Published online Jul 26, 2023. doi: 10.4330/wjc.v15.i7.328
Peer-review started: May 4, 2023
First decision: June 1, 2023
Revised: June 9, 2023
Accepted: June 21, 2023
Article in press: June 21, 2023
Published online: July 26, 2023
Processing time: 82 Days and 6.5 Hours
Heart failure with reduced ejection fraction (HFrEF) and nonalcoholic fatty liver disease (NAFLD) are two common comorbidities that share similar pathophy
Core Tip: This manuscript provides an overview of potential therapies for patients with coexisting heart failure with reduced ejection fraction and nonalcoholic fatty liver disease (NAFLD). The authors discuss the current research of pathogenesis in heart failure and NAFLD, as well as pharmacological therapies that have been shown benefits. We also discuss the potential role of diet, physical activity and novel therapies in managing these conditions.
- Citation: Arriola-Montenegro J, Beas R, Cerna-Viacava R, Chaponan-Lavalle A, Hernandez Randich K, Chambergo-Michilot D, Flores Sanga H, Mutirangura P. Therapies for patients with coexisting heart failure with reduced ejection fraction and non-alcoholic fatty liver disease. World J Cardiol 2023; 15(7): 328-341
- URL: https://www.wjgnet.com/1949-8462/full/v15/i7/328.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i7.328
Heart failure (HF) is a major clinical, economic, and public health concern worldwide. The prevalence of HF in the United States and Europe is estimated to be 1.5% to 1.9% of the population, reaching a considerable number among people aged > 65 years[1]. The predominant etiologies of HF include coronary artery disease (CAD), hypertension, tachyarrhythmia, and valvular disease, and as an additional emerging risk factor, non-alcoholic fatty liver disease (NAFLD)[2].
NAFLD represents evidence of hepatic steatosis (via imaging or histology) with a lack of secondary causes of hepatic fat accumulation and can be categorized as non-alcoholic fatty liver (Without evidence of hepatocellular injury) or Non-alcoholic Steatohepatitis (NASH: Hepatocellular injury with or without fibrosis)[3]. Most patients with NAFLD have associated conditions, such as obesity, diabetes mellitus, hypertension, and dyslipidemia. Recently, NAFLD has been associated with other conditions such as chronic kidney disease, osteoporosis, obstructive sleep apnea, psoriasis, colorectal cancer, and HF.
Some authors have mentioned that the association between NAFLD and cardiovascular disease (CVD) is inconsistent. They hypothesized that this connection might disappear after controlling for modifiable CVD risk factors[4]. However, there are strong arguments connecting NAFLD to CVD. Chiang et al[5] demonstrated that non-obese and relatively healthy subjects with NAFLD have an increased risk of developing cardiovascular events[5]. In addition to Chiang et al[5], other studies have shown that NAFLD patients have an increased risk of CVD after adjusting for major demographic, clinical, and metabolic confounders[6].
Furthermore, emerging epidemiological studies support a strong and independent association between NAFLD and HF. These studies estimated that the prevalence of HF in patients diagnosed with NAFLD is 6.4%, with a higher risk for HF preserved ejection fraction (HFpEF) than HF reduced ejection fraction (HFrEF)[7].
The association between HF and NAFLD involves similar processes in both HFpEF and HFrEF, which are mediated by inflammatory and fibrotic processes. The pathophysiological relationship between NAFLD and HFpEF is attributable, at least in part, to the secretion of adipokines and proinflammatory cytokines, such as leptin, which, at the level of the liver tissue, has profibrotic activity, and in the heart, it produces cardiac hypertrophy and endothelial dysfunction. Other important factors are tumor necrosis factor-α (TNF-α) and interleukin (IL) -6, which contribute to hepatocyte injury and NAFLD, whereas damaged hepatocytes release IL-33, which promotes a profibrotic effect. In the heart, IL-33 is released in response to myocardial fiber stretching[8].
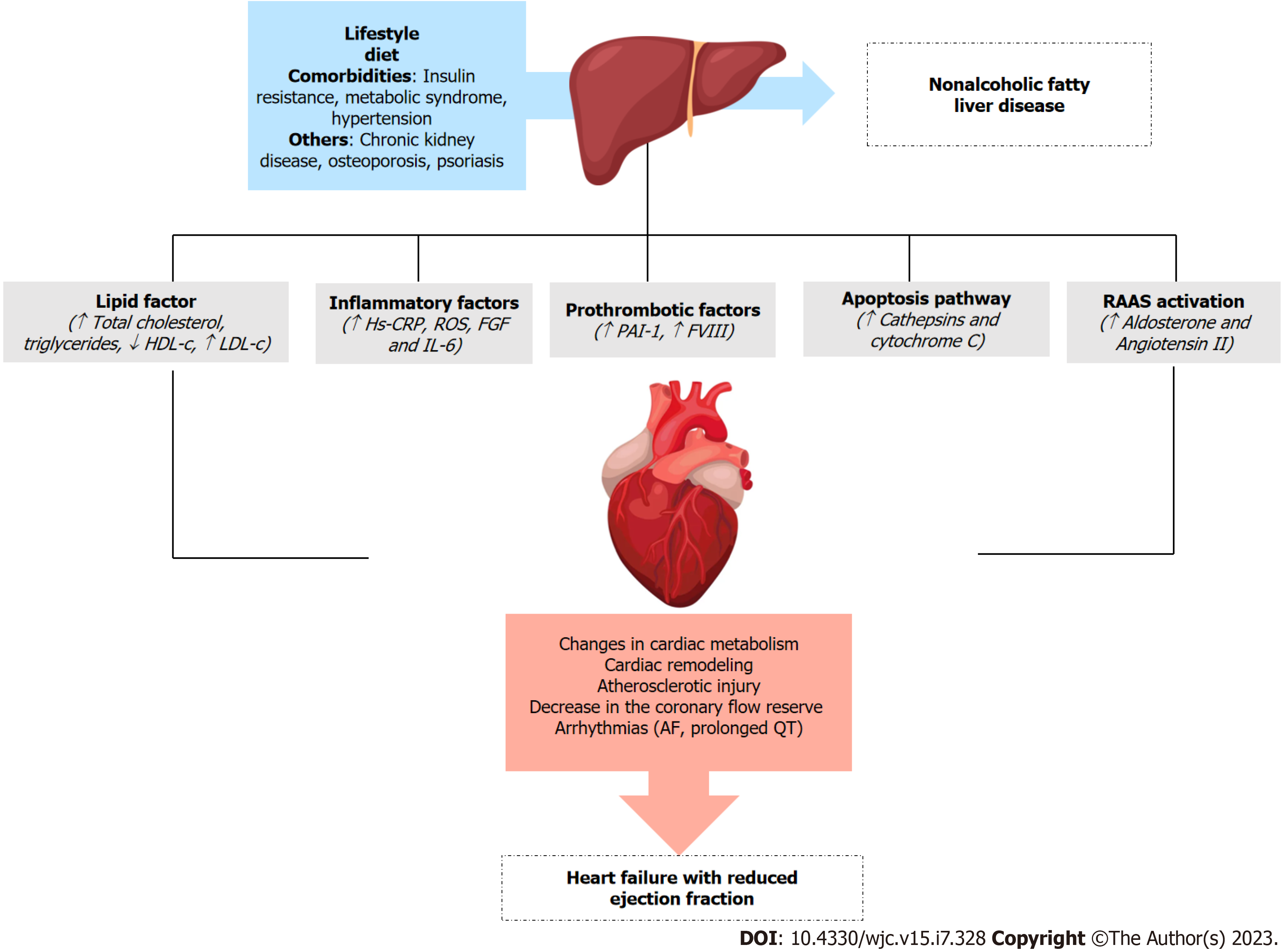
Several pathophysiological mechanisms have been proposed to explain this relationship. One potential mechanism by which fatty liver may increase the risk of HF is an increased prothrombotic state and systemic inflammation[8]. The hypercoagulable state of NAFLD is multifactorial and complex. Some studies have suggested that oxidative injury to lipids and lipoproteins may underlie thrombophilia[9]. Plasminogen activator inhibitor type 1 (PAI-1), the most thrombophilic factor reported, significantly increases with exposure to non-oxidized low-density lipoprotein (LDL) and is directly related to hepatic steatosis[10]. Another important process in this proinflammatory stress is the apoptotic pathway, which is activated in NASH as a result of fatty acid-mediated changes in the permeability of lysosomes and mitochondria with the release of cathepsins and cytochrome C, respectively. This activates the proapoptotic caspase cascade (fatty acid lipotoxicity), thereby resulting in a procoagulant state and contributing to atherosclerotic injury[11]. This process may explain why patients with NAFLD have a higher rate of major cardiovascular (CV) events (30% vs 8%)[12]. Additionally, NAFLD is associated with increased production of proinflammatory cytokines, such as IL-6 and high-sensitivity C-reactive protein (Hs-CRP), mitochondrial dysfunction eliciting reactive oxygen species (ROS) production, and stress biomarkers, such as fibroblast growth factors (FGFs), which increase the risk of CV and liver-related mortality[13,14] (Figure 1).
NAFLD is associated with high serum levels of total cholesterol, triglyceride, and LDL-cholesterol levels[15]. This dyslipidemia profile plays an important role in the pathogenesis of atherosclerosis. Nevertheless, existing data suggest that NAFLD per se might be an independent risk factor for CAD, even after adjusting for age, sex, traditional coronary risk factors, and visceral adipose tissue[16].
Cardiac structural and functional alterations are pivotal processes in HF in NAFLD patients. Most studies showed echocardiographic changes suggestive of left ventricular (LV) diastolic dysfunction, such as LV hypertrophy, increased left atrial volume, impaired LV relaxation, and higher left-sided filling pressures[17,18]. Furthermore, another study showed that hepatic steatosis and fibrosis are associated with diastolic dysfunction and are correlated with altered myocardial glucose uptake[19]. In addition, patients with NAFLD experience epicardial fat thickness, and both are at increased risk of coronary artery calcification[20].
Patients with NAFLD are also at an increased risk for cardiac arrhythmias, which can further increase the risk of LV dysfunction and HF. Cai et al[21] showed that NAFLD is associated with an increased risk of atrial fibrillation, and the strength of the association increases partially with the coexistence of cardiometabolic risk factors. In addition, Hung et al[22] found that mild, moderate, and severe NAFLD was associated with a high risk of heart rate-corrected QT (QTc) interval prolongation in both diabetic and nondiabetic subgroups. This mechanism is supported by the systemic inflammation and oxidative stress associated with NAFLD, which may trigger cardiac electrical and autonomic remodeling of the heart[23].
NAFLD and clinical CVD share similar risk factors (i.e., sedentary lifestyle, smoking, physiological stress, and sleep deprivation/disorders). Accumulation of visceral and ectopic fat leads to the release of toxic metabolites and the activation of inflammatory pathways, ultimately leading to both entities[24]. With progressive NAFLD, factors such as insulin resistance, activated renin-angiotensin-aldosterone system (RAAS), and oxidative stress markers have the potential to increase the risk of cardiac disease and HF[25]. Specifically, the RAAS system is activated as a compensatory mechanism in early HF owing to hypoperfusion and sympathetic activation, leading to a cascade of angiotensin II and aldosterone, which are responsible for increased preload and afterload at the expense of salt and water retention, cardiac remodeling, and vasoconstriction[26,27].
It has been found that liver disease, renal failure, and diabetes contribute to greater mortality in patients with HFrEF compared to patients with HFpEF[28]. However, a recent cohort study and meta-analysis demonstrated that patients with NAFLD are at an increased risk of incident HFpEF rather than HFrEF[7,29].
Simon et al[30] showed that patients with biopsy-proven NAFLD had a significantly higher incidence of HF across all stages of NAFLD. Likewise, in a recent meta-analysis, NAFLD patients had a lower ejection fraction than non-NAFLD patients and increased left ventricular mass and epicardial adipose thickness[31].
These findings support the notion that NAFLD is a “multisystem” disease with multiple potential pathophysiological mechanisms that may increase the risk of HF. Herein, we discuss the possible mechanism of ventricular dysfunction and its impact on the patient's lifestyle. Therefore, our review provides an overview of novel therapies for patients with coexisting HFrEF and NAFLD with the aim of developing future interventions to prevent and treat both diseases.
Lifestyle modifications, such as dietary changes, physical activity, and weight loss, are first-line treatments for NAFLD. These modifications affect body fat adipose deposits, which also influences the development of CV comorbidities[18]. It has been studied that HF is associated with splanchnic circulation congestion, which leads to bowel wall edema and impaired intestinal barrier function, which concomitantly promotes bacterial translocation and inflammation[32]. For example, trimethylamine N-oxide, an organic compound from gut bacteria, is an independent predictor of poor prognosis in patients with HF and is strongly linked to the pathogenesis of CVD[33].
Dietary change is one of the most important factors for the treatment of NAFLD and HF. Montemayor et al[34] concluded that customized hypocaloric dietary and enhanced physical activity interventions may be useful in ameliorate NAFLD[34]. The Mediterranean Diet, rich in vegetables, fruits, legumes, potatoes, non-refined cereals, fish, white meat, and red wine, seems to have a favorable association with NAFLD in Iranian adults, especially in women and patients with or without abdominal obesity[35]. The DASH diet, which is rich in antioxidants, micronutrients, fiber, and nitrates and has low saturated and trans fats, has been shown to decrease proinflammatory cytokines and ROS, restore micronutrient status, and promote endothelial function[36]. Belanger et al[37] demonstrated that a DASH diet progressively reduced high-sensitivity cardiac troponin I and Hs-CRP over 12 wk[37].
Bariatric surgery (BS) is one of the most effective treatments for obesity and its comorbidities[38]. The literature suggests that BS has also been associated with long-term improvements or even resolution of NAFLD in both clinical and histological features and has been shown to reduce CVD risk in patients with obesity by improving glucose tolerance and lipid panels[39-41]. Additionally, a recent meta-analysis showed that BS was associated with lower incidences of HF and myocardial infarction (MI)[42]. Another study showed that 96 months after BS, the cumulative incidence of HF was 4.2% and 11.5% in the surgical and non-surgical groups, respectively[43]. All these effects of BS seem to be related to changes triggered by gastrointestinal hormones such as Glucagon-like peptide 1 (GLP-1), gastric inhibitory polypeptide, leptin, gut hormone peptide YY, and ghrelin after anatomical intervention.
Additionally, changes in the gut microbiota are crucial for NAFLD[38]. Studies have shown a relationship between the gut microbiome and HF development[44]. This interplay involves gut microbial metabolites (which serve as mediators in HF pathophysiology), immune responses, and a vicious cycle caused by gut hypoperfusion in HF and subsequent additional microbiome alterations[33,44].
Currently, several therapies with strong evidence of benefit for HFrEF have also been reported to have an effect on NAFLD. Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) have been shown to reduce fibrosis and fat deposition in the liver. Mineralocorticoids receptor antagonist (MRA), such as spironolactone, have been reported to have a clear effect on the combination of this diuretic with vitamin E. Sodium-glucose cotransporter 2 inhibitors (SGLT-2i) have been reported to reduce liver stiffness and improve steatosis. Unfortunately, there are currently no data showing the beneficial effects of sacubitril/valsartan, ivabradine, hydralazine, isosorbide nitrates, digoxin, or beta-blockers (BB) on NAFLD (Table 1).
| Drug | HF phenotype with evidence of benefit | Effect on NAFLD Population | Comparator | Study |
| ACEI | HFrEF | Reduced liver-related events, liver cancer, and cirrhosis complications[47] | Placebo | Cohort |
| ARB | HFrEF | Trial failed to evidence that losartan 50 mg has antifibrotic effects on NASH due to widespread use[102] | Placebo | Trial |
| Patients with CKD-NAFLD taking ACEI or ARB had significantly lower liver stiffness degrees in comparison to those without those drugs[103] | Placebo | Cohort | ||
| Losartan 100 mg in children reduced alkaline phosphatase, but not ALT at 24 wk[104] | Placebo | Trial | ||
| Losartan 50 mg in children reduced ALT more frequently than those patients with placebo[105] | Placebo | Trial | ||
| Telmisartan 40 mg reduced free fatty acid level and increased liver-to-spleen ratio in diabetic patients with NAFLD[106] | Losartan | Trial | ||
| Telmisartan had similar effects to vitamin E in NAFLD histology[107] | Vitamin E | Trial | ||
| Telmisartan 40/80 mg improved NAFLD activity score and fibrosis in NASH[108] | Lifestyle modification | Trial | ||
| Telmisartan and olmesartan improved HOMA-IR and ALT levels[48] | Before-after comparison | Quasiexperimental | ||
| Losartan significantly decreased steatosis degree and visceral adipose tissue, addition of simvastatin further decreased those parameters[109] | Amlodipine and simvastatin | Trial | ||
| Amlodipine, lisinopril and rosuvastatin decreased ALT and alkaline phosphatase[110] | Therapy without rosuvastatin | Trial | ||
| Diuretics | HFrEF and congested HFpEF | In patients with NAFLD and diabetes lisinopril and hydrochlorothiazide were associated with less likelihood of advanced fibrosis, while furosemide and spironolactone had higher likelihood of it[111] | Other therapies | Cohort |
| Spironolactone and vitamin E reduced NAFLD liver fat score, insulin, and HOMA-IR[55,112] | Vitamin E alone | Trial | ||
| Five subjects received eplerenone. The study stopped early due to an unexpected increase in hepatic fat at 24 wk[113] | Open-label proof-of-concept study | |||
| SGLT2 inhibitors | HFrEF and HFpEF | Empagliflozin reduced liver stiffness measurement and steatosis (in patients with significant steatosis at baseline), liver fat level, AST, ALT and insulin in patients with NAFLD without diabetes[114] | Placebo | Trial |
| Tofogliflozin significantly improved the fibrosis scores, steatosis, hepatocellular ballooning, and lobular inflammation[115] | Glimepiride | Trial | ||
| Empagliflozin plus diabetes therapy better-improved liver fat in NAFLD patients with diabetes[116] | Diabetes therapy without empagliflozin | Trial | ||
| Dapagliflozin and omega-3 carboxylic acids reduced liver fat[117] | Placebo | Trial | ||
| Ipragliflozin as add-on diabetes therapy reduced liver steatosis in NAFLD patients with diabetes[118] | Metformin and pioglitazone | Trial | ||
| Empagliflozin was associated with reduction of ALT, liver stiffness and controlled attenuation parameter in patients with NAFLD and diabetes[119] | Before-after comparison | Cohort | ||
| Luseogliflozin improved liver-to-spleen ratio and liver fat in NAFLD patients with diabetes[120] | Metformin | Trial | ||
| Dapagliflozin and pioglitazone significantly increased liver-to-spleen ratio. Only dapagliflozin decreased visceral fat area in patients with NAFLD and diabetes[121] | Pioglitazone and glimepiride | Trial | ||
| Ipragliflozin reduced visceral fat area, but not AST or ALT, in patients with NAFLD and diabetes[122] | Pioglitazone | Trial |
ACEIs and ARBs block the effects of angiotensin II. These drugs are commonly prescribed to treat high blood pressure and HF. However, recent studies have suggested that ACEIs and ARBs may have beneficial effects on NAFLD[45]. Angiotensin II is a key contributor to abnormal lipid metabolism in NAFLD. Angiotensin II can worsen insulin sensitivity, generate ROS, and trigger the production of inflammatory cytokines such as TNF-α, IL-6, and PAI-1, all of which contribute to NAFLD progression[46]. For this reason, Zhang et al[47], in their retrospective cohort study of over 12000 patients with NAFLD, found that treatment with ACEIs for at least six months was associated with a lower risk of liver cancer and cirrhosis. Nevertheless, this effect was not seen with ARBs[42]. These data are surprising for Enjoji et al[48], who showed that ARBs can restore intracellular insulin signaling and facilitate the movement of excess fat from non-adipose tissues to adipocytes, which may improve markers of liver function such as transaminases, hepatic steatosis, and inflammation[48,49]. Furthermore, several clinical trials and meta-analyses have suggested that ACEIs and ARBs are effective in reducing mortality and hospitalization in patients with HFrEF and Advanced Kidney Disease[50]. Gilstrap et al[51] conducted a study to investigate the impact of BB and renin-angiotensin system inhibitors (RASi) on the outcomes of patients aged over 65 years with HFrEF. The study found that the use of BB and/or RASi at hospital discharge was associated with lower 30-d and 1-year mortality rates, even among patients aged > 85 years[51]. Similarly, the CHARM-Alternative trial investigated the use of candesartan vs placebo in patients with HF who were intolerant of ACE inhibitors. The study found that during a median follow-up of 3 years, hospitalization or cardiovascular-related death was reported in 33% of candesartan patients vs 40% of placebo patients[52].
Patients with NAFLD and HFrEF may experience beneficial outcomes with the use of aldosterone antagonists such as spironolactone and eplerenone. Wada et al[53] investigated the effects of eplerenone on nonalcoholic steatohepatitis and metabolic syndrome in a mouse model. The results showed that Eplerenone effectively ameliorated insulin resistance, blood pressure, and hepatic steatosis with fibrotic changes by inhibiting the inflammatory response in Kupffer cells and macrophages[53]. Similarly, spironolactone effectively improves the accumulation of triglycerides in the liver, reduces inflammation, and downregulates gluconeogenic and lipogenic gene expression[54]. Furthermore, combination therapy with spironolactone and vitamin E appears to have a positive effect on serum insulin levels in individuals with NAFLD[55].
Patients with HF and reduced ejection fraction may benefit from the use of MRA such as spironolactone, as they have been shown to reduce mortality when administered at low doses of 25 mg to prevent hyperkalemia[56]. However, the ATHENA-HF Trial found that a high dose of spironolactone or eplerenone may be a safe and effective treatment option for patients with HFrEF because it is associated with a reduction in NT-pro brain natriuretic peptide (BNP) levels, reduction in body weight, and improved symptoms of HF, such as dyspnea and fatigue, by reducing myocardial fibrosis and improving ventricular function[57,58]. Thus, evidence suggests that RAAS inhibitors, regardless of dose, may be particularly beneficial in patients with HFrEF.
SGLT-2i suppress glucose reabsorption in the proximal tubule of the kidney, resulting in excretion of glucose in the urine and improvement of insulin resistance. Initially developed as a diabetes mellitus therapy strategy independent of insulin[59]. The improvement in hyperglycemia and insulin resistance may be related to the control of lipogenesis through transcriptional regulation of lipogenic genes, including acetyl-CoA carboxylase and fatty acid synthase, and the development of hepatic steatosis[60].
Several studies have reported that SGLT-2i can inhibit the development of NAFLD and improve histological hepatic steatosis or steatohepatitis in experimental animal models[61]. Another possible mechanism of action of SGLT-2i in NAFLD is the weight-and visceral fat-dependent effects and inhibition of de novo lipogenesis in the liver[62].
SGLT-2i have been shown to reduce the risk of cardiovascular death or hospitalization in patients with HFrEF with or without type 2 diabetes mellitus (T2DM). There are several randomized controlled trials such as DAPA-HF, EMPEROR-Reduced, EMPULSE[63], EMPIRE-HF, SOLOIST-WHF[64], and CANVAS trials[65]. In addition to the most updated American College of Cardiology (ACC) guidelines for the management of HF, SGLT-2i has become a mainstay in the treatment of HFrEF and HFpEF.
A recent meta-analysis, including 1950 patients, evaluated liver structure and function in patients taking SGLT-2i with placebo or other oral antidiabetic drugs. It revealed a decrease in liver function tests (LFT), such as serum alanine and aspartate aminotransferases and gamma-glutamyl transferase, and a decrease in liver steatosis[66]. Another meta-analysis showed that SGLT-2i also reduced liver fat content and improved LFT in patients with NAFLD, as estimated by cardiac magnetic resonance proton density fat fraction[67].
These findings imply that SGLT-2i may be an effective treatment for patients with both NAFLD and HFrEF.
GLP-1 is an incretin hormone secreted in the gut in response to meal ingestion, which increases insulin secretion and inhibits glucagon production, targeting pancreatic β-cells. Consequently, GLP-1 receptor agonists improve hyperglycemia and delay gastric emptying, thereby promoting weight loss[68]. They can be an attractive therapeutic option for treating patients with NAFLD, particularly those with associated diabetes mellitus and obesity.
A multicenter, randomized, double-blind, placebo-controlled trial showed that liraglutide was associated with the resolution of NASH with no worsening of fibrosis score, improvement in steatosis, and hepatocyte ballooning score[69]. Liraglutide and exenatide have been attributed to decreases in trunk fat content, especially in the android region, which is associated with NAFLD and is closely associated with CVD risk[70,71].
In preclinical studies, some of the well-described effects of GLP-1 may reflect indirect mechanisms in the heart, such as augmentation of ventricular function in animals with HF or ischemia-induced ventricular dysfunction, attenuation of the development or progression of atherosclerosis or plaque formation, augmented myocardial or coronary artery blood flow rate control, reduced blood pressure, increased secretion of atrial natriuretic factor, and inhibition of platelet aggregation[72].
According to current guidelines, GLP-1 receptor agonists have no effect on the risk of HF hospitalization, which suggests that they are safe to use but are not beneficial in preventing HF in at-risk patients. Therefore, it should be used cautiously during acute decompensation[73].
Three small randomized controlled trials of GLP-1 receptor agonists were conducted in patients with HFrEF. The LIVE and FIGHT trials studied liraglutide vs placebo and showed no changes in left ventricular ejection fraction (LVEF), quality of life, or functional class at 24 wk. Albiglutide has also been studied and showed no significant differences in LVEF, BNP, 6-min walk test, myocardial glucose, or oxygen use[74]. GLP-1 RAs have a positive chronotropic effect, causing an increase in heart rate and induced increases in cAMP levels, which may worsen HF and increase the risk of death[75].
Although observations from treatment with GLP-1RAs and NAFLD suggest beneficial data, observations from randomized trials suggest no clear benefit in HF-related outcomes and even uncertainty regarding safety in patients with HFrEF. Larger studies of patients with HFrEF are recommended.
A novel medication for the treatment of T2DM, tirzepatide, a dual glucose-dependent insulinotropic polypeptide and a GLP-1 receptor agonist, has shown promising results in ongoing clinical trials, not only for T2DM but also for improving body weight and steatosis[76]. They compared its effects with those of dulaglutide on NAFLD biomarkers and fibrosis in patients with diabetes mellitus and found that a higher tirzepatide dose significantly decreased NAFLD biomarkers and increased adiponectin levels[77]. Additionally, SURPASS-3, using magnetic resonance imaging, demonstrated that tirzepatide significantly reduced liver fat content, visceral adipose tissue volume, and abdominal subcutaneous adipose tissue[78].
Currently, the efficacy and safety of tirzepatide in patients with HFpEF and obesity are being assessed[79]. No current data supports the use of tirzepatide in patients with HFrEF. Studies on patients with HFrEF are recommended.
Metformin is a biguanide that can improve insulin sensitivity and regulate glucose utilization by the liver[80]. Metformin treatment has been shown to be effective in alleviating hepatic lipogenesis in animal models of NAFLD through various mechanisms. However, in clinical studies, metformin modestly reduced body mass index, liver fat content, and liver enzyme levels in patients with NAFLD and diabetes. Despite these reports on the benefits of metformin, some contradictory results still exist. Despite these reports on the benefits of metformin, conflicting results remain. Combination treatments with other antidiabetic drugs, especially thiazolidinedione, GLP-1 receptor agonists, and SGLT2 inhibitors, demonstrated greater efficacy. Further research with a larger sample size is required to confirm these findings[81].
Left ventricular hypertrophy is a common finding in patients with ischemic heart disease and is associated with mortality in those with CVD. Metformin has been shown to reduce oxidative stress and left ventricular mass index. These results suggest a favorable effect of metformin on the left ventricular mass index and LVEF in patients with or without preexisting CVD[82]. In a recent report of diabetic patients with advanced HFrEF, patients treated with metformin demonstrated better quality of life and improved outcomes than patients not receiving metformin. Metformin remains one of the frontline drugs for the treatment of patients with HFrEF and Diabetes Mellitus[83].
The long-term clinical impact of metformin on HFrEF requires additional research despite its potential therapeutic effects on NAFLD.
Thizolidenidiones act as peroxisome proliferator-activated receptor-g activators in adipose, muscle, and liver tissues, resulting in a decrease in glucose production and subsequent increase in glucose utilization[84].
A recent meta-analysis compared placebo and pioglitazone, a thiazolidinedione, and found that it significantly improved steatosis grade, inflammation grade, and ballooning grade, whereas in the fibrosis stage, there was no significant improvement in pioglitazone compared with placebo. In addition, pioglitazone significantly reduced fasting blood glucose, glycosylated hemoglobin, serum alanine, and aspartate aminotransferase levels. Owing to the lack of relevant randomized controlled trials and short intervention times, long-term studies are needed to verify its efficacy and safety[85]. Another systematic review showed that pioglitazone consistently improved histological parameters and normalized liver transaminases, although the evidence supporting the benefits of other drugs in this class is minimal. Thiazolidinediones, particularly pioglitazone, have proven efficacious in patients with NAFLD/NASH[86].
Rosiglitazone has been shown to increase the risk of MI and HF, whereas pioglitazone decreased the risk of major adverse cardiovascular events, such as MI and stroke, but increased the risk of HF[87]. Pioglitazone was associated with higher rates of HF hospitalization in a smaller randomized controlled trial of participants with more severe symptomatic HFrEF than placebo[88]. Patients with T2DM with New York Heart Association functional class I-II CHF and reduced LVEF were randomized to 52 wk of treatment with rosiglitazone, and there were significantly more confirmed events of new or worsening edema and increased HF medication in the rosiglitazone group[89]. According to current guidelines for the treatment of HF from the ACC, given the existing evidence, thiazolidinediones should be avoided in patients with reduced LVEF[90].
In conclusion, although thiazolidinediones have demonstrated efficacy in patients with NAFLD, they are not recommended for diabetic patients at a high risk of HF, as they have been proven to increase the risk of HF in this group of patients.
Statins are safe for patients with NAFLD across the disease spectrum, including those with advanced liver disease, and lead to a demonstrable reduction in cardiovascular morbidity and mortality. The management of dyslipidemia in NAFLD should include the use of moderate- to high-intensity statins as first-line therapy, based on lipid risk levels and atherosclerotic ASCVD risk scores[91].
These medications can impair insulin sensitivity and secretion by pancreatic β-cells and increase insulin resistance in the peripheral tissues. Statins may also contribute to statin-induced T2DM[92]. Moreover, statin use is associated with a significant reduction in cardiovascular mortality and morbidity in both primary and secondary prevention strategies. A reduction in the risk of new-onset HF in patients with a high cardiovascular risk and hospitalization for HF has also been reported[25]. Rosuvastatin did not reduce the primary outcome or the number of deaths from any cause in older patients with systolic HF, although it did reduce the number of cardiovascular hospitalizations[93].
Statins may have a beneficial effect on CV outcomes irrespective of HF etiology and LVEF. Lipophilic statins (e.g., atorvastatin) and non-hydrophilic statins (e.g., rosuvastatin or pravastatin) showed significant reductions in clinical outcomes; however, lipophilic statins seem to be much more favorable for patients with HF[94].
Statins appear to have beneficial effects in NAFLD. Although there is the possibility of triggering T2DM, statins have more benefits than inconveniences in the treatment of NAFLD and reduce the risk of HFrEF.
Similarly, potential beneficial molecules for NAFLD are currently being investigated. Niacin, a vitamin derived from tryptophan metabolism, is known for its effect on dyslipidemia; however, recent studies have indicated that niacin reduces hepatic fat accumulation and steatosis, inflammation, and fibrosis by inhibiting diacylglycerol acyltransferase 2, an enzyme responsible for the synthesis of triglycerides, blocking the activation of hepatic stellate cells, and decreasing the activity of matrix metalloproteinases 2 and 9[95].
Sesquiterpene glycoside, an extract of the dried root Codonopsis pilosula, is a common drug used in traditional Chinese medicine because of its affordable cost and anti-inflammatory effects[96]. Therefore, a recent study provided evidence that the use of sesquiterpene glycosides in mice could protect against NAFLD in patients with T2DM. These findings were related to the repair of insulin signaling and inhibition of cytochrome P450 2E1 (CYP2E1) and NOD-like receptor family 3 in vivo and in vitro; thus, reducing oxidative stress, inflammation, and inflammatory cytokines and preventing insulin resistance[97].
Flavonoids (i.e. Baicalein, silymarin, rutin, and quercetin) has also shown hepatic protection by modulating the function of CYP2E1. These molecules are usually found in fruits, vegetables, and plant-derived beverages, and are used as nutritional supplements. They can improve insulin resistance, endoplasmic reticulum stress, lipid peroxidation, and fibrosis[98].
FGF21 is a hormone that plays an important role in regulating metabolic pathways[99]. This hormone is mainly produced by the liver and its signaling is associated with NAFLD pathogenesis[100]. In addition, FGF21 regulates lipid and glucose metabolism, which is correlated with CVD and HF. In summary, FGF21 may be a potential biomarker for prognosis prediction and as a treatment target in the future. However, further studies are required to determine their precise roles[101].
HFrEF is a major public health problem worldwide. Additionally, due to the rising incidence of obesity and associated comorbid conditions, such as diabetes mellitus and metabolic syndrome, NAFLD has also become a common condition. Multiple recent studies have shown a strong association between HF, especially the HFrEF subtype, and NAFLD. Although there are multiple proposed pathophysiological mechanisms, most are common factors in the development of systemic inflammation. To date, several non-pharmacological, pharmacological, and surgical interventions have been studied in patients with concomitant HFrEF and NAFLD. Evidence shows the potential benefits of dietary changes; certain medications, such as ACEI, ARB, MRA, and SGLT-2i; and BS. However, there is still a lack of robust data and well-designed clinical trials investigating several other drugs or novel therapies that could benefit from these conditions and improve outcomes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Banerjee P, United Kingdom; Radhakrishnan K, South Korea; Wong CM, China S-Editor: Li L L-Editor: A P-Editor: Li L
| 1. | Ma L, Chua MS, Andrisani O, So S. Epigenetics in hepatocellular carcinoma: an update and future therapy perspectives. World J Gastroenterol. 2014;20:333-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 77] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 2. | McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599-3726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8225] [Cited by in RCA: 7266] [Article Influence: 1816.5] [Reference Citation Analysis (0)] |
| 3. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4946] [Article Influence: 706.6] [Reference Citation Analysis (9)] |
| 4. | McKimmie RL, Daniel KR, Carr JJ, Bowden DW, Freedman BI, Register TC, Hsu FC, Lohman KK, Weinberg RB, Wagenknecht LE. Hepatic steatosis and subclinical cardiovascular disease in a cohort enriched for type 2 diabetes: the Diabetes Heart Study. Am J Gastroenterol. 2008;103:3029-3035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Chiang CH, Huang CC, Chan WL, Chen JW, Leu HB. The severity of non-alcoholic fatty liver disease correlates with high sensitivity C-reactive protein value and is independently associated with increased cardiovascular risk in healthy population. Clin Biochem. 2010;43:1399-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Mellinger JL, Pencina KM, Massaro JM, Hoffmann U, Seshadri S, Fox CS, O'Donnell CJ, Speliotes EK. Hepatic steatosis and cardiovascular disease outcomes: An analysis of the Framingham Heart Study. J Hepatol. 2015;63:470-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 7. | Fudim M, Zhong L, Patel KV, Khera R, Abdelmalek MF, Diehl AM, McGarrah RW, Molinger J, Moylan CA, Rao VN, Wegermann K, Neeland IJ, Halm EA, Das SR, Pandey A. Nonalcoholic Fatty Liver Disease and Risk of Heart Failure Among Medicare Beneficiaries. J Am Heart Assoc. 2021;10:e021654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 8. | Itier R, Guillaume M, Ricci JE, Roubille F, Delarche N, Picard F, Galinier M, Roncalli J. Non-alcoholic fatty liver disease and heart failure with preserved ejection fraction: from pathophysiology to practical issues. ESC Heart Fail. 2021;8:789-798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Northup PG, Argo CK, Shah N, Caldwell SH. Hypercoagulation and thrombophilia in nonalcoholic fatty liver disease: mechanisms, human evidence, therapeutic implications, and preventive implications. Semin Liver Dis. 2012;32:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Alessi MC, Bastelica D, Mavri A, Morange P, Berthet B, Grino M, Juhan-Vague I. Plasma PAI-1 levels are more strongly related to liver steatosis than to adipose tissue accumulation. Arterioscler Thromb Vasc Biol. 2003;23:1262-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 137] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Li Z, Berk M, McIntyre TM, Gores GJ, Feldstein AE. The lysosomal-mitochondrial axis in free fatty acid-induced hepatic lipotoxicity. Hepatology. 2008;47:1495-1503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 218] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 12. | Laryea M, Watt KD, Molinari M, Walsh MJ, McAlister VC, Marotta PJ, Nashan B, Peltekian KM. Metabolic syndrome in liver transplant recipients: prevalence and association with major vascular events. Liver Transpl. 2007;13:1109-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 237] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 13. | Zimmermann E, Anty R, Tordjman J, Verrijken A, Gual P, Tran A, Iannelli A, Gugenheim J, Bedossa P, Francque S, Le Marchand-Brustel Y, Clement K, Van Gaal L, Sørensen TIA, Jess T. C-reactive protein levels in relation to various features of non-alcoholic fatty liver disease among obese patients. J Hepatol. 2011;55:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Chang E, Chang JS, Kong ID, Baik SK, Kim MY, Park KS. Multidimensional Biomarker Analysis Including Mitochondrial Stress Indicators for Nonalcoholic Fatty Liver Disease. Gut Liver. 2022;16:171-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Peng K, Mo Z, Tian G. Serum Lipid Abnormalities and Nonalcoholic Fatty Liver Disease in Adult Males. Am J Med Sci. 2017;353:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Kim D, Choi SY, Park EH, Lee W, Kang JH, Kim W, Kim YJ, Yoon JH, Jeong SH, Lee DH, Lee HS, Larson J, Therneau TM, Kim WR. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56:605-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 253] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 17. | Simon TG, Bamira DG, Chung RT, Weiner RB, Corey KE. Nonalcoholic Steatohepatitis is Associated with Cardiac Remodeling and Dysfunction. Obesity (Silver Spring). 2017;25:1313-1316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | VanWagner LB, Wilcox JE, Ning H, Lewis CE, Carr JJ, Rinella ME, Shah SJ, Lima JAC, Lloyd-Jones DM. Longitudinal Association of Non-Alcoholic Fatty Liver Disease With Changes in Myocardial Structure and Function: The CARDIA Study. J Am Heart Assoc. 2020;9:e014279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 19. | Lee H, Kim G, Choi YJ, Huh BW, Lee BW, Kang ES, Cha BS, Lee EJ, Lee YH, Huh KB. Association between Non-Alcoholic Steatohepatitis and Left Ventricular Diastolic Dysfunction in Type 2 Diabetes Mellitus. Diabetes Metab J. 2020;44:267-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Perdomo CM, Ezponda A, Núñez-Córdoba JM, Herrero JI, Bastarrika G, Frühbeck G, Escalada J. Transient elastography and serum markers of liver fibrosis associate with epicardial adipose tissue and coronary artery calcium in NAFLD. Sci Rep. 2022;12:6564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Cai X, Zheng S, Liu Y, Zhang Y, Lu J, Huang Y. Nonalcoholic fatty liver disease is associated with increased risk of atrial fibrillation. Liver Int. 2020;40:1594-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 22. | Hung CS, Tseng PH, Tu CH, Chen CC, Liao WC, Lee YC, Chiu HM, Lin HJ, Ho YL, Yang WS, Wu MS, Chen MF. Nonalcoholic Fatty Liver Disease Is Associated With QT Prolongation in the General Population. J Am Heart Assoc. 2015;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Chen Z, Liu J, Zhou F, Li H, Zhang XJ, She ZG, Lu Z, Cai J. Nonalcoholic Fatty Liver Disease: An Emerging Driver of Cardiac Arrhythmia. Circ Res. 2021;128:1747-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 24. | Kasper P, Martin A, Lang S, Kütting F, Goeser T, Demir M, Steffen HM. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol. 2021;110:921-937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 383] [Article Influence: 76.6] [Reference Citation Analysis (1)] |
| 25. | Mantovani A, Byrne CD, Benfari G, Bonapace S, Simon TG, Targher G. Risk of Heart Failure in Patients With Nonalcoholic Fatty Liver Disease: JACC Review Topic of the Week. J Am Coll Cardiol. 2022;79:180-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 26. | Iravanian S, Dudley SC Jr. The renin-angiotensin-aldosterone system (RAAS) and cardiac arrhythmias. Heart Rhythm. 2008;5:S12-S17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Orsborne C, Chaggar PS, Shaw SM, Williams SG. The renin-angiotensin-aldosterone system in heart failure for the non-specialist: the past, the present and the future. Postgrad Med J. 2017;93:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Ergatoudes C, Schaufelberger M, Andersson B, Pivodic A, Dahlström U, Fu M. Non-cardiac comorbidities and mortality in patients with heart failure with reduced vs. preserved ejection fraction: a study using the Swedish Heart Failure Registry. Clin Res Cardiol. 2019;108:1025-1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 29. | Li W, Wen W, Xie D, Qiu M, Cai X, Zheng S, Huang Y. Association between non-alcoholic fatty liver disease and risk of incident heart failure: a meta-analysis of observational studies. Ther Adv Chronic Dis. 2022;13:20406223221119626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Simon TG, Roelstraete B, Hagström H, Sundström J, Ludvigsson JF. Non-alcoholic fatty liver disease and incident major adverse cardiovascular events: results from a nationwide histology cohort. Gut. 2022;71:1867-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 179] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 31. | Yong JN, Ng CH, Lee CW, Chan YY, Tang ASP, Teng M, Tan DJH, Lim WH, Quek J, Xiao J, Chin YH, Foo R, Chan M, Lin W, Noureddin M, Siddiqui MS, Muthiah MD, Sanyal A, Chew NWS. Non-alcoholic fatty liver disease association with structural heart, systolic and diastolic dysfunction: a meta-analysis. Hepatol Int. 2022;16:269-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 32. | Tang WHW, Li DY, Hazen SL. Dietary metabolism, the gut microbiome, and heart failure. Nat Rev Cardiol. 2019;16:137-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 510] [Article Influence: 102.0] [Reference Citation Analysis (0)] |
| 33. | Chaikijurajai T, Tang WHW. Gut Microbiome and Precision Nutrition in Heart Failure: Hype or Hope? Curr Heart Fail Rep. 2021;18:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Montemayor S, Bouzas C, Mascaró CM, Casares M, Llompart I, Abete I, Angullo-Martinez E, Zulet MÁ, Martínez JA, Tur JA. Effect of Dietary and Lifestyle Interventions on the Amelioration of NAFLD in Patients with Metabolic Syndrome: The FLIPAN Study. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 35. | Doustmohammadian A, Clark CCT, Maadi M, Motamed N, Sobhrakhshankhah E, Ajdarkosh H, Mansourian MR, Esfandyari S, Hanjani NA, Nikkhoo M, Zamani F. Favorable association between Mediterranean diet (MeD) and DASH with NAFLD among Iranian adults of the Amol Cohort Study (AmolCS). Sci Rep. 2022;12:2131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 36. | Wickman BE, Enkhmaa B, Ridberg R, Romero E, Cadeiras M, Meyers F, Steinberg F. Dietary Management of Heart Failure: DASH Diet and Precision Nutrition Perspectives. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 37. | Belanger MJ, Kovell LC, Turkson-Ocran RA, Mukamal KJ, Liu X, Appel LJ, Miller ER 3rd, Sacks FM, Christenson RH, Rebuck H, Chang AR, Juraschek SP. Effects of the Dietary Approaches to Stop Hypertension Diet on Change in Cardiac Biomarkers Over Time: Results From the DASH-Sodium Trial. J Am Heart Assoc. 2023;12:e026684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 38. | Maestri M, Santopaolo F, Pompili M, Gasbarrini A, Ponziani FR. Gut microbiota modulation in patients with non-alcoholic fatty liver disease: Effects of current treatments and future strategies. Front Nutr. 2023;10:1110536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 39. | Cerreto M, Santopaolo F, Gasbarrini A, Pompili M, Ponziani FR. Bariatric Surgery and Liver Disease: General Considerations and Role of the Gut-Liver Axis. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 40. | Mummadi RR, Kasturi KS, Chennareddygari S, Sood GK. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2008;6:1396-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 352] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 41. | Doumouras AG, Wong JA, Paterson JM, Lee Y, Sivapathasundaram B, Tarride JE, Thabane L, Hong D, Yusuf S, Anvari M. Bariatric Surgery and Cardiovascular Outcomes in Patients With Obesity and Cardiovascular Disease:: A Population-Based Retrospective Cohort Study. Circulation. 2021;143:1468-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 140] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 42. | van Veldhuisen SL, Gorter TM, van Woerden G, de Boer RA, Rienstra M, Hazebroek EJ, van Veldhuisen DJ. Bariatric surgery and cardiovascular disease: a systematic review and meta-analysis. Eur Heart J. 2022;43:1955-1969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 163] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 43. | Elsaid MI, Li Y, Bridges JFP, Brock G, Minacapelli CD, Rustgi VK. Association of Bariatric Surgery With Cardiovascular Outcomes in Adults With Severe Obesity and Nonalcoholic Fatty Liver Disease. JAMA Netw Open. 2022;5:e2235003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 44. | Mamic P, Chaikijurajai T, Tang WHW. Gut microbiome - A potential mediator of pathogenesis in heart failure and its comorbidities: State-of-the-art review. J Mol Cell Cardiol. 2021;152:105-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 45. | Panigrahi MK, Anirvan P. Letter to the editor: Using angiotensin-converting enzyme inhibitors to prevent liver-related events in NAFLD-Revisiting the renin-angiotensin-aldosterone system pathways. Hepatology. 2022;76:E32-E33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 46. | Borém LMA, Neto JFR, Brandi IV, Lelis DF, Santos SHS. The role of the angiotensin II type I receptor blocker telmisartan in the treatment of non-alcoholic fatty liver disease: a brief review. Hypertens Res. 2018;41:394-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 47. | Zhang X, Wong GL, Yip TC, Tse YK, Liang LY, Hui VW, Lin H, Li GL, Lai JC, Chan HL, Wong VW. Angiotensin-converting enzyme inhibitors prevent liver-related events in nonalcoholic fatty liver disease. Hepatology. 2022;76:469-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 20.3] [Reference Citation Analysis (1)] |
| 48. | Enjoji M, Kotoh K, Kato M, Higuchi N, Kohjima M, Nakashima M, Nakamuta M. Therapeutic effect of ARBs on insulin resistance and liver injury in patients with NAFLD and chronic hepatitis C: a pilot study. Int J Mol Med. 2008;22:521-527. [PubMed] |
| 49. | Paschos P, Tziomalos K. Nonalcoholic fatty liver disease and the renin-angiotensin system: Implications for treatment. World J Hepatol. 2012;4:327-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 50. | Patel S, Lam PH, Kanonidis EI, Ahmed AA, Raman VK, Wu WC, Rossignol P, Arundel C, Faselis C, Kanonidis IE, Deedwania P, Allman RM, Sheikh FH, Fonarow GC, Pitt B, Ahmed A. Renin-Angiotensin Inhibition and Outcomes in HFrEF and Advanced Kidney Disease. Am J Med. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 51. | Gilstrap L, Solomon N, Chiswell K, James O'Malley A, Skinner JS, Fonarow GC, Bhatt DL, Yancy CW, Devore AD. The Association Between Beta-blocker and Renin-Angiotensin System Inhibitor Use After Heart Failure With Reduced Ejection Fraction Hospitalization and Outcomes in Older Patients. J Card Fail. 2023;29:434-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 52. | Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S; CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1412] [Cited by in RCA: 1327] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 53. | Wada T, Miyashita Y, Sasaki M, Aruga Y, Nakamura Y, Ishii Y, Sasahara M, Kanasaki K, Kitada M, Koya D, Shimano H, Tsuneki H, Sasaoka T. Eplerenone ameliorates the phenotypes of metabolic syndrome with NASH in liver-specific SREBP-1c Tg mice fed high-fat and high-fructose diet. Am J Physiol Endocrinol Metab. 2013;305:E1415-E1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 54. | Wada T, Kenmochi H, Miyashita Y, Sasaki M, Ojima M, Sasahara M, Koya D, Tsuneki H, Sasaoka T. Spironolactone improves glucose and lipid metabolism by ameliorating hepatic steatosis and inflammation and suppressing enhanced gluconeogenesis induced by high-fat and high-fructose diet. Endocrinology. 2010;151:2040-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 55. | Polyzos SA, Kountouras J, Zafeiriadou E, Patsiaoura K, Katsiki E, Deretzi G, Zavos C, Tsarouchas G, Rakitzi P, Slavakis A. Effect of spironolactone and vitamin E on serum metabolic parameters and insulin resistance in patients with nonalcoholic fatty liver disease. J Renin Angiotensin Aldosterone Syst. 2011;12:498-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 56. | Eng M, Bansal S. Use of natriuretic-doses of spironolactone for treatment of loop diuretic resistant acute decompensated heart failure. Int J Cardiol. 2014;170:e68-e69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Ellison DH, Felker GM. Diuretic Treatment in Heart Failure. N Engl J Med. 2017;377:1964-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 219] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 58. | Butler J, Hernandez AF, Anstrom KJ, Kalogeropoulos A, Redfield MM, Konstam MA, Tang WH, Felker GM, Shah MR, Braunwald E. Rationale and Design of the ATHENA-HF Trial: Aldosterone Targeted Neurohormonal Combined With Natriuresis Therapy in Heart Failure. JACC Heart Fail. 2016;4:726-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Kadokura T, Zhang W, Krauwinkel W, Leeflang S, Keirns J, Taniuchi Y, Nakajo I, Smulders R. Clinical pharmacokinetics and pharmacodynamics of the novel SGLT2 inhibitor ipragliflozin. Clin Pharmacokinet. 2014;53:975-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Dentin R, Benhamed F, Hainault I, Fauveau V, Foufelle F, Dyck JR, Girard J, Postic C. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes. 2006;55:2159-2170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 349] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 61. | Yabiku K. Efficacy of Sodium-Glucose Cotransporter 2 Inhibitors in Patients With Concurrent Type 2 Diabetes Mellitus and Non-Alcoholic Steatohepatitis: A Review of the Evidence. Front Endocrinol (Lausanne). 2021;12:768850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 62. | Jung CH, Mok JO. The Effects of Hypoglycemic Agents on Non-alcoholic Fatty Liver Disease: Focused on Sodium-Glucose Cotransporter 2 Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists. J Obes Metab Syndr. 2019;28:18-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 63. | Voors AA, Angermann CE, Teerlink JR, Collins SP, Kosiborod M, Biegus J, Ferreira JP, Nassif ME, Psotka MA, Tromp J, Borleffs CJW, Ma C, Comin-Colet J, Fu M, Janssens SP, Kiss RG, Mentz RJ, Sakata Y, Schirmer H, Schou M, Schulze PC, Spinarova L, Volterrani M, Wranicz JK, Zeymer U, Zieroth S, Brueckmann M, Blatchford JP, Salsali A, Ponikowski P. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28:568-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 516] [Article Influence: 172.0] [Reference Citation Analysis (0)] |
| 64. | Vaduganathan M, Docherty KF, Claggett BL, Jhund PS, de Boer RA, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Desai AS, McMurray JJV, Solomon SD. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet. 2022;400:757-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 512] [Article Influence: 170.7] [Reference Citation Analysis (0)] |
| 65. | Figtree GA, Rådholm K, Barrett TD, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Matthews DR, Shaw W, Neal B. Effects of Canagliflozin on Heart Failure Outcomes Associated With Preserved and Reduced Ejection Fraction in Type 2 Diabetes Mellitus. Circulation. 2019;139:2591-2593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 66. | Coelho FDS, Borges-Canha M, von Hafe M, Neves JS, Vale C, Leite AR, Carvalho D, Leite-Moreira A. Effects of sodium-glucose co-transporter 2 inhibitors on liver parameters and steatosis: A meta-analysis of randomized clinical trials. Diabetes Metab Res Rev. 2021;37:e3413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 67. | Mantovani A, Petracca G, Csermely A, Beatrice G, Targher G. Sodium-Glucose Cotransporter-2 Inhibitors for Treatment of Nonalcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials. Metabolites. 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 68. | Andrikou E, Tsioufis C, Andrikou I, Leontsinis I, Tousoulis D, Papanas N. GLP-1 receptor agonists and cardiovascular outcome trials: An update. Hellenic J Cardiol. 2019;60: 347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 69. | Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K; LEAN trial team, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1469] [Article Influence: 163.2] [Reference Citation Analysis (1)] |
| 70. | Feng WH, Bi Y, Li P, Yin TT, Gao CX, Shen SM, Gao LJ, Yang DH, Zhu DL. Effects of liraglutide, metformin and gliclazide on body composition in patients with both type 2 diabetes and non-alcoholic fatty liver disease: A randomized trial. J Diabetes Investig. 2019;10:399-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 71. | Liu L, Yan H, Xia M, Zhao L, Lv M, Zhao N, Rao S, Yao X, Wu W, Pan B, Bian H, Gao X. Efficacy of exenatide and insulin glargine on nonalcoholic fatty liver disease in patients with type 2 diabetes. Diabetes Metab Res Rev. 2020;36:e3292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 72. | Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular Actions and Clinical Outcomes With Glucagon-Like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors. Circulation. 2017;136:849-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 405] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 73. | Dunlay SM, Givertz MM, Aguilar D, Allen LA, Chan M, Desai AS, Deswal A, Dickson VV, Kosiborod MN, Lekavich CL, Mccoy RG, Mentz RJ, Pina IL; American Heart Association Heart Failure and Transplantation Committee of The Council on Clinical Cardiology Council on Cardiovascular and Stroke Nursing Heart Failure Society of America. Corrigendum to "Type 2 Diabetes Mellitus and Heart Failure, A Scientific Statement from the American Heart Association and Heart Failure Society of America" Journal of Cardiac Failure Vol. 25 No. 8, pp. 584-619. J Card Fail. 2019;25:851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 74. | Khan MS, Fonarow GC, McGuire DK, Hernandez AF, Vaduganathan M, Rosenstock J, Handelsman Y, Verma S, Anker SD, McMurray JJV, Kosiborod MN, Butler J. Glucagon-Like Peptide 1 Receptor Agonists and Heart Failure: The Need for Further Evidence Generation and Practice Guidelines Optimization. Circulation. 2020;142:1205-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 75. | DeVore AD, Schulte PJ, Mentz RJ, Hardy NC, Kelly JP, Velazquez EJ, Maya JF, Kielhorn A, Patel HK, Reed SD, Hernandez AF. Relation of Elevated Heart Rate in Patients With Heart Failure With Reduced Ejection Fraction to One-Year Outcomes and Costs. Am J Cardiol. 2016;117:946-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 76. | Valenzuela-Vallejo L, Guatibonza-García V, Mantzoros CS. Recent guidelines for Non-Alcoholic Fatty Liver disease (NAFLD)/ Fatty Liver Disease (FLD): Are they already outdated and in need of supplementation? Metabolism. 2022;136:155248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 77. | Hartman ML, Sanyal AJ, Loomba R, Wilson JM, Nikooienejad A, Bray R, Karanikas CA, Duffin KL, Robins DA, Haupt A. Effects of Novel Dual GIP and GLP-1 Receptor Agonist Tirzepatide on Biomarkers of Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes. Diabetes Care. 2020;43:1352-1355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 237] [Article Influence: 47.4] [Reference Citation Analysis (1)] |
| 78. | Gastaldelli A, Cusi K, Fernández Landó L, Bray R, Brouwers B, Rodríguez Á. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. 2022;10:393-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 284] [Article Influence: 94.7] [Reference Citation Analysis (0)] |
| 79. | Lilly E. A Study of Tirzepatide (LY3298176) in Participants with Heart Failure with Preserved Ejection Fraction and Obesity. [accessed 25 March 2023]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04847557 ClinicalTrials.gov Identifier: NCT04847557. |
| 80. | Thomas I, Gregg B. Metformin; a review of its history and future: from lilac to longevity. Pediatr Diabetes. 2017;18:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 81. | Pinyopornpanish K, Leerapun A, Pinyopornpanish K, Chattipakorn N. Effects of Metformin on Hepatic Steatosis in Adults with Nonalcoholic Fatty Liver Disease and Diabetes: Insights from the Cellular to Patient Levels. Gut Liver. 2021;15:827-840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 82. | Kamel AM, Sabry N, Farid S. Effect of metformin on left ventricular mass and functional parameters in non-diabetic patients: a meta-analysis of randomized clinical trials. BMC Cardiovasc Disord. 2022;22:405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 83. | Benes J, Kotrc M, Kroupova K, Wohlfahrt P, Kovar J, Franekova J, Hegarova M, Hoskova L, Hoskova E, Pelikanova T, Jarolim P, Kautzner J, Melenovsky V. Metformin treatment is associated with improved outcome in patients with diabetes and advanced heart failure (HFrEF). Sci Rep. 2022;12:13038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 84. | Nassif ME, Kosiborod M. A Review of Cardiovascular Outcomes Trials of Glucose-Lowering Therapies and Their Effects on Heart Failure Outcomes. Am J Cardiol. 2019;124 Suppl 1:S12-S19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 85. | Lian J, Fu J. Pioglitazone for NAFLD Patients With Prediabetes or Type 2 Diabetes Mellitus: A Meta-Analysis. Front Endocrinol (Lausanne). 2021;12:615409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 86. | Ndakotsu A, Vivekanandan G. The Role of Thiazolidinediones in the Amelioration of Nonalcoholic Fatty Liver Disease: A Systematic Review. Cureus. 2022;14:e25380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 87. | Zhu J, Yu X, Zheng Y, Li J, Wang Y, Lin Y, He Z, Zhao W, Chen C, Qiu K, Wu J. Association of glucose-lowering medications with cardiovascular outcomes: an umbrella review and evidence map. Lancet Diabetes Endocrinol. 2020;8:192-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 88. | Giles TD, Miller AB, Elkayam U, Bhattacharya M, Perez A. Pioglitazone and heart failure: results from a controlled study in patients with type 2 diabetes mellitus and systolic dysfunction. J Card Fail. 2008;14:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 89. | Dargie HJ, Hildebrandt PR, Riegger GA, McMurray JJ, McMorn SO, Roberts JN, Zambanini A, Wilding JP. A randomized, placebo-controlled trial assessing the effects of rosiglitazone on echocardiographic function and cardiac status in type 2 diabetic patients with New York Heart Association Functional Class I or II Heart Failure. J Am Coll Cardiol. 2007;49:1696-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 201] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 90. | Correction to: 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;146:e185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 91. | Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, Kleiner DE, Loomba R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77:1797-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 1157] [Article Influence: 578.5] [Reference Citation Analysis (1)] |
| 92. | Galicia-Garcia U, Jebari S, Larrea-Sebal A, Uribe KB, Siddiqi H, Ostolaza H, Benito-Vicente A, Martín C. Statin Treatment-Induced Development of Type 2 Diabetes: From Clinical Evidence to Mechanistic Insights. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 93. | Kjekshus J, Apetrei E, Barrios V, Böhm M, Cleland JG, Cornel JH, Dunselman P, Fonseca C, Goudev A, Grande P, Gullestad L, Hjalmarson A, Hradec J, Jánosi A, Kamenský G, Komajda M, Korewicki J, Kuusi T, Mach F, Mareev V, McMurray JJ, Ranjith N, Schaufelberger M, Vanhaecke J, van Veldhuisen DJ, Waagstein F, Wedel H, Wikstrand J; CORONA Group. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248-2261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1085] [Cited by in RCA: 1142] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 94. | Bielecka-Dabrowa A, Bytyçi I, Von Haehling S, Anker S, Jozwiak J, Rysz J, Hernandez AV, Bajraktari G, Mikhailidis DP, Banach M. Association of statin use and clinical outcomes in heart failure patients: a systematic review and meta-analysis. Lipids Health Dis. 2019;18:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 95. | Kashyap ML, Ganji S, Nakra NK, Kamanna VS. Niacin for treatment of nonalcoholic fatty liver disease (NAFLD): novel use for an old drug? J Clin Lipidol. 2019;13:873-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 96. | Jiang Y, Liu Y, Guo Q, Xu C, Zhu C, Shi J. Sesquiterpene glycosides from the roots of Codonopsis pilosula. Acta Pharm Sin B. 2016;6:46-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 97. | Chen J, Ding X, Wu R, Tong B, Zhao L, Lv H, Meng X, Liu Y, Ren B, Li J, Jian T, Li W. Novel Sesquiterpene Glycoside from Loquat Leaf Alleviates Type 2 Diabetes Mellitus Combined with Nonalcoholic Fatty Liver Disease by Improving Insulin Resistance, Oxidative Stress, Inflammation, and Gut Microbiota Composition. J Agric Food Chem. 2021;69: 14176-14191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 98. | Wang K, Tan W, Liu X, Deng L, Huang L, Wang X, Gao X. New insight and potential therapy for NAFLD: CYP2E1 and flavonoids. Biomed Pharmacother. 2021;137:111326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 99. | Tezze C, Romanello V, Sandri M. FGF21 as Modulator of Metabolism in Health and Disease. Front Physiol. 2019;10:419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 238] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 100. | Falamarzi K, Malekpour M, Tafti MF, Azarpira N, Behboodi M, Zarei M. The role of FGF21 and its analogs on liver associated diseases. Front Med (Lausanne). 2022;9:967375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 101. | Zhang Y, Liu D, Long XX, Fang QC, Jia WP, Li HT. The role of FGF21 in the pathogenesis of cardiovascular disease. Chin Med J (Engl). 2021;134:2931-2943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 102. | McPherson S, Wilkinson N, Tiniakos D, Wilkinson J, Burt AD, McColl E, Stocken DD, Steen N, Barnes J, Goudie N, Stewart S, Bury Y, Mann D, Anstee QM, Day CP. A randomised controlled trial of losartan as an anti-fibrotic agent in non-alcoholic steatohepatitis. PLoS One. 2017;12:e0175717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 103. | Orlic L, Mikolasevic I, Lukenda V, Anic K, Jelic I, Racki S. Nonalcoholic fatty liver disease and the renin-angiotensin system blockers in the patients with chronic kidney disease. Wien Klin Wochenschr. 2015;127:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 104. | Vos MB, Van Natta ML, Blondet NM, Dasarathy S, Fishbein M, Hertel P, Jain AK, Karpen SJ, Lavine JE, Mohammad S, Miriel LA, Molleston JP, Mouzaki M, Sanyal A, Sharkey EP, Schwimmer JB, Tonascia J, Wilson LA, Xanthakos SA; NASH Clinical Research Network. Randomized placebo-controlled trial of losartan for pediatric NAFLD. Hepatology. 2022;76:429-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 105. | Vos MB, Jin R, Konomi JV, Cleeton R, Cruz J, Karpen S, Rodriguez DS, Frediani JK, McCracken C, Welsh J. A randomized, controlled, crossover pilot study of losartan for pediatric nonalcoholic fatty liver disease. Pilot Feasibility Stud. 2018;4:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 106. | Hirata T, Tomita K, Kawai T, Yokoyama H, Shimada A, Kikuchi M, Hirose H, Ebinuma H, Irie J, Ojiro K, Oikawa Y, Saito H, Itoh H, Hibi T. Effect of Telmisartan or Losartan for Treatment of Nonalcoholic Fatty Liver Disease: Fatty Liver Protection Trial by Telmisartan or Losartan Study (FANTASY). Int J Endocrinol. 2013;2013:587140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 107. | Alam S, Abrar M, Islam S, Kamal M, Hasan MJ, Khan MAS, Ahmad N. Effect of telmisartan and vitamin E on liver histopathology with non-alcoholic steatohepatitis: A randomized, open-label, noninferiority trial. JGH Open. 2020;4:663-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 108. | Alam S, Kabir J, Mustafa G, Gupta U, Hasan SK, Alam AK. Effect of telmisartan on histological activity and fibrosis of non-alcoholic steatohepatitis: A 1-year randomized control trial. Saudi J Gastroenterol. 2016;22:69-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 109. | Fogari R, Maffioli P, Mugellini A, Zoppi A, Lazzari P, Derosa G. Effects of losartan and amlodipine alone or combined with simvastatin in hypertensive patients with nonalcoholic hepatic steatosis. Eur J Gastroenterol Hepatol. 2012;24:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 110. | Baranova EI, Berezina AV, Melioranskaya EI, Polyakova EA. [Safety and Efficacy of Amlodipine, Lisinopril and Rosuvastatin Therapy in Patients With Metabolic Syndrome and Nonalcoholic Fatty Liver Disease]. Kardiologiia. 2015;55:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 111. | Siddiqui MT, Amin H, Garg R, Chadalavada P, Al-Yaman W, Lopez R, Singh A. Medications in type-2 diabetics and their association with liver fibrosis. World J Gastroenterol. 2020;26:3249-3259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 112. | Polyzos SA, Kountouras J, Mantzoros CS, Polymerou V, Katsinelos P. Effects of combined low‐dose spironolactone plus vitamin E vs vitamin E monotherapy on insulin resistance, non‐invasive indices of steatosis and fibrosis, and adipokine levels in non‐alcoholic fatty liver disease: a randomized controlled trial. Diabetes Obes Metab. 2017;19:1805-1809. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 113. | Chaudhury CS, Purdy JB, Liu CY, Morse CG, Stanley TL, Kleiner D, Hadigan C. Unanticipated increases in hepatic steatosis among human immunodeficiency virus patients receiving mineralocorticoid receptor antagonist eplerenone for non-alcoholic fatty liver disease. Liver Int. 2018;38:797-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 114. | Taheri H, Malek M, Ismail-Beigi F, Zamani F, Sohrabi M, Reza Babaei M, Khamseh ME. Effect of Empagliflozin on Liver Steatosis and Fibrosis in Patients With Non-Alcoholic Fatty Liver Disease Without Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Adv Ther. 2020;37:4697-4708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 115. | Takeshita Y, Honda M, Harada K, Kita Y, Takata N, Tsujiguchi H, Tanaka T, Goto H, Nakano Y, Iida N, Arai K, Yamashita T, Mizukoshi E, Nakamura H, Kaneko S, Takamura T. Comparison of Tofogliflozin and Glimepiride Effects on Nonalcoholic Fatty Liver Disease in Participants With Type 2 Diabetes: A Randomized, 48-Week, Open-Label, Active-Controlled Trial. Diabetes Care. 2022;45:2064-2075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 116. | Kuchay MS, Krishan S, Mishra SK, Farooqui KJ, Singh MK, Wasir JS, Bansal B, Kaur P, Jevalikar G, Gill HK, Choudhary NS, Mithal A. Effect of Empagliflozin on Liver Fat in Patients With Type 2 Diabetes and Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial (E-LIFT Trial). Diabetes Care. 2018;41:1801-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 431] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 117. | Eriksson JW, Lundkvist P, Jansson PA, Johansson L, Kvarnström M, Moris L, Miliotis T, Forsberg GB, Risérus U, Lind L, Oscarsson J. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia. 2018;61:1923-1934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 278] [Article Influence: 39.7] [Reference Citation Analysis (1)] |
| 118. | Han E, Lee YH, Lee BW, Kang ES, Cha BS. Ipragliflozin Additively Ameliorates Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Controlled with Metformin and Pioglitazone: A 24-Week Randomized Controlled Trial. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 119. | Pokharel A, Kc S, Thapa P, Karki N, Shrestha R, Jaishi B, Paudel MS. The Effect of Empagliflozin on Liver Fat in Type 2 Diabetes Mellitus Patients With Non-Alcoholic Fatty Liver Disease. Cureus. 2021;13:e16687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 120. | Shibuya T, Fushimi N, Kawai M, Yoshida Y, Hachiya H, Ito S, Kawai H, Ohashi N, Mori A. Luseogliflozin improves liver fat deposition compared to metformin in type 2 diabetes patients with non-alcoholic fatty liver disease: A prospective randomized controlled pilot study. Diabetes Obes Metab. 2018;20:438-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 156] [Article Influence: 22.3] [Reference Citation Analysis (1)] |
| 121. | Kinoshita T, Shimoda M, Nakashima K, Fushimi Y, Hirata Y, Tanabe A, Tatsumi F, Hirukawa H, Sanada J, Kohara K, Irie S, Kimura T, Nakamura Y, Nishioka M, Obata A, Nakanishi S, Mune T, Kaku K, Kaneto H. Comparison of the effects of three kinds of glucose-lowering drugs on non-alcoholic fatty liver disease in patients with type 2 diabetes: A randomized, open-label, three-arm, active control study. J Diabetes Investig. 2020;11:1612-1622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 122. | Ito D, Shimizu S, Inoue K, Saito D, Yanagisawa M, Inukai K, Akiyama Y, Morimoto Y, Noda M, Shimada A. Comparison of Ipragliflozin and Pioglitazone Effects on Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes: A Randomized, 24-Week, Open-Label, Active-Controlled Trial. Diabetes Care. 2017;40:1364-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (1)] |