Published online Oct 26, 2024. doi: 10.4330/wjc.v16.i10.564
Revised: September 3, 2024
Accepted: September 27, 2024
Published online: October 26, 2024
Processing time: 223 Days and 2 Hours
Arterial cannulation sites for the surgical repair of type A aortic dissection (AAD) have evolved from right axillary artery (AA) cannulation to bilateral carotid artery (CA) based of femoral artery (FA) cannulation. Postoperative descending aorta remodeling is closely linked to the false lumen area ratio (FLAR), defined as false lumen area/aortic area, as well as to the incidence of renal replacement therapy (RRT).
To investigate the effect of the updated arterial cannulation strategy on des
A total of 443 AAD patients who received FA combined cannulation between March 2015 and March 2023 were included in the study. Of these, 209 received right AA cannulation and 234 received bilateral CA cannulation. The primary outcome was the change in FLAR, as calculated from computed tomography angiography in three segments of the descending aorta: Thoracic (S1), upper abdominal (S2), and lower abdominal (S3). Secondary outcomes were the incidence of RRT and the serum inflammation response, as observed by the levels of high sensitivity C reaction protein (hs-CRP) and Interleukin-6 (IL-6).
The postoperative/preoperative ratio of FLAR in S2 and S3 was higher in the AA group compared to the CA group (S2: 0.80 ± 0.08 vs 0.75 ± 0.07, P < 0.001; S3: 0.57 ± 0.12 vs 0.50 ± 0.12, P < 0.001, respectively). The AA group also had a significantly higher incidence of RRT (19.1% vs 8.5%, P = 0.001; odds ratio: 2.533, 95%CI: 1.427-4.493) and higher levels of inflammation cytokines 24 h after the procedure [hr-CRP: 117 ± 17 vs 104 ± 15 mg/L; IL-6: 129 (103, 166) vs 83 (69, 101) pg/mL; both P < 0.001] compared to the CA group.
The CA cannulation strategy was associated with better abdominal aorta remodeling after AAD repair compared to AA cannulation, as observed by a greater change in FLAR and lower incidence of RRT.
Core Tip: Arterial cannulation sites for the surgical repair of type A acute aortic dissection have gradually evolved from right axillary artery cannulation to bilateral carotid artery cannulation based on femoral artery cannulation. This retrospective observational study found the carotid artery cannulation strategy was associated with better postoperative abdominal aorta remodeling with a higher false lumen area ratio, a lower incidence of renal replacement therapy, and lower levels of inflammation cytokines compared with the axillary artery cannulation mode.
- Citation: Jiang Q, Yu T, Huang KL, Liu K, Li X, Hu SS. Carotid versus axillary artery cannulation for descending aorta remodeling in type A acute aortic dissection. World J Cardiol 2024; 16(10): 564-573
- URL: https://www.wjgnet.com/1949-8462/full/v16/i10/564.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i10.564
With the improved survival rate following surgery for acute type A aortic dissection (AAD), the therapeutic focus amongst cardiac surgeons has switched from mortality to morbidity[1]. It is now widely accepted that total aortic arch replacement concomitant with the frozen elephant trunk technique is the standard procedure for eliminating the risk of rupture of the proximal segment of AAD[2]. In theory, the perfect repair of AAD should result in elimination of the false lumen throughout the entire aorta and its branches. However, the aortic remodeling that is characteristic of a completely thrombosed false lumen is usually incomplete, especially in the distal descending aorta[3].
The onset and progression of AAD endangers blood perfusion of the involved branches in the descending aorta, leading to poor organ perfusion and triggering systemic inflammation responses[4]. The anatomical morphology of the descending aorta continues to change following repair of the proximal aorta, and poor aortic remodeling increases the risk of unfavorable events and the need for aortic reintervention[5]. Computed tomography angiography (CTA) is the standard imaging tool used to evaluate the preoperative and postoperative morphology of the whole aorta. The false lumen area ratio (FLAR), defined as false lumen area/aortic area, is an important quantitative index for determining the extent of aortic remodeling. The FLAR is evaluated using auxiliary software[6].
Different cannulation sites used to establish the cardiopulmonary bypass (CPB) have distinct hemodynamic characteristics, including the velocity, stress, and shear stress at the tear site[7]. Although the triple artery cannulation mode is used in clinical practice, few studies have used medical imaging results to explore the hemodynamic effects of more recent arterial cannulation modes. A study on AAD patients who received total arch replacement in addition to the frozen elephant trunk implantation technique compared bilateral carotid artery (CA) cannulation and right axillary artery (AA) cannulation in terms of neurologic protection[8]. However, the effect of using bilateral CA cannulation on descending aorta modeling was not examined, nor was it compared with AA cannulation in combination with femoral artery (FA) cannulation. Hence, the aim of the present study was to compare CA cannulation and AA cannulation in terms of changes in FLAR in the descending aorta.
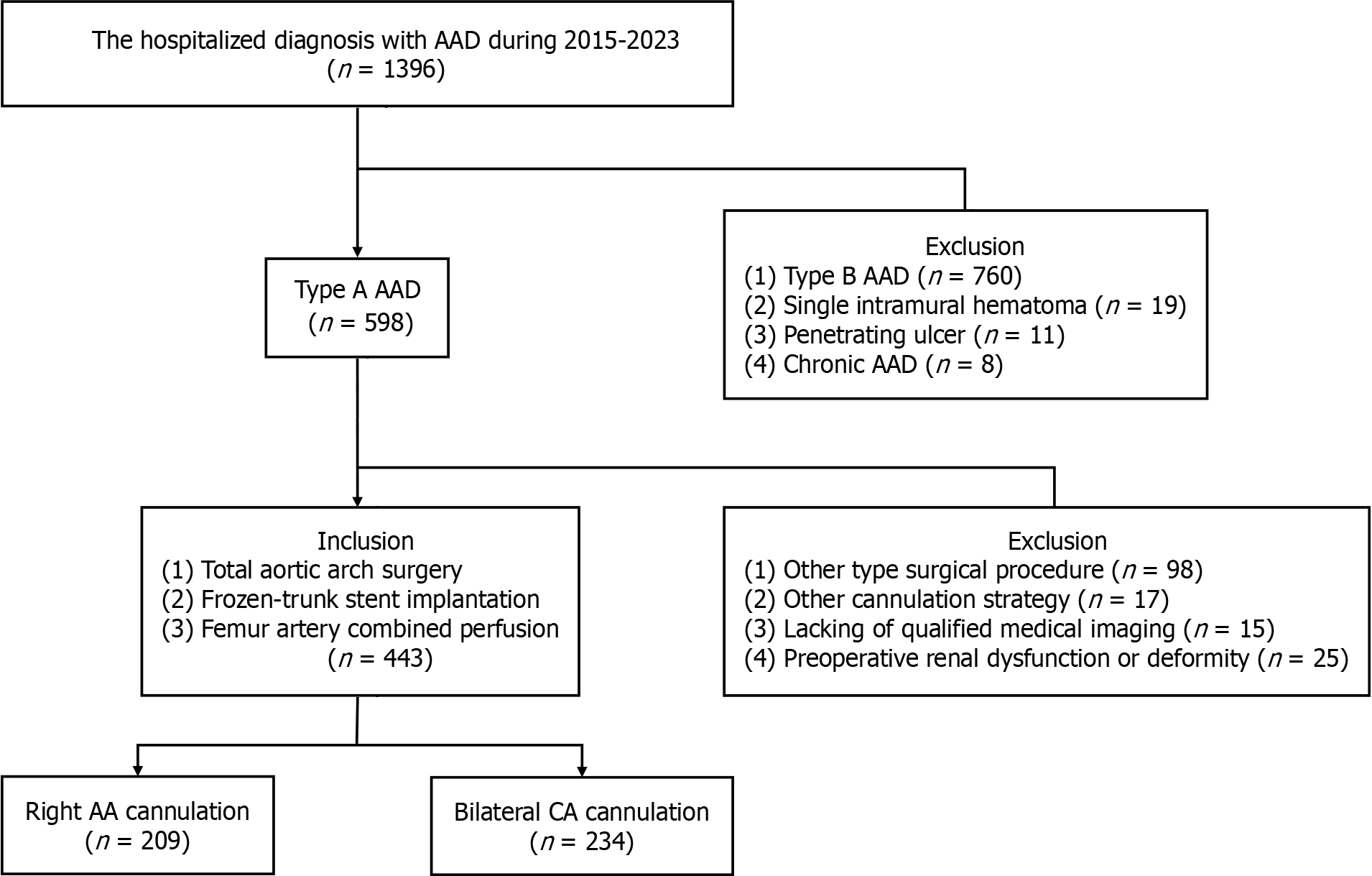
This retrospective observational study was carried out on AAD patients treated between March, 2015 and March, 2023 in the Cardiac Surgery Department of one tertiary hospital (Figure 1). Patients who received total aortic arch replacement and intraoperative frozen-trunk stent implantation surgery using femoral and right axillary/bilateral carotid artery cannulation were reviewed. The inclusion criteria included the availability of preoperative and postoperative CTA data on the entire aorta in the imaging system. The exclusion criteria were preoperative kidney disease or deformity, other surgical or cannulation type, and unqualified images or datasets. Patients were assigned to the AA or CA groups according to the primary arterial cannulation mode. The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Medical Ethics Committee of our institute (Approval number: 2021-215; March 1, 2021). Patient consent was waived due to the retrospective nature of the study. The primary endpoint of the study was the postoperative/preoperative FLAR, as calculated by CTA software. Secondary outcomes were the incidence of renal replacement treatment (RRT), and the levels of two serum inflammation response markers: high sensitivity C reaction protein (hs-CRP), and interleukin-6 (IL-6)[9].
All procedures were carried out by the same surgeon under intravenous and inhaled anesthesia. Arterial cannulation was administered with the “Seldinger” technique on the normal region in order to enter into the true lumen[10]. The use of bilateral CA cannulation was updated from right AA cannulation due to the high incidence of stroke and mortality with the latter[8]. In the AA group, right AA cannulation and FA cannulation jointly provided perfusion for the entire body during the CPB. Cerebral perfusion was achieved via right AA cannulation by blocking the proximal end of the innominate artery. In the CA group, right and left CA cannulation in addition to FA cannulation was used to sustain systemic circulation during the CPB. The aortic arch was replaced by a tetra-furcated graft (Maquet, Rastatt, Baden-Württemberg, Germany), and an endovascular frozen-trunk stent with a soft edge (CRONUS, MicroPort Scientifc Corporation, Shanghai, China) was implanted into the descending aorta during the hypothermia circulatory arrest (HCA) period.
Aortic CTA extending from the CA to FA was routinely prescribed at hospital admission and before discharge[11]. FLAR was calculated from the preoperative and postoperative CTA results using an area measurement tool (Neusoft PACS/ RIS Version 5.5 Workstation) on three segments of the descending aorta: thoracic segment (S1), upper abdominal segment (S2), and lower abdominal segment below the ostium of the renal artery(S3)[12]. The aortic area (mm2) was quantified along the inside contours of the aortic wall in the axial plane. To ensure objectivity and consistency, FLAR was determined at the maximum false lumen cross-section by a single radiologist who was not directly involved in the study. In addition, the condition of the ostium of abdominal aortic branches was recorded preoperatively and postoperatively.
Venous blood samples for laboratory tests were drawn at the time of admission and 12 hours, 24 hours and 48 hours after the procedure. Inflammatory response markers such as white blood cells, neutrophils, and hs-CRP were evaluated[13]. Human cytokines including IL-6 were also measured by flow cytometry at 24 hours postoperatively.
Blood gas analysis was performed regularly during stay in the intensive care unit (ICU) to inform the pulmonary ventilation function and electrolyte balance. The plasma creatinine level was monitored each day and the urine output each hour during the ICU stay. The indications for RRT included an increase in the creatinine level to > 26.5 μmol/L within 48 hours after surgery, a urine output of < 0.5 mL/kg/h and lasting for 6 hours, a serum potassium concentration of > 6.0 mmol/L, or a serum HCO3− concentration of < 10 mmol/L[14].
Continuous variables were expressed as the mean ± SD, and categorical values as a number (frequency). Continuous parameters with a non-normal distribution as determined by the Kolmogorov-Smirnov test were expressed as the median (1st quartile, 3rd quartile). Statistical differences between two groups were assessed using the Student independent samples t test for normally distributed continuous variables, the Mann-Whitney U non-parametric test for non-normally distributed variables, and Fisher’s χ2 test for categorical variables. The likelihood of RRT in the two groups was determined by binary logistic regression analysis. Receiver Operating Characteristic (ROC) curve analysis was used to determine the ability of FLAR to predict the incidence of RRT. The cutoff value was determined as the maximum value of sensitivity and specificity, minus one. Statistical analyses were performed using SPSS software (version 25.0 SPSS, Inc, Chicago, IL, United States) and a P value of < 0.05 was set for statistical significance.
A total of 1396 patients were identified in the medical records as being admitted with a diagnosis of AAD. Amongst these were 598 cases of type A AAD. Patients who underwent other types of surgical procedures, such as hemiarch replacement (n = 98), or other cannulation strategies (n = 17) were excluded. Also excluded were patients with incomplete preoperative or postoperative CTA images (n = 15), or with preoperative kidney injury or deformity (n = 25). A total of 209 cases with AA cannulation and 234 cases with CA cannulation were included in the final study cohort (Figure 1). The baseline characteristics of the two groups were not significantly different (Table 1).
| Characteristics | AA group (n = 209) | CA group (n = 234) |
| Age (years) | 53 ± 9 | 53 ± 9 |
| Male | 147 (70.3) | 163 (69.7) |
| BMI (kg/m2) | 23.3 ± 2.2 | 23.1 ± 2.3 |
| Previous medical history | ||
| Hypertension | 158(75.6) | 183 (78.2) |
| Dyslipidemia | 46 (22.0) | 47 (20.0) |
| Involved branch arteries | ||
| Coronary artery | 20 (10.0) | 25 (10.7) |
| Brachiocephalic artery | 43 (20.6) | 51 (21.8) |
| Coeliac trunk artery | 80 (38.3) | 92 (39.3) |
| Superior mesenteric artery | 73 (34.9) | 83 (35.5) |
| Renal artery | 156 (74.6) | 169 (72.2) |
| Cardiac function | ||
| LAD (mm) | 37 [35,43] | 36 [34,40] |
| LVEDD (mm) | 51.6 ± 6.0 | 51.5 ± 5.8 |
| LVEF (%) | 55.6 ± 7.0 | 56.2 ± 7.1 |
| Laboratory findings | ||
| hs-CRP (mg/L) | 27.0 ± 12.4 | 27.0 ± 10.9 |
| hs-troponin I (ug/L) | 0.09 [0.04, 0.25] | 0.11 [0.06, 0.18] |
| Lactate (mmol/L) | 0.34 ± 0.13 | 0.32 ± 0.19 |
| Hb (g/L) | 134.2 ± 18.3 | 132.1 ± 17.1 |
| WBC (109/L) | 10.6 ± 2.2 | 10.9 ± 2.6 |
| Neutrophil (109/L) | 8.7 [7.5, 10.2] | 8.6 [7.8, 10.4] |
The operative characteristics (operative type, duration, blood product use) between the two groups were not significantly different (Table 2). However, the serum hs-CRP level at 12, 24, and 48 hours after the procedure was significantly higher in the AA group than in the CA group (all P < 0.01). The serum IL-6 level at 24 hours after the procedure was also significantly higher in the AA group than in the CA group [129 (103, 166) pg/mL vs 83 (69, 100) pg/mL, P < 0.001]. In addition, the AA group had a higher APACHE II score (18 ± 6 vs 17 ± 5, P = 0.028; Table 3).
| Characteristics | AA group (n = 209) | CA group (n = 234) |
| Surgical type on the root of aorta | ||
| Sinus repair (commissure suspension) | 113 (54.1) | 118 (50.4) |
| Bentall | 36 (17.2) | 38 (16.2) |
| Wheat | 18 (8.6) | 21 (9.0) |
| Carbrol (modified) | 42 (20.1) | 57 (24.4) |
| operative duration (min) | ||
| PT | 403 ± 25 | 402 ± 26 |
| CPB | 189 ± 20 | 186 ± 20 |
| ACC | 89 [79,102] | 88 [79,95] |
| HCA | 20 [18,22] | 20 [18,22] |
| Intraoperative allogenic transfusion | ||
| RBC (U) | 4 [4,6] | 4 [4,6] |
| PLT (U) | 0.6 ± 0.6 | 0.5 ± 0.6 |
| FFP (ml) | 621 ± 222 | 610 ± 196 |
| Cryoprecipitation (U) | 1.4 ± 1.9 | 1.2 ± 1.9 |
| Characteristics | AA group (n = 209) | CA group (n = 234) | P value |
| Inflammation response indexes | |||
| hs-CRP (12 hours, mg/L) | 70 ± 16 | 65 ± 15 | 0.001 |
| hs-CRP (24 hours, mg/L) | 117 ± 17 | 104 ± 15 | < 0.001 |
| hs-CRP (48 hours, mg/L) | 190 ± 16 | 183 ± 18 | < 0.001 |
| IL-6 (24 hours, pg/mL) | 129 [103,166] | 83 [69,101] | < 0.001 |
| Cardiac injury indexes | |||
| TnI (12 hours, ng/mL) | 10.6 ± 2.8 | 10.5 ± 2.5 | 0.606 |
| TnI (24 hours, ng/mL) | 5.7 [4.8, 6.7] | 5.7 [5.0, 6.9] | 0.612 |
| TnI (48 hours, ng/mL) | 3.0 ± 1.2 | 3.2 ± 1.0 | 0.227 |
| Anaerobic metabolism | |||
| Lactate (12 hours, mmol/L) | 9.0 ± 1.7 | 8.5 ± 1.6 | 0.003 |
| Lactate (24 hours, mmol/L) | 3.5 ± 0.8 | 3.2 ± 0.9 | 0.001 |
| Lactate (48 hours, mmol/L) | 2.1 ± 0.7 | 1.8 ± 0.6 | < 0.001 |
| ICU recovery | |||
| APACHE II score (24 hours) | 18 ± 6 | 17 ± 5 | 0.028 |
| Ventilation time (hour) | 49 ± 20 | 36 ± 18 | < 0.001 |
| Duration in ICU stay (day) | 4 [3,5] | 3 [2,3] | < 0.001 |
| Chest tube drainage (mL) | 906 ± 168 | 836 ± 163 | < 0.001 |
| Involved branch arteries | |||
| Coeliac trunk artery | 51 (24.4) | 37 (15.8) | 0.014 |
| Superior mesenteric artery | 56 (26.8) | 42 (17.9) | 0.025 |
| Renal artery | 121 (57.9) | 105 (44.9) | 0.006 |
| 30-day recovery | |||
| Death | 25 (12.0) | 13 (5.6) | 0.018 |
| Stroke | 35 (16.7) | 22 (9.4) | 0.023 |
| RRT | 40 (19.1) | 20 (8.5) | 0.001 |
| Duration in hospital stay (d) | 16 [14,19] | 14 [12,15] | < 0.001 |
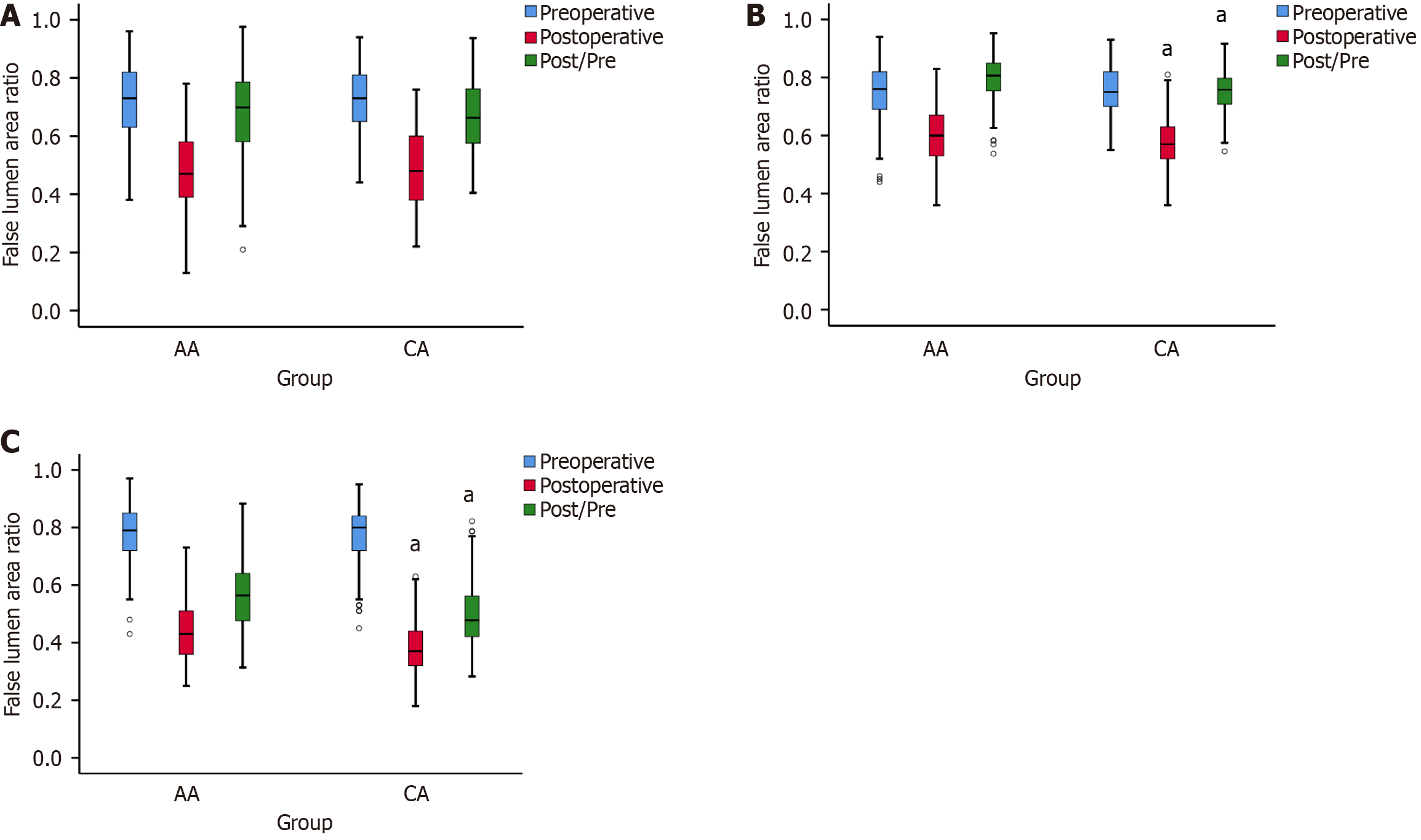
Preoperative CTA revealed no significant differences in FLAR between the AA and CA groups in three segments of the descending aorta (S1: 0.71 ± 0.12 vs 0.73 ± 0.11; S2: 0.75 ± 0.10 vs 0.76 ± 0.08; S3: 0.78 ± 0.10 vs 0.77 ± 0.10; all P > 0.05). However, a significant difference between the two groups was observed in S2 and S3 of the descending aorta (S2: 0.60 ± 0.10 vs 0.57 ± 0.08, P = 0.002; S3: 0.44 ± 0.11 vs 0.39 ± 0.10; P < 0.001; Figure 2 and Figure 3). The postoperative/preoperative ratio of FLAR in the S2 and S3 segments was higher in the AA group compared to the CA group (S2: 0.80 ± 0.08 vs 0.75 ± 0.07, P < 0.001; S3: 0.57 ± 0.12 vs 0.50 ± 0.12, P < 0.001; Figure 2 and Figure 3). The percentage of postoperative involvement of the coeliac trunk artery, superior mesenteric artery, and renal artery was markedly lower in the CA group compared to the AA group (Table 3).
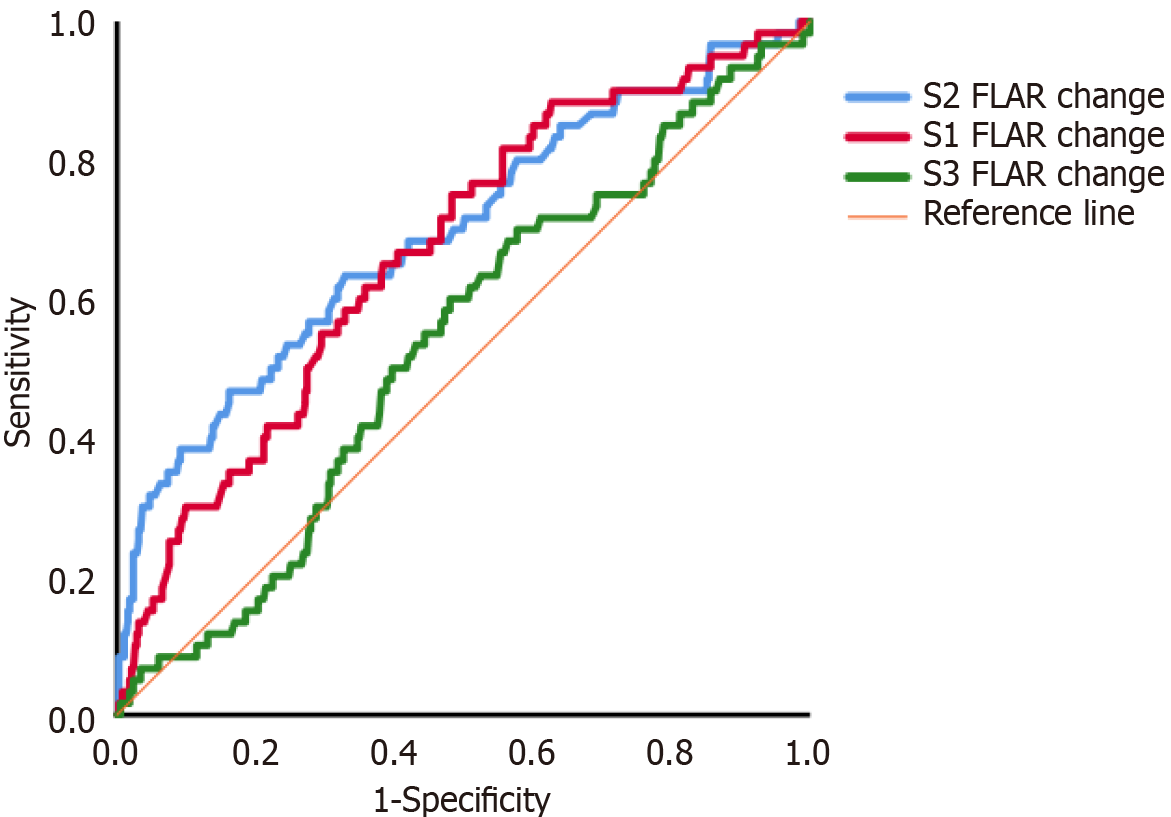
The incidence of RRT was 19.1% in the AA group and 8.5% in the CA group (P = 0.001), with an odds ratio of 2.533 (95%CI: 1.427-4.493). ROC analysis showed that postoperative/preoperative FLAR in S1, S2 and S3 was significantly better at predicting RRT than both the preoperative and postoperative FLAR. The ROC curve results for postoperative/preoperative FLAR in S1, S2 and S3 were 0.668 (95%CI: 0.595-0.741, P < 0.001), 0.693 (95%CI: 0.615-0.771, P < 0.001), and 0.535 (95%CI: 0.459-0.610, P > 0.05), respectively, for the prediction of RRT (Figure 4). The postoperative/preoperative FLAR cutoff value for the prediction of RRT was 0.712 for S1 (sensitivity of 0.650, specificity of 0.616), and 0.775 for S2 (sensitivity of 0.70, specificity of 0.514).
The three major findings of this study were: (1) Following surgical repair of AAD, the CA cannulation strategy resulted in a significantly lower FLAR in S2 and S3 of the descending aorta compared with AA cannulation in combination with FA cannulation; (2) The CA cannulation strategy also showed a lower incidence of RRT and inflammation response compared with AA cannulation in combination with FA cannulation; and (3) the change in FLAR in the upper abdominal aorta was predictive of postoperative RRT in patients who underwent total arch replacement and the frozen elephant trunk technique.
For the repair of AAD, it is widely accepted that the frozen elephant trunk technique gives superior results for descending aortic remodeling than total arch replacement alone. Tochii et al[15] reported that the ratio of the true lumen area and false lumen complete thrombosis rate in the segment of thoracic descending aorta were significantly higher in patients who underwent the frozen elephant trunk technique. For type A aortic dissection in Marfan syndrome, the frozen elephant trunk technique can also induce favorable remodeling in the distal aorta by expanding the true lumen[16]. However, a decreasing trend of aortic remodeling was observed in the descending aorta, with 85% at the middle of the frozen elephant trunk, 70% at the distal end of the frozen elephant trunk, 50% at the unstented thoracic aorta, and 35% proximal to the renal artery[17]. Failure to reduce the false lumen volume after endovascular repair of type B AAD predicted the likelihood of aortic distention and re-do surgery[18]. Therefore, modification of the arterial cannulation mode was one of the strategies used by the cardiovascular surgery community to enhance postoperative aortic remodeling.
The most commonly used arterial cannulation strategies used for type A aortic dissection are right AA, FA and both. In a previous study, combined cannulation with AA and FA provided advantages over a single cannulation strategy in terms of vascular wall injury, organ perfusion, and rapid cooling[19]. Patients undergoing unilateral cerebral perfusion have a higher risk of permanent cerebral dysfunction. This procedure is usually performed via right AA cannulation and by blocking the origin of the innominate artery after HCA. In a previous study, we suggested bilateral cerebral perfusion via bilateral CA cannulation[8]. This cannulation mode not only provides sufficient cerebral blood perfusion, particularly for patients without an intact Willis artery circuit, but also allows good blood supply of the branches in the descending aorta from FA, resulting in a lower incidence of RRT and low inflammation response indexes. Single FA perfusion in the descending aorta using this combined cannulation mode can eliminate the collision effect downward from AA perfusion by reducing the blood supply to the brachiocephalic trunk artery. Using an in vitro model, Heo et al[20] demonstrated that FA perfusion could compensate for the partial blood to the celiac and renal arteries when the intimal flap motion blocked the ostium of these branches in the descending aorta only in the axillary cannulation mode.
By monitoring the maximal cross-section diameter and false lumen area, CTA is the main imaging tool used to follow aortic modeling. Yamashita et al[21] found the predischarge maximal aortic diameter was a predictor of late aortic dilation in patients with residual dissected aorta after aortic replacement for AAD. Squizzato et al[22] demonstrated that aortic branch involvement and dilatation were associated with the patency of aortic false lumen and poor perfusion in patients with aortic dissection. Volume measurement was a much more sensitive indicator for identifying lumen expansion/shrinkage in the distal stented region[23]. However, specialized software is required to quantify the geometric area of the false lumen along the length of the aorta. The circumferential ratio of dissection at the cross-section was also associated with positive remodeling of the descending thoracic aorta following tear-oriented replacement for acute type I aortic dissection[24].
A previous study reported that the postoperative false lumen presented as an olive shape in the descending aorta during FA and AA cannulation mode, indicating the postoperative change in FLAR in S2 was less than that in S1 and S3[12]. In the present study, FLAR in S1 was not obviously different between the AA and CA cannulation modes. Moreover, the postoperative/preoperative FLAR values in S2 and S3 were lower with CA cannulation compared to the AA mode. Accordingly, the percentage involvement of the abdominal aorta branch ostium was also lower with CA cannulation. Furthermore, ROC analysis showed that postoperative/preoperative FLAR in S1 and S2 was predictive of RRT, with the strongest predictive ability found for S2. Other critical care indexes such as the APACHEⅡscore, duration of ICU stay, inflammation response[25] and anaerobic metabolism[26] were also lower with the bilateral CA and FA cannulation approach. We speculate that the sufficient bloodstream resulting from isolated FA perfusion in the descending aorta exerts a higher radially outward expanding force on the vessel wall compared with bilateral face-to-face perfusion in the AA and FA mode. This occurs especially at the abdominal aortic segment, which usually experiences inferior remodeling after surgical repair of AAD.
The main limitation of this study is its statistical reliability due to the single-center, retrospective study design. The CA approach was mostly utilized in the last four years, giving rise to selection bias. Another limitation was the lack of long-term follow-up for FLAR as a predictor of aortic remodeling. A longer follow-up period is therefore required to accumulate more meaningful data. Finally, FLAR was calculated based on CTA and was an independent predictor of hospitalized RRT events. This quantitative result was derived from the work of an experienced expert who gave a relatively precise evaluation after browsing the entire aorta. Calculation of the false lumen using deep learning-based reconstruction, or specialized software analysis of the irregular dissected aorta, may prove to be more accurate than manual analysis.
In patients undergoing AAD repair based on the FA cannulation strategy, we conclude the CA cannulation mode results in greater changes in FLAR in the S2 and S3 segments and a lower incidence of RRT compared with AA cannulation. Computational fluid dynamics should be used in future research to investigate how FLAR improvement is associated with the beneficial outcomes associated with bilateral CA and FA cannulation.
| 1. | Zhu Y, Lingala B, Baiocchi M, Tao JJ, Toro Arana V, Khoo JW, Williams KM, Traboulsi AA, Hammond HC, Lee AM, Hiesinger W, Boyd J, Oyer PE, Stinson EB, Reitz BA, Mitchell RS, Miller DC, Fischbein MP, Woo YJ. Type A Aortic Dissection-Experience Over 5 Decades: JACC Historical Breakthroughs in Perspective. J Am Coll Cardiol. 2020;76:1703-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 150] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 2. | Okada K. Total arch replacement: When and how? Asian Cardiovasc Thorac Ann. 2023;31:42-47. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Ikeno Y, Yokawa K, Yamanaka K, Inoue T, Tanaka H, Okada K, Okita Y. The fate of the downstream aorta after total arch replacement. Eur J Cardiothorac Surg. 2022;62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Xu H, Wang H, Wu L, Xu T, Han L, Lu F, Li B, Sun Y, Xu Z. Prognostic Value of Systemic Inflammation Response Index in Acute Type A Aortic Dissection. World J Surg. 2023;47:2554-2561. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Ismaguilova A, Martufi G, Gregory AJ, Appoo JJ, Herget EJ, Kotha V, Di Martino ES. Multidimensional Analysis of Descending Aortic Growth After Acute Type A Aortic Dissection. Ann Thorac Surg. 2021;111:615-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Kim JH, Lee SH, Lee S, Youn YN, Yoo KJ, Joo HC. Role of False Lumen Area Ratio in Late Aortic Events After Acute Type I Aortic Dissection Repair. Ann Thorac Surg. 2022;114:2217-2224. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Deng L, Qin H, Guan Z, Mu Q, Xia Q, Wang M, Huang WH, Gu K. Computational numerical analysis of different cannulation methods during cardiopulmonary bypass of type A aortic dissection model based on computational fluid dynamics. Ann Transl Med. 2021;9:667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 8. | Jiang Q, Huang K, Wang D, Xia J, Yu T, Hu S. A comparison of bilateral and unilateral cerebral perfusion for total arch replacement surgery for non-marfan, type A aortic dissection. Perfusion. 2023;2676591231161919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Jiang Q, Xiang B, Wang H, Huang K, Kong H, Hu S. Remote ischaemic preconditioning ameliorates sinus rhythm restoration rate through Cox maze radiofrequency procedure associated with inflammation reaction reduction. Basic Res Cardiol. 2019;114:14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Jiang Q, Yu T, Huang K, Liu L, Zhang X, Hu S. Feasibility, safety, and short-term outcome of totally thoracoscopic mitral valve procedure. J Cardiothorac Surg. 2018;13:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Jiang Q, Du J, Yu T, Huang X, Zuo M, Huang K. Ascending Aortic Aneurysm and Dissection Secondary to Bicuspid Aortic Valve with Concomitant Coarctation of Descending Aorta Successfully Repaired with Extracorporeal Membrane Oxygenation Support: A Case Report. CD. 2022;2:124-126. [DOI] [Full Text] |
| 12. | Jiang Q, Du J, Lei Y, Gu C, Hong L, Hu S. The relationship between false-lumen area ratio and renal replacement therapy after acute aortic dissection repair on bilateral artery cannulation: a cross-sectional study. Quant Imaging Med Surg. 2023;13:3104-3114. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Jiang Q, Liu SZ, Jiang L, Huang KL, Guo J, Hu SS. Comparison of two radiofrequency ablation devices for atrial fibrillation concomitant with a rheumatic valve procedure. Chin Med J (Engl). 2019;132:1414-1419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Wang Z, Ge M, Chen T, Chen C, Zong Q, Lu L, Wang D. Independent risk factors and the long-term outcomes for postoperative continuous renal replacement treatment in patients who underwent emergency surgery for type a acute aortic dissection. J Cardiothorac Surg. 2020;15:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Tochii M, Takami Y, Ishikawa H, Ishida M, Higuchi Y, Sakurai Y, Amano K, Takagi Y. Aortic remodeling with frozen elephant trunk technique for Stanford type A aortic dissection using Japanese J-graft open stent graft. Heart Vessels. 2019;34:307-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Chen Y, Ma WG, Zhi AH, Lu L, Zheng J, Zhang W, Liu YM, Zhu JM, Elefteriades JA, Sun LZ. Fate of distal aorta after frozen elephant trunk and total arch replacement for type A aortic dissection in Marfan syndrome. J Thorac Cardiovasc Surg. 2019;157:835-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Sato H, Fukada J, Tamiya Y, Mikami T. Morphometric Predictors of Aortic Remodeling after Frozen Elephant Trunk Repair of Type A Dissection. Ann Vasc Surg. 2022;84:179-186. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Kim TH, Ko YG, Kwon SW, Choi D, Lee DY, Shim WH, Hyon MS. Large false lumen area is a predictor of failed false lumen volume reduction after stent-graft repair in type B aortic dissection. J Endovasc Ther. 2014;21:697-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Kusadokoro S, Kimura N. Double arterial cannulation: a classical yet useful cannulation strategy-comment on cannulation strategy in frozen elephant trunk for type A aortic dissection: double arterial cannulation approach. Eur J Cardiothorac Surg. 2022;62. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Heo W, Lee GH, Kim TH, Lee Y, Huh H, Ha H, Song SW, Yoo KJ. Quantification of visceral perfusion and impact of femoral cannulation: in vitro model of aortic dissection. Eur J Cardiothorac Surg. 2022;62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Yamashita Y, Joo K, Okamoto K, Nakata Y, Ochiai Y, Tokunaga S. Postoperative Maximal Aortic Diameter is a Significant Predictor of Dilation of the Residual Dissected Aorta after Aortic Replacement for Acute Debakey Type I Aortic Dissection. Ann Vasc Surg. 2022;81:121-128. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Squizzato F, Oderich GS, Bower TC, Mendes BC, Kalra M, Shuja F, Colglazier J, DeMartino RR. Long-term fate of aortic branches in patients with aortic dissection. J Vasc Surg. 2021;74:537-546.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Wan Ab Naim WN, Sun Z, Liew YM, Chan BT, Jansen S, Lei J, Ganesan PB, Hashim SA, Sridhar GS, Lim E. Comparison of diametric and volumetric changes in Stanford type B aortic dissection patients in assessing aortic remodeling post-stent graft treatment. Quant Imaging Med Surg. 2021;11:1723-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Kim SY, Chang HW, Lee JH, Kim JS, Lim C, Park KH. Can preoperative features predict residual false lumen remodelling after tear-oriented limited resection for acute type I dissection? Eur J Cardiothorac Surg. 2022;62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Jiang Q, Yu T, Huang K, Huang X, Zhang Q, Hu S. The impact of medical insurance reimbursement on postoperative inflammation reaction in distinct cardiac surgery from a single center. BMC Health Serv Res. 2022;22:494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 26. | Jiang Q, Li H, Huang X, Yu L, Lueck S, Hu S. Postnatal exposure to hypobaric hypoxia and its impact on inflammation and injury indexes after a cardiac valve procedure. Interact Cardiovasc Thorac Surg. 2020;31:789-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |