Published online May 26, 2023. doi: 10.4330/wjc.v15.i5.205
Peer-review started: December 27, 2022
First decision: January 9, 2023
Revised: January 19, 2023
Accepted: April 24, 2023
Article in press: April 24, 2023
Published online: May 26, 2023
Processing time: 142 Days and 13.8 Hours
Hospitalizations for heart failure exceed 1 million per year in both the United States and Europe and more than 90% are due to symptoms and signs of fluid overload. Rates of rehospitalizations or emergency department visit at 60 days are remarkable regardless of whether loop diuretics were administered at low vs high doses or by bolus injection vs continuous infusion. Ultrafiltration (UF) has been considered a promising alternative to stepped diuretic therapy and it consists in the mechanical, adjustable removal of iso-tonic plasma water across a semipermeable membrane with the application of hydrostatic pressure gradient generated by a pump. Fluid removal with ultrafiltration presents several advantages such as elimination of higher amount of sodium with less neurohormonal activation. However, the conflicting results from UF studies highlight that patient selection and fluid removal targets are not completely understood. The best way to assess fluid status and therefore establish the fluid removal target is also still a matter of debate. Herein, we provide an up-to-date systematic review about the role of ultrafiltration among patients with fluid overload and its gaps in daily practice.
Core Tip: This mini review aimed to evaluate the role of ultrafiltration in congestive heart failure and to compare this approach to standard therapy essentially based on diuretics. Evidences are still controversial and matter of debate, however it is clear that the use of ultrafiltration has beneficial effects on outcomes such as rehospitalization for heart failure and symptoms attenuation. This review of the literature also highlighted the pivotal role of a non-invasive multiparametric assessment of fluid overload to guide physicians through tailoring patient's decongestion.
- Citation: Urbani A, Pensotti F, Provera A, Galassi A, Guazzi M, Castini D. Extracorporeal veno-venous ultrafiltration in congestive heart failure: What’s the state of the art? A mini-review. World J Cardiol 2023; 15(5): 205-216
- URL: https://www.wjgnet.com/1949-8462/full/v15/i5/205.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i5.205
Hospitalizations for heart failure (HF) exceed 1 million per year in both the United States and Europe and more than 90% are due to symptoms and signs of fluid overload. In addition, up to 1 in 4 patients (24%) is readmitted within 30 d and 50% of patients are readmitted within 6 mo[1,2]. Recurrent fluid overload in HF has uniformly been associated with worse outcomes independently of age and renal function[3]. Data from the Diuretic Optimization Strategies Evaluation trial show that 42% of patients with acutely decompensated HF reached the composite end point of death, rehospitalizations or emergency department visit at 60 d regardless of whether loop diuretics were administered at low vs high doses or by bolus injection vs continuous infusion[4]. Therefore, the need for adjunctive treatment strategies to standard stepped diuretic therapy in patients presenting with fluid overload in the context of decompensated HF is critical. One promising therapy is extracorporeal veno-venous ultrafiltration (UF). Ultrafiltration consists in the mechanical, adjustable removal of iso-tonic plasma water across a semipermeable membrane with the application of hydrostatic pressure gradient generated by a pump[5].
The fluid removed from the intravascular compartment is constantly replaced by fluid from the third space configurating the so called “intra-vascular refill” phenomenon, thus allowing gradual and controlled fluid overload resolution[6]. Despite fluid removal with UF presents several advantages such as elimination of higher amount of sodium with less neurohormonal activation, results from clinical studies regarding efficacy and safety have been variable.
Herein, we provide an up-to-date systematic review about the role of UF among patients with fluid overload and its gaps in daily practice.
The initial trigger of fluid overload is a reduced cardiac output that results from failing myocardium. This process causes an arterial hypovolemia which triggers a cascade of events designed to increase intra-arterial blood volume. The main mechanism involved is a neurohumoral activation of the renin-angiotensin-aldosterone (RAAS) axis that increases renal and sodium avidity, thereby resulting in an increase of effective blood volume. In a setting of HF, proximal sodium and water retention are so elevated that distal nephron chronically undergoes low sodium delivery, maintaining persistent RAAS activation[7]. Also, increased sympathetic tone leads to splanchnic arterial and venous constriction resulting in blood redistribution from the splanchnic capacitance vasculature to the circulatory volume. This expands the effective circulating volume by redistribution in a setting where volume expansion is already ongoing[8]. At the beginning, these changes occur as compensatory mechanisms to maintain effective circulating blood volume, over time they become harmful with the development of pathological inappropriate blood volume and interstitial fluid expansion contributing to fluid overload and organ congestion. An excessive effective circulating blood volume leads to hemodynamic congestion with increased central filling pressures[9]. Deranged hemodynamics and neurohormonal activation leading to excessive tubular reabsorption produce long-standing venous congestion. Elevation of central venous pressure is rapidly transmitted to the renal veins, causing increased interstitial and tubular hydrostatic pressure that decrease net glomerular filtration[10]. Hence, in this context, venous congestion of the kidneys rather than arterial underfilling is associated with decreased renal blood flow and an increase in creatinine[6,11]. Moreover, congestion within peripheral vascular tissues can produce endothelial activation followed by up-regulation of inflammatory cytokines, which promotes additional fluid retention[12,13]. Therefore, reducing congestion should be the foremost goal in patients with HF and fluid congestion[6].
Historically, the gold standard to evaluate fluid overload has been pulmonary artery catheterization (PAC) that allows a direct measurement of right atrial pressure and pulmonary capillary wedge pressure (PCWP)[14]. For several years it has been widely used but then, Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness trial showed that the use of PAC to guide diuretic therapy in comparison with serial clinical assessment did not improve mortality[15]. Currently, due to its invasive nature and the lack of evidence, the use of Swan Ganz catheterization is restricted to a selected group of critically ill patients in tertiary hospitals with high level of user competence.
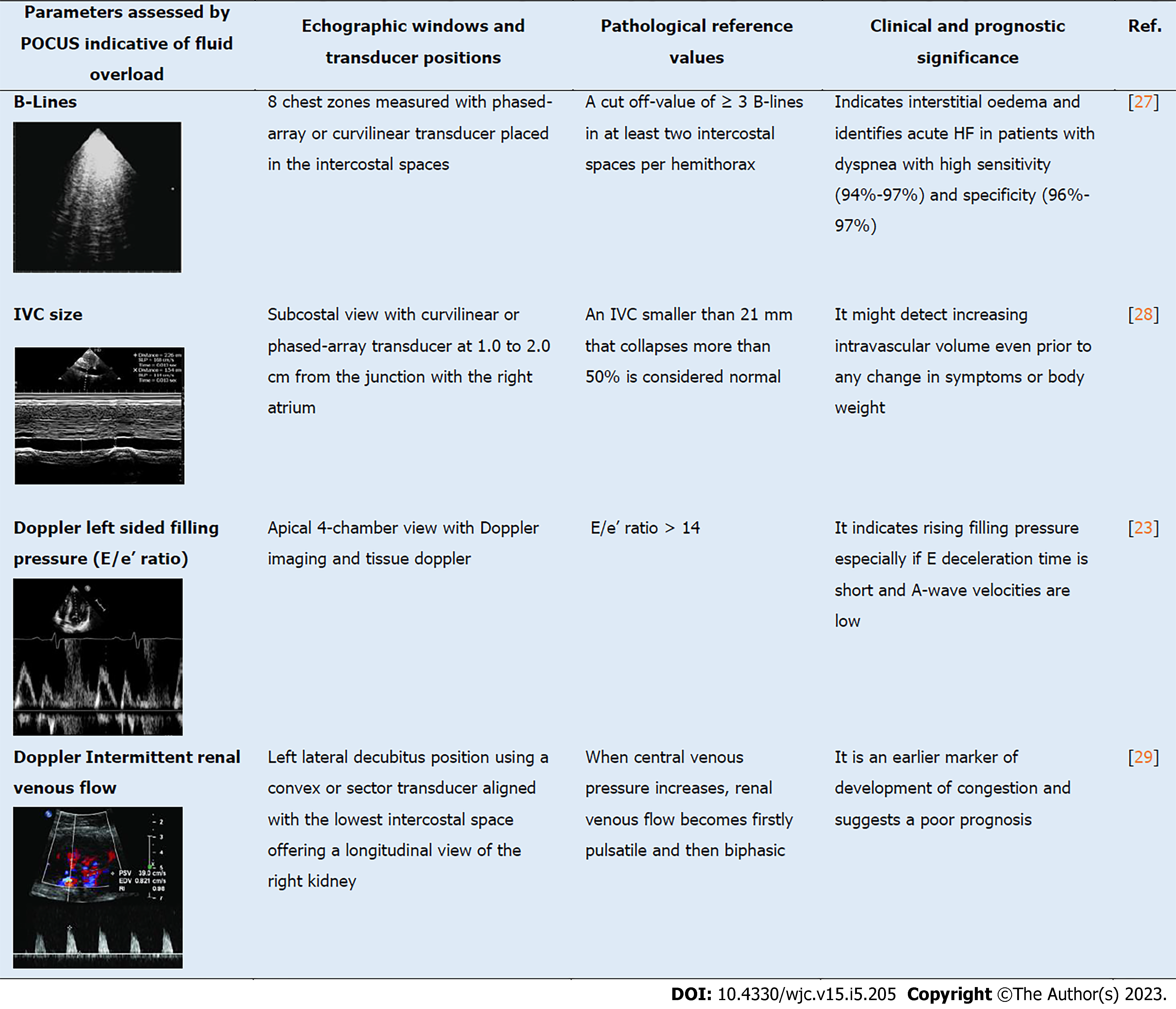
For what concern the non-invasive assessments of congestion, a multiparametric point of care ultrasound (POCUS) can play a key role. In the Figure 1, we make a comprehensive list of all the most used POCUS parameters.
Lung ultrasound (LUS) can be used to assess interstitial oedema and pleural effusion in patients with known or suspected HF and detects the so-called B-lines that originates from the extravascular fluid[16,17]. B-lines are hyperechoic artefacts which appear as vertical lines originating from the pleural surface[18]. More than three B-lines in more than two intercostal spaces bilaterally are considered diagnostic of interstitial and alveolar oedema in acute HF[8,19]. In comparison to chest X-ray, lung ultrasound is more accurate for the diagnosis of interstitial oedema and HF, although B-lines can occur in other condition such as interstitial lung disease and non-cardiogenic pulmonary oedema[16,20,21]. Echocardiography can, also, be used to non-invasively and quickly estimate right and left-sided filling pressure. An appraisal of the right atrial pressure can be performed by evaluating the collapsibility and width of the inferior vena cava (IVC)[22]. Any variation in right atrial pressure is transferred backward, and modifies IVC size, in fact a significant increase in right atrial pressure, as seen in HF, would eventually result in IVC distention. Pulsed doppler and tissue Doppler are useful tools in estimating left-sided filling pressure. The E/e’ ratio > 14 has high specificity for increased filling pressures, especially if E-wave deceleration time is short and A-wave velocities are low[23,24]. Furthermore, congestion and high central venous pressure lead to increased renal interstitial pressures that affects primarily renal venous flow which can be evaluated with Doppler ultrasound. Specifically, the presence of an intermittent renal venous flow rather than continuous as in healthy subjects has been strongly related with an increased central venous pressure measured invasively and has been associated with a worse prognosis in both acutely decompensated HF and among stable patients with chronic HF[25,26]. Despite diagnosis of congestion is currently made with the combination of signs and symptoms, a suggestive X-ray and the measurement of elevated natriuretic peptides (NPs), these additional non-invasive POCUS-guided approach can increase the accuracy of the diagnosis and, more importantly, can be helpful in tailoring patient‘s decongestion during the hospitalization (Table 1).
| Loop diuretics | Isolated ultrafiltration |
| Hypotonic urine | Isotonic plasma water |
| Direct neurohormonal activation | No direct neurohormonal activation |
| Unpredictable elimination of sodium and water | Precise control of rate and amount of fluid removal |
| Diuretic resistance | Restoration of diuretic responsiveness |
| Hypokalemia and hypomagnesemia | No effect on plasma concentration of potassium and magnesium |
| No need for anticoagulation | Need for anticoagulation |
| No extracorporeal circuit | Need for extracorporeal circuit |
Diuretic agents, especially loop diuretics, have been for decades the backbone of the therapy for fluid overload[30,31]. Guidelines recommend the use of intravenous loop diuretic rather than oral as first line therapy and an early as possible administration because of its association with reduced in-hospital mortality[32]. The initial diuretic regimen depends on whether the patient is diuretic naïve or not. In fact, diuretic naïve patients should receive an initial dose of at least 20-40 mg of intravenous furosemide, whereas patients already on an ambulatory diuretic regimen should receive 1-2 times the 24-h home dose intravenously. A spot urine sodium content of > 50-70 mEq/L and an hourly urine output of > 100-150 mL during the first 6 h usually identifies patients with an initial acceptable diuretic response[33,34]. If these targets are not reached, a prompt doubling of loop diuretic dose is usually required and should be repeated until maximal dose of loop diuretics is administered; as maximal dose of loop diuretic is given, an addition of another diuretic agent should be considered as increasing the loop diuretic dose does not improve natriuresis any further. Despite diuretics are highly effective in the early stages of acute HF, in a significant subset of patients loop diuretics become increasingly ineffective with disease progression due to the onset of diuretic resistance[35]. A recent definition of diuretic resistance has been proposed which implies a failure to increase fluid and sodium (Na+) output sufficiently to relieve volume overload, edema, or congestion, despite escalating doses of a loop diuretic to a ceiling level (80 mg of furosemide once or twice daily or greater in those with reduced glomerular filtration rate or HF)[36]. Diuretic resistance is the result of several factors such as impaired absorption, decreased renal blood flow, hypoalbuminemia and proteinuria, all leading to a reduced levels of active diuretics in the tubular lumen[35,37]. Unfortunately, clinical signs and symptoms are often unreliable to detect diuretic resistance. A poor diuretic response predicts mortality rate after discharge, subsequent rehospitalization, or renal complications from congestive HF[36]. Therefore, finding more effective treatments for fluid removal is an unmet need.
As briefly described in the introduction, the mechanism of action of UF is based on the use of a transmembrane pressure gradient generated by a pump, which through a semi-permeable membrane causes the removal of plasma water from whole blood. The net effect at the end of the process is the achievement of an isotonic concentration between the ultrafiltrate and the plasma water removed from the circulation[10].
UF requires a venous access to fulfill the filtration process through the hemofilter and the subsequent reintroduction of the ultrafiltered plasma into the systemic circulation[38].
Optimal anticoagulant therapy by continuous infusion of heparin is also required to preserve the function of the filter during the whole process.
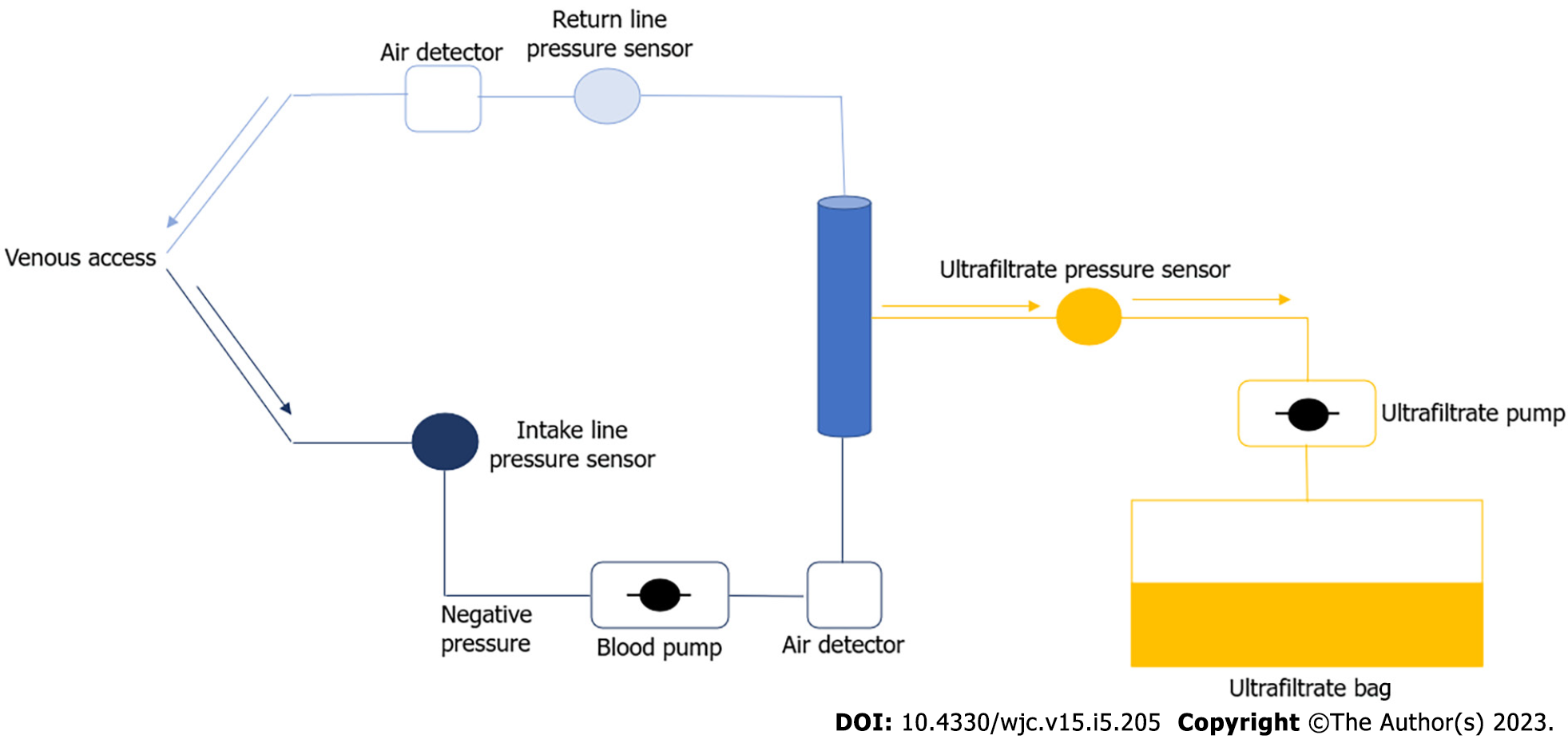
The Figure 2 schematically shows the UF process; the blood, after being extracted from the patient by venous access, is transferred to the extracorporeal circuit of the UF and then reintroduced into the bloodstream.
Newer devices allow, via a double-lumen venous catheter placed in the jugular or basilic vein, a blood draw with minimal recirculation.
There are various methods by which the UF process can be achieved: isolated UF, hemofiltration and UF in combination with dialysis.
Isolated UF is only a method of fluid control, whereas all the others can simultaneously achieve a certain degree of blood purification with different mechanisms. For example hemodialysis accomplishes blood purification with solutes moving from high concentration to low concentration along the electrochemical gradient whether UF, as stated above, the substances travel due to a pressure gradient.
According to a duration-based classification, UF techniques can be classified as acute or isolated, intermittent or continuous (< 24 h) and slow continuous (> 24 h)[39].
With pure UF, an extracorporeal blood pump, either by suction applied to the ultrafiltrate compartment (negative pressure) or by resistance induced in the venous line (positive pressure) transfers the blood through the filter where the UF process is achieved.
Advantages of pure UF include the avoidance of arterial puncture, short exposure to systemic anticoagulation and, furthermore, it does not require specialized dialysis personnel[40].
Using an appropriate UF rate allows the extracellular fluid to gradually fill the removed intravascular space, thus keeping the volume constant; this effect differs UF from diuretics which remove intravascular volume without causing adequate filling from the extravascular space, the main site of congestion in patients with HF.
On the other hand, if the UF rate is too high, the rate by which the intravascular volume is removed exceeds the reabsorption of fluid from the interstitium into the vascular space, thus losing the real benefit of using UF.
Therefore, to obtain a real benefit from UF, the challenge is to find the correct rate of decongestion while maintaining an adequate circulating blood volume[41].
With the usage of loop diuretics, the reduction of net body water is achieved through the removal of hypotonic urine whereas UF determines the production of an iso osmotic and isonatremic diuresis. Thus, for any amount of fluid withdrawn, the net quantity of sodium removed is greater with UF than with diuretic agents.
The use of loop diuretics, moreover, causes also an inhibition of sodium chloride uptake in the macula densa thus enhancing the RAAS system activation.
On the other hand, UF through iso-osmotic and isonatremic diuresis maintains the same uptake of sodium chloride from the macula densa, avoiding RAAS system activation.
Thereby, the prolonged use of loop diuretics increases water and sodium retention in the proximal tubule with subsequent reduction of net sodium delivering in the loop of Henle decreasing the efficacy of loop diuretics to relieve the congestion.
The final goal of the ultrafiltrative process determines an euvolemic state which is obtained by withdrawing intravascular volume which is equal in size to that reabsorbed from the extracellular space[41].
Compared with standard intravenous diuretic therapy UF, by reduction of neurohormonal pathways, also improve functional capacity in patients with HF.
As explained in the study of Agostoni et al[42], UF unequivocally improved the functional performance of patients with HF. Cardiopulmonary exercise test obtained after 4 d shows higher values of peak VO2 and better VD/VT ratio in UF patients compared with standard diuretic therapy. The authors suggest that favorable influences of UF on the functional capacity are related to the protective role of vasopressin and atrial peptides during and after UF that allows a more “physiological" fluid metabolism and lung decongestion in patient with HF. The study also demonstrated that UF was a more powerful stimulus than loop diuretics on the release of norepinephrine, with much less effect on the RAAS system[42]. In Table 1 we highlighted main differences of using UF or diuretics.
The first two randomized clinical trial (RCT) in which an UF-based decongestion was compared to a diuretic-based approach have been the Relief for Acutely Fluid-Overloaded Patients With Decompensated Congestive Heart Failure (RAPID-CHF) and the Use of Nitroprusside in Left Ventricular Dysfunction and Obstructive Aortic Valve Disease (UNLOAD) trial[43,44].
In the RAPID-CHF, authors demonstrated the feasibility and the potential beneficial effects of UF (with Aquadex system) vs usual care among patients admitted to the hospital for an acute decompensation of congestive HF (20 UF, 20 usual care). These patients received a single, 8-h course of UF with fluid removal rates determined by the attending physician (to a maximum of 500 cc/h), whereas usual care patients were treated with diuretics according to the guidelines. Weight loss after 24 h from enrollment, used as primary end-point, was greater in the UF group although did not reach a statistical significance. This trial also highlighted that at 1-d and 30-d follow-up patients had a sustained and more significant symptoms relief (dyspnea) compared to the usual care. Furthermore, there were no differences between groups in terms of hemodynamic parameters (e.g., blood pressure, heart rate) and adverse events.
However, the authors concluded that the small sample size (n = 40) reduced the significance of this study and did not allow to draw definitive conclusions about the potential beneficial role of UF in this subset of patients.
The investigators of the UNLOAD trial compared early UF strategy (at 24 h from hospitalization) vs diuretics in a population of 100 patients admitted with acutely decompensated HF in a prospective, randomized, multicenter trial. In the UF group, fluid was removed at an average fixed rate of 241 mL/h for 12 h. In the standard-care group, average daily diuretic dose of intravenous furosemide or equivalent was 181 mg during the 48 h after randomization. The study demonstrated a clear trend of each outcome in favor of UF; weight loss (primary endpoint), dyspnea improvement and net fluid loss assessed at 48 h from enrollment resulted statistically significant (P = 0.001) in the UF arm of the study as compared to diuretics arm. Furthermore, the UF group had fewer patients re-hospitalized for HF at 90 d [16 of 89 (18%) vs 28 of 87 (32%), P = 0.037].
One of the main limits of both the RAPID-CHF and UNLOAD trial was to not include a comprehensive assessment of hemodynamic parameters during the study.
Giglioli et al[45], in their ULTRADISCO trial performed a standardized evaluation of hemodynamic status obtained using Pressure Recording Analytical Method monitoring system by radial artery cannulation. Stroke volume indexed, cardiac index, cardiac power output, systemic vascular resistance was measured during hospitalization, at discharge, and at 1 and 3-mo follow-up among patients with acute decompensated HF (ADHF) treated with UF (using PRISMA system) vs conventional diuretics strategy. As a result, authors demonstrated that UF benefits can go beyond the net fluid loss and clinical improvement by significantly ameliorating hemodynamic status[45].
A different kind of population was target of the CARRESS-HF trial. In this study Bart et al[46] compared UF with a diuretic-based stepped pharmacologic therapy in patients hospitalized with ADHF with signs of congestion and worsening renal function (defined as an increase in the serum creatinine level of at least 0.3 mg per deciliter between 12 wk before and 10 d after the index admission for HF.) Ultrafiltration was performed at a fixed fluid-removal rate of 200 mL per hour. Both the primary endpoints (weight loss and serum creatinine variation) and secondary endpoints (clinical and laboratoristic) resulted statistically not significant. This trial also showed more adverse events in the UF arm such as bleeding, catheter thrombosis and advanced kidney failure. The reasons of these results remain still unclear although it is possible that the patients involved are not the subpopulation in which UF achieve its potential beneficial effect[46].
In the CUORE trial, investigators randomized highly selected patients with severe systolic congestive HF to UF (using Dedyca system) or standard therapy. Those patients randomized to UF had a significantly lower frequency of rehospitalization for congestive HF than control subjects and this result was maintained for up to 1 year. Furthermore, the overall reduction in rehospitalizations was linked to more significant maintenance of a body weight, renal function and lower diuretic dose in the first 6 mo after discharge[47].
The AVOID-HF trial, in contrast with the CARRESS-HF trial, remains faithful to the findings of other studies mentioned above in which UF is beneficial when applied early during the episode of HF decompensation. The AVOID-HF has the largest sample size with a total of 224 patients randomized to UF arm (using Aquadex system) or standard diuretic therapy and has a predefined decongestion dose adjustment protocol. The trial was interrupted prematurely for slow enrollment rate. Despite only one third of the sample size was achieved from the investigators, this trial showed a trend toward reduction in rehospitalization for HF in the first 90-d of discharge[48].
Lastly, Hu et al[49], in their single center experience trial has demonstrated that early UF effectively and safely reduces volume overload in patients with ADHF. Patient were enrolled in the first 24 h of admission randomlyassigned into early UF (n = 40) or torasemide plus tolvaptan (n = 60) groups. Criteria of inclusion were acutely decompensated HF patients of age more than 18 years old and who had 1 or more sign of congestion (lung rales on auscultation, chest X ray documenting pulmonary congestion, congestive hepatomegaly and/or ascites, jugular venous pulse > 10 cm; lower limb edema, B-type NP > 400 pg/mL). Primary and secondary efficacy endpoint were increase in urine output, weight loss, reduction of dyspnea and brain natriuretic peptide; each endpoint reached statistical significance[49].
In conclusion, Table 2 summarize the different RCTs designs and outcomes.
| RCTs | Target population | UF device | Primary and secondary endpoint | Results |
| RAPID-CHF (2005) | ADHF, n = 40 | Aquadex system, 8-h course | Weight loss at 24 h of treatment (Primary endpoint); Volume removal after 24 h | Weight reduction resulted not statistically significant (P = 0.24); Volume removal was significantly more in UF arm (P < 0.001) |
| UNLOAD (2007) | ADHF, n = 200 | Aquadex System, Mean fluid removal rate 241 mL/h | Weight loss at 48 h; Dyspnea score at 48 h | Weight loss resulted significantly increased in UF arm (P < 0.001), whereas there were no differences between groups in Dyspnea score (P = 0.588) |
| ULTRADISCO (2011) | ADHF, n = 30 | PRISMA, treatment duration 46 h | Changes in hemodynamics assessed using PRAM: SVi, CI, CPO, SVR were measured during hospitalization, at discharge, and at 1 and 3-mo follow-up | UF arm as compare to standard care had a significant improvement of global hemodynamic status |
| CARRESS-HF (2012) | ADHF, n = 188; Recent increase in serum creatinin >/= 0.3 mg/dL | Aquadex System at a fixed rate of 200 mL/h | Bivariate changes in sCr and change in weight 96 h after randomization | |
| CUORE (2014) | ADHF, LVEF </=40%, n = 56 | Dedyca system | HF rehospitalization at 1 yr | UF arm has a significant lower endpoint incidence (P = 0.002) |
| AVOID-HF (2016) | ADHF, n = 224 | Aquadex system at an ajdusted rate on a per protocol established basis | Time to first HF event (HF rehospitalization or unscheduled outpatient or emergency treatment with intravenous loop diuretic agents or UF) within 90 days of hospital discharge | 30-d HF rehospitalizations: 11 of 2876 (UF arm) vs 24 of 2882 (diuretics arm), P = 0.06 |
| Hu et al[49], 2021 | ADHF, n = 100 | FQ-16 type HF ultrafiltration dehydration device (Beijing Hartcare Medical Technology Co., Ltd) | Weight loss and an increase in urine output on days 4 and 8 of treatment; Secondary outcome evaluated: BNP, NYHA class, IVC collapse index, JVP | Early ultrafiltration group had a significantly greater weight loss (P < 0.001) than the torasemide + tolvaptan group and urine increase (P < 0.001); Secondary outcomes that were followed up demonstated a clear trend towards benefits of UF as compared to diuretics arm |
Given current data, it has not been yet clearly defined which subpopulation of patients suffering from acute HF refractory to diuretic therapy can benefit from UF. The conflicting results from UF studies highlight that patient selection and fluid removal targets are not completely understood.
Heterogeneity of HF patients (e.g., baseline clinical characteristics, hemodynamic profile, severity of renal functional impairment), the timing and the UF protocols used in the trials contributed to these inconsistent results.
As mentioned, guidelines recommend this therapeutic option in patients with a lack of hemodynamic and laboratory response despite maximal diuretic therapy[30,31]. Unfortunately, data regarding which patients may benefit the most from this strategy are scarce.
However, despite the conflicting results of the RCTs, we can still assume some broad indications from them. According to CARRESS-HF, UF may not be useful among patients with ADHF and worsening renal function[46]. Furthermore, in contrast with all other trials, the median time from the index hospital admission (the admission qualifying the patient for enrollment in the study) to randomization was 34 h. This data reinforce the belief that a more effective UF process could be related to an earlier beginning of treatment.
From some of the trials that demonstrated an effectiveness of UF, we can suggest how continuous, or at least frequent, assessment of hemodynamic stability and fluid overload are essential prerogatives before and during the treatment[45,48].
Baseline clinical characteristics of the patient and protocol used in these trials suggest in which clinical setting UF may be used.
Ultrafiltration has been principally used in decompensated HF patients as an escalation after diuretic failure or in the presence of cardiorenal syndrome. Earlier utilization of UF can expedite and maintain the compensation of acute HF by simultaneously reducing volume overload without causing intravascular volume depletion and re-establishing acid base and electrolyte balance. Despite the crucial need of alternatives to diuretics-based decongestive strategy there are still several gaps of knowledge about the correct use of UF. What clearly emerges from the literature is the lack of strong evidences able to support the routine use of UF as first and early step of treatment whereas the overall potential and beneficial effect remains clear.
To draw definitive conclusions, we need more data comings from new RCTs. At the moment, REVERSE-HF, a multicenter randomized controlled trial, is ongoing across the United States.
An important target of decongestive therapies to achieve is the so-called dry weight; however, the best way to assess fluid status and dry weight is still a matter of debate.
POCUS can, potentially, be a helpful tool for a quick and objective fluid status assessment. There are several echographic markers of the high pressures associated with congestive process, as described above, which have been proposed[50].
However, there is still a paucity of combined POCUS scoring systems able to predict adverse outcomes of patients with clinical and laboratoristic signs of congestion. One of the proposed scoring systems is Venous Excess Ultrasound grading system of the severity of venous congestion[51].
Bioelectrical impedance (BIA) is, also, an attractive non-invasive method for assessing the total body water. Measurements of bioimpedance vector require 2 pairs of electrodes to be placed on the wrist and ankles. This method, potentially, could help the physician to guide the reduction of patient’s fluid overload as showed in some trials[52].
Several authors have investigated if an objective tool such as BIA is better than clinical findings for guiding UF in hemodialysis patients. As a result of several RCTs, BIA-based interventions in hemodialysis patients for correction of overhydration have little to no effect on all-cause mortality, whereas BIA improved systolic blood pressure control. These results should be interpreted with caution as the size and power of the studies are low. Further studies, larger or with a longer follow-up period, should be performed to better describe the effect of BIA-based strategies on survival[53].
In a study by Hanna et al[54], they proposed that a protocol driven-UF with invasive PCWP as hemodynamic parameter can guide the physician for a safe and effective interruption of ultrafiltrative system, reaching the goal of sustained value </= 18 mmHg for more than 4 h.
The use of biomarkers able to show acute kidney injury can help physician to assess fluid status and guide decongestion. At the state of art, serum creatinine is the sole biomarker used in daily practice to guide fluid removal. However, serum creatinine can be elevated also in the context of volume depletion without acute tubular damage. Conversely, this parameter can be normal in documented tubular injury due to the delayed achievement of detectable changes of this analyte. It is therefore evident that we need to undercover new useful biomarkers able to be more specific for kidney damage. Neutrophil gelatinase-associated lipocalin (NGAL) attracts as a newly more specific biomarker of acute kidney injury; NGAL is not elevated in case of volume depletion as serum creatinine. In vitro studies found other genes expressed only after brief dose of ischemia as kidney injury molecule-1, tissue inhibitor of metalloproteinase-1, and clusterin, although none of these genes were expressed after volume depletion, despite the rise in serum creatinine in both models[55].
In conclusion, ultrafiltration represents an attractive alternative to pharmacologic therapy. More long-term data about safety, incidence of rehospitalization for HF and cost-effectiveness are crucial to definitely allocate this treatment also as a main option. Furthermore, we need more comprehensive and non-invasive tools to guide physicians in the fluid status management of congested patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Charach L, Israel; Sangani V, United States S-Editor: Wang JL L-Editor: A P-Editor: Yu HG
| 1. | Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1248] [Cited by in RCA: 1570] [Article Influence: 142.7] [Reference Citation Analysis (0)] |
| 2. | Crespo-Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka F, Ferrari R, Piepoli MF, Delgado Jimenez JF, Metra M, Fonseca C, Hradec J, Amir O, Logeart D, Dahlström U, Merkely B, Drozdz J, Goncalvesova E, Hassanein M, Chioncel O, Lainscak M, Seferovic PM, Tousoulis D, Kavoliuniene A, Fruhwald F, Fazlibegovic E, Temizhan A, Gatzov P, Erglis A, Laroche C, Mebazaa A; Heart Failure Association (HFA) of the European Society of Cardiology (ESC). European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur J Heart Fail. 2016;18:613-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 571] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 3. | Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 502] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 4. | Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O'Connor CM; NHLBI Heart Failure Clinical Research Network. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797-805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1359] [Cited by in RCA: 1226] [Article Influence: 87.6] [Reference Citation Analysis (0)] |
| 5. | Ronco C, Ricci Z, Bellomo R, Bedogni F. Extracorporeal ultrafiltration for the treatment of overhydration and congestive heart failure. Cardiology. 2001;96:155-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Somaschini A, Casirati A, Cornara S, Demarchi A, Mandurino-Mirizzi A, Androulakis E, Lioudaki E. Extracorporeal veno-venous ultrafiltration in patients with acute heart failure. Rev Cardiovasc Med. 2021;22:1311-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | Gologorsky RC, Roy S. Ultrafiltration for management of fluid overload in patients with heart failure. Artif Organs. 2020;44:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner-La Rocca HP, Martens P, Testani JM, Tang WHW, Orso F, Rossignol P, Metra M, Filippatos G, Seferovic PM, Ruschitzka F, Coats AJ. The use of diuretics in heart failure with congestion - a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21:137-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 696] [Article Influence: 116.0] [Reference Citation Analysis (0)] |
| 9. | Miller WL. Fluid Volume Overload and Congestion in Heart Failure: Time to Reconsider Pathophysiology and How Volume Is Assessed. Circ Heart Fail. 2016;9:e002922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 199] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 10. | Costanzo MR, Ronco C, Abraham WT, Agostoni P, Barasch J, Fonarow GC, Gottlieb SS, Jaski BE, Kazory A, Levin AP, Levin HR, Marenzi G, Mullens W, Negoianu D, Redfield MM, Tang WHW, Testani JM, Voors AA. Extracorporeal Ultrafiltration for Fluid Overload in Heart Failure: Current Status and Prospects for Further Research. J Am Coll Cardiol. 2017;69:2428-2445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | F Gnanaraj J, von Haehling S, Anker SD, Raj DS, Radhakrishnan J. The relevance of congestion in the cardio-renal syndrome. Kidney Int. 2013;83:384-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Colombo PC, Onat D, Harxhi A, Demmer RT, Hayashi Y, Jelic S, LeJemtel TH, Bucciarelli L, Kebschull M, Papapanou P, Uriel N, Schmidt AM, Sabbah HN, Jorde UP. Peripheral venous congestion causes inflammation, neurohormonal, and endothelial cell activation. Eur Heart J. 2014;35:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 13. | Ganda A, Onat D, Demmer RT, Wan E, Vittorio TJ, Sabbah HN, Colombo PC. Venous congestion and endothelial cell activation in acute decompensated heart failure. Curr Heart Fail Rep. 2010;7:66-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Vincent JL, Rhodes A, Perel A, Martin GS, Della Rocca G, Vallet B, Pinsky MR, Hofer CK, Teboul JL, de Boode WP, Scolletta S, Vieillard-Baron A, De Backer D, Walley KR, Maggiorini M, Singer M. Clinical review: Update on hemodynamic monitoring--a consensus of 16. Crit Care. 2011;15:229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 258] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 15. | Binanay C, Califf RM, Hasselblad V, O'Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW; ESCAPE Investigators and ESCAPE Study Coordinators. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294:1625-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1062] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 16. | Pellicori P, Platz E, Dauw J, Ter Maaten JM, Martens P, Pivetta E, Cleland JGF, McMurray JJV, Mullens W, Solomon SD, Zannad F, Gargani L, Girerd N. Ultrasound imaging of congestion in heart failure: examinations beyond the heart. Eur J Heart Fail. 2021;23:703-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 17. | Platz E, Merz AA, Jhund PS, Vazir A, Campbell R, McMurray JJ. Dynamic changes and prognostic value of pulmonary congestion by lung ultrasound in acute and chronic heart failure: a systematic review. Eur J Heart Fail. 2017;19:1154-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 18. | Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, Dean A, Tsung JW, Soldati G, Copetti R, Bouhemad B, Reissig A, Agricola E, Rouby JJ, Arbelot C, Liteplo A, Sargsyan A, Silva F, Hoppmann R, Breitkreutz R, Seibel A, Neri L, Storti E, Petrovic T; International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1739] [Cited by in RCA: 1882] [Article Influence: 144.8] [Reference Citation Analysis (0)] |
| 19. | Martindale JL, Secko M, Kilpatrick JF, deSouza IS, Paladino L, Aherne A, Mehta N, Conigiliaro A, Sinert R. Serial Sonographic Assessment of Pulmonary Edema in Patients With Hypertensive Acute Heart Failure. J Ultrasound Med. 2018;37:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Wang Y, Gargani L, Barskova T, Furst DE, Cerinic MM. Usefulness of lung ultrasound B-lines in connective tissue disease-associated interstitial lung disease: a literature review. Arthritis Res Ther. 2017;19:206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 21. | Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008;6:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 369] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 22. | Simonson JS, Schiller NB. Sonospirometry: a new method for noninvasive estimation of mean right atrial pressure based on two-dimensional echographic measurements of the inferior vena cava during measured inspiration. J Am Coll Cardiol. 1988;11:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 152] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2879] [Cited by in RCA: 3840] [Article Influence: 426.7] [Reference Citation Analysis (0)] |
| 24. | Caballero L, Kou S, Dulgheru R, Gonjilashvili N, Athanassopoulos GD, Barone D, Baroni M, Cardim N, Gomez de Diego JJ, Oliva MJ, Hagendorff A, Hristova K, Lopez T, Magne J, Martinez C, de la Morena G, Popescu BA, Penicka M, Ozyigit T, Rodrigo Carbonero JD, Salustri A, Van De Veire N, Von Bardeleben RS, Vinereanu D, Voigt JU, Zamorano JL, Bernard A, Donal E, Lang RM, Badano LP, Lancellotti P. Echocardiographic reference ranges for normal cardiac Doppler data: results from the NORRE Study. Eur Heart J Cardiovasc Imaging. 2015;16:1031-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 25. | Iida N, Seo Y, Sai S, Machino-Ohtsuka T, Yamamoto M, Ishizu T, Kawakami Y, Aonuma K. Clinical Implications of Intrarenal Hemodynamic Evaluation by Doppler Ultrasonography in Heart Failure. JACC Heart Fail. 2016;4:674-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 219] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 26. | Puzzovivo A, Monitillo F, Guida P, Leone M, Rizzo C, Grande D, Ciccone MM, Iacoviello M. Renal Venous Pattern: A New Parameter for Predicting Prognosis in Heart Failure Outpatients. J Cardiovasc Dev Dis. 2018;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Pivetta E, Goffi A, Nazerian P, Castagno D, Tozzetti C, Tizzani P, Tizzani M, Porrino G, Ferreri E, Busso V, Morello F, Paglieri C, Masoero M, Cassine E, Bovaro F, Grifoni S, Maule MM, Lupia E; Study Group on Lung Ultrasound from the Molinette and Careggi Hospitals. Lung ultrasound integrated with clinical assessment for the diagnosis of acute decompensated heart failure in the emergency department: a randomized controlled trial. Eur J Heart Fail. 2019;21:754-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 141] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 28. | Dovancescu S, Pellicori P, Mabote T, Torabi A, Clark AL, Cleland JGF. The effects of short-term omission of daily medication on the pathophysiology of heart failure. Eur J Heart Fail. 2017;19:643-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Costanzo MR. Ultrafiltration in Acute Heart Failure. Card Fail Rev. 2019;5:9-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599-3726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8225] [Cited by in RCA: 7266] [Article Influence: 1816.5] [Reference Citation Analysis (0)] |
| 31. | Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895-e1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1289] [Cited by in RCA: 1196] [Article Influence: 398.7] [Reference Citation Analysis (0)] |
| 32. | Matsue Y, Damman K, Voors AA, Kagiyama N, Yamaguchi T, Kuroda S, Okumura T, Kida K, Mizuno A, Oishi S, Inuzuka Y, Akiyama E, Matsukawa R, Kato K, Suzuki S, Naruke T, Yoshioka K, Miyoshi T, Baba Y, Yamamoto M, Murai K, Mizutani K, Yoshida K, Kitai T. Time-to-Furosemide Treatment and Mortality in Patients Hospitalized With Acute Heart Failure. J Am Coll Cardiol. 2017;69:3042-3051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 224] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 33. | Singh D, Shrestha K, Testani JM, Verbrugge FH, Dupont M, Mullens W, Tang WH. Insufficient natriuretic response to continuous intravenous furosemide is associated with poor long-term outcomes in acute decompensated heart failure. J Card Fail. 2014;20:392-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 34. | Testani JM, Hanberg JS, Cheng S, Rao V, Onyebeke C, Laur O, Kula A, Chen M, Wilson FP, Darlington A, Bellumkonda L, Jacoby D, Tang WH, Parikh CR. Rapid and Highly Accurate Prediction of Poor Loop Diuretic Natriuretic Response in Patients With Heart Failure. Circ Heart Fail. 2016;9:e002370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 35. | Wilcox CS, Testani JM, Pitt B. Pathophysiology of Diuretic Resistance and Its Implications for the Management of Chronic Heart Failure. Hypertension. 2020;76:1045-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 36. | Kiernan MS, Stevens SR, Tang WHW, Butler J, Anstrom KJ, Birati EY, Grodin JL, Gupta D, Margulies KB, LaRue S, Dávila-Román VG, Hernandez AF, de Las Fuentes L; NHLBI Heart Failure Clinical Trials Network Investigators. Determinants of Diuretic Responsiveness and Associated Outcomes During Acute Heart Failure Hospitalization: An Analysis From the NHLBI Heart Failure Network Clinical Trials. J Card Fail. 2018;24:428-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 37. | Gheorghiade M, Filippatos G. Reassessing treatment of acute heart failure syndromes: the ADHERE Registry. Eur Heart J Suppl. 2005;7 suppl_B:B13-B19. [RCA] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | Milazzo V, Cosentino N, Marenzi G. Extracorporeal ultrafiltration for acute heart failure: patient selection and perspectives. Vasc Health Risk Manag. 2017;13:449-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 39. | Costanzo MR. The role of ultrafiltration in the management of heart failure. Congest Heart Fail. 2008;14:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Costanzo MR. Ultrafiltration in the management of heart failure. Curr Opin Crit Care. 2008;14:524-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Neri M, Villa G, Garzotto F, Bagshaw S, Bellomo R, Cerda J, Ferrari F, Guggia S, Joannidis M, Kellum J, Kim JC, Mehta RL, Ricci Z, Trevisani A, Marafon S, Clark WR, Vincent JL, Ronco C; Nomenclature Standardization Initiative (NSI) alliance. Nomenclature for renal replacement therapy in acute kidney injury: basic principles. Crit Care. 2016;20:318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 42. | Agostoni P, Marenzi G, Lauri G, Perego G, Schianni M, Sganzerla P, Guazzi MD. Sustained improvement in functional capacity after removal of body fluid with isolated ultrafiltration in chronic cardiac insufficiency: failure of furosemide to provide the same result. Am J Med. 1994;96:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 156] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Bart BA, Boyle A, Bank AJ, Anand I, Olivari MT, Kraemer M, Mackedanz S, Sobotka PA, Schollmeyer M, Goldsmith SR. Ultrafiltration versus usual care for hospitalized patients with heart failure: the Relief for Acutely Fluid-Overloaded Patients With Decompensated Congestive Heart Failure (RAPID-CHF) trial. J Am Coll Cardiol. 2005;46:2043-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 297] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 44. | Costanzo MR, Guglin ME, Saltzberg MT, Jessup ML, Bart BA, Teerlink JR, Jaski BE, Fang JC, Feller ED, Haas GJ, Anderson AS, Schollmeyer MP, Sobotka PA; UNLOAD Trial Investigators. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49:675-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 741] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 45. | Giglioli C, Landi D, Cecchi E, Chiostri M, Gensini GF, Valente S, Ciaccheri M, Castelli G, Romano SM. Effects of ULTRAfiltration vs. DIureticS on clinical, biohumoral and haemodynamic variables in patients with deCOmpensated heart failure: the ULTRADISCO study. Eur J Heart Fail. 2011;13:337-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 46. | Bart BA, Goldsmith SR, Lee KL, Givertz MM, O'Connor CM, Bull DA, Redfield MM, Deswal A, Rouleau JL, LeWinter MM, Ofili EO, Stevenson LW, Semigran MJ, Felker GM, Chen HH, Hernandez AF, Anstrom KJ, McNulty SE, Velazquez EJ, Ibarra JC, Mascette AM, Braunwald E; Heart Failure Clinical Research Network. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367:2296-2304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 740] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 47. | Marenzi G, Muratori M, Cosentino ER, Rinaldi ER, Donghi V, Milazzo V, Ferramosca E, Borghi C, Santoro A, Agostoni P. Continuous ultrafiltration for congestive heart failure: the CUORE trial. J Card Fail. 2014;20:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 48. | Costanzo MR, Negoianu D, Jaski BE, Bart BA, Heywood JT, Anand IS, Smelser JM, Kaneshige AM, Chomsky DB, Adler ED, Haas GJ, Watts JA, Nabut JL, Schollmeyer MP, Fonarow GC. Aquapheresis Versus Intravenous Diuretics and Hospitalizations for Heart Failure. JACC Heart Fail. 2016;4:95-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 135] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 49. | Hu J, Wan Q, Zhang Y, Zhou J, Li M, Jiang L, Yuan F. Efficacy and safety of early ultrafiltration in patients with acute decompensated heart failure with volume overload: a prospective, randomized, controlled clinical trial. BMC Cardiovasc Disord. 2020;20:447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 50. | Beaubien-Souligny W, Bouchard J, Desjardins G, Lamarche Y, Liszkowski M, Robillard P, Denault A. Extracardiac Signs of Fluid Overload in the Critically Ill Cardiac Patient: A Focused Evaluation Using Bedside Ultrasound. Can J Cardiol. 2017;33:88-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 51. | Beaubien-Souligny W, Rola P, Haycock K, Bouchard J, Lamarche Y, Spiegel R, Denault AY. Quantifying systemic congestion with Point-Of-Care ultrasound: development of the venous excess ultrasound grading system. Ultrasound J. 2020;12:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 331] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 52. | Onofriescu M, Mardare NG, Segall L, Voroneanu L, Cuşai C, Hogaş S, Ardeleanu S, Nistor I, Prisadă OV, Sascău R, Covic A. Randomized trial of bioelectrical impedance analysis versus clinical criteria for guiding ultrafiltration in hemodialysis patients: effects on blood pressure, hydration status, and arterial stiffness. Int Urol Nephrol. 2012;44:583-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Covic A, Ciumanghel AI, Siriopol D, Kanbay M, Dumea R, Gavrilovici C, Nistor I. Value of bioimpedance analysis estimated "dry weight" in maintenance dialysis patients: a systematic review and meta-analysis. Int Urol Nephrol. 2017;49:2231-2245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 54. | Hanna MA, Tang WH, Teo BW, O'Neill JO, Weinstein DM, Lau SM, Van Lente F, Starling RC, Paganini EP, Taylor DO. Extracorporeal ultrafiltration vs. conventional diuretic therapy in advanced decompensated heart failure. Congest Heart Fail. 2012;18:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 55. | Xu K, Rosenstiel P, Paragas N, Hinze C, Gao X, Huai Shen T, Werth M, Forster C, Deng R, Bruck E, Boles RW, Tornato A, Gopal T, Jones M, Konig J, Stauber J, D'Agati V, Erdjument-Bromage H, Saggi S, Wagener G, Schmidt-Ott KM, Tatonetti N, Tempst P, Oliver JA, Guarnieri P, Barasch J. Unique Transcriptional Programs Identify Subtypes of AKI. J Am Soc Nephrol. 2017;28:1729-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |