Published online Apr 26, 2023. doi: 10.4330/wjc.v15.i4.174
Peer-review started: December 27, 2022
First decision: February 20, 2023
Revised: March 5, 2023
Accepted: April 12, 2023
Article in press: April 12, 2023
Published online: April 26, 2023
Processing time: 113 Days and 23.2 Hours
Bradyarrhythmias are typically treated with permanent pacemakers (PM). The elimination of bradyarrhythmia by PM implantation improves the patient's quality of life and prognosis, but it can also result in a number of sequalae. It is still unclear how PM implantation affects the hemostasis system's parameters and how such parameters relate to different consequences after PM placement.
To assess the blood coagulation factor activity in PM patients throughout the perioperative period.
Patients treated in the Department of Surgical Therapy of Cardiac Arrhythmias and Pacing at the Ryazan State "Regional Clinical Cardiology Dispensary" from April 2020 to December 2021 were included in the study. Before surgery, 7 and 30 d after PM placement, peripheral venous blood samples were withdrawn to measure the level of blood coagulation factor I (FI) and the activity of blood coagulation factors II (FII), V (FV), VII (FVII), VIII (FVIII), IX (FIX), X (FX), XI (FXI), XII (FXII). We used an automatic coagulometer Sysmex CA 660 (Sysmex Europe, Germany) and reagents from Siemens (Siemens Healthcare Diagnostics Products GmbH, Germany).
The study included 146 patients. The activity of factors FV [147.7 (102.1-247.55)% vs 103.85 (60-161.6)% vs 81.8 (67.15-130.65)%, P = 0.002], FVIII [80.4 (60.15-106.25)% vs 70.3 (48.5-89.1)% vs 63.7 (41.6-88.25)%, P = 0.039], FIX [86.2 (70.75-102.95)% vs 75.4 (59.2-88.3)% vs 73.9 (56.45-93.05)%, P = 0.014], FX [188.9 (99.3-308.18)% vs 158.9 (83.3-230)% vs 127.2 (95.25-209.35)%, P = 0.022], FXI [82.6 (63.9-103.6)% vs 69.75 (53.8-97.6)% vs 67.3 (54.25-98.05)%, P = 0.002], FXII [87.6 (67.15-102.3)% vs 78.9 (63.4-97.05)% vs 81.2 (62.15-97.4)%, P < 0.001] decreased at 7 and 30 d after surgery; FII activity [157.9 (109.7-245.25)% vs 130 (86.8-192.5)% vs 144.8 (103.31-185.6)%, P = 0.021] decreased at 7 d and increased at 30 d postoperatively. There were no statistically significant changes in the FVII activity within 30 d after PM placement [182.2 (85.1-344.8)% vs 157.2 (99.1-259)% vs 108.9 (74.9-219.8)%, P = 0.128]. Subgroup analysis revealed similar changes only in patients on anticoagulant therapy. FXII activity decreased in patients on antiplatelet therapy [82 (65.8-101.9)% vs 79.9 (63.3-97.1)% vs 89.7 (75.7-102.5)%, P = 0.01] 7 d after surgery, returning to baseline values at 30 d postoperatively.
PM placement and anticoagulant therapy were associated with decreased activity of clotting factors FV, FVIII, FIX, FX, FXI, FXII in the postoperative period. FVII activity did not decrease within 30 d after PM placement, which may indicate endothelial injury caused by lead placement.
Core Tip: Permanent pacemaker placement and anticoagulant therapy are associated with decreased activity of factors V (FV), FVIII, FIX, FX, FXI, FXII in the postoperative period. FVII activity does not decrease within 30 d after PM placement, which may be suggestive of ongoing endothelial injury.
- Citation: Kalinin R, Suchkov I, Povarov V, Mzhavanadze N, Zhurina O. Perioperative coagulation activation after permanent pacemaker placement. World J Cardiol 2023; 15(4): 174-183
- URL: https://www.wjgnet.com/1949-8462/full/v15/i4/174.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i4.174
Cardiac implantable electronic devices (CIED), which include pacemakers (PM), are widely used to treat patients with arrhythmias and chronic heart failure[1,2]. Bradyarrhythmias are typically treated with permanent PM. The elimination of bradyarrhythmia by PM implantation improves the patient's quality of life and prognosis, but it can also result in a number of sequalae[1,3,4]. It is still unclear how PM implantation affects the hemostasis system's parameters and how those parameters relate to different problems and other consequences. The aim of our study was to assess the blood coagulation factor activity in PM patients throughout the perioperative period.
The study included patients treated in the Department of Surgical Treatment of Cardiac Arrhythmias and Cardiac Pacing at the Ryazan State "Regional Clinical Cardiology Dispensary" from April 2020 to December 2021. Inclusion criteria for the study were indications for pacemaker implantation and age over 40 years; non-inclusion criteria were presence of a previously implanted pacemaker, contraindications to antithrombotic therapy, pregnancy or breastfeeding, and active cancer or remission for less than 5 years. After the patient consented to participate in the study and signed the informed consent form, the following data were collected: Age, sex, height, weight, underlying disease, comorbidities, history of surgical interventions, the type of antithrombotic therapy used. Peripheral venous blood samples were taken to analyze the activity of the studied blood coagulation factors on the day of surgery.
PM placement was carried out in accordance with the “European Heart Rhythm Association expert consensus statement and practical guide on optimal implantation technique for conventional PM and implantable cardioverter-defibrillators”[1]. Endocardial leads were implanted via cephalic vein; subclavian vein was used as vascular access only when the cephalic vein was not suitable. All patients had the same PM models, single- and dual-chamber, and leads. Atrial leads with active-fixation systems were implanted in the right atrial appendage, whereas all ventricular leads with passive-fixation systems were placed in the right ventricle's apex. The PM was placed either in the pectoralis major muscle or in the subcutaneous tissues above the fascia of the muscle.
After the PM placement, the patients were allowed to stay in bed for 12 h. Moreover, an ice load was administered to the surgical site for 2 h in order to prevent PM pocket hematoma. The patients spent an average of 6 d at the hospital. Venous blood sampling was repeated on the 7th and 30th days after PM placement.
Venous blood samples were centrifuged; the resulting plasma was used to assess the studied parameters: The level of blood coagulation factor I (FI) and the activity of blood coagulation factors II (FII), V (FV), VII (FVII), VIII (FVIII), IX (FIX), X (FX), XI (FXI), XII (FXII). We used an automatic coagulometer Sysmex CA 660 (Sysmex Europe, Germany) and reagents from Siemens (Siemens Healthcare Diagnostics Products GmbH, Germany).
Statistical analysis was performed using IBM SPSS Statistics 26.0 for Windows (SPSS Inc. Chicago, IL, United States). Numbers and percentages were used to express categorical data. The χ2 test and Fisher exact test were used to analyze categorical data. Shapiro-Wilk test was used to assess normality. Most data were expressed as medians since they were not normally distributed. Wilcoxon test, Mann-Whitney test, Friedman test, Kruskal-Wallis test, and post-hoc tests were used as non-parametric tests for data comparison between two groups. Several cases with normal distribution were analyzed using parametric statistical analysis techniques.
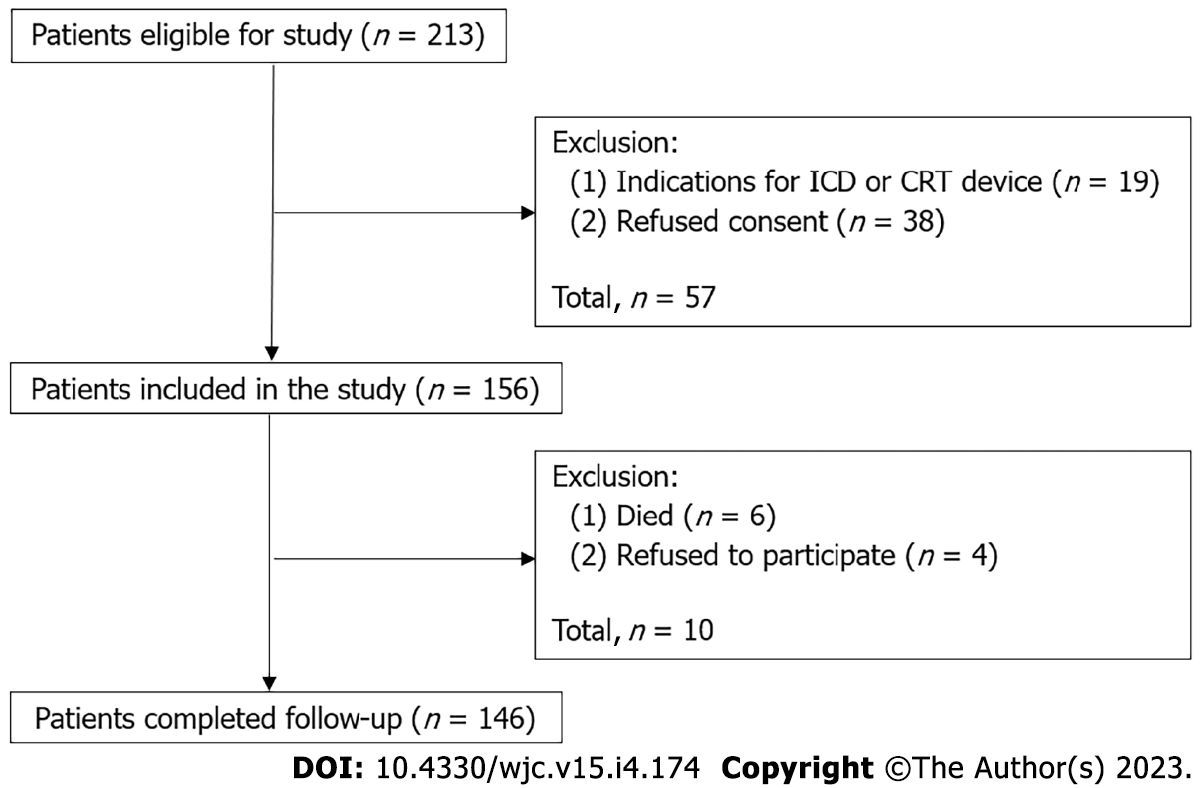
A total of 213 patients were screened to participate in the study. At the screening stage, 57 (26.7%) patients were withdrawn from the study: 38 (17.8%) refused to participate in the study, 19 (8.9%) had indications for placement of the other types CIED rather than a PM. As a result, 156 patients were included in the study, and 146 patients successfully completed the trial. Among 10 patients who dropped out of the study, 6 died and 4 withdrew their consent (Figure 1).
All patients signed an informed consent. This study was approved by the Local Ethics Committee of the Ryazan State Medical University. The clinical characteristics of the patients are shown in Table 1.
| Variable ( n = 146) | Data | |
| Age, years | 73 (67-81) | |
| Body mass index, kg/m2 | 27.5 (25-31) | |
| Gender | ||
| Male | 77 (52.7) | |
| Female | 69 (47.3) | |
| Pacemaker placement indication | ||
| Atrioventricular block | 49 (33.6) | |
| Sick sinus syndrome | 47 (32.2) | |
| Atrial fibrillation with impaired atrioventricular conduction | 50 (34.2) | |
| Comorbidity | ||
| Ischemic heart disease | 146 (100) | |
| Exertional angina | 44 (30.1) | |
| Arterial hypertension | 143 (97.9) | |
| Atrial fibrillation | 96 (65.8) | |
| Congestive heart failure | 146 (100) | |
| NYHA Class I | 7 (4.8) | |
| NYHA Class II | 60 (41.1) | |
| NYHA Class III | 79 (54.1) | |
| NYHA Class IV | 0 (0) | |
| History of myocardial infarction | 28 (19.2) | |
| History of stroke | 12 (8.2) | |
| Atherosclerotic peripheral arterial disease | 4 (2.7) | |
| Varicose veins | 31 (21.2) | |
| History of venous thromboembolism | 8 (5.5) | |
| Type 2 diabetes mellitus | 39 (21.2) | |
| History of coronavirus disease | 4 (2.7) | |
| Antithrombotic therapy | ||
| Antiplatelet therapy (aspirin) | 55 (37.7) | |
| Oral anticoagulants | 91 (62.3) | |
| Rivaroxaban | 57 (39) | |
| Apixaban | 20 (13.7) | |
| Dabigatran etexilate | 7 (4.8) | |
| Warfarin | 7 (4.8) | |
| Surgery features | ||
| Pacemaker | ||
| Single-chamber | 50 (34.2) | |
| Dual-chamber | 96 (65.8) | |
| Pacemaker placement side | ||
| Left side | 142 (97.3) | |
| Right side | 4 (2.7) | |
| Vascular access | ||
| Cephalic vein (section) | 132 (90.4) | |
| Subclavian vein (puncture) | 14 (9.6) | |
| Pacemaker pocket localization | ||
| Above the pectoral fascia | 133 (91.1) | |
| Inside the pectoralis major muscle | 13 (8.9) | |
| Mean surgery time, min | 54 (41-60) | |
All patients in the study received antithrombotic therapy (Table 1). All patients with atrial fibrillation received anticoagulants in accordance with clinical guidelines. Dabigatran etexilate was provided at a dose of 150 (110) mg twice day, apixaban at a dose of 5 (2.5) mg twice daily, and rivaroxaban at a dose of 20 (15) mg once daily. Warfarin was provided once daily; warfarin dosage was adjusted to achieve international normalized ratio of 2 to 3. The rest of the patients received acetylsalicylic acid at a dose of 100 mg once daily due to ischemic heart disease. None of the patients received anticoagulants and antiplatelets simultaneously. Antithrombotic therapy was not canceled or changed during the perioperative period.
There was a decrease in the activity of factors FV, FVIII, FIX, FX, FXI, and FXII at 7 and 30 d after the procedure, while the activity of FII decreased after 7 d and increased after 30 d. During the observation period, changes in FI levels and FVII activity were not statistically significant (Table 2).
| Variable | Before implantation | 7 d after implantation | 30 d after implantation | P value |
| FI | 2.61 (2.05-3.11) | 2.76 (2.08-3.42) | 2.54 (2.16-2.91) | 0.669 |
| FII | 157.9 (109.7-245.25)a | 130 (86.8-192.5) | 144.8 (103.31-185.6) | 0.021 |
| FV | 147.7 (102.1-247.55)a,b | 103.85 (60-161.6) | 81.8 (67.15-130.65) | 0.002 |
| FVII | 182.2 (85.1-344.8) | 157.2 (99.1-259) | 108.9 (74.9-219.8) | 0.128 |
| FVIII | 80.4 (60.15-106.25)a,b | 70.3 (48.5-89.1) | 63.7 (41.6-88.25) | 0.039 |
| FIX | 86.2 (70.75-102.95)a,b | 75.4 (59.2-88.3) | 73.9 (56.45-93.05) | 0.014 |
| FX | 188.9 (99.3-308.18)a,b | 158.9 (83.3-230) | 127.2 (95.25-209.35) | 0.022 |
| FXI | 82.6 (63.9-103.6)a,b | 69.75 (53.8-97.6) | 67.3 (54.25-98.05) | 0.002 |
| FXII | 87.6 (67.15-102.3)a,b | 78.9 (63.4-97.05) | 81.2 (62.15-97.4) | < 0.001 |
A subgroup analysis was conducted in order to identify the variables impacting the investigated parameters. The type of antithrombotic medication the patient received had the greatest impact on the variables in this study (Table 3).
| Variable | Antithrombotic therapy | Before implantation | 7 d after implantation | 30 d after implantation | P value |
| FI | Antiplatelet | 2.66 (2.13-2.99) | 2.85 (2.47-3.3) | 2.56 (2.19-3.16) | 0.513 |
| Anticoagulant | 2.55 (1.9-3.19) | 2.58 (1.93-3.44) | 2.49 (2.16-2.85) | 0.957 | |
| P value | 0.675 | 0.092 | 0.599 | - | |
| FII | Antiplatelet | 156.9 (94.5-237.3) | 139 (86.8-192.5) | 163.5 (112.4-203) | 0.289 |
| Anticoagulant | 186.2 (113.8-256.8) | 118.5 (86.8 -173) | 128.4 (99.6-170.5) | 0.067 | |
| P value | 0.458 | 0.609 | 0.263 | - | |
| FV | Antiplatelet | 164.9 (103.4-267.5) | 115.5 (92.8-198.9) | 98.6 (82.6-155.3) | 0.245 |
| Anticoagulant | 133.3 (96.6-187.9)a,b | 80.1 (45.9-152.8) | 73.4 (55.4-84.3) | 0.005 | |
| P value | 0.196 | 0.033 | 0.004 | - | |
| FVII | Antiplatelet | 203.4 (113-352.1) | 200 (116.4-438.3) | 223 (107.9-376.6) | 0.683 |
| Anticoagulant | 182.1 (87.3-384.6) | 122.3 (80.3-209.9) | 83.7 (64.6-154.8) | 0.153 | |
| P value | 0.691 | 0.024 | 0.002 | - | |
| FVIII | Antiplatelet | 82.1 (62.5-114.3) | 81.6 (63.8-97.5) | 75.5 (59.1-100.5) | 0.104 |
| Anticoagulant | 78.5 (58.7-99.7)a,b | 59.6 (41.7-82) | 55.1 (40.8-84.4) | 0.001 | |
| P value | 0.21 | 0.001 | 0.033 | - | |
| FIX | Antiplatelet | 85.4 (74.8-106.9) | 84.2 (78-105.8) | 96.7 (87.2-104) | 0.438 |
| Anticoagulant | 87 (68.4-99.6)a,b | 69.6 (55.6-81.8) | 63.2 (45.5-76.8) | 0.004 | |
| P value | 0.331 | <0.001 | <0.001 | - | |
| FX | Antiplatelet | 200 (105.8-308.2) | 163.8 (81.7-228.4) | 171.6 (120.2-240) | 0.708 |
| Anticoagulant | 187.8 (98.6-286.1)a,b | 152.6 (89-248.2) | 109.8 (82-163.5) | 0.007 | |
| P value | 0.837 | 0.983 | 0.03 | - | |
| FXI | Antiplatelet | 87.2 (69.8-100.8) | 93.8 (63.1-108.2) | 96.7 (84.2-108) | 0.957 |
| Anticoagulant | 74.8 (62.5-106.9)a,b | 61 (49.9-82.6) | 59.5 (47.5-86.5) | < 0.001 | |
| P value | 0.377 | 0.001 | < 0.001 | - | |
| FXII | Antiplatelet | 82 (65.8-101.9)a | 79.9 (63.3-97.1) | 89.7 (75.7-102.5) | 0.01 |
| Anticoagulant | 80.7 (69.4-110.2)a,b | 78.9 (63.4-97) | 73.8 (69.8-90.3) | 0.001 | |
| P value | 0.989 | 0.629 | 0.027 | - |
Patients with dual-chamber PMs on anticoagulant therapy 7 d after surgery had lower values of FI (P = 0.033), and lower activity of FV (P = 0.045), FVIII (P < 0.001), FIX (P < 0.001), FXI (P = 0.004); lower activity of FVIII (P = 0.049), FIX (P < 0.001), FXI (P = 0.003) was seen at 30 d after surgery as compared with patients on antiplatelet therapy. There were no differences in the studied parameters between patients receiving anticoagulant therapy with single-chamber and dual-chamber PM as well as different indications for PM placement (P > 0.05).
When evaluating the effect of each individual anticoagulant on the studied parameters, we found that patients who took apixaban had lower FIX (P = 0.049) activity at 7 d after surgery, and lower activity of FV (P = 0.046), FIX (P = 0.015), and FXI (P = 0.014) at 30 d after surgery as compared with patients who received acetylsalicylic acid. Patients who took rivaroxaban had lower activity of FIX (P = 0.004), FXI (P = 0.02) at 7 d after surgery, and lower activity of FIX (P = 0.006), FXI (P = 0.004) at 30 d after surgery as compared with patients who took acetylsalicylic acid. Patients who took dabigatran etexilate had lower FIX activity at 7 d (P = 0.023) and 30 d (P = 0.024) after surgery as compared with patients who took acetylsalicylic acid. Patients who took warfarin had lower FIX activity at 7 d (P = 0.023) and 30 d (P = 0.001) after surgery as compared to patients who received acetylsalicylic acid.
Female patients had higher baseline FVII (P = 0.001) and FIX (P = 0.003) activity, regardless of antithrombotic therapy type as compared with males.
Our aim was to study coagulation in patients with PM in the perioperative period. As a result, we discovered that at 7 and 30 d following surgery, the activity of coagulation factors V, VIII, IX, X, XI, and XII diminished. A more extensive statistical analysis revealed that patients on anticoagulant therapy experienced such changes more frequently. FXII activity in individuals who received acetylsalicylic acid decreased at 7 d after surgery before returning to baseline levels at 30 d after surgery. Patients undergoing antiplatelet and anticoagulant therapy did not show statistically significant changes in FVII activity or FI levels within 30 d of PM implantation.
Coagulation is one of the components of the human hemostasis system. Blood coagulation factors such as transglutaminases, glycoproteins, and serine proteases are part of the coagulation system[5]. The cascade model was once regarded as the primary coagulation model. This paradigm distinguishes between intrinsic and extrinsic coagulation pathways, which include the successive activation of blood coagulation components. Both pathways merge into a common coagulation pathway, which results in the formation of fibrin, which strengthens the thrombus[6,7].
The modern concept of coagulation is a cell-based model that describes the close relationship between the blood coagulation factors, platelets and endothelial cells. The coagulation process is broken down into three parts by the cell model: initiation, amplification, and propagation. When the vascular endothelium is injured during the initiation phase, cells that express tissue factor (such as smooth muscle cells) interact with FVII (initiation phase). This complex triggers the activation of FII, FIX, and FX. In the amplification phase, FII interfaces with the platelet membrane, where FXI, FVIII, and FV activation start. The propagation phase starts when activated FVIII and FIX combine to generate a complex capable of activating a significant amount of FX. Afterwards, FII and FI are activated, much like in the cascade model. Other interactions of blood coagulation factors in the cell-based model of hemostasis are also described, in addition to those described above. Coagulation is controlled by the anticoagulant system of blood. A cell-based model of hemostasis shifts our understanding of the blood clotting process to a different level, not excluding the cascade model[5,6,8].
The majority of PM patients are elderly people who frequently have a variety of comorbidities and illnesses linked to a hypercoagulable state of the hemostasis system. Atrial fibrillation, arterial hypertension, coronary heart disease, chronic heart failure, obesity, and other disorders fall under this category. Prior to PM implantation, bradyarrhythmia significantly influences the development of chronic heart failure and hypercoagulability in such patients[9,10]. Participants in the study who received anticoagulants displayed a decrease in the activity of intrinsic pathway factors such as FV, FVIII, FIX, FX, FXI, or amplification and propagation phases factors (according to the cell-based model). The baseline values of the examined parameters in these patients would likewise be lower than in patients receiving antiplatelet medications, but this was not the case in our study. In this instance, the use of anticoagulants and the elimination of bradyarrhythmia by PM implantation both likely contributed to the decline in the activity of the examined parameters. Elimination of bradyarrhythmia in patients receiving antiplatelets only temporarily reduced FXII activity.
Vascular access to the right ventricle of the heart is necessary for PM implantation operation. The lead is passed through the venous system after the subclavian vein is punctured or the cephalic vein is sectioned during surgery. Conditions are produced at the damaged area to enable the hemostasis system to function. A rise in tissue factor and von Willebrand factor in patients following pacemaker implantation supports this idea[3,9,11]. The second place of activation of the hemostasis system is the area of contact of the lead with endocardium. Gjesdal et al[12] showed high platelet activity in vitro when stimulated with PM bipolar leads. Palatianos et al[13] in an experiment on pigs noted that the largest accumulation of platelets was detected at the distal end of the PM lead. Although it is thought that lead has a low thrombogenicity, blood clots could still form for a variety of reasons, including a disruption of the laminar blood flow through the vein[13,14]. In our work, FVII activity does not decline in patients throughout the course of the 30-d observation period. This might be because tissue factor continues to activate FVII at the locations where the electrode caused endothelium damage. The persistence of FII activity shows that this coagulation route (extrinsic pathway of the cascade model, initiation phase of the cell-based model) is active during the entire observation time.
Our study did not aim to assess each individual anticoagulant medication's effect on the coagulation hemostasis measures. Amplification phase factors FV, FIX, and FXI's activity was shown to be decreased by apixaban and rivaroxaban when compared to acetylsalicylic acid due to FX's inhibition. Patients taking dabigatran etexilate had lower FIX activity because FII was inhibited as compared to patients receiving acetylsalicylic acid[4,5,15].
Many studies on the topic of coagulation in PM patients have been published in the international literature. The majority of these studies focus on how these patients' coagulation patterns relate to deep vein thrombosis (DVT) of the upper extremities and venous thromboembolism in general[3,11,14,16,17]. Zhang et al[3] noted an increase in FVIII activity 7 d after surgery. Lelakowski et al[11] observed an increase in FVII activity at the same period. The findings of our previous studies have demonstrated the predictive value of D-dimer levels in relation to the occurrence of DVT in the upper extremities following the initial implantation of the PM and the association between a high level of D-dimer and impaired patency of the veins in the upper extremities in patients with already implanted PM[16,18].
One of the limitations of our study was inability to assess the changes of the studied parameters in patients with single-chamber PM who require antiplatelet therapy. Currently, single-chamber PMs in the vast majority of cases are implanted in patients with permanent atrial fibrillation who require anticoagulant therapy. Placement of a single-chamber PM in the atrial position in patients with sick sinus syndrome, who could potentially receive antiplatelet agents and be investigated in this regard, is not common these days[1,19]. The study was also characterized by a limited number of postoperative visits and a certain choice of antiplatelet therapy (acetylsalicylic acid), non-inclusion of certain categories of patients such as younger patients, children, patients with leadless PM, etc.
PM placement and anticoagulant therapy were associated with decreased activity of clotting factors FV, FVIII, FIX, FX, FXI, FXII in the postoperative period. FVII activity did not decrease within 30 d after PM placement, which may indicate endothelial injury caused by lead placement. We think that further investigation of hemostasis system will contribute to the creation of newer approaches to the detection, prognosis, management, and prevention of numerous hemorrhagic and thromboembolic complications in patients requiring PM implantation.
Bradyarrhythmias are typically treated with permanent pacemakers (PM). The elimination of bradyarrhythmia by PM implantation improves the patient's quality of life and prognosis, but it can also result in a number of sequalae.
It is still unclear how PM implantation affects the hemostasis system's parameters and how such parameters relate to different complications after PM placement.
To assess the blood coagulation factor activity in PM patients throughout the perioperative period.
Patients treated in the Department of Surgical Therapy of Cardiac Arrhythmias and Pacing at the Ryazan State "Regional Clinical Cardiology Dispensary" from April 2020 to December 2021 were included in the study. Before surgery, 7 and 30 d after PM placement, peripheral venous blood samples were withdrawn to measure the level of blood coagulation factor I (FI) and the activity of blood coagulation factors II (FII), V (FV), VII (FVII), VIII (FVIII), IX (FIX), X (FX), XI (FXI), XII (FXII). We used an automatic coagulometer Sysmex CA 660 (Sysmex Europe, Germany) and reagents from Siemens (Siemens Healthcare Diagnostics Products GmbH, Germany).
The study included 146 patients. The activity of factors FV [147.7 (102.1-247.55)% vs 103.85 (60-161.6)% vs 81.8 (67.15-130.65)%, P = 0.002], FVIII [80.4 (60.15-106.25)% vs 70.3 (48.5-89.1)% vs 63.7 (41.6-88.25)%, P = 0.039], FIX [86.2 (70.75-102.95)% vs 75.4 (59.2-88.3)% vs 73.9 (56.45-93.05)%, P = 0.014], FX [188.9 (99.3-308.18)% vs 158.9 (83.3-230)% vs 127.2 (95.25-209.35)%, P = 0.022], FXI [82.6 (63.9-103.6)% vs 69.75 (53.8-97.6)% vs 67.3 (54.25-98.05)%, P = 0.002], FXII [87.6 (67.15-102.3)% vs 78.9 (63.4-97.05)% vs 81.2 (62.15-97.4)%, P < 0.001] decreased at 7 and 30 d after surgery; FII activity [157.9 (109.7-245.25)% vs 130 (86.8-192.5)% vs 144.8 (103.31-185.6)%, P = 0.021] decreased at 7 d and increased at 30 d postoperatively. There were no statistically significant changes in the FVII activity within 30 d after PM placement [182.2 (85.1-344.8)% vs 157.2 (99.1-259)% vs 108.9 (74.9-219.8)%, P = 0.128]. Subgroup analysis revealed similar changes only in patients on anticoagulant therapy. FXII activity decreased in patients on antiplatelet therapy [82 (65.8-101.9)% vs 79.9 (63.3-97.1)% vs 89.7 (75.7-102.5)%, P = 0.01] 7 d after surgery, returning to baseline values at 30 d postoperatively.
PM placement and anticoagulant therapy were associated with decreased activity of clotting factors FV, FVIII, FIX, FX, FXI, FXII in the postoperative period. FVII activity did not decrease within 30 d after PM placement, which may indicate endothelial injury caused by lead placement.
We think that further investigation of hemostasis system will contribute to the creation of newer approaches to the detection, prognosis, management, and prevention of numerous hemorrhagic and thromboembolic complications in patients requiring PM implantation.
The authors would like to thank the members of the Scientific and Clinical Center of Hematology, Oncology and Immunology, Ryazan State Medical University for their technical support.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Abrignani MG, Italy; Shen F, China S-Editor: Liu XF L-Editor: A P-Editor: Yu HG
| 1. | Burri H, Starck C, Auricchio A, Biffi M, Burri M, D'Avila A, Deharo JC, Glikson M, Israel C, Lau CP, Leclercq C, Love CJ, Nielsen JC, Vernooy K; Reviewers:, Dagres N, Boveda S, Butter C, Marijon E, Braunschweig F, Mairesse GH, Gleva M, Defaye P, Zanon F, Lopez-Cabanillas N, Guerra JM, Vassilikos VP, Martins Oliveira M. EHRA expert consensus statement and practical guide on optimal implantation technique for conventional pacemakers and implantable cardioverter-defibrillators: endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), and the Latin-American Heart Rhythm Society (LAHRS). Europace. 2021;23:983-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 119] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 2. | Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009--a World Society of Arrhythmia's project. Pacing Clin Electrophysiol. 2011;34:1013-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 671] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 3. | Zhang X, Li Y, Wang N, Zhang C, Zhang D, Li Q. Effects of permanent cardiac pacemaker implantation on vascular endothelial function, blood coagulation and cardiac function in patients with bradycardia. Exp Ther Med. 2018;16:4717-4721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Creta A, Finlay M, Hunter RJ, Chow A, Sporton S, Muthumala A, Dhillon G, Papageorgiou N, Waddingham P, Ahsan S, Dhinoja M, Earley MJ, Khan F, Lowe M, Ahmad M, Ricciardi D, Grigioni F, Di Sciascio G, Lambiase PD, Schilling RJ, Providência R. Non-vitamin K oral anticoagulants at the time of cardiac rhythm device surgery: A systematic review and meta-analysis. Thromb Res. 2020;188:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Sang Y, Roest M, de Laat B, de Groot PG, Huskens D. Interplay between platelets and coagulation. Blood Rev. 2021;46:100733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 230] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 6. | Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev. 2013;93:327-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 727] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 7. | Davie EW, Ratnoff OD. Waterfall sequence for intrinsic blood clotting. Science. 1964;145:1310-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 633] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 8. | Podoplelova NA, Sveshnikova AN, Kotova YN, Eckly A, Receveur N, Nechipurenko DY, Obydennyi SI, Kireev II, Gachet C, Ataullakhanov FI, Mangin PH, Panteleev MA. Coagulation factors bound to procoagulant platelets concentrate in cap structures to promote clotting. Blood. 2016;128:1745-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Cacko A, Kozyra-Pydyś E, Gawałko M, Opolski G, Grabowski M. The role of hemostatic markers as venous stenosis or occlusion predictors following first transvenous cardiac device implantation. Cardiol J. 2021;28:690-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Cugno M, Mari D, Meroni PL, Gronda E, Vicari F, Frigerio M, Coppola R, Bottasso B, Borghi MO, Gregorini L. Haemostatic and inflammatory biomarkers in advanced chronic heart failure: role of oral anticoagulants and successful heart transplantation. Br J Haematol. 2004;126:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Lelakowski J, Domagała TB, Rydlewska A, Januszek R, Kotula Horowitz K, Majewski J, Ząbek A, Małecka B, Musiał J. Effect of selected prothrombotic and proinflammatory factors on the incidence of venous thrombosis after pacemaker implantation. Kardiol Pol. 2012;70:260-267. [PubMed] |
| 12. | Gjesdal G, Hansen AB, Brandes A. Does bipolar pacemaker current activate blood platelets? Pacing Clin Electrophysiol. 2009;32:627-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Palatianos GM, Dewanjee MK, Panoutsopoulos G, Kapadvanjwala M, Novak S, Sfakianakis GN. Comparative thrombogenicity of pacemaker leads. Pacing Clin Electrophysiol. 1994;17:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Safi M, Akbarzadeh MA, Azinfar A, Namazi MH, Khaheshi I. Upper extremity deep venous thrombosis and stenosis after implantation of pacemakers and defibrillators; A prospective study. Rom J Intern Med. 2017;55:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Altiok E, Marx N. Oral Anticoagulation. Dtsch Arztebl Int. 2018;115:776-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Kalinin RE, Suchkov IA, Mzhavanadze ND, Povarov VO. Dynamics of coagulation parameters and its relationship with venous thromboembolism in patients with cardiac implantable electronic devices. Flebologiya. 2019;13:21-27 (In Russ.). [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Ma J, Cui L, Huo W, Wang G, Quan X, Zhang J. Correlation between Deep Venous Thrombosis and Inflammation in Patients after Implantation of Permanent Pacemaker. Iran J Public Health. 2020;49:30-36. [PubMed] |
| 18. | Kalinin RE, Suchkov IA, Povarov VO, Mzhavanadze ND, Zhurina ON. Venous obstruction of the upper extremities in patients with pacemakers: D-dimer testing. Flebologiya. 2022;16:262 269 (In Russ.). [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Vogler J, Keelani A, Traub A, Tilz RR. [ESC guidelines 2021 on cardiac pacing and cardiac resynchronization therapy: What's new? Herz. 2022;47:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |