Published online May 26, 2023. doi: 10.4330/wjc.v15.i5.244
Peer-review started: February 11, 2023
First decision: March 28, 2023
Revised: April 10, 2023
Accepted: April 25, 2023
Article in press: April 25, 2023
Published online: May 26, 2023
Processing time: 96 Days and 20.7 Hours
Non-A non-B aortic dissection (AAD) is an infrequently documented condition, comprising of only a small proportion of all AADs. The unique anatomy of the aortic arch and the failure of the existing classifications to adequately define individuals with non-A non-B AAD, have led to an ongoing controversy around the topic. It seems that the clinical progression of acute non-A non-B AAD diverges from the typical type A and B dissections, frequently leading to serious complications and thus mandating early intervention. Currently, the available treatment methods in the surgical armamentarium are conventional open, endovascular techniques and combined hybrid methods. The optimum approach is tailored in every individual case and may be determined by the dissection’s location, extent, the aortic diameter, the associated complications and the patient’s status. The management of non-A non-B dissections still remains challenging and a unanimous consensus defining the gold standard treatment has yet to be reached. In an attempt to provide further insight into this perplexing entity, we performed a minireview of the literature, aiming to elucidate the epidemiology, clinical course and the optimal treatment modality.
Core Tip: The available treatment options in the surgical armamentarium are conventional open surgery with standard aortic arch replacement or frozen elephant trunk (FET), interventional therapies such as the thoracic endovascular aortic repair (TEVAR) and hybrid techniques combining TEVAR with debranching of the supra-aortic vessels. In the case of a favorable arch anatomy, TEVAR is the preferable treatment option. Alternatively, when the entry tear is located in the proximal segment of the aortic arch, a hybrid arch repair, aortic arch replacement or even FET should be given thorough consideration.
- Citation: Christodoulou KC, Karangelis D, Efenti GM, Sdrevanos P, Browning JR, Konstantinou F, Georgakarakos E, Mitropoulos FA, Mikroulis D. Current knowledge and contemporary management of non-A non-B aortic dissections. World J Cardiol 2023; 15(5): 244-252
- URL: https://www.wjgnet.com/1949-8462/full/v15/i5/244.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i5.244
As part of the cluster of the clinical entities related to acute aortic syndromes[1], acute aortic dissection (AAD) is a challenging and life-threatening cardiovascular emergency[2], with approximately 6 new cases per 100000 population per annum[3]. In an AAD, an intimal tear compromises the medial layer’s structural integrity, separating the aortic wall layers. Subsequently, a “new” false lumen is formed, allowing blood to enter the tunica media[3]. If not addressed, it has a significant propensity for developing into a fatal disorder due to rupture, myocardial infarction, cardiac tamponade, aortic valve insufficiency[4], or even end-organ malperfusion[5]. Since the second half of the previous century, the Stanford and DeBakey classifications are the primary systems, which have consistently determined the patients’ management. However, by default, they both fail to distinguish and address dissections confined or involving the aortic arch[5]. von Segesser et al[6] were the first to introduce the term “non-A non-B” when referring to intima tears sparing the ascending aorta.
Non-A non-B AAD can be defined as an entry intimal tear, located beyond the left subclavian artery (LSA), with the dissection extending retrogradely into the aortic arch (descending entry type), or as an entry tear located between the innominate and the LSA (arch entry type), with or without distal extension of the dissection[7]. It seems that there is scarcity of data regarding their natural history, clinical course and management[8]. Accordingly, we performed a minireview of the literature, to unveil the details of the topic with regards to epidemiology, contemporary classification systems as well as the available surgical armamentarium and possible treatment options.
Non-A non-B AAD is an infrequently documented condition, comprising only a small proportion of all AADs. Its comparatively elevated mortality, hinders the ascertainment of its true incidence, since a considerable number of patients die prior to the diagnosis[2]. Regardless of the divergent results across different studies, a recent review exhibited that the incidence of non-A non-B AAD lies between the respective of type A and type B, varying from 2.8% to 16.5%[5]. The affected individuals tend to be 5 to 10 years younger in comparison to patients with other AADs[9], while some disagreement exists as to whether or not there is a prevailing type of location for the entry tear (arch or descending)[10,11].
There are several risk factors associated with non-A non-B AADs, with chronic and mainly poorly controlled hypertension being the most frequently reported. In the majority of the published cases, roughly 80% of the patients have a medical history of hypertension[5,8,10,12,13]. Tobacco addiction, hypercholesterolemia and diabetes mellitus were also found to pose a great risk for AAD. In addition, connective tissue disorders like Ehlers-Danlos and Marfan Syndromes, inflammatory vasculitis, pregnancy, trauma, previous heart surgery, the presence of bicuspid aortic valve, stimulant usage, infections and some very rare genetic disorders are among the predisposing medical conditions linked to AAD[2]. Additionally, a non-A non-B dissection can be attributed to an intramural hematoma and penetration aortic ulceration[1].
Furthermore, the role of other factors has also been investigated. Recent papers have exhibited a correlation between this specific type of AAD and anatomic characteristics of the aortic arch. Rylski et al[12] demonstrated that arch types are distributed variably among different dissections. Type I in which the vertical distance between innominate artery and the top of the aortic arch is less than the diameter of the left common carotid artery (LCCA), was usually seen in type A AADs, while in non-A non-B AADs arch type II (vertical distance between innominate artery and aortic arch is equal or twice the diameter of the LCCA) prevailed (49%). In approximately 1/3 of the non-A non-B patients (28%), two instead of the three branches arise from the arch, since the innominate and the LCCA were found to share a common trunk and in 16% of the cases the left vertebral artery arose directly from the arch[10]. Irrespective of these variations, the aortic arch’s curved shape and its branches, form two natural barriers, which can prevent a dissection from spreading further. The origin of the innominate artery serves as the proximal barrier, while the distal barrier is considered to be the LSA. As a result, these margins may be incriminated as the two very distinctive arch and descending entry dissection types[11].
In most cases of AAD, the most common symptom is sudden, acute chest pain and/or back discomfort[3]. Nevertheless, in rare cases a subtle or discrete type of AAD[3] has been reported, which is typically detected intraoperatively, despite being misdiagnosed during the patient’s initial assessment[14]. Wang et al[15] in their retrospective study, stated that all non-A non-B patients reported an abrupt onset of severe chest pain for at least a six-hour duration. Notably, a recent meta-analysis showed that nearly 50% of the patients were admitted with or developed over time, signs of at least one organ malperfusion and that 6% were at risk of an impending rupture[8]. In the same paper, the authors highlighted the surprisingly large proportion of patients (88%), who had a complicated clinical course, as distinct from type B dissections. Hence, cardiogenic shock, cardiac arrest, cardiac tamponade, periaortic hematoma, acute renal failure, stroke/neurologic deficits or even aortic rupture are some of the baseline characteristics of non-A non-B dissection patients[13].
Over the years and with the exploitation of the emerging imaging modalities, several classification systems have been proposed and deployed to facilitate the triage and enhance the post-treatment clinical outcomes. The two traditional and widely known DeBakey and Stanford classifications were introduced in the 1950s and 1960s, respectively, and are based on the intimal tear’s location and extension[16,17]. It is evident, that since the aforementioned systems focus primarily on the ascending and descending part of the aorta, they lack clarity regarding aortic arch involvement[4].
The European Society of Cardiology guidelines about aortic diseases fail to include the arch dissection as a distinct entity. The American Heart Association guidelines propose the term “proximal type B aortic dissection” for patients with entry tears in the arch, expanding antegradely to the descending aorta[18,19]. The 2019 consensus of the European Association for Cardio-Thoracic Surgery and the European Society for Vascular Surgery, referred to non-A non-B AAD, as an arch involvement either by the most proximal tear or by retrograde extension, but sparing the ascending aorta[20].
Since the introduction of the term non-A non-B AAD back in 1994[6], several studies have proposed the modification of the already existing classifications, by adding this type of dissection. Based on the entry tear’s location, in 2017, non-A non-B AADs were divided into two distinct groups; arch and descending entry tear[10]. Recently, Qanadli et al[4] driven by the Stanford classification, proposed the incorporation of “type C” AAD, referring to the corresponding non-A non-B dissection suggested earlier by Rylski et al[10]. For the first time, they also mention, grades of malperfusion syndrome (MPS grade 0-3). More specifically, the absence of malperfusion is classified as grade 0, compression of the true lumen as grade 1, while the extension of the dissection to renal artery is categorized as grade 2. According to the authors grade 3 incorporates both the absence of malperfusion and the extension of the dissection to the renal artery. Therefore, the MPS grade dictates the need for additional therapy to maintain perfusion of vital organs.
Sievers et al[9] extended the Stanford classification by including all types of AAD, the location of primary aortic entry tear and the malperfusion status (TEM). According to their proposal, T refers to type A, type B or non-A non-B dissections, E includes E0: No visible entry site, E1: Entry tear in ascending aorta, E2: Tear in arch, E3: Tear in descending aorta and M includes M0, M1, M2, M3 when no malperfusion, coronary artery, subra-aortic arteries, renal/visceral ± lower extremity arteries were involved, respectively. They also mention the presence (+) and absence (-) of clinical symptoms of organ malperfusion offering a more complete picture of AADs in an attempt to achieve optimal therapeutic planning and outcome[9].
Decision on optimal medical treatment of AADs is determined based on the dissection’s location, extent, the aortic diameter, the accompanying complications, the adjacent anatomy, the patient’s status and comorbodities[21]. Stanford type A/DeBakey type I or II dissections, when left untreated, typically have a mortality rate exceeding 50% the first 48 h, so open surgery on emergency basis with replacement of the ascending aorta is highly recommended[3,19]. On the other hand, when the dissection involves solely the descending thoracic aorta (DTA) (Stanford type B/DeBakey type III dissections), the clinical course is usually uncomplicated. When that is the case, the 30-d mortality rate is approximately 10%, thus, conservative medical therapy or interventional, endovascular treatment (for complicated type B dissections) are the most common, yet not exclusive, treatment options[3,19]. However, aortic arch dissections have not been accurately classified, which leads to uncertainty and disagreement on treatment planning[4]. In addition, the contribution of the imaging modalities, towards an accurate diagnosis should not be omitted. The transthoracic echocardiography, although mostly employed in nonemergency cases, could potentially provide useful details regarding the proximal DTA, the aortic arch branches and detect some of the dissection’s complications[19]. Yet, the valuable role of the preoperative computed tomography is constantly increasing, as it can sometimes detect the dissection and its extent, delineate the anatomy of the area, and even depict clinically significant incidental findings[22], enhancing the diagnostic accuracy and tailoring the treatment plan[19].
Aiming for the closure of the proximal entry tear, a great number of research papers have suggested the implementation of surgical treatment for non-A non-B AADs, explicitly exhibiting that conservative treatment is inferior to any type of intervention, presenting a 30-d mortality rate of 14% as compared to 3.6% for surgically or endovascularly treated patients[8]. According to recent data, it seems that the clinical manifestation and progression of acute non-A non-B AAD diverge from the typical type A and B dissections and especially from the acute type B AAD, which is confined to the DTA[23]. Valentine et al[24] noted that dissections including the arch had a worse outcome than those affecting only the DTA. Equally important results from an expert consensus state that the arch’s involvement in the dissecting process has an immense impact on the patients’ outcome, including but not limited to the prolonged hospital stay, the increased probability of cardiac and neurologic complications such as rupture, congestive heart failure, MPS, stroke, spinal cord injury (SCI) and the need for reintervention if not addressed betimes[20,25].
Because non-A non-B AADs are associated with an increased likelihood of a complicated course, the optimal management orders early intervention[5]. In spite of the fact that, during the past years, many therapeutic plans have been proposed, there remains a dearth of literature regarding pertinent studies comparing different modalities for the treatment of non-A, non-B AAD. Moreover, the existing studies have limited patient samples and lack long-term follow-up[21]. Therefore, a unanimous consensus defining the gold standard treatment has yet to be reached[5]. Currently, the available treatment options in the surgical armamentarium are conventional open surgery with standard aortic arch replacement or frozen elephant trunk (FET), interventional therapies such as the thoracic endovascular aortic repair (TEVAR) with extra-thoracic surgical transposition or chimney stent graft and hybrid techniques combining TEVAR with debranching of the supra-aortic vessels[8]. Lately, a new hybrid technique was launched by Wang et al[15] with satisfactory short-term outcomes, called the “inclusion aortic arch technique”, as an alternative to the traditional hybrid surgery, so to avoid endoleaks and retrograde type A dissection, which may complicate the procedure.
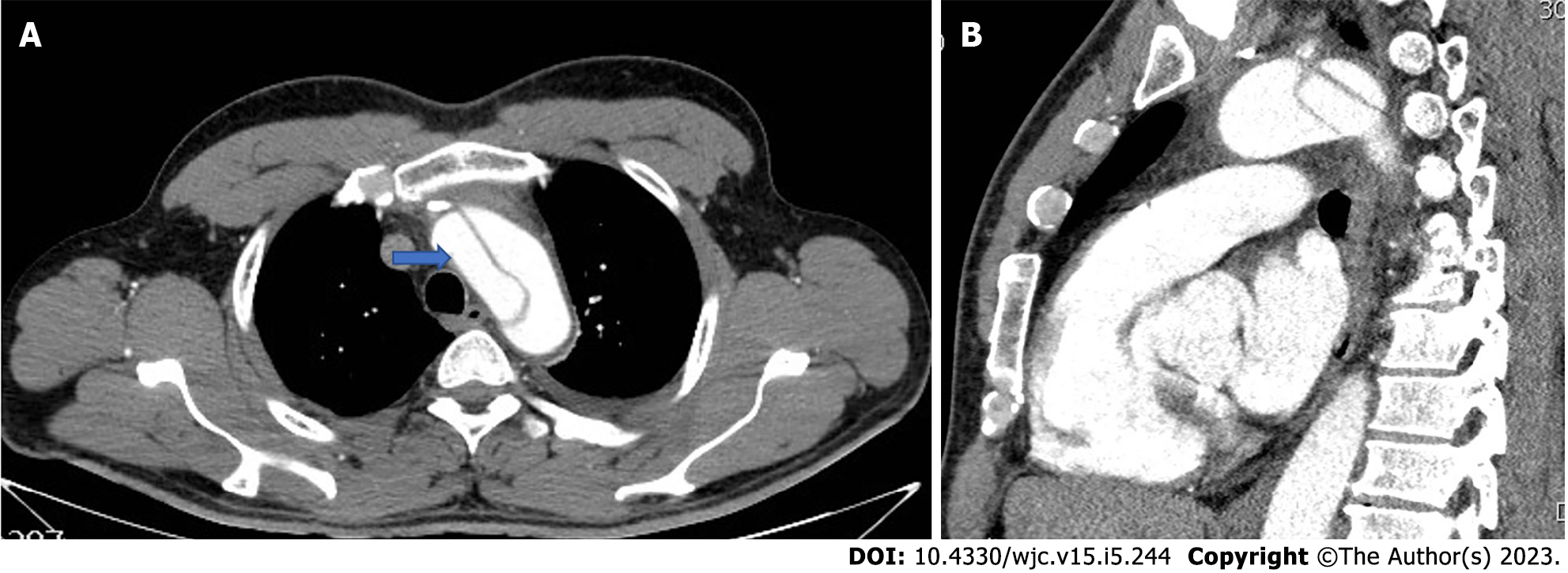
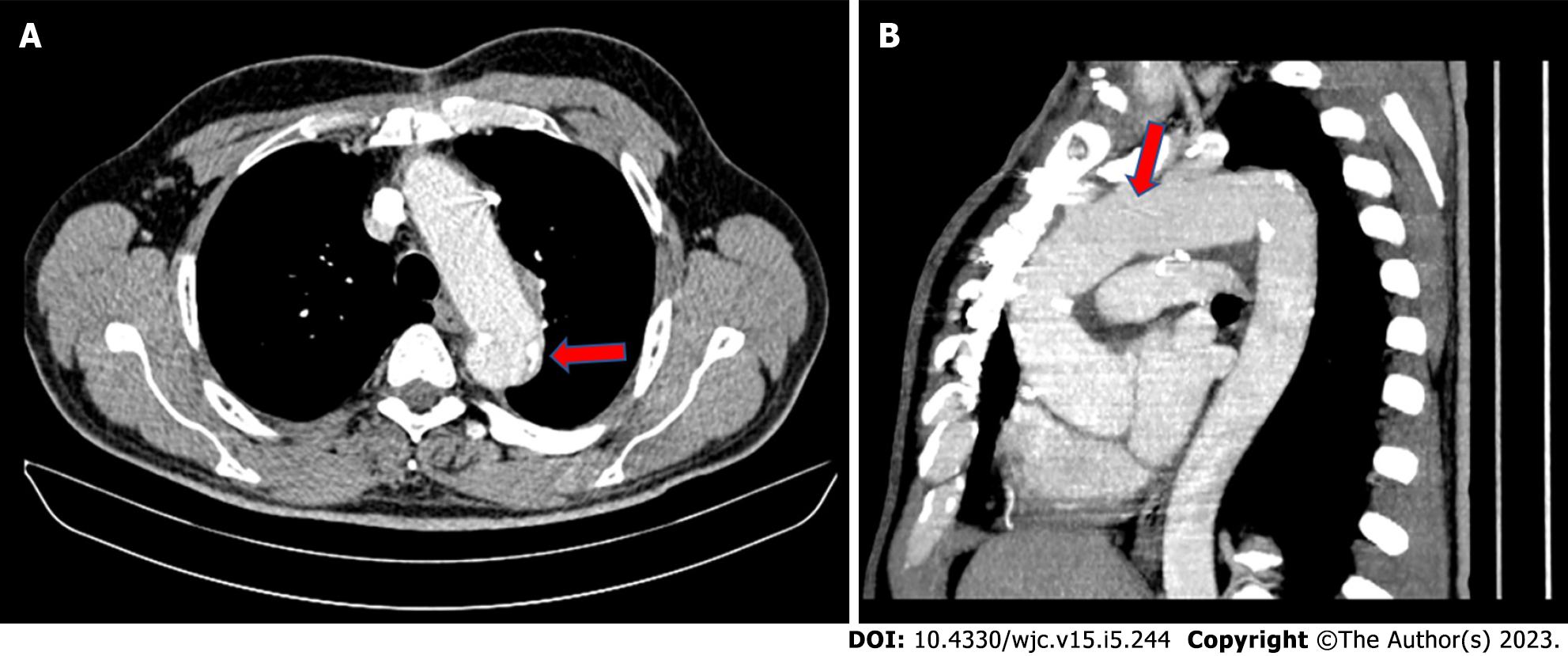
According to the International Registry of Acute Aortic Dissection study findings, arch entry and DTA entry type dissections differ from one another in terms of their course. More specifically, a DTA dissection, expanding retrogradely to the arch, has no impact on either early or late mortality or the management plan, with its clinical course resembling that of type B AAD. Whereas complicated arch entry dissections with antegrade expansion to the DTA, were found to have an elevated in-hospital mortality, requiring an even more urgent intervention[13,25]. Trimarchi et al[13] also presented data which argue that non-A non-B AAD should perhaps warrant a more aggressive approach. In their analysis, 14% of patients with an “uncomplicated” arch tear had progression of the dissection, which likely contributed to a 30% incidence of lethal stroke. In the same frame, Kosiorowska et al[7] stressed/pointed out the contemporary tendency towards an earlier intervention on the altar of a favorable aortic remodeling. In arch entry type patients, open aortic arch repair is preferable compared to endovascular techniques in term of survival and post-operative complications rate[7]. In these cases, there are several arch replacement grafts commercially available (Figures 1 and 2).
The arch entry dissections, can be managed with standard aortic arch replacement combined with FET, as the procedure is associated with low peri and post-operative mortality and complication rates[7]. Hybrid methods have been proposed as an effective alternative, which can prevent the need for cardiopulmonary bypass, hypothermic cardiac arrest and any related problems, but their efficacy has not yet been proven in the long run[26]. However, the study conducted by Tian et al[27], employing different hybrid methods (type I-III) to treat 46 patients, has demonstrated promising outcomes with respect to overall mortality and complication rates, in comparison to surgical or endovascular repair. Taking into account the lack of guidelines regarding postoperative thromboprophylaxis, caution must be exerted to avoid the graft’s occlusion[28]. Irrespective of the entry type, evidence shows that TEVAR is a valid treatment option provided that the aortic arch has favorable anatomy[8]. Alternatively, when the entry tear is located in zone 1, a hybrid arch repair, aortic arch replacement or even FET should be given thorough consideration[8]. This minireview focuses primarily on the FET and TEVAR techniques.
Treatment of non-A non-B AAD can be performed with a two-stage surgical technique called “ET”[29]. The first stage of ET includes a sternotomy and a reconstruction of the ascending aorta and the aortic arch with a graft while in the second stage, the floating extension of the graft (ET) in the descending aorta is utilized in order to extend the repair of the DTA. The second stage per se includes a lateral thoracotomy to approach the descending aorta. The evolution of surgical and endovascular techniques has resulted in the development of a composite prosthesis, known as the “Frozen ET”[20]. The use of FET instead of the conventional ET has been increasing over the last years[20].
Combining the benefits of both open and interventional repair, FET is a viable solution in cases of a proximal entry tear as well as for patients with non-A non-B AAD or complicated chronic type B, who are not suitable for TEVAR[30,31]. This one-stage procedure allows a total aortic arch replacement with antegrade delivery of a descending aortic stent-graft[32]. The stent-graft may serve as a proximal landing zone to facilitate future endovascular intervention in the distal aorta[30]. The FET technique has been extensively applied in cases of acute dissections to restore true lumen patency, seal intimal tears in the descending aorta and promote false lumen thrombosis, as well as repairing chronic degenerative arch aneurysms[19,32]. FET implantation has the potential to prevent secondary thoracic and abdominal aortic replacement by enlarging the true lumen and promoting favorable remodeling of the distal aorta[19,33,34]. Yet, if replacement of the thoracic and abdominal aorta is required, its additional suture margins at each side, facilitate anastomosis[5,30]. The two most common commercially available hybrid FET prostheses namely the Thoraflex hybrid and E-vita open are associated with comparable outcomes[20].
Carino et al[8] mention that 7% of the non-A non-B patients underwent the FET procedure, pointing out that it should be considered in complicated cases, since it alleviates the compressed true lumen and covers any newly formed entry tears in the proximal DTA, applying pressure to the false lumen. Zhao et al[35] reported the in-hospital mortality rate of their 24 non-A non-B AAD patients treated with FET, to be 4.1%, while the 5-year survival rate was approximately 92%. The authors concluded that, this surgical technique represents a feasible option with acceptable short and long-term outcomes. Another study cohort, whilst exhibiting similar early results, stresses the importance of the continuous monitoring and follow-up, due to the potential need for aortic reintervention[31]; results in accordance with Tian et al[27] paper.
Although the technique is more invasive than TEVAR, it minimizes the risk of type Ia endoleaks and retrograde type A dissection[30]. On the other hand, FET’s primary disadvantage is the increased risk of surgical trauma brought on by the necessity for extended periods of extracorporeal circulation, circulatory arrest, myocardial ischemia, stroke and SCI, due to the extensive coverage of the descending aorta with the occlusion of intercostal arteries[8]. Additionally, aortic valve insufficiency, bovine aortic arch, the dissection of the LCCA and preoperative cardiopulmonary resuscitation have all been linked to an increased risk for postoperative stroke[36,37]. Notably, in many studies, including a meta-analysis with 3000 “aortic surgical” patients, SCI was documented in 4% of the cases. By diminishing the circulatory arrest time and FET maneuvers above the 8th thoracic vertebra, SCIs could be substantially reduced[38]. Cerebrospinal fluid drainage and neuromonitoring should also be carried out for high-risk patients[39].
TEVAR, given its minimal invasive character, has recently been shown to be the most widely used technique to treat non-A non-B dissections, comprising 55% of the treatment[8]. The aim of the stent-graft’s deployment in the thoracic aorta is the successful closure of the primary entry tear. As a result, the false lumen is excluded from the systemic circulation, thrombosed and shrank, thus it no more compresses the true lumen and organ perfusion is maintained. According to the location of the primary entry tear, different TEVAR sealing zones exist (zone 0, 1, 2, 3). Zone 3 TEVAR refers to entry tears located in the DTA with retrograde extension, with the stent-graft deployed distal to the LSA. For more proximal entry tears, zone 2 TEVAR is employed, which includes a sealing zone between the origin of the LCCA and the LSA. TEVAR zone 1 refers to graft apposition between the innominate artery and LCCA, while TEVAR zone 0 includes a sealing zone proximal to the innominate artery[3,5,10,20].
In order to establish a disease-free sealing zone, the sacrifice of important aortic arch branches can be, in some cases, unavoidable. Nonetheless, there are several techniques to maintain perfusion; supra-aortic branch bypass (hybrid technique), apposition of extra stent-grafts (chimney technique) or both[40]. TEVAR zone 2 can be accompanied with carotid-subclavian (CS) bypass/transposition or with chimney graft to the LSA. Similarly, TEVAR zone 1 can be followed by two chimney grafts to the LCCA and LSA, but more frequently, a single chimney graft to the LCCA and a CS bypass/transposition are used. Finally, TEVAR zone 0 can entail two chimney grafts to the innominate artery and LCCA followed by a left CS bypass, or alternatively a chimney graft to the innominate artery, right to left carotid bypass and CS bypass[20]. Compared to more distal landing zones, zone 0 is more likely to be associated with retrograde type A dissection, especially when accompanied by excessive oversizing of the stent-graft[41]. Yet, it has also been demonstrated that the use of chimney grafts in the aortic arch, increases the risk of post-operative complications[40].
Liu et al[21] studied 215 patients with a non-A non-B AAD, treated with TEVAR, open surgical techniques or conservatively. TEVAR was used in 127 patients (59.1%) with a success rate of 85.9%. The 30-d mortality rate was 1.6%, compared to 7.1% in patients treated with open techniques. However, during the follow up, 9 deaths occurred in the endovascular group, 5 of which were related to the dissection, while no deaths occurred in the surgical group. Supplementary, following TEVAR, patients had a worse clinical outcome in terms of aortic rupture, retrograde type A dissection, distal stent graft induced new entry, major stroke and the need for reintervention. Likewise, a study published in 2022, observed that 67% of the initial TEVAR cases (10/15), demanded reintervention, with eventually 4 patients deceasing during the first 30 d[7].
Management of non-A non-B dissections remains challenging. The paucity of relevant studies reflects the fact that non-A non-B AADs account for a relatively modest percentage of all AADs. Although little is known about their natural history and course of treatment, it has been explicitly shown that surgical intervention surpasses medical treatment in the case of a non-A non-B dissection[5,8,42]. Current data advocate that this clinical entity does not behave like an uncomplicated type B AAD, and perhaps a more aggressive approach is warranted. Open repair is mostly employed in arch entry, while TEVAR with hybrid procedures in descending entry dissections both of which are thought to be preferable as opposed to conservative treatment. Secondary analysis with long-term follow up of the existing studies as well as large prospective clinical trials will enlighten the field around non-A non-B AAD which still remains an obscure and perplexing variation of acute AAD. Future research is anticipated to determine the most optimal surgical method as well as the time to intervene, ultimately helping surgeons to navigate through these uncharted waters.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sef D, United Kingdom; Ueda H, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Yu HG
| 1. | Vilacosta I, San Román JA, di Bartolomeo R, Eagle K, Estrera AL, Ferrera C, Kaji S, Nienaber CA, Riambau V, Schäfers HJ, Serrano FJ, Song JK, Maroto L. Acute Aortic Syndrome Revisited: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021;78:2106-2125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 2. | Sayed A, Munir M, Bahbah EI. Aortic Dissection: A Review of the Pathophysiology, Management and Prospective Advances. Curr Cardiol Rev. 2021;17:e230421186875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 3. | Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, Grabenwöger M, Haverich A, Iung B, Manolis AJ, Meijboom F, Nienaber CA, Roffi M, Rousseau H, Sechtem U, Sirnes PA, Allmen RS, Vrints CJ; ESC Committee for Practice Guidelines. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873-2926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2374] [Cited by in RCA: 3104] [Article Influence: 282.2] [Reference Citation Analysis (0)] |
| 4. | Qanadli SD, Malekzadeh S, Villard N, Jouannic AM, Bodenmann D, Tozzi P, Rotzinger DC. A New Clinically Driven Classification for Acute Aortic Dissection. Front Surg. 2020;7:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Howard C, Ponnapalli A, Shaikh S, Idhrees M, Bashir M. Non-A non-B aortic dissection: A literature review. J Card Surg. 2021;36:1806-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | von Segesser LK, Killer I, Ziswiler M, Linka A, Ritter M, Jenni R, Baumann PC, Turina MI. Dissection of the descending thoracic aorta extending into the ascending aorta. A therapeutic challenge. J Thorac Cardiovasc Surg. 1994;108:755-761. [PubMed] |
| 7. | Kosiorowska M, Berezowski M, Widenka K, Kreibich M, Beyersdorf F, Czerny M, Rylski B. Non-A non-B acute aortic dissection with entry tear in the aortic arch. Interact Cardiovasc Thorac Surg. 2022;34:878-884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 8. | Carino D, Singh M, Molardi A, Agostinelli A, Goldoni M, Pacini D, Nicolini F. Non-A non-B aortic dissection: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2019;55:653-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Sievers HH, Rylski B, Czerny M, Baier ALM, Kreibich M, Siepe M, Beyersdorf F. Aortic dissection reconsidered: type, entry site, malperfusion classification adding clarity and enabling outcome prediction. Interact Cardiovasc Thorac Surg. 2020;30:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (1)] |
| 10. | Rylski B, Pérez M, Beyersdorf F, Reser D, Kari FA, Siepe M, Czerny M. Acute non-A non-B aortic dissection: incidence, treatment and outcome. Eur J Cardiothorac Surg. 2017;52:1111-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Urbanski PP, Wagner M. Acute non-A-non-B aortic dissection: surgical or conservative approach? Eur J Cardiothorac Surg. 2016;49:1249-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Rylski B, Schofer F, Beyersdorf F, Kondov S, Kreibich M, Schlett CL, Czerny M. Aortic Arch Anatomy in Candidates for Aortic Arch Repair. Semin Thorac Cardiovasc Surg. 2022;34:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Trimarchi S, de Beaufort HWL, Tolenaar JL, Bavaria JE, Desai ND, Di Eusanio M, Di Bartolomeo R, Peterson MD, Ehrlich M, Evangelista A, Montgomery DG, Myrmel T, Hughes GC, Appoo JJ, De Vincentiis C, Yan TD, Nienaber CA, Isselbacher EM, Deeb GM, Gleason TG, Patel HJ, Sundt TM, Eagle KA. Acute aortic dissections with entry tear in the arch: A report from the International Registry of Acute Aortic Dissection. J Thorac Cardiovasc Surg. 2019;157:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Sef D, Brown S, Jarral OA, Hussain A, Haslam E, Rajakaruna C, McAloon CJ. Subtle aortic dissection in a patient with severe aortic regurgitation and undiagnosed bicuspid aortic valve: A case report with a literature review. J Card Surg. 2021;36:3417-3420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 15. | Wang W, Piao H, Wang Y, Li B, Zhu Z, Wang T, Liu K. Early outcomes with a hybrid technique for repair of a non-A non-B aortic dissection. J Thorac Cardiovasc Surg. 2022;163:1766-1774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 16. | DEBAKEY ME, HENLY WS, COOLEY DA, MORRIS GC Jr, CRAWFORD ES, BEALL AC Jr. SURGICAL MANAGEMENT OF DISSECTING ANEURYSMS OF THE AORTA. J Thorac Cardiovasc Surg. 1965;49:130-149. [PubMed] |
| 17. | Daily PO, Trueblood HW, Stinson EB, Wuerflein RD, Shumway NE. Management of acute aortic dissections. Ann Thorac Surg. 1970;10:237-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 726] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 18. | Spanos K, Nana P, von Kodolitsch Y, Behrendt CA, Kouvelos G, Panuccio G, Athanasiou T, Matsagkas M, Giannoukas A, Detter C, Kölbel T. Management of Ascending Aorta and Aortic Arch: Similarities and Differences Among Cardiovascular Guidelines. J Endovasc Ther. 2022;29:667-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 19. | Isselbacher EM, Preventza O, Hamilton Black J 3rd, Augoustides JG, Beck AW, Bolen MA, Braverman AC, Bray BE, Brown-Zimmerman MM, Chen EP, Collins TJ, DeAnda A Jr, Fanola CL, Girardi LN, Hicks CW, Hui DS, Schuyler Jones W, Kalahasti V, Kim KM, Milewicz DM, Oderich GS, Ogbechie L, Promes SB, Gyang Ross E, Schermerhorn ML, Singleton Times S, Tseng EE, Wang GJ, Woo YJ. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022;146:e334-e482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 831] [Article Influence: 277.0] [Reference Citation Analysis (0)] |
| 20. | Czerny M, Schmidli J, Adler S, van den Berg JC, Bertoglio L, Carrel T, Chiesa R, Clough RE, Eberle B, Etz C, Grabenwöger M, Haulon S, Jakob H, Kari FA, Mestres CA, Pacini D, Resch T, Rylski B, Schoenhoff F, Shrestha M, von Tengg-Kobligk H, Tsagakis K, Wyss TR; EACTS/ESVS scientific document group. Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the European Association for Cardio-Thoracic surgery (EACTS) and the European Society for Vascular Surgery (ESVS). Eur J Cardiothorac Surg. 2019;55:133-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 327] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 21. | Liu J, Yang F, Chen L, Xie E, Su S, Liu Y, Geng Q, Fan R, Li J, Luo J. Management and Outcomes of Non-A Non-B Aortic Dissection. Eur J Vasc Endovasc Surg. 2022;64:497-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Sef D, Birdi I. Clinically significant incidental findings during preoperative computed tomography of patients undergoing cardiac surgery. Interact Cardiovasc Thorac Surg. 2020;31:629-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Kimura N, Nakamura M, Takagi R, Mieno MN, Yamaguchi A, Czerny M, Beyersdorf F, Kari FA, Rylski B. False lumen/true lumen wall pressure ratio is increased in acute non-A non-B aortic dissection. Interact Cardiovasc Thorac Surg. 2022;35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 24. | Valentine RJ, Boll JM, Hocking KM, Curci JA, Garrard CL, Brophy CM, Naslund TC. Aortic arch involvement worsens the prognosis of type B aortic dissections. J Vasc Surg. 2016;64:1212-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Tsai TT, Isselbacher EM, Trimarchi S, Bossone E, Pape L, Januzzi JL, Evangelista A, Oh JK, Llovet A, Beckman J, Cooper JV, Smith DE, Froehlich JB, Fattori R, Eagle KA, Nienaber CA; International Registry of Acute Aortic Dissection. Acute type B aortic dissection: does aortic arch involvement affect management and outcomes? Insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2007;116:I150-I156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Xiong Z, Yang P, Li D, Qiu Y, Zheng T, Hu J. A computational fluid dynamics analysis of a patient with acute non-A-non-B aortic dissection after type I hybrid arch repair. Med Eng Phys. 2020;77:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Tian C, Chen D, Zhao J, Zhang Y, Luo M, Fang K, Tian C, Sun X, Guo H, Qian X, Shu C. Surgical treatment patterns and clinical outcomes of type B aortic dissection involving the aortic arch. J Vasc Surg. 2023;77:1016-1027.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 28. | Saltarocchi S, De Orchi P, Saade W, D'Abramo M, Chourda E, Romiti S, Vinciguerra M, Greco E, Miraldi F, Mazzesi G. Acute thrombotic occlusion of a brachiocephalic branch graft and pseudoaneurysm formation after debranching surgery for a "non-A non-B" aortic dissection. J Card Surg. 2022;37:2879-2883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 29. | Borst HG, Walterbusch G, Schaps D. Extensive aortic replacement using "elephant trunk" prosthesis. Thorac Cardiovasc Surg. 1983;31:37-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 465] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 30. | Luo C, Qi R, Zhong Y, Chen S, Liu H, Guo R, Ge Y, Sun L, Zhu J. Early and Long-Term Follow-Up for Chronic Type B and Type Non-A Non-B Aortic Dissection Using the Frozen Elephant Trunk Technique. Front Cardiovasc Med. 2021;8:714638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 31. | Kreibich M, Siepe M, Berger T, Kondov S, Morlock J, Pingpoh C, Beyersdorf F, Rylski B, Czerny M. The Frozen Elephant Trunk Technique for the Treatment of Type B and Type Non-A Non-B Aortic Dissection. Eur J Vasc Endovasc Surg. 2021;61:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | Shrestha M, Bachet J, Bavaria J, Carrel TP, De Paulis R, Di Bartolomeo R, Etz CD, Grabenwöger M, Grimm M, Haverich A, Jakob H, Martens A, Mestres CA, Pacini D, Resch T, Schepens M, Urbanski PP, Czerny M. Current status and recommendations for use of the frozen elephant trunk technique: a position paper by the Vascular Domain of EACTS. Eur J Cardiothorac Surg. 2015;47:759-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 211] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 33. | Iafrancesco M, Goebel N, Mascaro J, Franke UFW, Pacini D, Di Bartolomeo R, Weiss G, Grabenwöger M, Leontyev SA, Mohr FW, Sioris T, Jakob H, Tsagakis K; International E-vita Open Registry Group. Aortic diameter remodelling after the frozen elephant trunk technique in aortic dissection: results from an international multicentre registry. Eur J Cardiothorac Surg. 2017;52:310-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 34. | Dohle DS, Tsagakis K, Janosi RA, Benedik J, Kühl H, Penkova L, Stebner F, Wendt D, Jakob H. Aortic remodelling in aortic dissection after frozen elephant trunk†. Eur J Cardiothorac Surg. 2016;49:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 35. | Zhao HP, Zhu JM, Ma WG, Zheng J, Liu YM, Sun LZ. Total arch replacement with stented elephant trunk technique for acute type B aortic dissection involving the aortic arch. Ann Thorac Surg. 2012;93:1517-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Dumfarth J, Kofler M, Stastny L, Plaikner M, Krapf C, Semsroth S, Grimm M. Stroke after emergent surgery for acute type A aortic dissection: predictors, outcome and neurological recovery. Eur J Cardiothorac Surg. 2018;53:1013-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 37. | Zhao H, Ma W, Wen D, Duan W, Zheng M. Computed tomography angiography findings predict the risk factors for preoperative acute ischaemic stroke in patients with acute type A aortic dissection. Eur J Cardiothorac Surg. 2020;57:912-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Preventza O, Liao JL, Olive JK, Simpson K, Critsinelis AC, Price MD, Galati M, Cornwell LD, Orozco-Sevilla V, Omer S, Jimenez E, LeMaire SA, Coselli JS. Neurologic complications after the frozen elephant trunk procedure: A meta-analysis of more than 3000 patients. J Thorac Cardiovasc Surg. 2020;160:20-33.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 39. | Ma WG, Zhang W, Wang LF, Zheng J, Ziganshin BA, Charilaou P, Pan XD, Liu YM, Zhu JM, Chang Q, Rizzo JA, Elefteriades JA, Sun LZ. Type A aortic dissection with arch entry tear: Surgical experience in 104 patients over a 12-year period. J Thorac Cardiovasc Surg. 2016;151:1581-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Ahmad W, Mylonas S, Majd P, Brunkwall JS. A current systematic evaluation and meta-analysis of chimney graft technology in aortic arch diseases. J Vasc Surg. 2017;66:1602-1610.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 41. | Chen Y, Zhang S, Liu L, Lu Q, Zhang T, Jing Z. Retrograde Type A Aortic Dissection After Thoracic Endovascular Aortic Repair: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 42. | Karangelis D, Tagarakis G. Non-A non-B aortic dissections: are we still in uncharted waters? Eur J Cardiothorac Surg. 2019;56:423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |