Published online Oct 26, 2024. doi: 10.4330/wjc.v16.i10.550
Revised: August 25, 2024
Accepted: September 6, 2024
Published online: October 26, 2024
Processing time: 200 Days and 5.7 Hours
Heart failure (HF) is a chronic disease associated with high morbidity and mor
Core Tip: Heart failure (HF) is associated with high morbidity and mortality rates. Sodium glucose cotransporter-2 inhibitors (SGLT-2is) are approved for diabetes mellitus (DM), and have also demonstrated improvement in renal and cardiovascular (CV) outcomes along with good glycemic control. Two landmark studies of SGLT-2is in patients with HF demonstrated improvement in HF hospitalization and CV mortality, irrespective of DM status. Subsequent clinical trials proved that SGLT-2is also benefit patients with HF with preserved ejection fraction with/without DM. Emerging positive data for SGLT-2is in acute HF and post-myocardial infarction scenarios have bolstered their pivotal role in the full diapason of HF.
- Citation: Bhandari M, Pradhan A, Vishwakarma P, Singh A, Sethi R. Sodium glucose cotransporter 2 inhibitors in the management of heart failure: Veni, Vidi, and Vici. World J Cardiol 2024; 16(10): 550-563
- URL: https://www.wjgnet.com/1949-8462/full/v16/i10/550.htm
- DOI: https://dx.doi.org/10.4330/wjc.v16.i10.550
Heart failure (HF) is the chronic outcome of several cardiac illnesses such as coronary artery disease, hypertension, repaired cyanotic congenital defects, and cardiomyopathies. HF is caused by the functional or structural impairment of ventricular filling or ejection[1]. Worldwide, there are approximately 37.7 million cases of HF, and the prevalence of this condition is increasing[2]. The classification of HF includes HF with reduced ejection fraction (HFrEF) (i.e. HF with a left ventricular ejection fraction [LVEF] of ≤ 40%), HF with mildly reduced ejection fraction (HFmrEF) (i.e. HF with LVEF of 41%-49%), and HF with preserved ejection fraction (HFpEF) (i.e. HF with LVEF of ≥ 50%). A new entity recently described in literature is HF with improved ejection fraction (i.e. HF with a baseline LVEF of ≤ 40%, a ≥ 10-point increase from baseline LVEF, and a second measurement of LVEF > 40%)[3]. The standard treatment for HFrEF comprises beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers with mineralocorticoid receptor blockers, and diuretics[4]. These medications have offered immense clinical benefits to patients with HFrEF and are supported by evidence from large clinical trials. All of these drugs were introduced into clinical practice in the early 1990s and 2000s. Since then, only angiotensin receptor neprilysin inhibitor (ARNI)-sacubitril-valsartan has shown superiority over angiotensin-converting enzyme (ACE) inhibitors in reducing cardiovascular (CV) mortality and HF hospitalization (HHF) in 2014[5]. Although strong evidence is available for the benefits of these drugs in HFrEF, they have failed to offer comparable benefits in patients with HFpEF. Table 1 depicts the number needed to treat (NNT) of major guideline-approved HF medications derived from the seminal trials. The much anticipated PARAGON-HF trial, which compared ARNI with valsartan, did not report significant benefits in patients with HFpEF. However, certain benefits were observed in women and in those with HFmrEF. Thus, evidence-based therapies to enhance the outcomes of patients with HFpEF are lacking[6].
| Study | Drug tested | Primary endpoints | Results | NNT | Year | Number of patients | Follow-up |
| EMPEROR-Reduced trial | Empagliflozin vs placebo | CV death or HF hospitalization | 19.4% vs 24.7% HR: 0.75 (95%CI: 0.65-0.86) | 19 | 2020 | 3730 | 16 months |
| DAPA-HF trial | Dapagliflozin vs placebo | CV death or HF hospitalization | 16.3% vs 21.2% HR: 0.74 (95%CI: 0.65-0.85) | 21 | 2019 | 4744 | 18.2 months |
| SOLOIST-HF trial | Sotagliflozin vs placebo | CV death or HF hospitalization | 70% vs 98% HR: 0.67 (95%CI: 0.52-0.85) | 4 | 2021 | 1222 | 9 months |
| PARADIGM-HF trial | ARNI vs enalapril | CV death or HF hospitalization | 21.8% vs 26.5% HR: 0.80 (95%CI: 0.73-0.87) | 21 | 2014 | 8442 | 27 months |
| RALES trial | Spironolactone vs placebo | Death from all causes | 35% vs 46% HR: 0.70 (95%CI: 0.60-0.82) | 9 | 1999 | 1663 | 24 months |
| EMPHASIS-HF | Eplerenone vs placebo | CV death or HF hospitalization | 18.3% vs 25.9% HR: 0.63 (95%CI: 0.54-0.74) | 19 | 2011 | 2737 | 1.8 years |
| EPHESUS | Eplerenone vs placebo | Death any cause CV death or HF hospitalization | HR: 0.85 (95%CI: 0.75-0.96); HR: 0.87 (95%CI: 0.79-0.95) | 50 to prevent 1 death; 33 to prevent 1 CV death or HF hospitalization | 2003 | 6642 | 16 months |
| MERIT-HF trial | Metoprolol vs placebo | All-cause death | 7.2% vs 11% HR: 0.66 (95%CI: 0.53-0.81) | 27 | 1999 | 3991 | 2.4 years |
| CIBIS II-HF trial | Bisoprolol vs placebo | All-cause death HF hospitalization | 11.8% vs 17.3%; 33% vs 39% | 18; 17 | 1999 | 2647 | 1.3 years |
| COPERNICUS trial | Carvedilol vs placebo | All-cause death and HF hospitalization | 36.8% vs 44.7% | 13 | 2001 | 2289 | 10 months |
| CHARM trial | Candesartan vs placebo | CV death and HF hospitalization | 22% vs 24% HR: 0.89 (95%CI: 0.77-1.03) | 3023 | 36.6 months | ||
| VA-HEFT Trial | Valsartan vs placebo | Mortality plus morbidity | No difference 28.8% vs 32.1% HR: 0.87 (95%CI: 0.77-0.97) | 2001 | 5010 | 23 months | |
| SHIFT trial | Ivabradine vs placebo | CV death and HF hospitalization | 24% vs 29% HR: 0.82 (95%CI: 0.75-0.90) | 27 | 2010 | 6558 | 22.9 months |
| SOLVD trial | Enalapril vs placebo | Mortality HF hospitalization | 1991 | 2569 | 22-55 months |
Sodium glucose cotransporter-2 inhibitors (SGLT-2is), which were initially approved as antidiabetic agents, are now used to treat HF and constitute one of the four pillars of HF pharmacotherapy. The indication for SGLT-2is as major HF medications came after the landmark trial of dapagliflozin in HFrEF, which proved the potential of the drug in reducing CV outcomes irrespective of the presence or absence of diabetes[7,8]. Subsequently, the efficacy of empagliflozin, another SGLT-2i, was confirmed in a major trial on empagliflozin outcome trial in patients with chronic HF and a reduced ejection fraction (EMPEROR-Reduced), which showed that empagliflozin significantly reduced HHF regardless of the presence of diabetes; however, mortality reduction was not noted[9]. Moreover, the most recently published articles on SGLT-2is in HFpEF (Empagliflozin outcome trial in patients with chronic HF with preserved ejection fraction [EMPEROR-Preserved] and dapagliflozin evaluation to improve the lives of patients with preserved ejection fraction HF [DELIVER trial]) have shown promising results. Based on these findings, the latest guidelines recommend their use in HFpEF therapy[10,11].
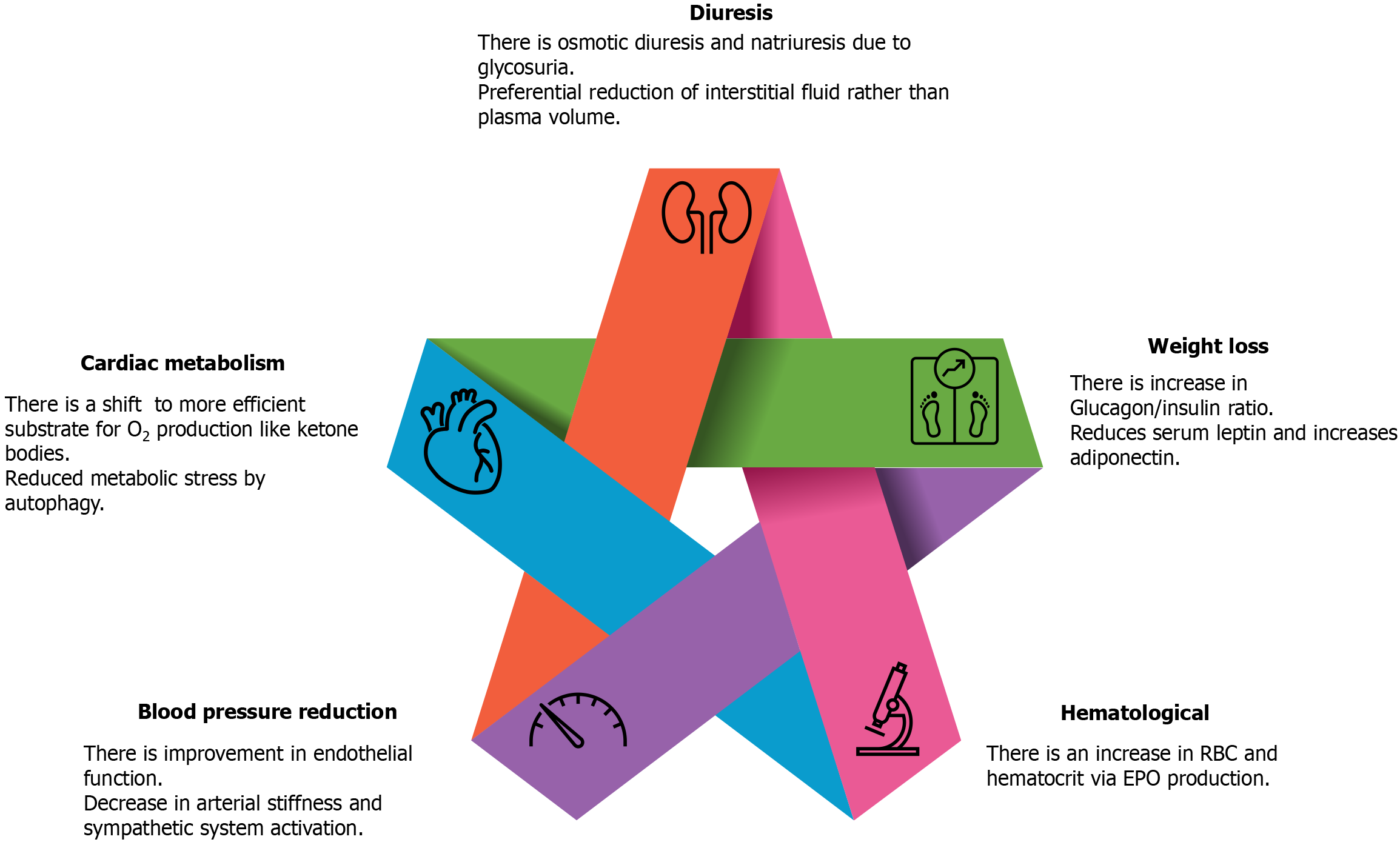
SGLT-2is are a class of drugs that inhibits SGLT-2 receptors in the proximal convoluted tubules of nephrons in the kidney, resulting in the failure of glucose reabsorption in the kidney. However, the benefits in CV outcomes cannot be explained by these simple mechanisms alone. Conventional and direct cardiac mechanisms of action of SGLT-2is can confer CV benefits (Figure 1).
Natriuresis and osmotic diuresis occur because of glucosuria. However, the coadministration of loop diuretics and dependence of the degree of osmotic diuresis and glycosuria on blood glucose levels indicate alternative mechanisms of benefit. Similar benefits were observed in patients without diabetes. Studies have suggested that SGLT-2is decrease only interstitial fluid and not plasma volume and thus can act synergistically[12,13]. SGLT-2is reduce blood pressure secondary to improvement in endothelial function, reduction in arterial stiffness, and alterations in sympathetic nervous activity[14,15]. However, these drugs exert only a modest antihypertensive effect. Weight loss occurs because of an increased glucagon: insulin ratio, which augments lipid mobilization[16,17]. Hematocrit and red blood cell mass increase with an elevation in erythropoietin production in the kidneys[18].
SGLT-2i therapies reverse adverse cardiac remodeling[19,20]. This effect has been demonstrated in patients with left ventricular hypertrophy and type 2 diabetes mellitus (T2DM) but not in those with HF, and a direct novel cardioprotective effect may be plausible[20,21]. Other effects include changes to more oxygen-efficient ketone bodies, cardiac metabolism of fatty acids, and glucose oxidation, which improve cardiac efficiency[22]. Furthermore, SGLT-2is inhibit sodium-hydrogen exchanger 1 and SGLT-1 transporters and improve the levels of cytosolic sodium[23,24]. Autophagy exerts a favorable effect on HF by alleviating metabolic stress. Continuous glycosuria simulates a state of nutrient depletion and catabolism, which induces autophagy[25,26]. SGLT-2is reduce the serum leptin-to-adiponectin ratio, exerting cardioprotective effects[27,28].
SGLT-2is were initially postulated to increase fasting ketone levels, which might act as an additional substrate for myocyte energy production; however, this theory was not supported by experimental data[29,30]. SGLT-2is maintain cytosolic calcium levels by inhibiting the sodium-hydrogen exchanger[23,24]. Certain preclinical studies have suggested that SGLT-2is induce a myocardial substrate switch, thereby improving myocardial energetics. However, in the EMPA-VISION trial (assessment of cardiac energy metabolism, function and physiology in patients with HF taking empagliflozin), treatment with 10 mg empagliflozin once daily for 12 weeks did not enhance cardiac energetics or alter the levels of circulating serum metabolites associated with energy metabolism compared with placebo. Thus, enhanced cardiac energy metabolism is unlikely to mediate the beneficial effects of SGLT-2is in HF[31].
The most common adverse effects of SGLT-2is are mycotic genital infections in women, urinary tract infections, nausea, and constipation. Other adverse events include lower limb amputation, which is especially seen with canagliflozin. Predisposing factors to limb amputation with the use of SGLT-2is are preexisting peripheral arterial disease, neuropathy, and diabetic foot ulcers. Hence, in patients with foot ulcers, SGLT-2is should be avoided or discontinued. The risk of euglycemic diabetic ketoacidosis (DKA) is also seen with SGLT-2is, which can be up to three-fold higher, and is again most noted with the use of canagliflozin. This finding could be attributed to noninsulin-dependent glucose clearance, hyperglucagonemia, and volume depletion. Thus, in patients with suspected DKA, the drug should be discontinued. A modest but reversible decrease in estimated glomerular filtration rate (eGFR) and rise in serum creatinine may also be noted in the initial period with the use of these drugs due to intravascular volume contraction. Therefore, the patient’s volume status should be corrected, especially in the elderly, before initiating treatment. Other rare side effects include bone fractures, bladder cancer (to be avoided in patients with active bladder cancer), and hyperkalemia.
The contraindications for therapy include T1DM, dialysis-dependent kidney disease and hypersensitivity reactions, such as anaphylaxis or angioedema, to any of the four agents.
The combined analysis of the canagliflozin cardiovascular assessment study (CANVAS) and CANVAS-renal trials, which compared CV events in patients with T2DM taking the SGLT-2i canagliflozin vs those taking placebo, was conducted as a part of the CANVAS program[32]. The findings showed a significant reduction in major adverse cardiac events (26.9 participants per 1000 patient-years in the canagliflozin group vs 31.5 per 1000 patient-years in the placebo arm). Equal benefits were observed in patients with HFrEF and those with HFpEF, with greater benefits in those with a history of HF.
The empagliflozin cardiovascular outcome event trial in T2DM patients (EMPA-REG outcome) was conducted to evaluate the CV safety of empagliflozin in patients with T2DM with atherosclerotic CV disease (ASCVD). The trial reported a 14% reduction in major adverse cardiovascular outcomes (MACEs) with empagliflozin compared with placebo, along with a relative risk (RR) reduction of 38% for CV deaths, 32% for all-cause deaths, and 35% for HHF[33].
The dapagliflozin effect on cardiovascular events-thrombolysis in myocardial infarction 58 (DECLARE-TIMI 58) study examined the CV safety of dapagliflozin in patients with T2DM having ASCVD or a high risk for the condition. Although dapagliflozin significantly improved glycemic control, it did not significantly reduce MACEs. Nonetheless, in patients with HFrEF, hospitalization and CV death rates significantly decreased[34].
In the effect of sotagliflozin on cardiovascular and renal events in patients with T2DM and moderate renal impairment who are at cardiovascular risk (SCORED) trial, 10584 patients with T2DM (glycated hemoglobin level ≥ 7%), chronic kidney disease (eGFR 25-60 mL/min/1.73 m2 of body surface area), and CV disease risk were randomly assigned in a 1:1 ratio to receive either sotagliflozin or placebo. The primary endpoint of this study was the composite of CV death, HHF, and urgent HF visits. The rates of primary endpoint events were 5.6 and 7.5 events per 100 patient-years in the sotagliflozin and placebo groups, respectively (hazard ratio [HR]: 0.74; 95% confidence interval [CI]: 0.63-0.88; P = 0.001). Diarrhea, DKA, genitourinary infections, and dehydration occurred more frequently in the sotagliflozin group than in the placebo group. Hence, in patients with diabetic nephropathy with or without albuminuria, sotagliflozin treatment reduced the rates of primary endpoints, especially HHF[35].
The meta-analysis performed by Zelniker et al[36] included data from 34322 patients (60.2% with established ASCVD) and three identified trials, namely, EMPA-REG outcomes, CANVAS program, and DECLARE-TIMI-58. A total of 3342 MACEs, 2028 CV deaths and HHF events, and 766 renal composite outcomes were documented. Although the rate of MACEs was reduced to 11% (HR: 0.89; 95%CI: 0.83-0.96; P = 0.0014), the benefit was evident only in patients with ASCVD (HR: 0.86; 95%CI: 0.80-0.93) and not in those without ASCVD (HR: 1.00; 95%CI: 0.87-1.16; P for interaction = 0.0501). However, the risk of CV death or HHF was decreased by 23% (HR: 0.77; 95%CI: 0.71-0.84; P = 0.0001) both in patients with and without established ASCVD and irrespective of the presence or absence of HF. Similarly, SGLT-2is alleviated the risk of renal disease progression by 45% (HR: 0.55; 95%CI: 0.48-0.64; P = 0.0001) regardless of the presence or absence of ASCVD. The extent of benefits offered by SGLT-2is in reducing HHF and the progression of renal impairment was the highest in patients with advanced renal disease[36].
Another meta-analysis of four trials of SGLT-2is in patients with diabetes conducted by Lo et al[37] demonstrated benefits in reducing CV events. This meta-analysis examined the results based on renal impairment. The pooled RR (95%CI) for the composite CV outcome was 0.93 (0.87-0.99) in the general study population (NNT: 167 and 0.89 (0.77-1.02) in patients with eGFR 60 mL/min/1.73 m2; that for all-cause mortality was 0.9 (0.84-0.97) with NNT = 143; that for CV death was 0.89 (0.81-0.99) in the general population and 0.82 (0.62-1.07) in patients with eGFR 60 mL/min/1.73 m2; and that for HHF was 0.71 (0.63-0.79) with NNT = 91. Regarding renal outcomes, the pooled RR (95%CI) for the composite renal outcome was 0.63 (0.56-0.71) with NNT = 67 in the general population and 0.67 with eGFR 60 mL/min/1.73 m2. In addition, the risk for albuminuria progression was reduced (RR = 0.80).
These meta-analyses confirm that SGLT-2is are associated with significantly lower MACEs, HHF, and all-cause mortality, with the strongest evidence for HHF reduction. Although the evidence was weaker in the population subset with eGFR 60 mL/min/1.73 m2, SGLT-2is significantly reduced the number of adverse renal events and also possibly retarded the progression of renal disease, with these effects being obvious even in the population with eGFR 60 mL/min/1.73 m2[37].
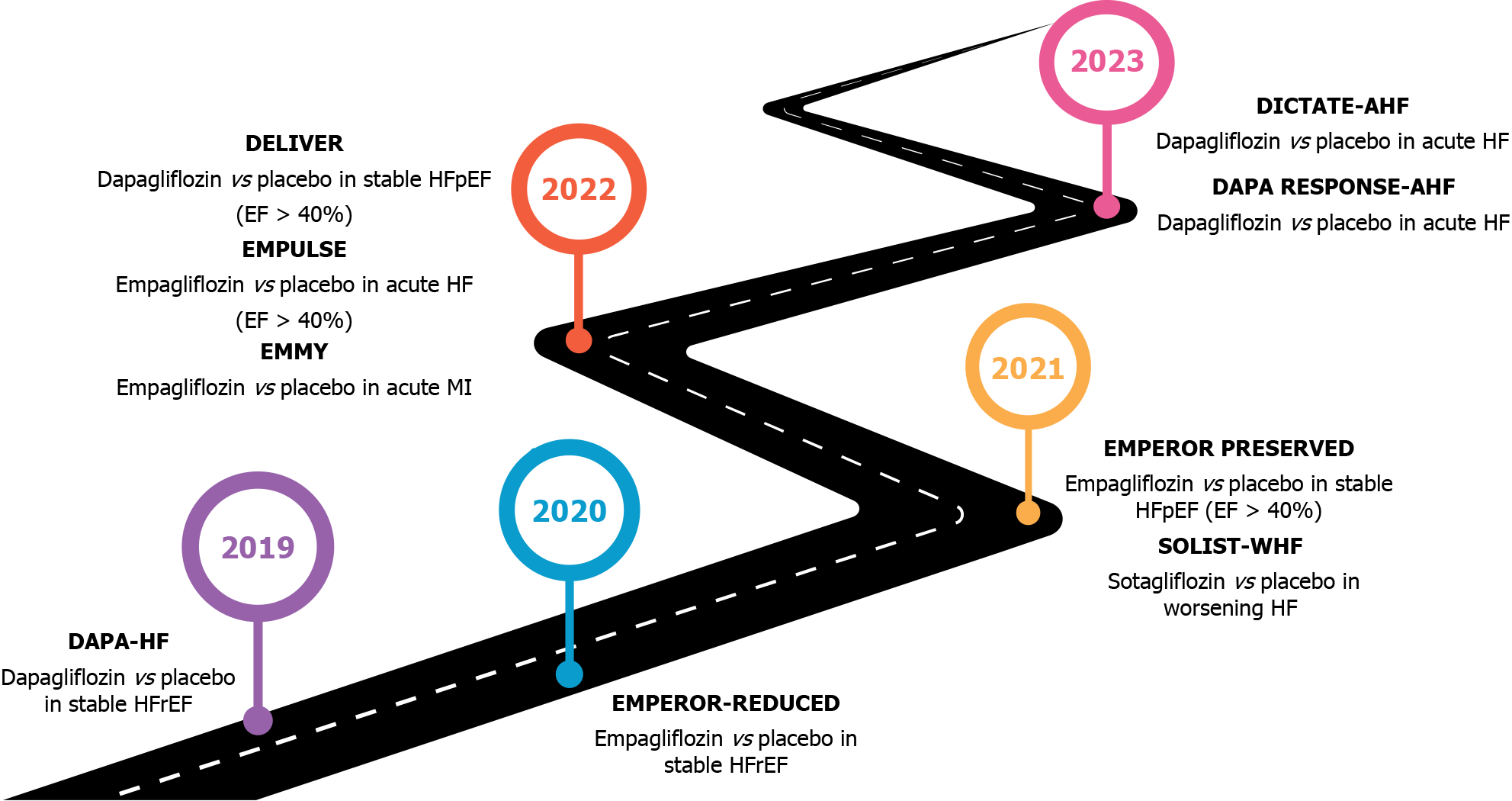
These trials are shown in Table 2 and Figure 2.
| Trial | Year | Number of patients | SGLT-2i used vs placebo | Endpoints | P value | |
| SGLT-2 arm | Placebo arm | |||||
| EMPAREG outcomes | 2015 | 7028 | Empagliflozin 10 or 25 mg | CV death, non-fatal MI or stroke: 10.5% | CV death, non-fatal MI or stroke: 12.1% | < 0.001 |
| All-cause mortality: 3.8% | All-cause mortality 5.1% | < 0.01 | ||||
| HHF: 2.7% | HHF: 4.1% | 0.002 | ||||
| DECLARE TIMI 58 | 2018 | 17160 | Dapagliflozin 10 mg | CV death, MI, stroke: 8.8% | CV death, MI, stroke: 9.4% | 0.17 |
| CV death or HHF: 4.9% | CV death or HHF: 5.8% | 0.005 | ||||
| CANVAS | 2017 | 10142 | Canagliflozin | Composite of CV death, non-fatal MI or stroke: 26.9% | Composite of CV death, non-fatal MI or stroke: 31.5% | < 0.0001 |
| CV death or HHF: 16.3% | CV death or HHF: 20.8% | |||||
| DAPA-HF | 2019 | 4744 | Dapagliflozin 10 mg | CV death or WHF: 16.3% | CV death or WHF: 21.2% | 0.001 |
| EMPEROR-Reduced | 2020 | 3730 | Empagliflozin 10 mg | CV death or HHF: 19.4% | CV death or HHF: 24.7% | < 0.001 |
| EMPEROR-Preserved | 2021 | 5988 | Empagliflozin 10 mg | CV death or HHF: 13.8% | CV death or HHF: 17.1% | < 0.001 |
| DELIVER | 2022 | 6263 | Dapagliflozin 10 mg | CV death or WHF: 16.4% | CV death or WHF: 19.5% | < 0.001 |
| SOLOIST WHF | 2021 | 1222 | Sotagliflozin | CV death, HHF, urgent visit for HF: 51% | CV death, HHF, urgent visit for HF: 76% | 0.001 |
| EMPA RESPONSE | 2020 | 80 | Empagliflozin 10 mg | Change in VAS dyspnea score, wt. change, change in NT-proBNP, hospital stay length: 10% | Change in VAS dyspnea score, wt. change, change in NT-pro-BNP, hospital stay length: 13% | 0.014 |
| EMPULSE | 2022 | 530 | Empagliflozin 10 mg | Net clinical benefit: 53.9% | Net clinical benefit: 39.7% | 0.0054 |
| CV death: 4.2% | CV death: 8.3% | |||||
| HF events: 10.6% | HF events: 14.7% | |||||
| Change in KCCQ-TSS: 4.5 | Change in KCCQ-TSS | 0.035 | ||||
| Wt. change: -1.5 Kg | Wt. change | 0.014 | ||||
| EMMY | 2022 | 476 | Empagliflozin 10 mg | Change in NT-pro-BNP: 15% lower vs placebo | 0.026 | |
| LVEF: 1.5% vs placebo | 0.029 | |||||
| E/e’: 6.8% vs placebo | 0.015 | |||||
| LVESV: 7.5 mL vs placebo | 0.0003 | |||||
| LVEDV: 9.7 mL vs placebo | 0.0015 | |||||
Dapagliflozin and prevention of adverse outcomes in HF (DAPA-HF): This trial randomized 4744 patients with New York Heart Association (NYHA) class II-IV symptoms and LVEF 40% to either dapagliflozin (10 mg once a day) or placebo along with guideline-directed medical therapy. The primary endpoint was a composite of CV death and hospitalization because of worsening HF symptoms. After a follow-up of 18.2 months, dapagliflozin significantly reduced the primary outcome (16.3% vs 21.2%, HR: 0.74, 95%CI: 0.65-0.85; P = 0.001). The first HF event occurred at a significantly lower rate with dapagliflozin than with placebo (10.0% vs 13.7%, HR: 0.70, 95%CI: 0.59-0.83). The incidence rate of CV death was 9.6% in the dapagliflozin group and 11.5% in the placebo arm (HR: 0.82, 95%CI: 0.69-0.98), whereas those of non-CV death were 11.6% and 13.9%, respectively (HR: 0.83, 95%CI: 0.71-0.97). These results remained the same irrespective of the patients’ diabetes status. The frequency of adverse events was comparable in both treatment groups[7].
EMPEROR-Reduced: In this trial, patients with NYHA II-IV HF and LVEF 40% were randomized to receive either empagliflozin (10 mg once daily) or placebo. The major endpoint was a composite of hospitalization, worsening HF symptoms, and CV death. After 16 months of follow-up, the primary outcome event occurred at a significantly lower rate with empagliflozin than with placebo (19.4% vs 24.7%, HR: 0.75, 95%CI: 0.65-0.86; P = 0.001). These benefits were noted irrespective of the patients’ glycemic status. Moreover, the rate of HHF was significantly lower with empagliflozin than with placebo (HR: 0.70, 95%CI: 0.58-0.85; P = 0.001)[8].
EMPEROR-Preserved: In this double-blind trial, 5988 patients with NYHA class II-IV HF and LVEF > 40% were randomized to receive either empagliflozin (10 mg once daily) or placebo in addition to the routine therapy and followed up for 2 years. The primary outcome was a combination of hospitalization for worsening HF symptoms and CV death. The findings indicated that empagliflozin significantly reduced the primary endpoints compared with placebo (13.8% vs 17.1%, HR: 0.79, 95%CI: 0.69-0.90; P = 0.001). These outcomes were predominantly driven by a reduction in the rate of HHF with empagliflozin (HR: 0.73, 95%CI: 0.61-0.88; P = 0.001) and were similar in patients with and without diabetes. Uncomplicated genital and urinary tract infections and hypotension were reported more often with empagliflozin than with placebo[10].
DELIVER trial: This trial comprised 6263 stable patients with HF who had LVEF of > 40% with or without diabetes. The patients received dapagliflozin 10 mg once daily or placebo in addition to guideline-directed medical therapy. Those with LVEF 40% and elevated natriuretic peptide levels with structural heart disease were eligible for the study. The time to first CV death or the worsening of HF events (HHF or urgent HF visits) was the primary endpoint. After a median follow-up of 2.3 years, the primary outcome occurred in 16.4% of the patients in the dapagliflozin arm and 19.5% in the placebo group (HR: 0.82, 95%CI: 0.73-0.92; P = 0.001). The rate of HF worsening was 11.8% vs 14.5% (HR: 0.79, 95%CI: 0.69-0.91) and that of CV death was 7.4% vs 8.3% (HR: 0.88, 95%CI: 0.74-1.05) in the dapagliflozin vs placebo group. Comparable results were obtained in the prespecified subgroups, including patients with and without diabetes, and the incidence of adverse events was also similar[11].
Effect of empagliflozin on exercise ability and HF symptoms in patients with chronic HF (EMPERIAL) trial: Patients with HFrEF (EF < 40%) (EMPERIAL-Reduced, n = 312) or HFpEF (EF 40%) (EMPERIAL-Preserved, n = 315), with and without T2DM, were randomized to receive either empagliflozin 10 mg or placebo for 12 weeks. The primary endpoint was a 6-minute walk test distance change at week 12. Key secondary endpoints included the Kansas city cardiomyopathy questionnaire total symptom score (KCCQ-TSS) and Chronic HF questionnaire self-administered standardized format dyspnea score. The 6-minute walk test distance median differences (95%CI) for the empagliflozin and placebo groups at week 12 were -4.0 meters (-16.0 to 6.0; P = 0.42) and 4.0 m (-5.0 to 13.0; P = 0.37) in EMPERIAL-Reduced and EMPERIAL-Preserved, respectively, which were nonsignificant. All secondary endpoints were considered exploratory, indicating an improvement only in the EMPERIAL-Reduced trial[38].
Although HFpEF is not as malignant as HFrEF, considerable morbidity and mortality are associated with it because of comorbidities. In a retrospective study of HFpEF, patients who were admitted for acute or chronic HF exhibited a readmission rate of 21%, which led to increased mortality and resource consumption. Thus, there is a need for better management of these patients, for which SGLT2-is can be helpful[39].
Sotagliflozin is a combined of SGLT-2 and SGLT-1 receptor inhibitor. SGLT-1 inhibition reduces postprandial glucose levels by delaying intestinal glucose absorption. In this trial, 1222 patients with HF and recent HF worsening were randomized in a 1:1 ratio to receive either sotagliflozin or placebo, with a median follow-up of 9 months. The patients received either sotagliflozin or placebo before discharge (48.8%) and at a median of 2 days after discharge (51.2%). The primary endpoint was a combination of CV death, hospitalization, and urgent visits for worsening HF (first and subsequent).
In total, 600 primary endpoint events were reported (245 in the sotagliflozin group and 355 in the placebo group). The rate of primary endpoint events was significantly reduced in the sotagliflozin group compared with the placebo group (51.0 vs 76.3, HR: 0.67, 95%CI: 0.52-0.85; P = 0.001). Moreover, the rates of CV death were 10.6% with sotagliflozin and 12.5% with placebo (HR: 0.84, 95%CI: 0.58-1.22) and those of non-CV death were 13.5% in the sotagliflozin group and 16.3% in the placebo group (HR: 0.82, 95%CI: 0.59-1.14). However, more episodes of diarrhea (6.1% vs 3.4%) and severe hypoglycemia (1.5% vs 3.0%) were reported in the sotagliflozin group than in the placebo group. Furthermore, the percentages of patients with hypotension (6.0% vs 4.6%) and acute renal injury (4.1% vs 4.6%) were slightly higher in the sotagliflozin group[40].
Effects of empagliflozin on clinical outcomes in patients with acute decompensated HF (EMPA-RESPONSE-AHF) trial: In this randomized, placebo-controlled, double-blind, parallel-group, multicenter pilot study, 80 patients with AHF with and without diabetes were randomized to receive either empagliflozin 10 mg/day or placebo for 30 days. The primary endpoints were alterations in the visual analog scale (VAS) dyspnea score, diuretic response (weight change per 40 mg furosemide), change in N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels, and the length of stay. The secondary outcomes comprised safety and clinical endpoints. The mean age was 76 years, 33% were women, 47% had de novo HF, and the median NT-proBNP level was 5236 pg/mL. Differences were not observed in the primary endpoints between the empagliflozin and placebo groups. Nevertheless, empagliflozin decreased the combined endpoint of in-hospital HF worsening, rehospitalization for HF, or death at 60 days compared with placebo (4 [10%] vs 13 [33%]; P = 0.014). Moreover, urinary output was higher in the empagliflozin group than in the placebo group. Empagliflozin was well tolerated, safe, and did not exert any adverse effects on the patients’ blood pressure or renal function[41].
Effects on clinical benefit, safety, and tolerability of once daily oral empagliflozin 10 mg compared to placebo, initiated in patients hospitalized for acute heart failure who have been stabilized (EMPULSE) trial: In this trial, patients with AHF who exhibited a systolic blood pressure of 100 mmHg and did not receive inotropic support during the last 24 hours were randomized to receive either empagliflozin 10 mg (n = 265) or placebo (n = 265). Any intravenous (IV) diuretic or vasodilator use was discontinued within the last 6 hours of randomization. Patients with NT-proBNP of ≥ 1600 pg/mL or BNP of ≥ 400 pg/mL during hospitalization or within 72 hours prior to admission were included. The median LVEF was 31%. The primary endpoints were the composite of death, number of HF events, time to first HF event, and the KCCQ-TSS score from baseline to 90 days (P = 0.0054). In patients with acute decompensated HF (ADHF), empagliflozin was linked to a significant clinical benefit at 90 days and resulted in improved weight loss (decongestion) compared with placebo[42].
Efficacy and safety of dapagliflozin in AHF (DICTATE-AHF): The DICTATE-AHF trial investigated the efficacy and safety of dapagliflozin initiated within 24 hours of hospital admission on the diuretic response in patients with hypervolemic ADHF. Adult patients with T2DM admitted to the hospital with ADHF and underwent current or planned treatment with IV loop diuretics were included in this study. The findings were presented at the European Society of Cardiology (ESC) congress 2023. Early initiation of dapagliflozin did not significantly improve the diuretic efficiency compared with structured routine care in patients with ADHF. However, it did not worsen any prespecified safety outcomes. Exploratory analyses revealed that the drug alleviated decongestion and resulted in early discharge from the hospital[43].
Effect of adjuvant dapagliflozin on improving the treatment of congestion in patients with AHF (DAPA-RESPONSE AHF): This randomized double-blind study included 87 patients with ADHF who presented with dyspnea. The patients were randomized to receive either dapagliflozin (10 mg/day, n = 45) or placebo (n = 42) for 30 days within 24 hours of admission. The primary outcome was the difference in the area under the curve (AUC) of the VAS dyspnea score between the groups over the first 4 days. The secondary endpoints were urinary sodium concentration 2 hours after randomization, percent change in NT-proBNP, cumulative urine output (UOP), and differences in mortality and hospital readmission rates. The results revealed that dapagliflozin significantly reduced the AUC of the VAS dyspnea score compared with placebo (3192.2 ± 1631.9 mm × hr vs 4713.1 ± 1714.9 mm × hr; P < 0.001). Moreover, the relative change in NT-proBNP compared with baseline was larger with dapagliflozin than with placebo (-34.89% vs -10.085%; P = 0.001). In addition, a higher cumulative UOP was observed with dapagliflozin on day 4 (18600 mL vs 13700 mL; P = 0.031). Dapagliflozin also reduced the rehospitalization rates within 30 days after discharge; however, it did not affect spot urinary sodium concentration, incidence of HF worsening, or mortality rates[44].
Empagliflozin in patients with acute myocardial infarction (EMMY) trial: In this randomized, double-blind trial, 476 patients with acute myocardial infarction (MI) were randomly assigned to receive either empagliflozin 10 mg or a matching placebo once daily within 72 hours of percutaneous coronary intervention (PCI). The primary endpoint was the change in NT-proBNP level over 26 weeks, and the secondary endpoint was alterations in echocardiographic parameters. The baseline median (interquartile range) NT-proBNP level was 1294 (757-2246) pg/mL. NT-proBNP reduction was significantly higher in the empagliflozin group than in the placebo group, which was 15% lower (95%CI: -4.4 to -23.6) after adjusting for baseline NT-proBNP level, sex, and diabetes status (P = 0.026). In addition, significant improvements were noted in LVEF, E/e’, and left ventricle volume. Seven patients (three in the empagliflozin group) were hospitalized for HF[45].
Sudden cardiac death (SCD) is the most devastating complication of HF. Current guidelines recommend intracardiac defibrillator device implantation in patients with HFrEF who have LVEF of ≤ 35% even after receiving optimized HF treatment for at least 3 months. ACE inhibitors/angiotensin II receptor blockers, beta-blockers, mineralocorticoid receptor antagonists, and ARNIs prevent adverse cardiac remodeling and thus reduce the risk of SCD. Moreover, SGLT-2is also reduce the risk of ventricular arrhythmia, thereby preventing SCD.
In a population-based cohort study involving 399810 patients with recently diagnosed T2DM, SGLT-2is decreased the risk of all-cause mortality and new-onset arrhythmias (17% lower risk of new-onset arrhythmia) compared with placebo[46]. Furthermore, the EMPA-REG outcome study reported a significant reduction in CV deaths, including SCD, with empagliflozin[47].
Moreover, post-hoc analysis of the DAPA-HF (dapagliflozin and prevention of adverse outcomes in HF) study indicated that patients on dapagliflozin [140/2373 patients (5.9%)] exhibited significantly fewer arrhythmic events and SCD than those on placebo [175/2371 patients (7.4%); HR: 0.79; 95%CI: 0.63-0.99; P = 0.037)[48]. The mechanism was, in this case too, a reduction in wall stress and adverse remodeling.
In a recent meta-analysis of 22 trials that comprised 52115 patients, SGLT-2is were found to alleviate the risks of atrial fibrillation and ventricular tachyarrhythmia by 18% and 28%, respectively[49].
The pathogenesis of hyperuricemia and gout is intricately linked to that of T2DM and HF. Visceral obesity, diabetes, and HF increase the incidence of hyperuricemia, which in turn exacerbates the risk of diabetes and HF. Hyperuricemia worsens glucose tolerance in patients with diabetes and causes ventricular dysfunction in those with HF. Nutrient surplus and signal deprivation are deranged, which leads to urate overproduction and underexcretion. SGLT-2is induce starvation mimicry in a state of nutrient surplus and decrease flux via the pentose phosphate pathway. These changes attenuate purine and urate synthesis and promote renal urate excretion, thus alleviating hyperuricemia and gout. Hence, the use of SGLT-2is may reduce the need for gout medications in patients with HF[50].
A multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy of empagliflozin in ADHF.
To evaluate the efficacy and safety of in-hospital initiation of empagliflozin in patients hospitalized for new-onset AHF, regardless of LVEF, for up to 90 days of follow-up.
A prospective, single arm, cohort study to evaluate the synergistic empagliflozin and furosemide in acutely decompensated HF patients complicated by hypovolemia and diuretic resistance.
To evaluate whether SGLT-2is is effective in reducing the size of infarction and myocardial remodeling in patients with AMI and at high risk of heart failure. SGLT-2is will be administered before PCI in patients with ST-elevation MI (STEMI) or non-STEMI, and infract size as well as LV end systolic volume will be assessed using cardiac magnetic resonance imaging.
To examine whether dapagliflozin use in patients waiting for heart transplant has any effect on soluble urokinase type plasminogen activation receptor-a biomarker useful both in acute kidney injury and HF.
To investigate the effects of dapagliflozin on volume status (assessed by change in relative plasma volume and blood volume) and vascular function (flow mediated dilatation and pulse wave velocity) in patients with congestive HF.
Although SGLT-2is were initially recommended for HFrEF, latest guidelines have extended their use to HFpEF. The 2022 American College of Cardiology/American Heart Association/Heart Failure Society of America guidelines for HF provide SGLT-2i class IA indications for HFrEF treatment. In patients with HFmrEF (41%-49%) and HFpEF, SGLT-2is are given a class 2A indication, which is also supported by the 2023 American College of Cardiology expert consensus decision pathway for the management of HFpEF. This guideline recommends that SGLT-2is should be started in all patients in the absence of any contraindications[51,52]. The 2023 Focused Update of ESC provides SGLT-2is a class IA indication in patients with HFrEF and HFmrEF to alleviate the risk of HHF or CV death. In addition, ESC provides a strong class IA indication for SGLT-2is in patients for HFpEF in this recent update[53].
SGLT-2is are a class of drugs that were initially introduced as antidiabetic medications but have recently become one of the four essential pillars of HF management. The efficacy of these inhibitors has been proven in the entire HF spectrum, irrespective of LVEF and diabetic status. The mechanisms underlying these benefits, although not well established, are believed to involve various cardiometabolic and biomolecular targets, in addition to the diuretic effects. These inhibitors offer early and sustained benefits without substantial side effects and should therefore be initiated at the earliest in HF management to reduce morbidity and mortality.
| 1. | Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13:368-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 791] [Cited by in RCA: 1231] [Article Influence: 136.8] [Reference Citation Analysis (0)] |
| 2. | Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gosselin R, Grainger R, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Ma J, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Donnell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA 3rd, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leòn FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5305] [Cited by in RCA: 5716] [Article Influence: 439.7] [Reference Citation Analysis (0)] |
| 3. | Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, Anker SD, Atherton J, Böhm M, Butler J, Drazner MH, Felker GM, Filippatos G, Fonarow GC, Fiuzat M, Gomez-Mesa JE, Heidenreich P, Imamura T, Januzzi J, Jankowska EA, Khazanie P, Kinugawa K, Lam CSP, Matsue Y, Metra M, Ohtani T, Francesco Piepoli M, Ponikowski P, Rosano GMC, Sakata Y, SeferoviĆ P, Starling RC, Teerlink JR, Vardeny O, Yamamoto K, Yancy C, Zhang J, Zieroth S. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 435] [Article Influence: 108.8] [Reference Citation Analysis (0)] |
| 4. | WRITING COMMITTEE MEMBERS; Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 1554] [Article Influence: 129.5] [Reference Citation Analysis (0)] |
| 5. | McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4078] [Cited by in RCA: 4656] [Article Influence: 423.3] [Reference Citation Analysis (0)] |
| 6. | Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin-Colet J, Cleland J, Düngen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP; PARAGON-HF Investigators and Committees. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2019;381:1609-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1097] [Cited by in RCA: 1540] [Article Influence: 256.7] [Reference Citation Analysis (0)] |
| 7. | McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381:1995-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 4284] [Article Influence: 714.0] [Reference Citation Analysis (0)] |
| 8. | Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383:1413-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1875] [Cited by in RCA: 3043] [Article Influence: 608.6] [Reference Citation Analysis (0)] |
| 9. | Sha S, Polidori D, Heise T, Natarajan J, Farrell K, Wang SS, Sica D, Rothenberg P, Plum-Mörschel L. Effect of the sodium glucose co-transporter 2 inhibitor canagliflozin on plasma volume in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2014;16:1087-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 195] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 10. | Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M; EMPEROR-Preserved Trial Investigators. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021;385:1451-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3009] [Cited by in RCA: 2601] [Article Influence: 650.3] [Reference Citation Analysis (0)] |
| 11. | Solomon SD, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Lindholm D, Wilderäng U, Öhrn F, Claggett B, Langkilde AM, Petersson M, McMurray JJV. Dapagliflozin in heart failure with preserved and mildly reduced ejection fraction: rationale and design of the DELIVER trial. Eur J Heart Fail. 2021;23:1217-1225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 206] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 12. | Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab. 2018;20:479-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 341] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 13. | Cherney DZ, Perkins BA, Soleymanlou N, Har R, Fagan N, Johansen OE, Woerle HJ, von Eynatten M, Broedl UC. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014;13:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 378] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 14. | Pfeifer M, Townsend RR, Davies MJ, Vijapurkar U, Ren J. Effects of canagliflozin, a sodium glucose co-transporter 2 inhibitor, on blood pressure and markers of arterial stiffness in patients with type 2 diabetes mellitus: a post hoc analysis. Cardiovasc Diabetol. 2017;16:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 15. | Nakayama H, Ohtsuka Y, Kawahara M, Nakamura Y, Iwata S, Yoshinobu S, Soga R, Oshige T, Kawano S, Kakino S, Tsuruta M, Tajiri Y, Yamada K. Changes in body composition during SGLT2 inhibitor treatment and their relevance to the improvement of insulin sensitivity. Diabetes Res Clin Pract. 2016;120:S50-S51. [DOI] [Full Text] |
| 16. | Iemitsu K, Iizuka T, Takihata M, Takai M, Nakajima S, Minami N, Umezawa S, Kanamori A, Takeda H, Kawata T, Ito S, Kikuchi T, Amemiya H, Kaneshiro M, Mokubo A, Takuma T, Machimura H, Tanaka K, Asakura T, Kubota A, Aoyagi S, Hoshino K, Ishikawa M, Obana M, Sasai N, Kaneshige H, Miyakawa M, Tanaka Y, Terauchi Y, Matsuba I. Factors Influencing Changes in Hemoglobin A1c and Body Weight During Treatment of Type 2 Diabetes With Ipragliflozin: Interim Analysis of the ASSIGN-K Study. J Clin Med Res. 2016;8:373-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Pinto LC, Rados DV, Remonti LR, Kramer CK, Leitao CB, Gross JL. Efficacy of SGLT2 inhibitors in glycemic control, weight loss and blood pressure reduction: a systematic review and meta-analysis. Diabetol Metab Syndr. 2015;7:A58. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Shi L, Zhu D, Wang S, Jiang A, Li F. Dapagliflozin Attenuates Cardiac Remodeling in Mice Model of Cardiac Pressure Overload. Am J Hypertens. 2019;32:452-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 19. | Lee HC, Shiou YL, Jhuo SJ, Chang CY, Liu PL, Jhuang WJ, Dai ZK, Chen WY, Chen YF, Lee AS. The sodium-glucose co-transporter 2 inhibitor empagliflozin attenuates cardiac fibrosis and improves ventricular hemodynamics in hypertensive heart failure rats. Cardiovasc Diabetol. 2019;18:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 20. | Verma S, Mazer CD, Yan AT, Mason T, Garg V, Teoh H, Zuo F, Quan A, Farkouh ME, Fitchett DH, Goodman SG, Goldenberg RM, Al-Omran M, Gilbert RE, Bhatt DL, Leiter LA, Jüni P, Zinman B, Connelly KA. Effect of Empagliflozin on Left Ventricular Mass in Patients With Type 2 Diabetes Mellitus and Coronary Artery Disease: The EMPA-HEART CardioLink-6 Randomized Clinical Trial. Circulation. 2019;140:1693-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 385] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 21. | Singh JSS, Mordi IR, Vickneson K, Fathi A, Donnan PT, Mohan M, Choy AMJ, Gandy S, George J, Khan F, Pearson ER, Houston JG, Struthers AD, Lang CC. Dapagliflozin Versus Placebo on Left Ventricular Remodeling in Patients With Diabetes and Heart Failure: The REFORM Trial. Diabetes Care. 2020;43:1356-1359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 22. | Verma S, Rawat S, Ho KL, Wagg CS, Zhang L, Teoh H, Dyck JE, Uddin GM, Oudit GY, Mayoux E, Lehrke M, Marx N, Lopaschuk GD. Empagliflozin Increases Cardiac Energy Production in Diabetes: Novel Translational Insights Into the Heart Failure Benefits of SGLT2 Inhibitors. JACC Basic Transl Sci. 2018;3:575-587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 276] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 23. | Lee TI, Chen YC, Lin YK, Chung CC, Lu YY, Kao YH, Chen YJ. Empagliflozin Attenuates Myocardial Sodium and Calcium Dysregulation and Reverses Cardiac Remodeling in Streptozotocin-Induced Diabetic Rats. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 24. | Uthman L, Baartscheer A, Bleijlevens B, Schumacher CA, Fiolet JWT, Koeman A, Jancev M, Hollmann MW, Weber NC, Coronel R, Zuurbier CJ. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na(+)/H(+) exchanger, lowering of cytosolic Na(+) and vasodilation. Diabetologia. 2018;61:722-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 423] [Cited by in RCA: 444] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 25. | Santulli G. Cardioprotective effects of autophagy: Eat your heart out, heart failure! Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Hawley SA, Ford RJ, Smith BK, Gowans GJ, Mancini SJ, Pitt RD, Day EA, Salt IP, Steinberg GR, Hardie DG. The Na+/Glucose Cotransporter Inhibitor Canagliflozin Activates AMPK by Inhibiting Mitochondrial Function and Increasing Cellular AMP Levels. Diabetes. 2016;65:2784-2794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 298] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 27. | Ghantous CM, Azrak Z, Hanache S, Abou-Kheir W, Zeidan A. Differential Role of Leptin and Adiponectin in Cardiovascular System. Int J Endocrinol. 2015;2015:534320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 28. | Wu P, Wen W, Li J, Xu J, Zhao M, Chen H, Sun J. Systematic Review and Meta-Analysis of Randomized Controlled Trials on the Effect of SGLT2 Inhibitor on Blood Leptin and Adiponectin Level in Patients with Type 2 Diabetes. Horm Metab Res. 2019;51:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 29. | Deng Y, Xie M, Li Q, Xu X, Ou W, Zhang Y, Xiao H, Yu H, Zheng Y, Liang Y, Jiang C, Chen G, Du D, Zheng W, Wang S, Gong M, Chen Y, Tian R, Li T. Targeting Mitochondria-Inflammation Circuit by β-Hydroxybutyrate Mitigates HFpEF. Circ Res. 2021;128:232-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 261] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 30. | Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, Ishikawa K, Watanabe S, Picatoste B, Flores E, Garcia-Ropero A, Sanz J, Hajjar RJ, Fuster V, Badimon JJ. Empagliflozin Ameliorates Adverse Left Ventricular Remodeling in Nondiabetic Heart Failure by Enhancing Myocardial Energetics. J Am Coll Cardiol. 2019;73:1931-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 437] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 31. | Hundertmark MJ, Adler A, Antoniades C, Coleman R, Griffin JL, Holman RR, Lamlum H, Lee J, Massey D, Miller JJJJ, Milton JE, Monga S, Mózes FE, Nazeer A, Raman B, Rider O, Rodgers CT, Valkovič L, Wicks E, Mahmod M, Neubauer S. Assessment of Cardiac Energy Metabolism, Function, and Physiology in Patients With Heart Failure Taking Empagliflozin: The Randomized, Controlled EMPA-VISION Trial. Circulation. 2023;147:1654-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 51] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 32. | Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, Barrett TD, Weidner-Wells M, Deng H, Matthews DR, Neal B. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6:691-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 461] [Article Influence: 65.9] [Reference Citation Analysis (0)] |
| 33. | Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7124] [Cited by in RCA: 8133] [Article Influence: 813.3] [Reference Citation Analysis (1)] |
| 34. | Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4652] [Cited by in RCA: 4141] [Article Influence: 690.2] [Reference Citation Analysis (0)] |
| 35. | Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Inzucchi SE, Kosiborod MN, Cherney DZI, Dwyer JP, Scirica BM, Bailey CJ, Díaz R, Ray KK, Udell JA, Lopes RD, Lapuerta P, Steg PG; SCORED Investigators. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N Engl J Med. 2021;384:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 762] [Article Influence: 190.5] [Reference Citation Analysis (0)] |
| 36. | Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1634] [Cited by in RCA: 1882] [Article Influence: 313.7] [Reference Citation Analysis (0)] |
| 37. | Lo KB, Gul F, Ram P, Kluger AY, Tecson KM, McCullough PA, Rangaswami J. The Effects of SGLT2 Inhibitors on Cardiovascular and Renal Outcomes in Diabetic Patients: A Systematic Review and Meta-Analysis. Cardiorenal Med. 2020;10:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 38. | Abraham WT, Lindenfeld J, Ponikowski P, Agostoni P, Butler J, Desai AS, Filippatos G, Gniot J, Fu M, Gullestad L, Howlett JG, Nicholls SJ, Redon J, Schenkenberger I, Silva-Cardoso J, Störk S, Krzysztof Wranicz J, Savarese G, Brueckmann M, Jamal W, Nordaby M, Peil B, Ritter I, Ustyugova A, Zeller C, Salsali A, Anker SD. Effect of empagliflozin on exercise ability and symptoms in heart failure patients with reduced and preserved ejection fraction, with and without type 2 diabetes. Eur Heart J. 2021;42:700-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 39. | Jha AK, Ojha CP, Krishnan AM, Paul TK. Thirty-day readmission in patients with heart failure with preserved ejection fraction: Insights from the nationwide readmission database. World J Cardiol. 2022;14:473-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (3)] |
| 40. | Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B; SOLOIST-WHF Trial Investigators. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N Engl J Med. 2021;384:117-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 753] [Cited by in RCA: 1225] [Article Influence: 306.3] [Reference Citation Analysis (0)] |
| 41. | Damman K, Beusekamp JC, Boorsma EM, Swart HP, Smilde TDJ, Elvan A, van Eck JWM, Heerspink HJL, Voors AA. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur J Heart Fail. 2020;22:713-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 299] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 42. | Kosiborod MN, Angermann CE, Collins SP, Teerlink JR, Ponikowski P, Biegus J, Comin-Colet J, Ferreira JP, Mentz RJ, Nassif ME, Psotka MA, Tromp J, Brueckmann M, Blatchford JP, Salsali A, Voors AA. Effects of Empagliflozin on Symptoms, Physical Limitations, and Quality of Life in Patients Hospitalized for Acute Heart Failure: Results From the EMPULSE Trial. Circulation. 2022;146:279-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 43. | ESC. DICTATE-AHF trial fails to meet primary endpoint with dapagliflozin in acute heart failure. ESC, 2023. [cited 2 September 2024] Available from: https://www.escardio.org/The-ESC/Press-Office/Press-releases/DICTATE-AHF-trial-fails-to-meet-primary-endpoint-with-dapagliflozin-in-acute-heart-failure. |
| 44. | Emara AN, Wadie M, Mansour NO, Shams MEE. The clinical outcomes of dapagliflozin in patients with acute heart failure: A randomized controlled trial (DAPA-RESPONSE-AHF). Eur J Pharmacol. 2023;961:176179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 45. | von Lewinski D, Kolesnik E, Tripolt NJ, Pferschy PN, Benedikt M, Wallner M, Alber H, Berger R, Lichtenauer M, Saely CH, Moertl D, Auersperg P, Reiter C, Rieder T, Siller-Matula JM, Gager GM, Hasun M, Weidinger F, Pieber TR, Zechner PM, Herrmann M, Zirlik A, Holman RR, Oulhaj A, Sourij H. Empagliflozin in acute myocardial infarction: the EMMY trial. Eur Heart J. 2022;43:4421-4432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 169] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 46. | Chen HY, Huang JY, Siao WZ, Jong GP. The association between SGLT2 inhibitors and new-onset arrhythmias: a nationwide population-based longitudinal cohort study. Cardiovasc Diabetol. 2020;19:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 47. | Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME® trial investigators. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J. 2016;37:1526-1534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 669] [Cited by in RCA: 719] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 48. | Curtain JP, Docherty KF, Jhund PS, Petrie MC, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Bengtsson O, Langkilde AM, Sjöstrand M, Solomon SD, McMurray JJV. Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA-HF. Eur Heart J. 2021;42:3727-3738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 141] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 49. | Li HL, Lip GYH, Feng Q, Fei Y, Tse YK, Wu MZ, Ren QW, Tse HF, Cheung BY, Yiu KH. Sodium-glucose cotransporter 2 inhibitors (SGLT2i) and cardiac arrhythmias: a systematic review and meta-analysis. Cardiovasc Diabetol. 2021;20:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 50. | Packer M. Hyperuricemia and Gout Reduction by SGLT2 Inhibitors in Diabetes and Heart Failure: JACC Review Topic of the Week. J Am Coll Cardiol. 2024;83:371-381. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 51. | Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e876-e894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 211] [Article Influence: 70.3] [Reference Citation Analysis (1)] |
| 52. | Kittleson MM, Panjrath GS, Amancherla K, Davis LL, Deswal A, Dixon DL, Januzzi JL Jr, Yancy CW. 2023 ACC Expert Consensus Decision Pathway on Management of Heart Failure With Preserved Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2023;81:1835-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 183] [Article Influence: 91.5] [Reference Citation Analysis (0)] |
| 53. | Marx N, Federici M, Schütt K, Müller-Wieland D, Ajjan RA, Antunes MJ, Christodorescu RM, Crawford C, Di Angelantonio E, Eliasson B, Espinola-Klein C, Fauchier L, Halle M, Herrington WG, Kautzky-Willer A, Lambrinou E, Lesiak M, Lettino M, McGuire DK, Mullens W, Rocca B, Sattar N; ESC Scientific Document Group. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023;44:4043-4140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 536] [Article Influence: 268.0] [Reference Citation Analysis (0)] |