Published online Apr 26, 2023. doi: 10.4330/wjc.v15.i4.142
Peer-review started: January 3, 2023
First decision: March 15, 2023
Revised: March 27, 2023
Accepted: April 7, 2023
Article in press: April 7, 2023
Published online: April 26, 2023
Processing time: 106 Days and 21.3 Hours
The recent shift of the concept of cardiovascular disease as a chronic progressive condition, potentially involving multiple districts, has driven attention to the optimal management of patients with concomitant coronary and peripheral artery disease, representing a subset of patients with an increased risk of events and impaired survival. Recent pharmacological achievements in terms of antithrombotic therapy and lipid-lowering drugs allow multiple therapeutical combinations, thus requiring optimizing the treatment in a tailored fashion according to patients’ risk profiles. Nevertheless, data dedicated to this specific subset of patients are still modest. We summarize currently available strategies and indications for the management of antithrombotic and lipid-lowering drugs in patients with the poly-vascular disease.
Core Tip: Patients with concomitant coronary and peripheral artery disease, i.e. poly-vascular disease, represent a subset of patients with higher risk and worse prognosis. In these patients antithrombotic and antilipidemic drugs should be tailored in order to achieve the most aggressive combination tolerated for each patient. Multidisciplinary approach, involving both a cardiologist and vascular surgeon, combining different therapeutic goals and perspectives, could provide additional benefits in the correct management of poly-vascular patients.
- Citation: Gioscia R, Castagno C, Verdoia M, Conti B, Forliti E, Rognoni A. Optimization of the pharmacological therapy in patients with poly-vascular disease: A multidisciplinary approach. World J Cardiol 2023; 15(4): 142-153
- URL: https://www.wjgnet.com/1949-8462/full/v15/i4/142.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i4.142
The large efforts dedicated in the last decades to reducing the burden of cardiovascular disease (CVD) worldwide have allowed a better identification of causal risk factors and pathophysiological mechanisms, thus leading to progressive shift in the concept of CVD as a chronic progressive condition[1].
In the 2019 European Society of Cardiology (ESC) guidelines[2], the term stable coronary artery disease (CAD) has been replaced with “chronic coronary syndrome”, focusing on CAD as a dynamic evolutive process, resulting from a myriad of interactions, both environmental and genetic.
A subsequent development has led to consider CAD as part of a wider spectrum of disease, sharing the same atherosclerotic pathogenesis, thus leading to the most recent concept of “cardio-cerebro-vascular continuum”[3,4], representing a condition with a high potential of progression to death or major acute ischemic events, in absence of interventions interrupting this pathological loop.
Recent advances in terms of more potent antithrombotic and lipid-lowering therapies, allowing prevention of a large proportion of acute ischemic events and the progression of the disease[5,6], have significantly modified the prognosis of these patients, therefore promoting attentive research of multidistrict involvement.
However, despite the rising attention, and especially in secondary prevention, among patients with an established diagnosis of CAD or peripheral arterial disease (PAD), still the indications for systematic pan-vascular screening and the appropriate management of the newly available therapies is debated, leading to the risk of underestimation of the risk and undertreatment[7].
Moreover, increasing evidence has emerged on the advantages of an earlier establishment of pharmacological anti-atherosclerotic measures, remarking the importance to identify the instruments for adequate assessment of the cardiovascular risk profile. The present review will aim to provide an overview of the management of patients with poly-vascular disease, with a particular focus on recent updates in the management of antithrombotic and lipid-lowering therapies.
PAD is defined, according to the European Society of Cardiology guidelines[8], as a blood circulation disorder of the arteries that supply all the districts excluding coronary circulation and aorta.
However, CAD and PAD or multidistrict PAD often tend to co-exist, representing different presentations of the same pathogenetic process, atherosclerosis, an inflammatory disease leading to the progressive occlusion of the vessel lumen[9]. Atherosclerosis within 2 or more arterial beds has been termed a poly-vascular disease and recent studies have documented that its prevalence could be even more common than previously considered[10].
In the Reduction of Atherothrombosis for Continued Health Registry (REACH)[11], an international registry including patients ≥ 45 years of age with established CAD, CVD, PAD, or ≥ 3 risk factors for atherosclerotic disease; 24.7% of patients with CAD and up to 61.5% of patients with PAD had the concomitant disease in other vascular beds, being associated with a severely worsened prognosis. In fact, in the same study, the strongest predictor for future ischemic events was a poly-vascular disease, which was associated with a 99% increased risk of major cardiovascular ischemic events (MACE) at 4-year follow-up, an almost doubled mortality (4.6% vs 2.4%) and markedly increased morbidity. A similar negative prognostic impact was also confirmed in several registries and trials[12,13]. However, routine screening for poly-vascular involvement in patients with disease in one arterial bed has not been recommended, so far, in guidelines, in consideration of multiple factors.
Indeed, the modest awareness of patients and physicians, being generally more focused on the prognostic weight of CAD, rather than PAD, and the more delayed presentation of the latter, remaining often symptomatic until the occurrence of critical or acute ischemia, certainly have represented a limitation for many years[14].
Moreover, whereas an early establishment of lifestyle counseling and medications was able to reduce the risk of cardiovascular events in primary prevention, no differential pharmacological management was advised, so far, among patients with CAD, PAD or both, due to the lack of evidence of potential additional benefits with more aggressive therapy[1,8].
Nevertheless, the recently introduced antithrombotic and antilipidemic drugs have provided greater benefits among patients with more severe multidistrict vascular disease, thus raising the need to better define risk models and shared protocols.
In particular, the role of indirect indexes of vascular disease, which are widely validated in primary prevention, still needs to be defined among patients with an established diagnosis of CAD or PAD.
Among them, the ankle-brachial index (ABI) represents an easy-to-measure and widely available tool for objectifying CAD. Several studies have shown that patients with an ABI < 0.9 have more severe coronary artery disease[15].
Moreover, among patients with PAD, ABI has emerged as a strong predictor of mortality and major acute cardiovascular and limb-related ischemic events (MACE and MALE)[16].
A similar prognostic role of ABI, however, was also confirmed among asymptomatic patients undergoing screening for PAD, where an ABI < 0.85 increased the relative risk for total mortality to 2.36 (95%CI: 1.60, 3.48), being even enhanced with decreasing ABI (P < 0.0001). Specific causes of death, directly related to the magnitude of ABI were mainly due to myocardial infarctions, further reinforcing the importance of poly-vascular disease identification[17].
Similarly, pulse wave velocity (PWV), the most widely used measure of arterial stiffness, has emerged as a useful tool for risk stratification in CVD. Various studies and meta-analyses have shown the association between PWV and PAD or CAD. Moreover, PWV emerged as an independent risk factor for future cardiovascular events[18].
Other indirect markers of an ongoing atherosclerotic process, such as carotid intima-media thickness, as well as vasculogenic erectile dysfunction or coronary calcium score, have been addressed for the estimation of cardiovascular risk, although their exact predictive role and prognostic impact is still under debate. Recent studies suggested that carotid intima-media thickness (cIMT) evolution, and in particular cIMT reduction, rather than the baseline value, could predict the degree of CVD risk reduction[19].
Indeed, future dedicated studies are certainly deserved to define the most appropriate tools and criteria for the assessment of cardiovascular risk and for establishing the most appropriate preventive measures for the management of higher-risk patients.
Antiplatelet therapy is the cornerstone of the treatment of patients with acute and chronic coronary syndrome. Dual antiplatelet therapy (DAPT) consisting of aspirin (ASA) and an adenosine diphosphate inhibitor, such as clopidogrel, prasugrel or ticagrelor is currently indicated for the prevention of cardiovascular events in patients presenting with the acute coronary syndrome (ACS), after percutaneous coronary intervention for any indication and in particular subsets of higher-risk stable CAD patients.
Clinical practical guidelines in the United States and Europe recommend more potent P2Y12 inhibitors in ACS, such as ticagrelor and prasugrel, given that head-to-head comparison clinical trials have shown the superiority of these inhibitors over clopidogrel in reducing ischemic events[20,21].
In the acute setting, clopidogrel should be used only if these potent inhibitors are contraindicated or unavailable, while still representing the first choice in patients undergoing elective percutaneous coronary interventions[1].
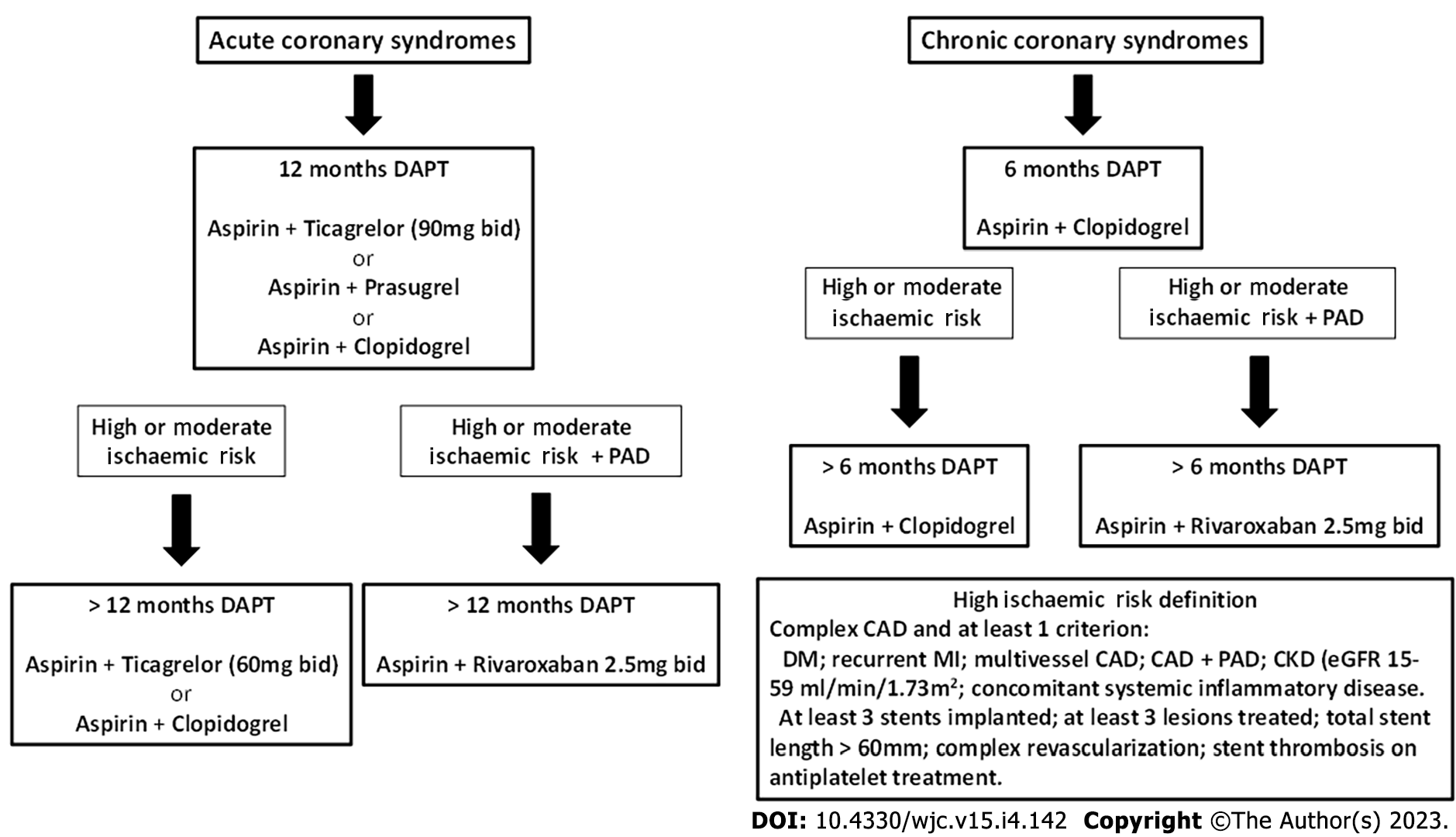
The recommended duration of DAPT is 12 mo after an acute coronary syndrome, unless there are contraindications, and 6 mo in CCS. In specific clinical scenarios, DAPT duration can be shortened (< 12 mo), extended (> 12 mo) or modified (switching DAPT, DAPT de-escalation) and these decisions depend on individual clinical judgment being driven by the patient’s ischemic and bleeding risk[1,22].
Patients are defined as having “high ischemic risk" if presenting multivessel CAD and at least one additional risk factor, including diabetes, recurrent acute myocardial infarction (AMI), renal failure or PAD. On the other hand, patients with at least one criterion between multivessel CAD, diabetes, recurrent AMI, PAD, renal failure or heart failure are considered to be at “moderate” risk[23].
In these patients, the potential advantage of extending DAPT duration beyond the routine period of 6-12 mo was first suggested in the DAPT trial[24]. In this study dual antiplatelet therapy beyond 1 year and up to 30 mo after placement of a drug-eluting stent, as compared with aspirin alone, significantly reduced the risk of stent thrombosis and major adverse cardiovascular and cerebral events, but was associated with an increased rate of bleeding.
Subsequently, the PEGASUS-TIMI 54 trial documented the reduction of MACE with ASA+ ticagrelor 60 mg × 2 among moderate-high risk patients with a previous AMI who had tolerated DAPT for 1 year, with no difference in severe bleedings[25].
Among the patients at higher risk included in the study, patients with PAD are at heightened risk of MACE, including myocardial infarction (MI) and stroke. In a sub-analysis of the PEGASUS-TIMI 54 trial dedicated to this specific setting, it was observed that the benefit of ticagrelor for relative risk reduction of MACE was consistent, regardless of the presence or absence of known PAD; however, patients with PAD had a particularly robust risk reduction, due to the higher rate of events, furthermore, producing advantages on MALE. However, the low number of patients (only 5% of the overall study population) and the lack of any impact on mortality did not translate into a particular indication for ticagrelor in patients with previous MI and PAD[26].
More recently the COMPASS Trial compared rivaroxaban (2.5 mg bid) plus ASA 100 mg/d or rivaroxaban 5 mg bid alone vs ASA 100 mg/die in patients with stable CAD and/ or PAD. Rivaroxaban 2.5 mg bid plus ASA showed a significant reduction of stroke, total and cardiovascular mortality, in addition to reducing MACE and MALE[27]. This regimen was associated with an increase in major bleeding events primarily from gastrointestinal sites, but there was no increase in critical organ bleeding, non-fatal intracranial bleeding or fatal bleeding. The net clinical benefit analysis (inclusive of MACE, MALE and severe bleeding events) maintained significant benefits in favor of the rivaroxaban plus aspirin arm[28,29].
Therefore, given this evidence, the cardiological approach to antiplatelet therapy, should be to pursue the extension of antithrombotic therapy for the longest tolerated period (> 1 year after ACS; > 6 mo after CCS), balancing with the hemorrhagic risk and tailoring the different strategies according to the patient’s characteristics. Our proposed strategy is depicted in Figure 1. In patients with poly-vascular disease in whom the ischemic risk outweighs the risk of bleeding, the combination of antiplatelet therapy and low-dose anticoagulation currently appears supported by the most robust evidence and by the larger prescribing criteria, being allowed both in ACS and CCS patients and even in those who have discontinued DAPT at distance from an event.
Peripheral artery disease (PAD) is one of the manifestations of atherosclerosis, which is known to involve many different vascular districts. Indeed, PAD shares with CAD a common etiology and therefore therapeutical approaches are often similar. In this context, the management of antithrombotic therapy is the milestone of pharmacological strategy in vascular surgery, both in primary and secondary prevention after revascularization. Historically, PAD patients have often represented a subgroup in large RCTs assessing generic antithrombotic therapies in poly-vascular populations. These trials then led to more specific studies dedicated to vascular patients in the last years, both in the chronic and postoperative settings. MACE is often the pivotal outcome of these studies; however, in PAD patients, also MALE becomes a crucial parameter to assess the efficacy of antithrombotic therapy. Therefore, in all RCTs and metanalysis these two outcomes are usually evaluated, balanced with the bleeding risk of different antithrombotic approaches.
The benefit of aspirin in atherosclerotic disease is well established. In 2002, the Antithrombotic Trialists' Collaboration established a general benefit of aspirin in terms of prevention of death and different cardiovascular events, but many antiplatelet drugs and regimens were tested[30]. However, use of aspirin was afterward re-assessed in asymptomatic diabetic patients with ABI < 0.9[31].
Clopidogrel, a second-generation thienopyridine, showed better results in terms of MACE vs aspirin in the CAPRIE trial, mostly in a subgroup of patients with PAD; however, no benefit for MALE was found.
Ticagrelor is another thienopyridine often used in CAD. The EUCLID trial[13] compared clopidogrel with ticagrelor in PAD patients and showed no difference in MACE or bleeding events.
Nevertheless, the use of these two antiplatelet agents in monotherapy for PAD patients is limited, with only clopidogrel often being considered as an alternative in case of aspirin intolerance.
Aspirin has also been evaluated in combination with both clopidogrel and ticagrelor. The CHARISMA trial[32] compared a heterogeneous population with different cardiovascular diseases receiving aspirin vs aspirin plus clopidogrel. In the PAD subgroup no difference in MACE was found between the two groups, with a slightly higher risk of moderate/severe bleeding with the dual antiplatelet regimen.
The PEGASUS-TIMI 54 trial[25] was similar, but ticagrelor was used instead of clopidogrel. The study found a higher benefit of the dual therapy in patients with PAD, both for MACE and MALE.
In the past decades, the combination of antiplatelets and anticoagulants did not show favorable outcomes in PAD patients[33]. The advent of NOACs recently changed the scenario also in vascular surgery. In 2017 the COMPASS trial highlighted the effectiveness of low dose of rivaroxaban (i.e. 2.5 mg bid) in a large population of atherosclerotic patients[27]. This benefit was found to be even clearer in PAD patients. In a COMPASS subgroup analysis of PAD patients[28], both MACE and MALE were significantly lower in those assigned to rivaroxaban plus aspirin vs aspirin alone and this evidence was directly proportional to the Rutherford class and cardiovascular risk profile at baseline. Furthermore, critical bleedings were not significantly higher in patients with combined therapy.
The role of antithrombotic therapies is even more important after lower extremity revascularization[34]. While single antiplatelet therapy is essential after any kind of bypass, it is unclear whether the addition of warfarin is useful in venous grafts, due to a higher risk of bleeding.
Similarly, there is strong evidence to support single antiplatelet therapy after prosthetic bypass, while the role of DAPT is less clear. Indeed, the CASPAR trial[35] evidenced a benefit of aspirin plus clopidogrel only in a subgroup of patients after below-the-knee prosthetic bypasses (typically at risk of low patency), without significant increasing major bleeding.
More recently, based on the COMPASS trial findings[28], a randomized controlled trial was designed to assess the efficacy of aspirin plus a low dose of rivaroxaban in patients who underwent lower extremity revascularization[36]. Those assigned to the dual therapy had better outcomes in terms of both MACE and MALE, balanced by an acceptable risk of bleeding. A subgroup analysis that referred to only open-surgical patients confirmed these findings[37]. Recently, Bonaca et al[38] tried to compare a “CASPAR-like” population derived from a post hoc analysis of the VOYAGER PAD trial to assess whether aspirin plus rivaroxaban performed better than aspirin plus clopidogrel after LER. No trials so far directly compared these two postoperative antithrombotic regimens. Their findings confirmed that aspirin combined with rivaroxaban led to better postoperative outcomes compared with clopidogrel as adjunctive therapy. Furthermore, Hiatt et al[34] found that the addition of rivaroxaban to aspirin after LER was effective and safe even if concomitant clopidogrel was prescribed, suggesting the potential role of a triple antithrombotic therapy, which however should be further evaluated in a dedicated trial.
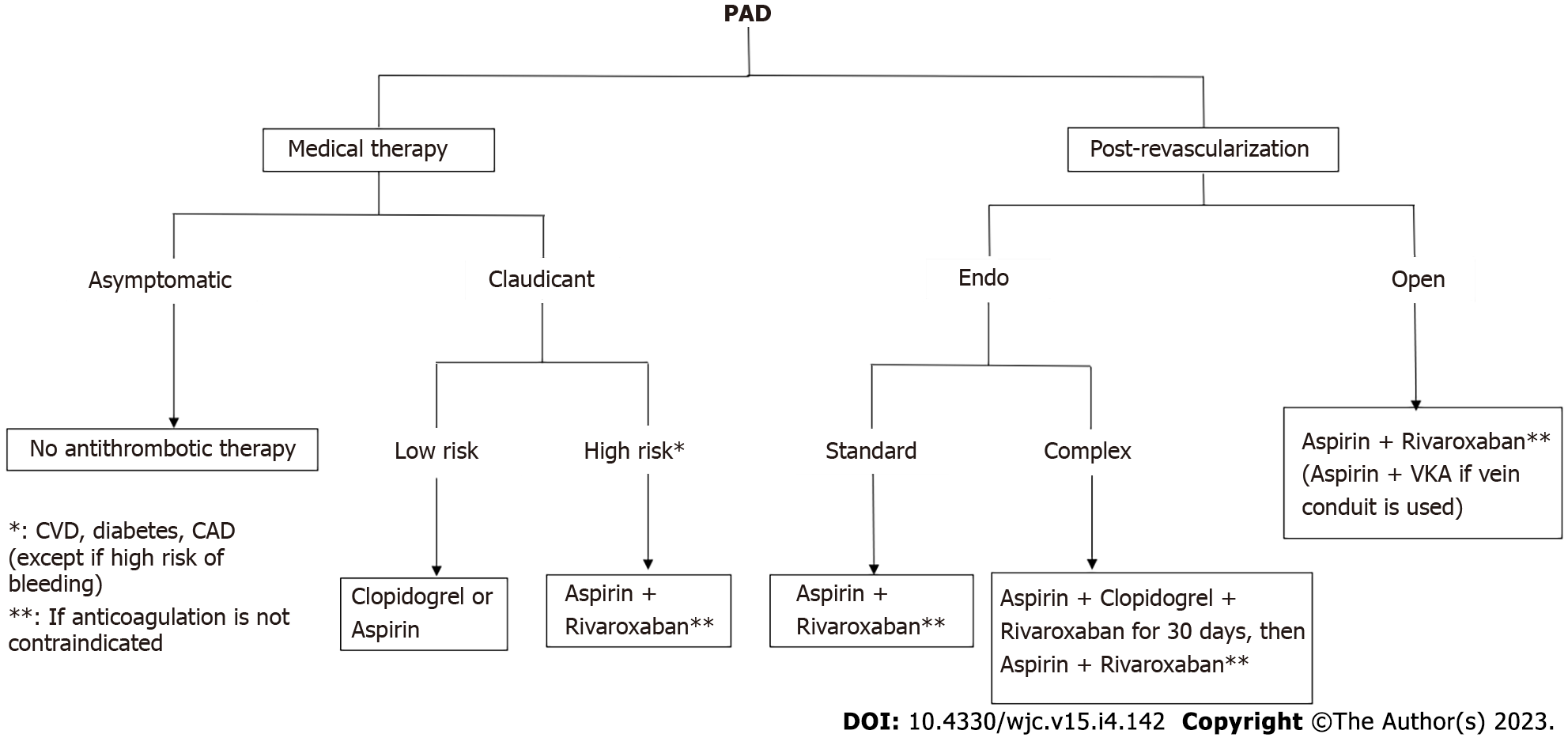
In the wake of all the aforementioned trials[28,34,36-38], four major guidelines have been published in the last years regarding PAD[8,39-41].
In 2016 the ACC/AHA reported their recommendations for PAD[39]. Single antiplatelet therapy was strongly recommended in symptomatic PAD patients and after revascularization, while its routine use in asymptomatic patients was uncertain, as well as the role of DAPT. Indeed, these guidelines were released before the COMPASS trial, therefore not considering anticoagulant drugs.
The European guidelines[8] were published one year later by the ESC in collaboration with the European Society for Vascular and endovascular Surgery. They recommended antiplatelet therapy for asymptomatic patients, while a single drug is mandatory for symptomatic patients. Of note, deferring from the American guidelines, the latter suggested clopidogrel as the preferred molecule over aspirin[40]. Regarding postoperative schemes, DAPT was recommended after percutaneous procedures for 1 mo (or longer if concomitant CAD), while single antiplatelet was deemed adequate after open surgery. According to these guidelines, standard anticoagulants (i.e. warfarin) could be associated with antiplatelet therapy only after endovascular procedures and in patients at low risk of bleeding who were preoperatively on this regimen for other reasons (i.e. atrial fibrillation, mechanical heart valve,
The Global vascular guidelines on critical limb ischemia (CLI)[41] have been published in 2019. They gave weak recommendations for DAPT after infrainguinal bypasses for a period of 6 to 24 mo postoperatively and at least 1 mo after endovascular procedures (or a longer period in case of multiple reinterventions). Of note, these are the first guidelines that recommend the association between aspirin and a low dose of rivaroxaban to reduce MACE and MALE in patients with CLI (having the COMPASS trial been published 1 year before, while the VOYAGER PAD trial was ongoing at the time of publication).
The Canadian guidelines[42] are the most recently published guidelines for PAD. They confirm most of the recommendations given by the previous ones, except for more precise indications for rivaroxaban associated with aspirin. Indeed, this represents the preferred antithrombotic strategy in symptomatic patients with high-risk comorbidities and higher stages of limb ischemia (“high-risk patients” and “high-risk limb”). Furthermore, they suggest the same approach after both open and endovascular interventions, with a restricted indication for DAPT only in patients unable to receive rivaroxaban. These conclusions, which are outlined in Figure 2, may represent a paradigm shift in the treatment of PAD patients, regardless of the need for surgical procedures. Of course, these statements have to be confirmed by further trials, but we can affirm that this new antithrombotic strategy represents one of the most interesting fields of research in vascular surgery for the next years.
Low-density lipoprotein cholesterol (LDL-C) is a well-established risk factor for cardiovascular disease, as evidenced by epidemiological and Mendelian randomization studies. LDL-C lowering is associated with a reduction of MACE in a linear way, without any plateau for lower LDL-C levels[43].
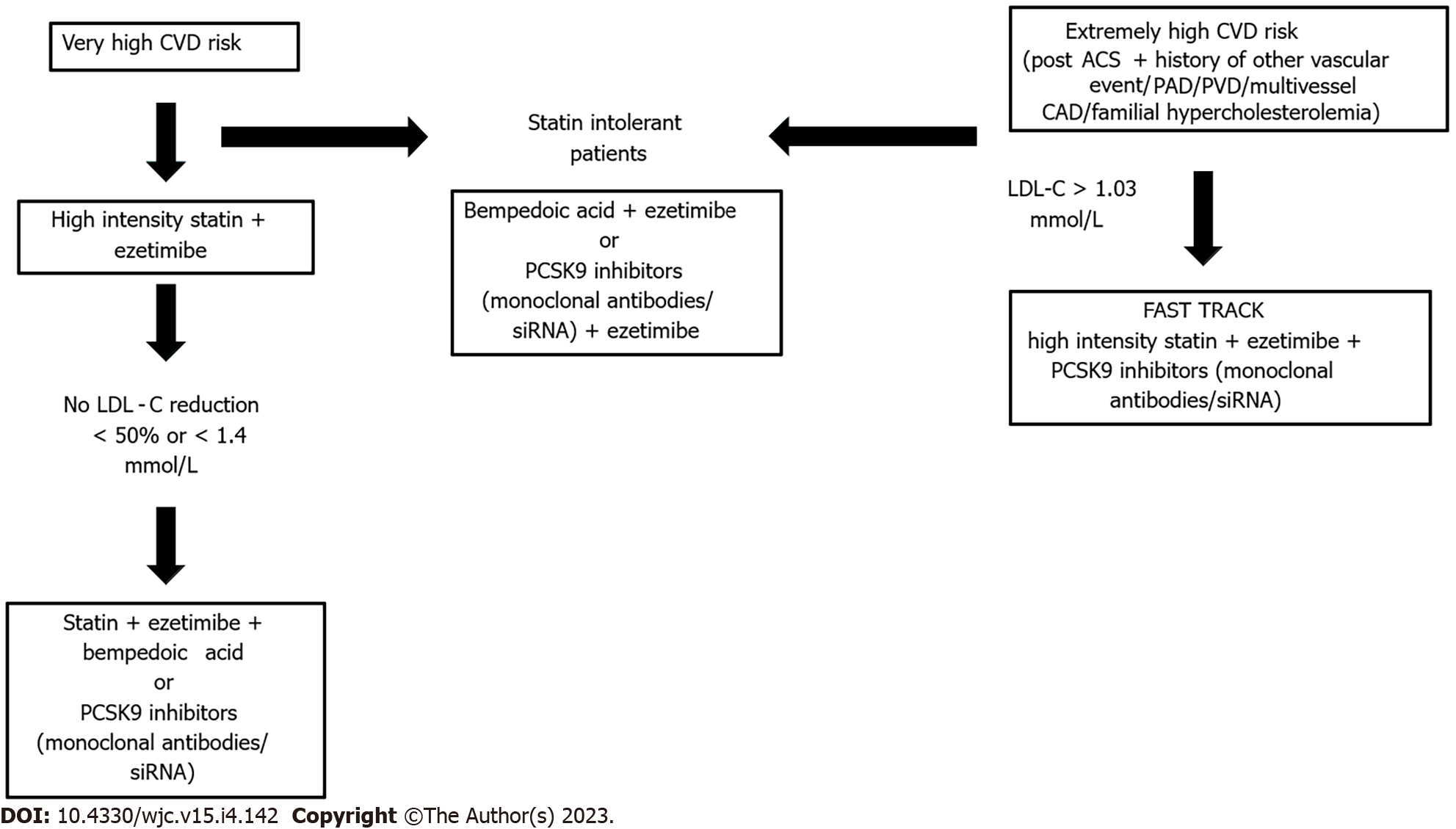
Therefore, a progressive lowering of the target of LDL-C has been observed in guidelines and consensus documents. The 2018 AHA/ACC/multisociety cholesterol guidelines[44], are more conservative in the definition of the LDL-C threshold to < 70 mg/dL and in the identification of very-high risk patients (not enclosing patients without established atherosclerotic cardiovascular disease, as those with diabetes or chronic kidney disease). On the contrary 2019 ESC/EAS Guidelines on the management of dyslipidemias[45] recommend, for very-high-risk patients, LDL-C target to be lower than 1.4 mmol/L (55 mg/dL), in addition to the goal of achieving a 50% reduction from baseline. Patients with any documented atherosclerotic cardiovascular disease, involving one or more districts are already considered at “very high” risk, whereas patients with repeated acute coronary syndrome events within 2 years should be considered at “extremely” high risk and pursue a lower threshold of 1.03 mmol/L. Patients with post-acute coronary syndrome and presence of peripheral artery disease or poly-vascular disease; post-acute coronary syndrome and coexistent multivessel coronary artery disease; and post-acute coronary syndrome and familial hypercholesterolemia have been recently paired in the latter category, according to certain European cardiological societies[46].
Lipid-lowering therapies modify the risk in patients with atherosclerosis and have been shown to exert larger absolute risk reductions in patients with the poly-vascular disease[47].
New evidence has raised the opportunity to start with a combination of statin therapy plus ezetimibe for very high-risk patients. If patients do not achieve the 2019 Guideline-recommend LDL-C goal, a third lipid-lowering therapy, such as bempedoic acid or proprotein convertase subtilisinkexin type 9 inhibitor (PCSK9-i) targeted therapies should be added. Furthermore, in extremely high-risk patients triple therapy (statin plus ezetimibe plus PCSK9-inhibitors) could be administered at the beginning[48].
As observed in a sub-analysis of the FOURIER Trial, Evolocumab significantly reduced the risk of MACE in symptomatic PAD, including those without prior MI or stroke. Furthermore, LDL-C lowering with evolocumab reduced the risk of MALE including acute limb ischemia and major amputation. Akin to what has been observed for MACE, there was a consistently lower risk of MALE with lower levels of achieved LDL-C, down to 10 mg/dL[49].
Similar results were found in an analysis of the ODISSEY-OUTCOME trial. In patients with recent ACS and dyslipidemia, the poly-vascular disease is associated with a high risk of MACE and death. The large absolute benefit of PCSK9 inhibition with alirocumab, when added to high-intensity statin therapy, is a potential benefit for this group of patients[50].
A new therapeutic weapon is represented by inclisiran, a small interfering RNA (siRNA) therapeutic agent which reduces hepatic synthesis of PCSK9. Inclisiran was approved in the European Union in 2020 for use in adults with primary hypercholesterolemia or mixed dyslipidemia based on the results of the ORION Trials. Inclisiran, administered subcutaneously every 6 mo, reduces LDL-C levels by approximately 50%[51].
In a post-hoc analysis of the Orion Trials, focused on patients with the polyvascular disease (PVD), two-yearly dosing with inclisiran provided an effective and sustained LDL-C lowering, irrespective of PVD status, with no relevant side effect[52].
The relationship between statins and PAD has been seldom investigated. As well as for antithrombotic therapies, the literature focused more on pleiotropic effects rather than limb-related benefits of statins in PAD patients. Moreover, PAD patients often represented a subgroup in large RCTs of atherosclerotic patients, with few dedicated studies published so far. In a meta-analysis by Antoniou[53], statins proved to significantly reduce mortality and stroke in symptomatic PAD patients when compared to placebo. These trends have been recently confirmed by Kokkinidis[54] in another meta-analysis, which summarized some observational studies regarding the impact of statin therapy in CLI patients. Of note, this study investigated almost 27000 patients with CLI, but only half of them were statin users; these patients had better patency rates and a lower risk of amputations after revascularization, besides better outcomes in terms of MACE and mortality. The issue of under-prescription of statins in PAD patients has also been raised by Parmar[55], in a population of 488 patients who underwent LER. These authors found that statins were the most impactful factor on MACE and MALE after revascularization, even more than all the antithrombotic strategies globally compared. Nevertheless, only 41% of patients in this study were on statin therapy. Furthermore, statins may induce muscle pain, which could affect the adherence to this therapy in PAD patients. In this scenario, the Global guidelines suggest to lower statins to the maximum tolerated dose, adding a non-statin molecule to reduce cholesterol blood levels to the optimum[42].
Recently, PCSK9-i and other new lipid-lowering therapies have been tested in large atherosclerotic populations, with only a small portion of them affected by PAD. The FOURIER trial[47] evaluated evolocumab, a PCSK9-I, in a large cohort of patients, while a PAD subgroup has been subsequently analyzed. The adjunct of evolocumab to statins significantly reduced MACE by 27% and MALE by 37% in this subgroup, regardless of the Rutherford class. On the other hand, the ODYSSEY OUTCOMES trial[50] provided different results with alirocumab. Once again, this was a RCT of patients with acute coronary syndrome (ACS) receiving either the placebo or alirocumab. This drug provided significantly fewer PAD events (i.e.: CLI, unplanned amputations, need for limb revascularization) in patients with ACS. Conversely, in a subanalysis of this trial by Jukema[50], patients with concomitant PAD had no benefit in terms of MACE, while the main trial showed positive results in the overall population. This confirms the need for studies specifically designed for PAD patients.
The aforementioned vascular guidelines[8,39-42] give fewer recommendations for lipid-lowering drugs than for antithrombotic therapies in PAD. Both the American and European guidelines generically recommend the use of statins in all PAD patients, with the latter specifying an optimal cut-off of 1.8 mmol/L (i.e.: < 70 mg/dL) for LDL-c levels. More specific indications are given by the Global guidelines in patients with CLI[39]. These guidelines suggest the use of moderate or high-intensity statin therapy with rosuvastatin 20-40 mg or atorvastatin 40-80 mg daily, with the same LDL-c target of 1.8 mmol/L. The Canadian guidelines[41] give more detailed indications for aggressive lipid-lowering therapies. All PAD patients should receive the higher tolerated dose of statins; the addition of PCSK9-i or ezetimibe should be considered if the cut-off of 1.8 mmol/L is not reached at the higher dose of statin. Furthermore, triglycerides levels are also mentioned, where the use of icosapent ethyl is suggested when statin therapy alone does not lead to a level of 1.8-5.6 mmol/L. Eventually, while all the previous guidelines give their recommendations for statins to reduce all-cause and CV mortality and morbidity (i.e.: Nonfatal MI, nonfatal stroke), these guidelines[41] are the only ones underlying the benefit of a more strict control of lipid levels also on MALEs.
Similarly, the recently released 2022 ACC Expert Consensus Decision Pathway[56] on nonstatin therapies firstly integrated the results of the trials with bempedoic acid, alone or in association with ezetimibe, in order to achieve the target of LDL-C in patients with ASCVD or extremely high cholesterol levels.
Therefore, as summarized in Figure 3, LDL-C reduction should be aimed at any vascular patient. However, the achievement of very low levels should be pursued in patients with CAD+PAD, by establishing an aggressive management, with the combination of all the available strategies, immediately after the evidence of the poly-vascular disease, seeking an earlier achievement of the required goal.
Patients with concomitant multidistrict artery disease, i.e. poly-vascular disease, represent a higher risk subset of patients with worse prognosis. In these patients, the most aggressive tolerated antithrombotic and antilipidemic therapy should be attempted, although accounting for the interindividual differences. Multidisciplinary approach, involving both a cardiologist and vascular surgeon, combining different therapeutic goals and perspectives, could provide additional benefits in the correct tailoring of pharmacological therapy among poly-vascular patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Forţofoiu MC, Romania; Shalaby MN, Egypt S-Editor: Liu JH L-Editor: A P-Editor: Yu HG
| 1. | Bauersachs R, Zeymer U, Brière JB, Marre C, Bowrin K, Huelsebeck M. Burden of Coronary Artery Disease and Peripheral Artery Disease: A Literature Review. Cardiovasc Ther. 2019;2019:8295054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 262] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 2. | Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2791] [Cited by in RCA: 4523] [Article Influence: 904.6] [Reference Citation Analysis (0)] |
| 3. | Dzau VJ, Antman EM, Black HR, Hayes DL, Manson JE, Plutzky J, Popma JJ, Stevenson W. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes: part I: Pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease). Circulation. 2006;114:2850-2870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 266] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 4. | Chrysant SG. Stopping the cardiovascular disease continuum: Focus on prevention. World J Cardiol. 2010;2:43-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Kubica J, Adamski P, Niezgoda P, Alexopoulos D, Badarienė J, Budaj A, Buszko K, Dudek D, Fabiszak T, Gąsior M, Gil R, Gorog DA, Grajek S, Gurbel PA, Gruchała M, Jaguszewski MJ, James S, Jeong YH, Jilma B, Kasprzak JD, Kleinrok A, Kubica A, Kuliczkowski W, Legutko J, Lesiak M, Siller-Matula JM, Nadolny K, Pstrągowski K, Di Somma S, Specchia G, Stępińska J, Tantry US, Tycińska A, Verdoia M, Wojakowski W, Navarese EP. Prolonged antithrombotic therapy in patients after acute coronary syndrome: A critical appraisal of current European Society of Cardiology guidelines. Cardiol J. 2020;27:661-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Verdoia M, Pergolini P, Rolla R, Nardin M, Schaffer A, Barbieri L, Daffara V, Marino P, Bellomo G, Suryapranata H, De Luca G; Novara Atherosclerosis Study Group (NAS). Impact of high-dose statins on vitamin D levels and platelet function in patients with coronary artery disease. Thromb Res. 2017;150:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Gutierrez JA, Aday AW, Patel MR, Jones WS. Polyvascular Disease: Reappraisal of the Current Clinical Landscape. Circ Cardiovasc Interv. 2019;12:e007385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 8. | Aboyans V, Ricco JB, Bartelink MEL, Björck M, Brodmann M, Cohnert T, Collet JP, Czerny M, De Carlo M, Debus S, Espinola-Klein C, Kahan T, Kownator S, Mazzolai L, Naylor AR, Roffi M, Röther J, Sprynger M, Tendera M, Tepe G, Venermo M, Vlachopoulos C, Desormais I; ESC Scientific Document Group. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39:763-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1435] [Cited by in RCA: 2257] [Article Influence: 376.2] [Reference Citation Analysis (0)] |
| 9. | Bergheanu SC, Bodde MC, Jukema JW. Pathophysiology and treatment of atherosclerosis: Current view and future perspective on lipoprotein modification treatment. Neth Heart J. 2017;25:231-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 166] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 10. | Weissler EH, Jones WS, Desormais I, Debus S, Mazzolai L, Espinola-Klein C, Nikol S, Nehler M, Sillesen H, Aboyans V, Patel MR. Polyvascular disease: A narrative review of current evidence and a consideration of the role of antithrombotic therapy. Atherosclerosis. 2020;315:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Steg PG, Bhatt DL, Wilson PW, D'Agostino R Sr, Ohman EM, Röther J, Liau CS, Hirsch AT, Mas JL, Ikeda Y, Pencina MJ, Goto S; REACH Registry Investigators. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Bhatt DL, Peterson ED, Harrington RA, Ou FS, Cannon CP, Gibson CM, Kleiman NS, Brindis RG, Peacock WF, Brener SJ, Menon V, Smith SC Jr, Pollack CV Jr, Gibler WB, Ohman EM, Roe MT; CRUSADE Investigators. Prior polyvascular disease: risk factor for adverse ischaemic outcomes in acute coronary syndromes. Eur Heart J. 2009;30:1195-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 13. | Gutierrez JA, Mulder H, Jones WS, Rockhold FW, Baumgartner I, Berger JS, Blomster JI, Fowkes FGR, Held P, Katona BG, Mahaffey KW, Norgren L, Hiatt WR, Patel MR. Polyvascular Disease and Risk of Major Adverse Cardiovascular Events in Peripheral Artery Disease: A Secondary Analysis of the EUCLID Trial. JAMA Netw Open. 2018;1:e185239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 14. | Chen Q, Li L, Chen Q, Lin X, Li Y, Huang K, Yao C. Critical appraisal of international guidelines for the screening and treatment of asymptomatic peripheral artery disease: a systematic review. BMC Cardiovasc Disord. 2019;19:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | de Oliveira DC, Correia A, Nascimento Neto J, Gurgel M, Sarinho FW, Victor EG. Association Between Ankle-Brachial Index and Coronary Lesions Assessed by Coronary Angiography. Cardiol Res. 2015;6:216-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Abola MTB, Golledge J, Miyata T, Rha SW, Yan BP, Dy TC, Ganzon MSV, Handa PK, Harris S, Zhisheng J, Pinjala R, Robless PA, Yokoi H, Alajar EB, Bermudez-Delos Santos AA, Llanes EJB, Obrado-Nabablit GM, Pestaño NS, Punzalan FE, Tumanan-Mendoza B. Asia-Pacific Consensus Statement on the Management of Peripheral Artery Disease: A Report from the Asian Pacific Society of Atherosclerosis and Vascular Disease Asia-Pacific Peripheral Artery Disease Consensus Statement Project Committee. J Atheroscler Thromb. 2020;27:809-907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 17. | Farkas K, Kolossváry E, Ferenci T, Paksy A, Kiss I, Járai Z. Ankle Brachial Index is a strong predictor of mortality in hypertensive patients: results of a five-year follow-up study. Int Angiol. 2022;41:517-524. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Kim HL, Kim SH. Pulse Wave Velocity in Atherosclerosis. Front Cardiovasc Med. 2019;6:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 261] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 19. | Willeit P, Tschiderer L, Allara E, Reuber K, Seekircher L, Gao L, Liao X, Lonn E, Gerstein HC, Yusuf S, Brouwers FP, Asselbergs FW, van Gilst W, Anderssen SA, Grobbee DE, Kastelein JJP, Visseren FLJ, Ntaios G, Hatzitolios AI, Savopoulos C, Nieuwkerk PT, Stroes E, Walters M, Higgins P, Dawson J, Gresele P, Guglielmini G, Migliacci R, Ezhov M, Safarova M, Balakhonova T, Sato E, Amaha M, Nakamura T, Kapellas K, Jamieson LM, Skilton M, Blumenthal JA, Hinderliter A, Sherwood A, Smith PJ, van Agtmael MA, Reiss P, van Vonderen MGA, Kiechl S, Klingenschmid G, Sitzer M, Stehouwer CDA, Uthoff H, Zou ZY, Cunha AR, Neves MF, Witham MD, Park HW, Lee MS, Bae JH, Bernal E, Wachtell K, Kjeldsen SE, Olsen MH, Preiss D, Sattar N, Beishuizen E, Huisman MV, Espeland MA, Schmidt C, Agewall S, Ok E, Aşçi G, de Groot E, Grooteman MPC, Blankestijn PJ, Bots ML, Sweeting MJ, Thompson SG, Lorenz MW; PROG-IMT and the Proof-ATHERO Study Groups. Carotid Intima-Media Thickness Progression as Surrogate Marker for Cardiovascular Risk: Meta-Analysis of 119 Clinical Trials Involving 100 667 Patients. Circulation. 2020;142:621-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 303] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 20. | Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4971] [Cited by in RCA: 4920] [Article Influence: 273.3] [Reference Citation Analysis (0)] |
| 21. | James S, Akerblom A, Cannon CP, Emanuelsson H, Husted S, Katus H, Skene A, Steg PG, Storey RF, Harrington R, Becker R, Wallentin L. Comparison of ticagrelor, the first reversible oral P2Y(12) receptor antagonist, with clopidogrel in patients with acute coronary syndromes: Rationale, design, and baseline characteristics of the PLATelet inhibition and patient Outcomes (PLATO) trial. Am Heart J. 2009;157:599-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 305] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 22. | Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM; ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2569] [Cited by in RCA: 3150] [Article Influence: 787.5] [Reference Citation Analysis (0)] |
| 23. | Patti G, Ghiglieno C. Prevention of ischaemic events in subjects with polydistrict vascular disease. Eur Heart J Suppl. 2021;23:E103-E108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 24. | Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, Holmes DR Jr, Krucoff MW, Hermiller J, Dauerman HL, Simon DI, Kandzari DE, Garratt KN, Lee DP, Pow TK, Ver Lee P, Rinaldi MJ, Massaro JM; DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371:2155-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1401] [Cited by in RCA: 1498] [Article Influence: 136.2] [Reference Citation Analysis (0)] |
| 25. | Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, Bengtsson O, Oude Ophuis T, Budaj A, Theroux P, Ruda M, Hamm C, Goto S, Spinar J, Nicolau JC, Kiss RG, Murphy SA, Wiviott SD, Held P, Braunwald E, Sabatine MS; PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1314] [Cited by in RCA: 1389] [Article Influence: 138.9] [Reference Citation Analysis (0)] |
| 26. | Bonaca MP, Bhatt DL, Storey RF, Steg PG, Cohen M, Kuder J, Goodrich E, Nicolau JC, Parkhomenko A, López-Sendón J, Dellborg M, Dalby A, Špinar J, Aylward P, Corbalán R, Abola MTB, Jensen EC, Held P, Braunwald E, Sabatine MS. Ticagrelor for Prevention of Ischemic Events After Myocardial Infarction in Patients With Peripheral Artery Disease. J Am Coll Cardiol. 2016;67:2719-2728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 286] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 27. | Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, Widimsky P, Hori M, Avezum A, Piegas LS, Branch KRH, Probstfield J, Bhatt DL, Zhu J, Liang Y, Maggioni AP, Lopez-Jaramillo P, O'Donnell M, Kakkar AK, Fox KAA, Parkhomenko AN, Ertl G, Störk S, Keltai M, Ryden L, Pogosova N, Dans AL, Lanas F, Commerford PJ, Torp-Pedersen C, Guzik TJ, Verhamme PB, Vinereanu D, Kim JH, Tonkin AM, Lewis BS, Felix C, Yusoff K, Steg PG, Metsarinne KP, Cook Bruns N, Misselwitz F, Chen E, Leong D, Yusuf S; COMPASS Investigators. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N Engl J Med. 2017;377:1319-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 1667] [Article Influence: 208.4] [Reference Citation Analysis (0)] |
| 28. | Anand SS, Bosch J, Eikelboom JW, Connolly SJ, Diaz R, Widimsky P, Aboyans V, Alings M, Kakkar AK, Keltai K, Maggioni AP, Lewis BS, Störk S, Zhu J, Lopez-Jaramillo P, O'Donnell M, Commerford PJ, Vinereanu D, Pogosova N, Ryden L, Fox KAA, Bhatt DL, Misselwitz F, Varigos JD, Vanassche T, Avezum AA, Chen E, Branch K, Leong DP, Bangdiwala SI, Hart RG, Yusuf S; COMPASS Investigators. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 597] [Article Influence: 85.3] [Reference Citation Analysis (0)] |
| 29. | McClure GR, Kaplovitch E, Narula S, Bhagirath VC, Anand SS. Rivaroxaban and Aspirin in Peripheral Vascular Disease: a Review of Implementation Strategies and Management of Common Clinical Scenarios. Curr Cardiol Rep. 2019;21:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4959] [Cited by in RCA: 4588] [Article Influence: 199.5] [Reference Citation Analysis (0)] |
| 31. | Belch J, MacCuish A, Campbell I, Cobbe S, Taylor R, Prescott R, Lee R, Bancroft J, MacEwan S, Shepherd J, Macfarlane P, Morris A, Jung R, Kelly C, Connacher A, Peden N, Jamieson A, Matthews D, Leese G, McKnight J, O'Brien I, Semple C, Petrie J, Gordon D, Pringle S, MacWalter R; Prevention of Progression of Arterial Disease and Diabetes Study Group; Diabetes Registry Group; Royal College of Physicians Edinburgh. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 652] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 32. | Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, Flather MD, Haffner SM, Hamm CW, Hankey GJ, Johnston SC, Mak KH, Mas JL, Montalescot G, Pearson TA, Steg PG, Steinhubl SR, Weber MA, Brennan DM, Fabry-Ribaudo L, Booth J, Topol EJ; CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2037] [Cited by in RCA: 1860] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 33. | Tang T, Zhang M, Li W, Hu N, Du X, Ran F, Li X. Oral Anticoagulant and Antiplatelet Therapy for Peripheral Arterial Disease: A Meta-analysis of Randomized Controlled Trials. Clin Appl Thromb Hemost. 2021;27:1076029621996810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Hiatt WR, Bonaca MP, Patel MR, Nehler MR, Debus ES, Anand SS, Capell WH, Brackin T, Jaeger N, Hess CN, Pap AF, Berkowitz SD, Muehlhofer E, Haskell L, Brasil D, Madaric J, Sillesen H, Szalay D, Bauersachs R. Rivaroxaban and Aspirin in Peripheral Artery Disease Lower Extremity Revascularization: Impact of Concomitant Clopidogrel on Efficacy and Safety. Circulation. 2020;142:2219-2230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 35. | Belch JJ, Dormandy J; CASPAR Writing Committee, Biasi GM, Cairols M, Diehm C, Eikelboom B, Golledge J, Jawien A, Lepäntalo M, Norgren L, Hiatt WR, Becquemin JP, Bergqvist D, Clement D, Baumgartner I, Minar E, Stonebridge P, Vermassen F, Matyas L, Leizorovicz A. Results of the randomized, placebo-controlled clopidogrel and acetylsalicylic acid in bypass surgery for peripheral arterial disease (CASPAR) trial. J Vasc Surg. 2010;52:825-833, 833.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 246] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 36. | Bonaca MP, Bauersachs RM, Anand SS, Debus ES, Nehler MR, Patel MR, Fanelli F, Capell WH, Diao L, Jaeger N, Hess CN, Pap AF, Kittelson JM, Gudz I, Mátyás L, Krievins DK, Diaz R, Brodmann M, Muehlhofer E, Haskell LP, Berkowitz SD, Hiatt WR. Rivaroxaban in Peripheral Artery Disease after Revascularization. N Engl J Med. 2020;382:1994-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 655] [Article Influence: 131.0] [Reference Citation Analysis (0)] |
| 37. | Debus ES, Nehler MR, Govsyeyev N, Bauersachs RM, Anand SS, Patel MR, Fanelli F, Capell WH, Brackin T, Hinterreiter F, Krievins D, Nault P, Piffaretti G, Svetlikov A, Jaeger N, Hess CN, Sillesen HH, Conte M, Mills J, Muehlhofer E, Haskell LP, Berkowitz SD, Hiatt WR, Bonaca MP. Effect of Rivaroxaban and Aspirin in Patients With Peripheral Artery Disease Undergoing Surgical Revascularization: Insights From the VOYAGER PAD Trial. Circulation. 2021;144:1104-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 38. | Bonaca MP, Szarek M, Debus ES, Nehler MR, Patel MR, Anand SS, Muehlhofer E, Berkowitz SD, Haskell LP, Bauersachs RM. Efficacy and safety of rivaroxaban versus placebo after lower extremity bypass surgery: A post hoc analysis of a "CASPAR like" outcome from VOYAGER PAD. Clin Cardiol. 2022;45:1143-1146. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 39. | Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RA, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D, Walsh ME. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e686-e725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 440] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 40. | Kithcart AP, Beckman JA. ACC/AHA Versus ESC Guidelines for Diagnosis and Management of Peripheral Artery Disease: JACC Guideline Comparison. J Am Coll Cardiol. 2018;72:2789-2801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 41. | Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, Mills JL, Ricco JB, Suresh KR, Murad MH; GVG Writing Group. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg. 2019;69:3S-125S.e40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 895] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 42. | Primary Panel:, Abramson BL, Al-Omran M, Anand SS, Albalawi Z, Coutinho T, de Mestral C, Dubois L, Gill HL, Greco E, Guzman R, Herman C, Hussain MA, Huckell VF, Jetty P, Kaplovitch E, Karlstedt E, Kayssi A, Lindsay T, Mancini GBJ, McClure G, McMurtry MS, Mir H, Nagpal S, Nault P, Nguyen T, Petrasek P, Rannelli L, Roberts DJ, Roussin A, Saw J, Srivaratharajah K, Stone J, Szalay D, Wan D; Secondary Panel:, Cox H, Verma S, Virani S. Canadian Cardiovascular Society 2022 Guidelines for Peripheral Arterial Disease. Can J Cardiol. 2022;38:560-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 43. | Patti G, Spinoni EG, Grisafi L, Mehran R, Mennuni M. Safety and efficacy of very low LDL-cholesterol intensive lowering: a meta-analysis and meta-regression of randomized trials. Eur Heart J Cardiovasc Pharmacother. 2023;9:138-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 44. | Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285-e350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1551] [Cited by in RCA: 1639] [Article Influence: 273.2] [Reference Citation Analysis (0)] |
| 45. | Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 5316] [Article Influence: 1063.2] [Reference Citation Analysis (0)] |
| 46. | Solnica B, Sygitowicz G, Sitkiewicz D, Cybulska B, Jóźwiak J, Odrowąż-Sypniewska G, Banach M. 2020 Guidelines of the Polish Society of Laboratory Diagnostics (PSLD) and the Polish Lipid Association (PoLA) on laboratory diagnostics of lipid metabolism disorders. Arch Med Sci. 2020;16:237-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 47. | Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA. 2016;316:1289-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1152] [Cited by in RCA: 1012] [Article Influence: 112.4] [Reference Citation Analysis (0)] |
| 48. | Ray KK, Reeskamp LF, Laufs U, Banach M, Mach F, Tokgözoğlu LS, Connolly DL, Gerrits AJ, Stroes ESG, Masana L, Kastelein JJP. Combination lipid-lowering therapy as first-line strategy in very high-risk patients. Eur Heart J. 2022;43:830-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 49. | Bonaca MP, Nault P, Giugliano RP, Keech AC, Pineda AL, Kanevsky E, Kuder J, Murphy SA, Jukema JW, Lewis BS, Tokgozoglu L, Somaratne R, Sever PS, Pedersen TR, Sabatine MS. Low-Density Lipoprotein Cholesterol Lowering With Evolocumab and Outcomes in Patients With Peripheral Artery Disease: Insights From the FOURIER Trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk). Circulation. 2018;137:338-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 555] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 50. | Jukema JW, Szarek M, Zijlstra LE, de Silva HA, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Karpov Y, Moryusef A, Pordy R, Prieto JC, Roe MT, White HD, Zeiher AM, Schwartz GG, Steg PG; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab in Patients With Polyvascular Disease and Recent Acute Coronary Syndrome: ODYSSEY OUTCOMES Trial. J Am Coll Cardiol. 2019;74:1167-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 51. | Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, Bisch JA, Richardson T, Jaros M, Wijngaard PLJ, Kastelein JJP; ORION-10 and ORION-11 Investigators. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N Engl J Med. 2020;382:1507-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 893] [Article Influence: 178.6] [Reference Citation Analysis (0)] |
| 52. | Ray KK, Raal FJ, Kallend DG, Jaros MJ, Koenig W, Leiter LA, Landmesser U, Schwartz GG, Lawrence D, Friedman A, Garcia Conde L, Wright RS; ORION Phase III investigators. Inclisiran and cardiovascular events: a patient-level analysis of phase III trials. Eur Heart J. 2023;44:129-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 113] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 53. | Antoniou GA, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg. 2015;61:519-532.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 54. | Kokkinidis DG, Arfaras-Melainis A, Giannopoulos S, Katsaros I, Jawaid O, Jonnalagadda AK, Parikh SA, Secemsky EA, Giri J, Kumbhani DJ, Armstrong EJ. Statin therapy for reduction of cardiovascular and limb-related events in critical limb ischemia: A systematic review and meta-analysis. Vasc Med. 2020;25:106-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 55. | Parmar GM, Novak Z, Spangler E, Patterson M, Passman MA, Beck AW, Pearce BJ. Statin use improves limb salvage after intervention for peripheral arterial disease. J Vasc Surg. 2019;70:539-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 56. | Writing Committee; Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Covington AM, DePalma SM, Minissian MB, Orringer CE, Smith SC Jr, Waring AA, Wilkins JT. 2022 ACC Expert Consensus Decision Pathway on the Role of Nonstatin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;80:1366-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 268] [Article Influence: 89.3] [Reference Citation Analysis (0)] |