inhibitors correlated with LVMi. In a multivariate regression analysis that included these parameters as independent variables, along with BMI and systolic blood pressure, the latter three were significantly correlated with LVMi (R2 = 10.77%, P < 0.0001): Age [β = 0.599 (95%CI: 0.227; 0.971), t ratio: 3.153, P = 0.0018], EATT [β = 1.997 (95%CI: 0.847; 3.146), t ratio: 3.405, P = 0.0008], and SHBP [β = -0.280 (95%CI: -0.469; -0.091), t ratio: 2.901, P = 0.0040].
To better understand the interrelationship between the liver and heart, we further investigated which liver-related and -independent factors had a significant impact on the LAD. Initially we performed bivariate correlation analyses and identified several parameters that were significantly associated with LAD (Table 6). Age, duration of diabetes, BMI, smoking status, alcohol intake and the other laboratory parameters mentioned in Table 2, as well as therapy with GLP-1RA and/or SGLT2 inhibitors were not correlated with LAD. Subsequently, the multiple regression analysis (which included as independent variables those factors found to be significantly correlated with LAD in the bivariate analysis) revealed that three of them remained independently correlated with LAD (R2 = 15.18%; P < 0.0001; Table 7). The presence of atrial fibrillation had the strongest impact, but higher serum values of GGT and lower SHBP concentrations also influenced LAD.
MASLD is frequently associated with T2DM and has emerged as a risk factor for CVD, yet the nature of the cardio-liver interaction, particularly in patients with T2DM is not completely understood. In this study, which included T2DM patients with MASLD, we found that a higher proportion of patients with markers of advanced liver fibrosis had a reduced EF, and the mean EF values were lower in this category. Literature indicates that MASLD is associated with an increased risk of heart failure (HF), although there is limited information regarding the association between MASLD severity/fibrosis and HF phenotypes[33,34]. A recent cohort study suggested a stronger association of MASLD with HF with preserved EF (HFpEF) rather than HF with reduced EF (HFrEF); however, this study involved a healthcare database interrogation and not a direct echocardiographic evaluation of MASLD patients[35]. Another recent study in hospitalized patients with T2DM also showed a higher risk of HFpEF in MASLD [OR: 1.59 (95%CI: 1.22-2.08)], independent of cardiometabolic risk factors, primarily associated with more advanced liver fibrosis; however, this study did not specifically investigate the correlations of liver fibrosis with EF (or the risk of HFrEF)[36]. To our knowledge this is the first report of lower EF in T2DM patients with markers of advanced MASLD-related fibrosis. Nevertheless, existing literature is congruent with our findings. A small imaging study in T2DM and in MASLD patients, that employed high-resolution magnetic resonance imaging (MRI), tagging, and spectroscopy, demonstrated alterations in cardiac structure and function[37]. T2DM patients exhibited significant systolic dysfunction (shown by reduced stroke index), and diastolic dysfunction (reduced E/A)[37]. Moreover, a recent meta-analysis of 41 papers (n = 33891 patients who underwent echocardiography) demonstrated that patients with liver biopsy- or imaging-defined MASLD had lower EF [mean difference: -0.693 (95%CI: -1.112 to -0.274); P = 0.001] compared with patients without MASLD[38]. These patients also presented indicators of diastolic dysfunction, higher LVM and increased EATT[38]. Our study population also presented markers of diastolic dysfunction: Over 70% of subjects had lateral e’ values < 10 cm/second, without differences between groups, while e’ septal values were lower in the more advanced liver fibrosis group. Additionally, we have found that more than 60% of T2DM-MASLD patients included in this study had increased LVMi, and LVMi values were higher in subjects with markers of advanced hepatic fibrosis. A recent meta-analysis of twenty studies showed higher LVMi in MASLD; however, in patients with T2DM the differences in LVMi according to MASLD were not significant[39]. The potentially attenuated impact of MASLD on LVMi in T2DM may be due to T2DM itself being associated with higher LVM, particularly in the context of insulin resistance, longer duration of diabetes, and the presence of hypertension[40,41]. Notably, our study group of T2DM patients with more advanced liver fibrosis also had higher HOMA-IR values, indicative of significant insulin-resistance. Nonetheless, another meta-analysis of ten studies that included 1,800 T2DM patients reported higher LVMi in the presence of MASLD, accompanied by other markers of diastolic dysfunction[42]. However, neither of the two meta-analyses evaluated the relationship between LVMi and the severity of liver fibrosis.
It has also been suggested that high LVMi might lead to early LV diastolic dysfunction, which in turn is related to changes in left atrial dimensions and function, partly due to increased filling pressures and preload[42,43]. In our study, LAD was positively correlated with markers of liver fibrosis, and was significantly higher in T2DM patients with advanced liver fibrosis. This finding aligns with the research conducted by Fan et al[22], which showed a positive cor
In our study, LVMi and LAD negatively correlated with serum SHBP concentrations. Emerging evidence suggests that lower SHBP levels are associated with higher cardiovascular risk in men, and with elevated LVMi in post-menopausal women[49,50]. SHBP is a glycoprotein secreted by the liver (and other tissues, including the myocardium), that acts as a carrier for steroid hormones (androgens and estrogens, potentially influencing their availability and activity in specific tissues[51,52]. Additionally, in vitro experiments have shown that SHBP can bind to membrane receptors and activate intracellular signaling pathways, either through a putative G-protein-coupled receptor that increases intracellular cAMP levels, or through the megalin receptor, which induces the internalization of SHBG[51,53]. While the cardioprotective effects of sex hormones (primarily estrogens) are well established, the role of circulating SHBP in cardiovascular pathophysiology remains incompletely understood and warrants further investigation[54,55]. The findings of this research pave the way for subsequent mechanistic studies exploring the potential role of SHBP in linking liver fibrosis and cardiac remodeling.
Indeed, a mechanistic explanation for the correlation between liver fibrosis and remodeling of left atrium and left ventricle is still greatly needed. Perhaps shared pathogenetic mechanisms of cardiac and liver fibrosis, triggered by similar factors (such as inflammation/activation of inflammasomes or oxidative stress), leading to fibroblast activation and increased collagen formation, might explain these correlations (although causality remains a possibility)[56,57]. Increased visceral adiposity (particularly increased EATT), associated with insulin resistance and dysregulated adi
We acknowledge several limitations of our study. First, the cross-sectional design did not permit a prospective evaluation of liver-heart cross-talk, and a cause-effect inference. Second, the liver fibrosis status was assessed using two fibrosis scores (FIB4 and NFS), resulting in a relatively high proportion of patients being classified as having indeter
T2DM patients with markers of MASLD-related liver fibrosis exhibit lower EF and present indicators of diastolic dysfunction, cardiac hypertrophy and dyskinesia. Additionally, LVMi and LAD negatively correlated with serum SHBP concentrations.
The authors would like to thank the members of the Department of Cardiology at the Emergency County Clinical Hospital, Târgu Mureş for their technical support. The research was carried out with the support of the Advanced Center for Medical and Pharmaceutical Research (CCAMF) of the George Emil Palade University of Medicine, Pharmacy, Sciences and Technology of Târgu Mureș.
| 1. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. 2024;81:492-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 379] [Article Influence: 379.0] [Reference Citation Analysis (1)] |
| 2. | Cernea S, Raz I. NAFLD in type 2 diabetes mellitus: Still many challenging questions. Diabetes Metab Res Rev. 2021;37:e3386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Cernea S, Cahn A, Raz I. Pharmacological management of nonalcoholic fatty liver disease in type 2 diabetes. Expert Rev Clin Pharmacol. 2017;10:535-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Roden M. Mechanisms of Disease: hepatic steatosis in type 2 diabetes--pathogenesis and clinical relevance. Nat Clin Pract Endocrinol Metab. 2006;2:335-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 304] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 5. | Targher G, Corey KE, Byrne CD, Roden M. The complex link between NAFLD and type 2 diabetes mellitus - mechanisms and treatments. Nat Rev Gastroenterol Hepatol. 2021;18:599-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 502] [Article Influence: 125.5] [Reference Citation Analysis (0)] |
| 6. | Cernea S. NAFLD Fibrosis Progression and Type 2 Diabetes: The Hepatic-Metabolic Interplay. Life (Basel). 2024;14. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Ballestri S, Zona S, Targher G, Romagnoli D, Baldelli E, Nascimbeni F, Roverato A, Guaraldi G, Lonardo A. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:936-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 540] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 8. | Mantovani A, Petracca G, Beatrice G, Tilg H, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta-analysis of 501 022 adult individuals. Gut. 2021;70:962-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 308] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 9. | Jarvis H, Craig D, Barker R, Spiers G, Stow D, Anstee QM, Hanratty B. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of population-based observational studies. PLoS Med. 2020;17:e1003100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 284] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 10. | Wild SH, Morling JR, McAllister DA, Kerssens J, Fischbacher C, Parkes J, Roderick PJ, Sattar N, Byrne CD; Scottish and Southampton Diabetes and Liver Disease Group; Scottish Diabetes Research Network Epidemiology Group. Type 2 diabetes and risk of hospital admission or death for chronic liver diseases. J Hepatol. 2016;64:1358-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Tada T, Toyoda H, Sone Y, Yasuda S, Miyake N, Kumada T, Tanaka J. Type 2 diabetes mellitus: A risk factor for progression of liver fibrosis in middle-aged patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2019;34:2011-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Mikolasevic I, Milic S, Turk Wensveen T, Grgic I, Jakopcic I, Stimac D, Wensveen F, Orlic L. Nonalcoholic fatty liver disease - A multisystem disease? World J Gastroenterol. 2016;22:9488-9505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 146] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 13. | Wild SH, Walker JJ, Morling JR, McAllister DA, Colhoun HM, Farran B, McGurnaghan S, McCrimmon R, Read SH, Sattar N, Byrne CD; Scottish Diabetes Research Network Epidemiology Group. Cardiovascular Disease, Cancer, and Mortality Among People With Type 2 Diabetes and Alcoholic or Nonalcoholic Fatty Liver Disease Hospital Admission. Diabetes Care. 2018;41:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 14. | Wu S, Wu F, Ding Y, Hou J, Bi J, Zhang Z. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: A systematic review and meta-analysis. Sci Rep. 2016;6:33386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 225] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 15. | Mantovani A, Csermely A, Petracca G, Beatrice G, Corey KE, Simon TG, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: an updated systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:903-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 355] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 16. | Zhou YY, Zhou XD, Wu SJ, Hu XQ, Tang B, Poucke SV, Pan XY, Wu WJ, Gu XM, Fu SW, Zheng MH. Synergistic increase in cardiovascular risk in diabetes mellitus with nonalcoholic fatty liver disease: a meta-analysis. Eur J Gastroenterol Hepatol. 2018;30:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 17. | Saokaew S, Kanchanasurakit S, Thawichai K, Duangprom P, Wannasri M, Khankham S, Kositamongkol C, Chaiyakunapruk N, Phisalprapa P. Association of non-alcoholic fatty liver disease and all-cause mortality in hospitalized cardiovascular disease patients: A systematic review and meta-analysis. Medicine (Baltimore). 2021;100:e24557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, Ishigami M, Toyoda H, Wai-Sun Wong V, Peleg N, Shlomai A, Sebastiani G, Seko Y, Bhala N, Younossi ZM, Anstee QM, McPherson S, Newsome PN. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology. 2020;158:1611-1625.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 724] [Article Influence: 144.8] [Reference Citation Analysis (0)] |
| 19. | Morrison AE, Zaccardi F, Khunti K, Davies MJ. Causality between non-alcoholic fatty liver disease and risk of cardiovascular disease and type 2 diabetes: A meta-analysis with bias analysis. Liver Int. 2019;39:557-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 20. | Shabbirhussain BV, Singh S, Dixit VK, Verma A, Singh SK. Carotid intima media as predictor of liver fibrosis in type 2 diabetes mellitus with NAFLD. Diabetes Metab Syndr. 2022;16:102560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 21. | Lee H, Kim G, Choi YJ, Huh BW, Lee BW, Kang ES, Cha BS, Lee EJ, Lee YH, Huh KB. Association between Non-Alcoholic Steatohepatitis and Left Ventricular Diastolic Dysfunction in Type 2 Diabetes Mellitus. Diabetes Metab J. 2020;44:267-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Fan N, Ding X, Zhen Q, Gu L, Zhang A, Shen T, Wang Y, Peng Y. Association of the Non-Alcoholic Fatty Liver Disease Fibrosis Score with subclinical myocardial remodeling in patients with type 2 diabetes: A cross-sectional study in China. J Diabetes Investig. 2021;12:1035-1041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne CD, Castro Narro GE, Chowdhury A, Cortez-Pinto H, Cryer DR, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot BG, Korenjak M, Kowdley KV, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell EE, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78:1966-1986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1212] [Cited by in RCA: 1260] [Article Influence: 630.0] [Reference Citation Analysis (0)] |
| 24. | Younossi ZM, Paik JM, Stepanova M, Ong J, Alqahtani S, Henry L. Clinical profiles and mortality rates are similar for metabolic dysfunction-associated steatotic liver disease and non-alcoholic fatty liver disease. J Hepatol. 2024;80:694-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 141] [Article Influence: 141.0] [Reference Citation Analysis (0)] |
| 25. | Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3281] [Cited by in RCA: 3703] [Article Influence: 176.3] [Reference Citation Analysis (0)] |
| 26. | Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, Crews DC, Doria A, Estrella MM, Froissart M, Grams ME, Greene T, Grubb A, Gudnason V, Gutiérrez OM, Kalil R, Karger AB, Mauer M, Navis G, Nelson RG, Poggio ED, Rodby R, Rossing P, Rule AD, Selvin E, Seegmiller JC, Shlipak MG, Torres VE, Yang W, Ballew SH, Couture SJ, Powe NR, Levey AS; Chronic Kidney Disease Epidemiology Collaboration. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N Engl J Med. 2021;385:1737-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1478] [Cited by in RCA: 2249] [Article Influence: 562.3] [Reference Citation Analysis (0)] |
| 27. | Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1205] [Cited by in RCA: 1159] [Article Influence: 72.4] [Reference Citation Analysis (1)] |
| 28. | Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, Lindor K, Sanderson SO, Lenzi M, Adams LA, Kench J, Therneau TM, Day CP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1917] [Cited by in RCA: 2276] [Article Influence: 126.4] [Reference Citation Analysis (1)] |
| 29. | Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1-39.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6446] [Cited by in RCA: 9249] [Article Influence: 924.9] [Reference Citation Analysis (0)] |
| 30. | Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79:1757-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 523] [Article Influence: 174.3] [Reference Citation Analysis (0)] |
| 31. | Foppa M, Duncan BB, Rohde LE. Echocardiography-based left ventricular mass estimation. How should we define hypertrophy? Cardiovasc Ultrasound. 2005;3:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 175] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 32. | Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5:303-11; discussion 312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3259] [Cited by in RCA: 3131] [Article Influence: 94.9] [Reference Citation Analysis (0)] |
| 33. | Li W, Wen W, Xie D, Qiu M, Cai X, Zheng S, Huang Y. Association between non-alcoholic fatty liver disease and risk of incident heart failure: a meta-analysis of observational studies. Ther Adv Chronic Dis. 2022;13:20406223221119626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Inciardi RM, Mantovani A, Targher G. Non-Alcoholic Fatty Liver Disease as an Emerging Risk Factor for Heart Failure. Curr Heart Fail Rep. 2023;20:308-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 35. | Fudim M, Zhong L, Patel KV, Khera R, Abdelmalek MF, Diehl AM, McGarrah RW, Molinger J, Moylan CA, Rao VN, Wegermann K, Neeland IJ, Halm EA, Das SR, Pandey A. Nonalcoholic Fatty Liver Disease and Risk of Heart Failure Among Medicare Beneficiaries. J Am Heart Assoc. 2021;10:e021654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 36. | Jiang W, Liu Z, Liu S, Du T. Associations of advanced liver fibrosis with heart failure with preserved ejection fraction in type 2 diabetic patients according to obesity and metabolic goal achievement status. Front Endocrinol (Lausanne). 2023;14:1183075. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 37. | Cassidy S, Hallsworth K, Thoma C, MacGowan GA, Hollingsworth KG, Day CP, Taylor R, Jakovljevic DG, Trenell MI. Cardiac structure and function are altered in type 2 diabetes and non-alcoholic fatty liver disease and associate with glycemic control. Cardiovasc Diabetol. 2015;14:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Yong JN, Ng CH, Lee CW, Chan YY, Tang ASP, Teng M, Tan DJH, Lim WH, Quek J, Xiao J, Chin YH, Foo R, Chan M, Lin W, Noureddin M, Siddiqui MS, Muthiah MD, Sanyal A, Chew NWS. Non-alcoholic fatty liver disease association with structural heart, systolic and diastolic dysfunction: a meta-analysis. Hepatol Int. 2022;16:269-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 39. | Goliopoulou A, Theofilis P, Oikonomou E, Anastasiou A, Pantelidis P, Gounaridi MI, Zakynthinos GE, Katsarou O, Kassi E, Lambadiari V, Tousoulis D, Vavuranakis M, Siasos G. Non-Alcoholic Fatty Liver Disease and Echocardiographic Parameters of Left Ventricular Diastolic Function: A Systematic Review and Meta-Analysis. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Ghosh AK, Hughes AD, Chaturvedi N, Francis DP, Pellerin D, Deanfield J, Pierce M, Kuh D, Mayet J, Hardy RJ. 136 Increase in left ventricular mass in type 2 diabetes is dependent on duration of diabetes. Heart. 2012;98 Suppl 1:A77. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 41. | Palmieri V, Bella JN, Arnett DK, Liu JE, Oberman A, Schuck MY, Kitzman DW, Hopkins PN, Morgan D, Rao DC, Devereux RB. Effect of type 2 diabetes mellitus on left ventricular geometry and systolic function in hypertensive subjects: Hypertension Genetic Epidemiology Network (HyperGEN) study. Circulation. 2001;103:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 234] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 42. | Wang S, Zhang X, Zhang Q, Zhang B, Zhao L. Is non-alcoholic fatty liver disease a sign of left ventricular diastolic dysfunction in patients with type 2 diabetes mellitus? A systematic review and meta-analysis. BMJ Open Diabetes Res Care. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 43. | Li X, Yu S, Li L, Han D, Dai S, Gao Y. Cirrhosis-related changes in left ventricular function and correlation with the model for end-stage liver disease score. Int J Clin Exp Med. 2014;7:5751-5757. [PubMed] |
| 44. | Decoin R, Butruille L, Defrancq T, Robert J, Destrait N, Coisne A, Aghezzaf S, Woitrain E, Gouda Z, Schino S, Klein C, Maboudou P, Brigadeau F, Klug D, Vincentelli A, Dombrowicz D, Staels B, Montaigne D, Ninni S. High liver fibrosis scores in metabolic dysfunction-associated fatty liver disease patients are associated with adverse atrial remodeling and atrial fibrillation recurrence following catheter ablation. Front Endocrinol (Lausanne). 2022;13:957245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 45. | van Kleef LA, Lu Z, Ikram MA, de Groot NMS, Kavousi M, de Knegt RJ. Liver stiffness not fatty liver disease is associated with atrial fibrillation: The Rotterdam study. J Hepatol. 2022;77:931-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 46. | Käräjämäki AJ, Kettunen O, Lepojärvi S, Koivurova OP, Kesäniemi YA, Huikuri H, Ukkola O. Presence of atrial fibrillation is associated with liver stiffness in an elderly Finnish population. PLoS One. 2017;12:e0173855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 47. | Nakashima M, Tanakaya M, Miyoshi T, Saito T, Katayama Y, Sakuragi S, Ito H. The Fibrosis-4 Index Predicts Cardiovascular Prognosis in Patients With Severe Isolated Tricuspid Regurgitation. Circ J. 2022;86:1777-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Shibata N, Ito T, Toyoda H, Tanaka A, Morita Y, Kanzaki Y, Watanabe N, Yoshioka N, Yasuda S, Morishima I. Predictability of noninvasive liver fibrosis score for cardiac events in patients with nonalcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2024;34:2115-2123. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 49. | Kalme T, Seppälä M, Qiao Q, Koistinen R, Nissinen A, Harrela M, Loukovaara M, Leinonen P, Tuomilehto J. Sex hormone-binding globulin and insulin-like growth factor-binding protein-1 as indicators of metabolic syndrome, cardiovascular risk, and mortality in elderly men. J Clin Endocrinol Metab. 2005;90:1550-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 50. | Jianshu C, Qiongying W, Ying P, Ningyin L, Junchen H, Jing Y. Association of free androgen index and sex hormone-binding globulin and left ventricular hypertrophy in postmenopausal hypertensive women. J Clin Hypertens (Greenwich). 2021;23:1413-1419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Basualto-Alarcón C, Llanos P, García-Rivas G, Troncoso MF, Lagos D, Barrientos G, Estrada M. Classic and Novel Sex Hormone Binding Globulin Effects on the Cardiovascular System in Men. Int J Endocrinol. 2021;2021:5527973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Laurent MR, Hammond GL, Blokland M, Jardí F, Antonio L, Dubois V, Khalil R, Sterk SS, Gielen E, Decallonne B, Carmeliet G, Kaufman JM, Fiers T, Huhtaniemi IT, Vanderschueren D, Claessens F. Sex hormone-binding globulin regulation of androgen bioactivity in vivo: validation of the free hormone hypothesis. Sci Rep. 2016;6:35539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 53. | Fissore F, Fortunati N, Comba A, Fazzari A, Gaidano G, Berta L, Frairia R. The receptor-mediated action of sex steroid binding protein (SBP, SHBG): accumulation of cAMP in MCF-7 cells under SBP and estradiol treatment. Steroids. 1994;59:661-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | dos Santos RL, da Silva FB, Ribeiro RF Jr, Stefanon I. Sex hormones in the cardiovascular system. Horm Mol Biol Clin Investig. 2014;18:89-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 55. | Barrientos G, Llanos P, Basualto-Alarcón C, Estrada M. Androgen-Regulated Cardiac Metabolism in Aging Men. Front Endocrinol (Lausanne). 2020;11:316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Nakashima M, Nakamura K, Nishihara T, Ichikawa K, Nakayama R, Takaya Y, Toh N, Akagi S, Miyoshi T, Akagi T, Ito H. Association between Cardiovascular Disease and Liver Disease, from a Clinically Pragmatic Perspective as a Cardiologist. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 57. | Zhang WJ, Chen SJ, Zhou SC, Wu SZ, Wang H. Inflammasomes and Fibrosis. Front Immunol. 2021;12:643149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 58. | Badmus OO, Hinds TD Jr, Stec DE. Mechanisms Linking Metabolic-Associated Fatty Liver Disease (MAFLD) to Cardiovascular Disease. Curr Hypertens Rep. 2023;25:151-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 38] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 59. | Gutiérrez-Cuevas J, Santos A, Armendariz-Borunda J. Pathophysiological Molecular Mechanisms of Obesity: A Link between MAFLD and NASH with Cardiovascular Diseases. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 139] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 60. | Ahmed B, Farb MG, Gokce N. Cardiometabolic implications of adipose tissue aging. Obes Rev. 2024;e13806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 61. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021;75:659-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 993] [Cited by in RCA: 1039] [Article Influence: 259.8] [Reference Citation Analysis (0)] |
| 62. | Cusi K, Isaacs S, Barb D, Basu R, Caprio S, Garvey WT, Kashyap S, Mechanick JI, Mouzaki M, Nadolsky K, Rinella ME, Vos MB, Younossi Z. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract. 2022;28:528-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 561] [Article Influence: 187.0] [Reference Citation Analysis (1)] |