Published online Apr 26, 2023. doi: 10.4330/wjc.v15.i4.119
Peer-review started: January 6, 2023
First decision: January 20, 2023
Revised: February 2, 2023
Accepted: April 10, 2023
Article in press: April 10, 2023
Published online: April 26, 2023
Processing time: 103 Days and 22.9 Hours
Syncope is a concerning symptom that affects a large proportion of patients. It can be related to a heterogeneous group of pathologies ranging from trivial causes to diseases with a high risk of sudden death. However, benign causes are the most frequent, and identifying high-risk patients with potentially severe etiologies is crucial to establish an accurate diagnosis, initiate effective therapy, and alter the prognosis. The term cardiac syncope refers to those episodes where the cause of the cerebral hypoperfusion is directly related to a cardiac disorder, while arrhy
Core Tip: In this review, we summarized the most important and novel data on arrhythmic syncope, the value of the different diagnostic tests, the management, and the specific characteristics in some particular populations such as patients with cardiomyopathies or channelopathies. The review emphasized the importance of an appropriate stepwise approach work-up and intervention.
- Citation: Francisco Pascual J, Jordan Marchite P, Rodríguez Silva J, Rivas Gándara N. Arrhythmic syncope: From diagnosis to management. World J Cardiol 2023; 15(4): 119-141
- URL: https://www.wjgnet.com/1949-8462/full/v15/i4/119.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i4.119
Syncope is a total loss of consciousness (T-LOC) secondary to cerebral hypoperfusion, characterized by rapid onset, short duration, and complete spontaneous recovery[1]. It must be differentiated from other T-LOC that do not meet these characteristics, such as T-LOC of traumatic origin, some types of epilepsy, or certain psychiatric disorders. It should be noted that syncope is a symptom that encompasses a heterogeneous group of pathologies ranging from trivial causes to diseases with a high risk of sudden death. Therefore, it should not constitute a final diagnosis. It is of great importance to stratify the risk and try to determine the cause.
The term cardiac syncope refers to those episodes where the cause of the cerebral hypoperfusion is directly related to a cardiac disorder, while arrhythmic syncope refers to cardiac syncope specifically due to rhythm disorders. Indeed, arrhythmias are the most common cause of cardiac syncope (Table 1). Both bradyarrhythmia and tachyarrhythmia can cause a sudden decrease in cardiac output, causing the syncope. Non-arrhythmic causes of cardiac syncope are usually related to structural heart diseases with obstruction of outflow and/or inflow of blood. These obstructions can restrict increases in cardiac output on exercise rendering this insufficient to maintain the circulation. Severe aortic stenosis (AoS), hypertrophic cardiomyopathy (HCM), mitral stenosis, atrial myxoma, or severe pulmonary hypertension are some examples of conditions that can cause cardiac syncope via this mechanism.
| Cardiac syncope | |||
| Arrhythmic causes | Bradyarrhythmia | Sick sinus syndrome/sinus node disfunction | |
| Atrioventricular block | |||
| Tachyarrhythmia | Supraventricular tachycardia (AVNRT, AVRT, AT, fast AF, etc.) | ||
| Ventricular arrhythmias | Related to structural heart disease | ||
| Channelopathies and inherited arrhythmia syndromes | |||
| Non-arrhythmic causes | Mechanical causes | Valvulopathies (aortic stenosis, mitral stenosis, etc.) | |
| HCM | |||
| Atrial myxoma | |||
| Pulmonary emboli | |||
| Tamponade | |||
| Severe pulmonary hypertension | |||
| Acute coronary syndrome |
Furthermore, myocardial ischemia and acute ischemic syndromes may also precipitate syncope through multiple mechanisms. It is important to highlight that most of these heart diseases can also be associated with arrhythmias or reflex syncope, and therefore it is often challenging to determine the main cause of syncope in structural cardiac syncope[1-5]. In other words, the mere presence of structural heart disease associated with obstruction does not allow us to conclude that the syncope is due to this mechanism. In many cases, it will be necessary to rule out other possible causes, especially arrhythmic ones.
It is estimated that almost one in two people will suffer at least one syncopal episode in their lifetime[1,6]. It is a front-line health problem with a high impact on the health system, even though it is known that only a small proportion of patients with syncope seek medical attention. An epidemiological study carried out in the United States showed that the prevalence of patients with syncope in the community requiring medical attention is 9.5 per 100 inhabitants, and that 1 in 10 required hospital admission[7].
The incidence of the first syncopal episode is distributed with a bimodal curve, with a first peak in youth (between 10-30 years of age) and a second peak over 65 years of age. Cardiac syncope is the third most common cause of syncope after reflex and orthostatic hypotension (OH)[1,8,9]. In the emergency department (ED), cardiac syncope accounts for 5%-21% of syncope. In the Framingham cohort, the prevalence of syncope and long-term prognosis were analyzed[10]. The incidence of a first report of syncope was 6.2 per 1000 person-years. Reflex or vasovagal syncope is the most common cause in the general population. In the Framingham cohort it represented 21.0% of the cases, while cardiac syncope made up only 9.5%[10]. It is remarkable that the prevalence of cardiac syncope increases with advancing age[1,9-11]. Cardiac syncope causes less than 1% of syncope in youth (< 40 years)[12] and up to one-third in those over 60 years of age[10,12].
The prognosis of syncope is mostly related to the underlying cause and the presence of structural heart disease. While reflex syncope has an excellent prognosis in terms of survival, cardiac syncope is associated with an increased risk of mortality, especially if it is not identified and treated properly. Patients with reflex syncope have similar survival to patients without syncope[10], with a mortality rate between 4%-12% after 1 year (depending on the patient’s age and comorbidities)[10,13-15]. By contrast, the 1-year mortality rate for cardiac syncope rises to 20%-30%[10,13-15]. In the Framingham cohort, cardiac syncope was associated with a two-fold increase in the risk of death compared with those without a history of syncope, with an approximately 50% 5-year survival[10]. In this study, patients with syncope of unknown origin also had an increased risk of all-cause mortality compared with the general population [hazard ratio = 1.32, 95% confidence interval (CI): 1.09-1.60]. This observation was also made in other studies focused on specific populations[5]. This may be due to the fact that there are potentially serious causes for syncope left untreated due to a lack of diagnosis.
Importantly, in patients with syncope of unknown origin, the mere presence of structural cardiac abnormalities or the evidence of a conduction system disorder is associated with a poor prognosis, increasing the risk of death by a factor of more than five[1,9,16-19]. On the other hand, a structurally normal heart with a normal electrocardiogram (ECG) is usually associated with a benign etiology for syncope and a favorable prognosis[1,20-22].
T-LOC is a relatively common cause of presentation to the ED, and half of these episodes can be attributed to syncope[23]. However, it is important to distinguish it from other causes of T-LOC, to avoid unnecessary investigations in patients with benign causes, and to correctly detect and treat patients with cardiac syncope, which can lead to serious outcomes. The most common condition that can be confused with syncope is probably epilepsy. This confusion is an important phenomenon leading to misdiagnosis with rates ranging from 6%-67%[24]. This misdiagnosis contributes significantly to the numbers of patients with a questionable diagnosis of epilepsy and to those with apparently drug-resistant epilepsy. Syncope can be accompanied with urinary incontinence and/or muscular contractions that can resemble epileptic seizures, making it difficult to differentiate between the diagnoses. While in epilepsy muscular movements are generalized and appear from the beginning of the T-LOC and continue for a few minutes, syncope can also be associated with muscular contractions, which often tend to appear a few seconds after the collapse. They tend to be pleiomorphic and last only a short period of time. Some clinical findings have been suggested to differentiate seizures from convulsive syncope. Tongue biting and confusion on awakening are the most useful in predicting an epileptic origin[25]. In addition, clinical clues that should raise the suspicion for psychogenic pseudosyncope include prolonged duration, eye closure during the episode, unusual triggers, no recognizable prodromes, and a high frequency of attacks[26].
Another common source of confusion in the ED is represented by falls, especially in the elderly population with non-witnessed T-LOC. On the one hand, elderly people with cognitive impairment and muscular weakness can present with falls as a manifestation of another illness, such as infections or metabolic disorders[27]. On the other hand, these populations are usually treated with medications that can lower blood pressure (BP) and heart rate (HR) and tend to be dehydrated due to reduced water consumption. This combination of factors can promote orthostatic syncope. Additionally, in the elderly, there is a high prevalence of sinus node dysfunction, conduction disturbances, and structural heart disease, putting these patients at high risk of presenting with cardiac syncope[2]. For all these reasons, current guidelines recommend that repeated falls in elderly people without a reasonable explanation should be approached like unexplained syncope[1].
Once the syncope diagnosis has been established, special attention should be paid to determining the underlying cause. Syncope can be caused by three main different etiologies: Reflex mechanism (also known as neural-mediated syncope); OH; or cardiac syncope, which can be due to arrythmia or structural heart disease. The diagnostic approach should focus on detecting potential cardiac syncope, as it could be clinical manifestation of a primary cardiac disease with high risk of events.
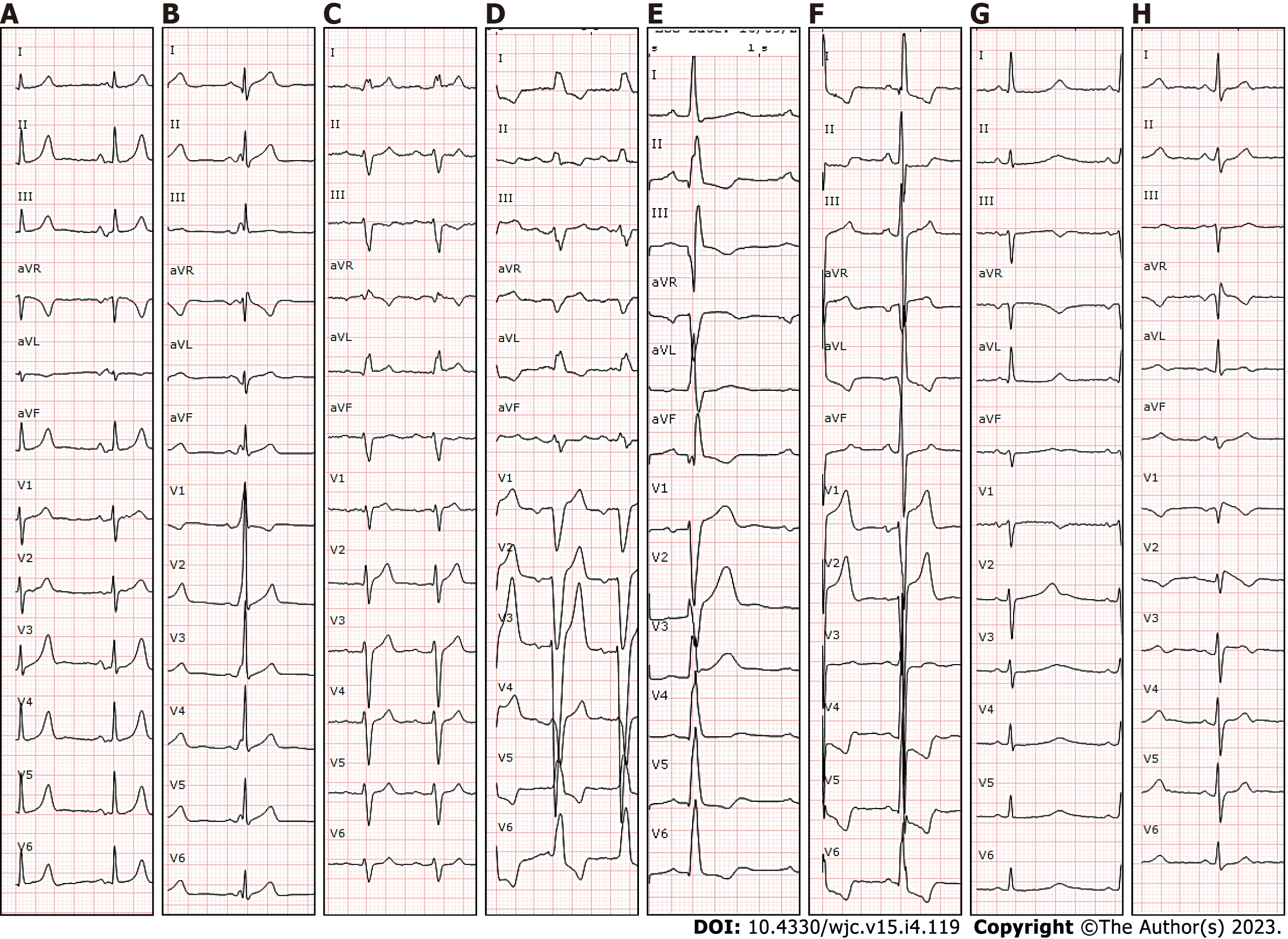
Initial evaluation of any patient presenting with syncope should include three basic elements: (1) Careful history taking regarding the current and previous episodes (including eyewitness accounts); (2) Physical examination; and (3) ECG. Clinical history is probably the most important one, and it should be focused on past medical history, especially previous cardiac conditions, and symptoms related to the episode. Syncope during exertion or in a supine position accompanied by chest pain or palpitations have been described as high-risk factors and should raise the suspicion of cardiac syncope[28,29]. In addition, a family history of sudden cardiac death (SCD) at a young age or personal history of structural heart disease or coronary artery disease (CAD) have been considered high-risk factors. Physical examination does not usually show relevant findings, but it could reveal signs of heart failure or a systolic murmur suggesting structural heart disease. Performing an ECG is crucial, as it can show conduction disturbances, pathological Q waves, or repolarization abnormalities reflecting an underlying cardiac disease[15,30-32] (Figure 1). It is important to mention that every patient with syncope should have an ECG even if there is clear evidence that is a reflex syncope since there are some channelopathies such as long QT syndrome (LQTS) that can present with ventricular arrhythmias after emotional stimulus that can be confused with reflex syncope. Additionally, it has been described that patients with Brugada syndrome (BrS) are more prone to vasovagal syncope[33].
There are several scores developed for risk stratification according to clinical and ECG findings[34]. However, some of them have been tested with external validation cohorts showing poor sensitivity and specificity for detecting cardiac syncope, and they perform no better than clinician judgement at predicting short-term serious outcomes. Therefore, current guidelines do not recommend using them alone to make decisions in the ED. Most items included on these scales are those suggesting cardiac syncope, such as ECG abnormalities or signs or symptoms of structural heart disease.
Carotid sinus massage (CSM) consists of applying external pressure to the area of the neck where the carotid sinus is located and is indicated in patients over 40 with syncope. According to current clinical guidelines, carotid sinus hypersensitivity is defined by a sinus pause longer than 3 s or a drop in systolic BP (SBP) higher than 50 mmHg[1,9]. However, this condition is very common among older individuals with cardiovascular disease, even in the absence of syncope. To avoid misdiagnoses, it has been proposed that the diagnosis of carotid sinus syndrome requires reproduction of patient’s symptoms and a sinus pause longer than 6 s or more or a drop in mean arterial pressure of 60 mmHg or more[35]. Patients fulfilling these criteria have been shown to have recurrent long pauses on monitoring and to respond well to cardiac pacing[36,37]. The worst complication of CSM is stroke, which is extremely uncommon.
Orthostatic challenge consists of measuring HR and BP changes between supine and upright positions. It is recommended to measure them during the first 3 min, but it can be extended to the first 10 min if there is a high suspicion of OH since retarded responses have been described[38]. OH is defined by a drop of more than 20 mmHg in SBP, or a drop of more than 10 mmHg of diastolic BP, or if SBP becomes lower than 90 mmHg, and always accompanied by symptoms[39]. OH is very common among elderly people, especially in patients taking anti-hypertensive medications and/or with autonomous nervous system diseases like Parkinson’s disease or diabetes, and it represents an important cause of syncope in this population[2,40].
Tilt testing is recommended in patients with suspected reflex syncope or autonomic failure, including delayed forms of OH or postural orthostatic tachycardia syndrome. The most frequently used protocol is the so called “Italian protocol,” which includes a 20-min stabilization phase, followed by administration of sublingual nitroglycerin[41]. It is useful in patients with true reflex syncope, as it has been demonstrated that a positive cardioinhibitory response is highly predictive of asystolic spontaneous syncope[42]. However, it can also be positive in a high percentage of patients with unexplained syncope and even in patients with cardiac arrhythmic syncope. Therefore, it offers little diagnostic value in these populations and should not be performed routinely[1]. It has also been tested to evaluate treatment effectiveness, showing little value in this aspect. Finally, in recent years it has been demonstrated that cardiac denervation of parasympathetic ganglia can be highly effective in reducing cardioinhibitory reflex syncope, a technique known as cardioneuroablation[43]. Tilt test might play a crucial role in detecting suitable patients for this promising procedure[44].
According to current European Guidelines[1], electrophysiological study (EPS) is indicated in patients with syncope and bifascicular block (BFB) or previous myocardial infarction or other scar-related conditions, when the etiology remains unexplained after non-invasive evaluation. It could also be considered when syncope is preceded by palpitations or in patients with sinus bradycardia, when the rest of the study has been negative. However, in patients with normal ECG and no structural heart disease, EPS is of poor diagnostic value, and other options like home monitoring are more appropriate. Additionally, a positive EPS is strongly predictive of the origin of the previous syncope, but a negative result cannot exclude arrhythmic events in the future. Therefore, it has a low negative predictive value[45,46].
Sick sinus syndrome is a heterogenous disease where sinus node does not function normally and includes some different kinds of bradycardia such as sinus pauses or junctional rhythm. However, these conditions are relatively common in elderly people, and it is crucial to correlate the bradycardia episodes with the patient’s symptoms. A sinus node recovery time (SNRT) longer than 1600 ms is considered abnormal [or corrected SNRT (cSNRT) longer than 525 ms] and has been correlated with sick sinus syndrome[45], but its prognostic value remains unclear. There are few data supporting the benefit of pacing in patients with an abnormal SNRT.
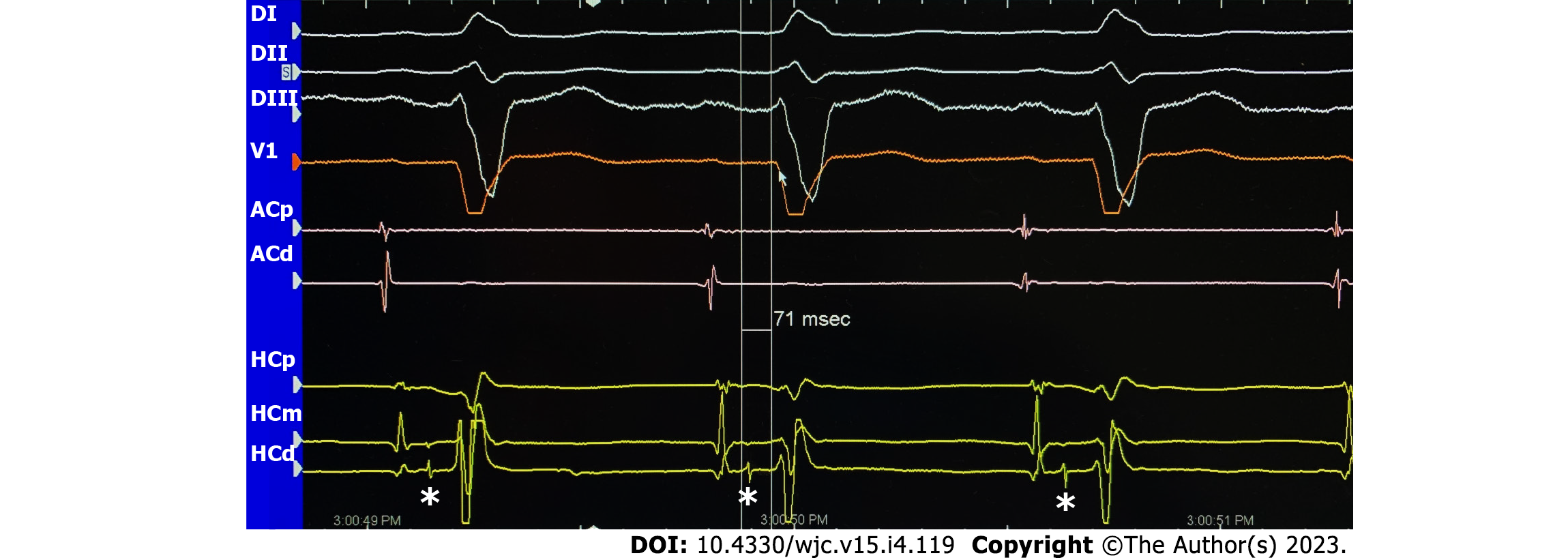
Patients with intraventricular conduction disturbances like BFB or nonspecific conduction disturbance with a QRS greater than 120 ms are at higher risk of arrhythmic events due to His-Purkinje system disease, and in this population paroxysmal atrioventricular block (AVB) is the most common cause of syncope[46-48]. In these patients with syncope suspected to be related to bradycardia, an HV interval longer than 70 ms or the development of second or third-degree AVB during incremental atrial pacing or pharmacological stress identifies a group with a high risk of developing AVB in the future[49], and pacing is recommended. In addition, some studies have evaluated the relationship between ECG conduction disturbance and the results of EPS, showing that PR interval prolongation and/or BFB patterns make a positive result in EPS more likely rather than a right bundle branch block (RBBB) pattern alone[50] (Figure 2).
Another important part of the EPS in the syncope work-up is programmed ventricular stimulation. In patients with previous myocardial infarction and syncope, the induction of monomorphic sustained ventricular tachycardia (MSVT) is strongly predictive of the cause of syncope and should be managed as spontaneous MSVT[51,52]. In contrast, the induction of polymorphic ventricular tachycardia (PVT) or ventricular fibrillation (VF) is considered a less specific finding, especially with aggressive stimulation protocols[53]. However, induction of PVT or VF may play a role in risk stratification of specific populations such as patients with repaired tetralogy of fallot[19,54-56] or BrS[57,58].
ECG cardiac monitoring is one of the cornerstones of the etiological diagnosis of arrhythmic syncope. In addition, there are several areas of interest other than unexplained syncope in which monitoring devices have been investigated[54,59-72]. The objective of ECG monitoring is to correlate the patient’s symptoms with the electrocardiographic recordings to reach an objective diagnosis. For this reason, the diagnostic yield of ECG monitoring is primarily related to the duration of monitoring and the frequency of symptoms. Since syncope is often an infrequent event, a long-term monitoring device is usually needed to have a chance of recording a syncopal episode. Moreover, the identification of significant asymptomatic arrhythmias (such as advanced AV block) can be important for the diagnosis. Therefore, as a general rule, ECG monitoring is indicated when there is a high pre-test probability of identifying an arrhythmia associated with syncope and after appropriate risk stratification. The choice of monitoring modality depends on the frequency of events.
In recent years, ECG monitoring systems have incorporated many technical upgrades allowing for improvement in several of the limitations presented by the 24-hr Holter monitor. This evolution of the ECG recording systems include, among other aspects, smaller devices, greater memory capacity for long-term monitoring, better quality of records, or remote monitoring capacity[70] (Table 2).
| Advantages | Disadvantages | Main indications | |
| 24-hr holter | Continuous recording: 12 leads with good correlation with surface ECG; low economic cost per study | Discomfort for the patient; artifacts; maximum recording of 24-48 h (low diagnostic yield); high economic cost per diagnosis | Very frequent (daily) symptoms; in-hospital monitoring (if ECG-telemetry not available) |
| Skin patches | Continuous recording of 7-14 d; good tolerability for patients | Single-use and greater economic cost; only one | Frequent (weekly) symptoms |
| External loop recorders | Loop recording (includes beginning and end of arrhythmic event); monitoring for 4 wk; low economic cost per study | Patient discomfort; requires education from healthcare professional on how to correctly place the electrodes; relatively low diagnostic yield | Frequent (weekly-monthly) symptoms |
| Implantable loop recorders | Loop recording; up to 3-yr monitoring (good diagnostic yield); patient does not have to do anything; remote monitoring | Invasiveness and associated complications (infection, bleeding, etc.); individual economic cost; single lead | Infrequent symptoms; most useful in syncope |
The main current ECG monitoring devices available are the following:
(1) In-hospital telemetry. In-hospital monitoring should be mandatory in patients with high-risk clinical features, especially if the monitoring is applied immediately after syncope. A recent study that evaluates the optimal ECG monitoring duration of ED patients with syncope found that a serious underlying arrhythmia was often identified within the first 2 h of ED arrival for low-risk patients and within 6 h for medium-risk and high-risk patients[73]. The diagnostic yield of ECG monitoring varies from 2%-20% depending on the patients’ characteristics[1,9,69,73-75].
(2) 24/48-hr Holter monitoring. Despite likely being the most frequently used device, the diagnostic yield is as low as 1%-2% in unselected patients due to its short monitoring time[69,70]. Even the newest devices with a longer recording capacity (7-14 d) offer a very limited diagnostic yield. In the opinion of the authors of this review, at the present time, the 24/48-hr Holter should only be considered in patients with daily or very frequent symptoms[69,70]. In different circumstances, other modalities offer not only a greater diagnostic yield but also better cost efficiency per diagnosis.
(3) Loop recorders. These allow for more prolonged monitoring since they do not store a continuous recording. Even though they continuously monitor the ECG, the device just stores a few minutes, which is subsequently overwritten with a newer recording. Only when the device is activated (be it via manual activation or through an automatic arrhythmia detection algorithm), it stores from a few minutes before the start of the event until its end in another part of the memory. These stored episodes are protected from overwriting and available for review. In this way, several minutes before activation are stored in the device memory, and the likelihood of recording the trace at the time of the syncope episode is relatively high. Within this category, we have differentiated between external and implantable devices.
External loop recorders. The device uses cutaneous electrodes to record, like the 24 hr Holter monitor. The patients themselves position the electrodes daily. Due to the characteristics of these devices, these systems tend to be worn by patients for no more than a few weeks (usually 3-4 wk, although there are reports of more prolonged periods of time[70,76]). For this reason, in the setting of syncope, the diagnostic yield is no greater than 10%. They are especially useful for the investigation of symptoms that occur every 2-3 wk. Significantly, it has been found in various studies that early recorder use increased the likelihood of diagnostic events during external ECG monitoring[73,77].
Implantable loop recorders (ILR). These are small devices that are implanted subcutaneously, usually in the left parasternal region. They have the disadvantage of being minimally invasive since the latest models have been made significantly smaller. However, these devices allow for a more prolonged continuous monitoring of up to 3 to 4 years, making them especially useful in patients with syncope. Numerous studies have evaluated the diagnostic value and the usefulness of ILRs for the work-up and the diagnostic yield increases up to 30%-50%[5,48,59,62,78-82]. In a meta-analysis of five randomized controlled trials, it was found that initial implantation of an ILR in the work-up provided a 3.7-fold (95%CI: 2.7-5.0) increase in the relative probability of a diagnosis compared with the conventional strategy[1,71,83,84]. Different studies have also demonstrated that ILR was more cost-effective than the conventional strategy[69,81,83-85].
(4) Skin patches. They consist of patches of different materials, which adhere to the skin and contain electrodes to obtain one (the most common) or two ECG leads that allow for a continuous ECG recording for 7-30 d of monitoring. Diagnostic yield and limitations are similar to external loop recorders. It should be noted that some new wearable devices like intelligent watches or other ECG prospective intermittent event recorders, which are quite popular nowadays, are generally not useful for syncope workup. These devices start recording only when the patient activates them. They have the limitation of not allowing for the recording of the onset of the episodes, which is often important for diagnosis. Furthermore, if the patient activates the device after recovering from the syncopal episode, in most cases the possible rhythm disorder would have resolved.
Autonomic function tests like the Valsalva maneuver or deep breathing test can be considered to diagnose autonomic disfunction, but there is weak evidence that these tests may be useful in patients with syncope. Echocardiography should be performed in all patients with suspected valvular or structural heart disease, as it can detect some conditions that could present with cardiac obstructive syncope (i.e., AoS or cardiac tamponade). Exercise testing is especially useful in patients that have experienced syncope during or shortly after exertion. The main purpose of these tests is to rule out ventricular arrythmias related to CAD or exercise-induced advanced AVB, which is usually located distally to the AV node. Cardiac biomarkers such as high sensitivity troponin and natriuretic peptides can be elevated in patients with syncope and have been associated with worse outcomes in some case series[86,87]. However, such determinations are highly non-specific and rarely contribute to a certain diagnosis, and they may indicate serious illness rather than myocardial ischemia or heart failure. Therefore, it remains unclear whether they should be determined on a routine basis[88].
Cardiac syncope is a life-threatening condition. By consequence, the main goal of risk stratification is to identify those low-risk patients with benign causes that can be discharged home and only require medical education from those high-risk patients with syncope likely related with cardiac arrythmias or structural heart disease who require hospital admission for further investigation. This initial evaluation is especially necessary in the ED, where most patients with syncope first consult (Table 3).
| High-risk features |
| Past medical history |
| Previous myocardial infarction |
| Previous cardiovascular condition (i.e., BrS, hypertrophic cardiomyopathy, Long QT syndrome, etc.) |
| Syncopal event |
| Syncope during exertion or in supine position |
| Syncope associated with chest pain, palpitations, breathless, or abdominal pain |
| Physical examination |
| Signs of heart failure |
| Cardiac murmur suggesting specific condition (i.e., aortic stenosis) |
| Signs of shock |
| Electrocardiogram |
| Conduction disturbance (AV block, bundle branch block) |
| Pathological Q waves |
| Long QT interval |
| Pre-excitation syndrome |
| Negative T waves |
For this purpose, several risk scores have been developed. In 2016, the Canadian Syncope Risk Score[34] was published. They included 4030 patients who presented to EDs of three centers in Canada for syncope and analyzed the occurrence of serious events including death, myocardial infarction, arrythmia, structural heart disease, pulmonary embolism, serious bleeding, and procedural intervention within 30 d from admission. Finally, they included nine predictors: (1) Predisposition to vasovagal syncope; (2) Heart disease; (3) Any systolic pressure reading in the ED < 90 or > 180 mmHg; (4) Troponin level above 99th percentile for the normal population; (5) Abnormal QRS axis (< -30° or > 100°); (6) QRS duration longer than 130 ms; (7) QTc interval longer than 480 ms; (8) ED diagnosis of cardiac syncope; and (9) ED diagnosis of vasovagal syncope. Those items suggesting reflex syncope conferred negative points, and those suggesting cardiac syncope conferred positive points. Each patient obtained a final score, with higher scores representing a greater risk of serious events (-3-0 points are considered low risk, while 0-3 points and 4-11 points are considered high and very high risk, respectively).
Recently, the same authors have validated this risk score in another large cohort of 3819 patients, showing very good correlation. Setting a threshold score of -1 point, they achieved very good sensitivity (97.8%) but poor specificity (44.3%) for serious events[89]. In addition, another group of researchers validated the same score in a cohort of 2283 patients from three continents also showing good correlation and better performance when compared with another European risk score[90]. However, they also observed that a simplified model including only the clinical classification (vasovagal, cardiac, or other), also achieved a similar degree of discrimination with regard to the primary outcome, showing that some of the predictors included may have a secondary role.
There are some other scales previously developed, such as the San Francisco Syncope Rule[91] or the EGSY score[29]. Both have shown similar results with good sensitivity but poor specificity. However, lack of reproducibility and remarkable heterogeneity in study design, variables, and outcome definitions of primary studies have prevented widespread use of these tools in clinical practice[92]. Moreover, recently some authors compared the EGSY score with clinical judgement, both alone and in addition to cardiac biomarkers, showing that clinical judgement has the highest diagnostic accuracy[93].
In summary, multiple risk scores have shown good sensitivity but poor specificity for predicting short-term serious outcomes, and they performed no better than clinical judgement. Therefore, they should not be used in isolation for the purposes of decision-making. It is also worth mentioning that, apart from risk scores, some other tests such as EPS, cardiac magnetic resonance (CMR), or stress test may be useful for risk stratification in selected groups of patients, as is discussed above in other sections of this article.
As previously mentioned, syncope could be the presenting symptom of an impending sudden cardiac arrest or can be related to more benign conditions such as neuro-mediated syncope or OH. Thus, it is important to correctly stratify the risk of each patient. For this reason, we need to understand the clinical scenario in which syncope takes place. Patients without overt structural heart diseases are at a lower risk of subsequent cardiac complications. Nonetheless, we must also consider some inherited heart diseases, which are primarily electrical, known as channelopathies and that can take place themselves in the absence of structural heart disease. In the following paragraphs we summarized some of those heart conditions that are associated with a higher risk of ventricular arrhythmias and sudden cardiac arrest.
Ischemic heart disease: Patients with ischemic heart disease (IHD) are at a higher risk of ventricular arrhythmias. It is necessary to differentiate between three stages in the ischemic evolution: (1) Acute ongoing ischemia. A patient suffering from an acute myocardial infarction might have VF and ventricular tachycardia related to the ischemic myocardium[1,19,94,95]. The acute ischemia induces a dispersion of the repolarization that may produce polymorphic ventricular arrhythmias and VF in the acute setting. In the same way, some patients might present with monomorphic ventricular arrhythmias during acute myocardial infarction, in which a macro re-entrant circuit involving the ischemic tissue is a more probable mechanism. This latter mechanism is much less frequent than the former[94]; (2) In the subacute phase of ischemia, comprising hours to days after the ischemic event, Purkinje-related ectopia is a frequent mechanism for VF and acute cardiac arrest. The premature ventricular complexes are characterized by their very short coupling intervals and by the presence of a normal QT interval. It is believed that the ischemia induces an abnormal calcium release to the cytosol of Purkinje cells, which causes such early post depolarization[94]; and (3) In the chronic setting, which accounts for most patients with syncope and IHD, a frequent mechanism is a ventricular arrhythmia due to macro re-entry in well-established ventricular scars[1,19]. The risk of ventricular arrhythmias is much higher among those patients with IHD with low ventricular ejection fraction[1,19,96].
Ventricular arrhythmias should be suspected in patients with syncope and IHD[1,6,9,97]. If the patient has a left ventricle ejection fraction (LVEF) of < 35% despite optimal medical treatment, an implantable cardiac defibrillator (ICD) is indicated[18,19,98]. These patients have solid evidence of high arrhythmic risk independently of the invasive risk stratification, and an ICD implantation is strongly indicated even if the etiology of the syncope is treated subsequently. This recommendation is strongly supported by large randomized clinical trials (SCD-HeFT, MADIT-II)[99,100] and class 1A recom
When the cause of the syncope remains unknown after an initial evaluation, and there is no apparent direct indication for ICD, an EPS with programmed ventricular stimulation should be performed. If MSVT are induced, the implantation of an ICD should be considered. The induction of polymorphic ventricular arrhythmias or VF has not been consistently related with ventricular arrhythmias or sudden cardiac arrest and no recommendation about ICD implantation can be made in this scenario. Despite the absence of solid evidence, the 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of SCD[19] recommends performing an EPS in patients with syncope and previous ST elevation myocardial infarction with a class IC recommendation. It is not clear if this recommendation is applicable to patients with a history of coronary revascularization without infarction or in the absence of late gadolinium enhancement in the CMR, and further studies are needed.
As we previously mentioned, the induction of a monomorphic VT in a patient with previous myocardial infarction presenting with syncope is an indication for an ICD implantation. On the other hand, the induction of VF has been traditionally considered as a non-specific result as these patients appear to have a similar prognosis as patients without any ventricular arrhythmia induction. Brugada et al[103] demonstrated that non-sustained PVT and VF are nonspecific responses to an aggressive stimulation protocol including three to four extra stimuli. Brodsky et al[104], presenting the results of the AVID trial, were not able to demonstrate that the induction of VF or fast VT (rate > 200 bpm) is related with death or ventricular arrhythmia recurrence (P = 0.07), but the induction of slow VTs (HR < 200 bpm) was independently related with recurrences as monomorphic VT.
Mittal et al[53] also evaluated the prognosis of ventricular arrhythmia induction in a cohort of 118 consecutive patients with CAD presenting with syncope. The mean LVEF of their cohort was 42% ± 13%. VF was the only arrhythmia induced in 20 patients (17% of the cohort). There was a survival rate of 89% and 81% at 1 year and 2 years consecutively in the entire cohort, and there were no differences between patients with VF induction or no induced arrhythmia (P = 0.39). By contrast, Link et al[105] found contradictory results in their cohort where they followed 274 consecutive patients with CAD and syncope or presyncope. The risk of arrhythmia occurrence was evaluated at the time of presentation with syncope by an EPS. VF was induced in 23 patients (8%) and ventricular flutter (monomorphic tachycardia with CL < 230 ms) in 24 patients (9%). Overall, 41 patients (15% of the cohort) were inducible for monomorphic ventricular tachycardias. After a follow-up of 37 ± 25 mo, 34 patients had ventricular arrhythmias. VF was induced in the initial EPS in 3 out of 23 patients (13% of this group) and in ventricular flutter in 7 out of 24 patients (30% of this group). Considering these results together, the induction of VF/ventricular flutter was predictive of ventricular arrhythmias during follow-up (P ≤ 0.001 vs non-inducible patients)[105].
Nonetheless, the 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of SCD[19] only have clear recommendations for the induction of sustained monomorphic ventricular tachycardia. Thus, an ICD is recommended in patients with CAD and unexplained syncope with MSVT induced during EPS with a IIa B level of recommendation. The induction of polymorphic VT, VF, or non-sustained ventricular arrhythmias are considered non-specific responses, and considering the absence of solid evidence, no specific recommendations can be made.
Despite the fact that VT should be ruled out in patients with IHD, many other causes may be present in this set of patients[3,9,106,107]. In fact, VT is not the most common cause of syncope. Patients with IHD have some factors that predispose them to other causes. For example, they are often on different hypotensive drugs that predispose to OH or reflex syncope[2]. Also, some conduction disturbances are more frequent in patients with IHD[30,48,50]. In the presence of conduction disturbances on the ECG, advanced AV block is a common cause of syncope[46,50,108]. Importantly, if the EPS is negative, VT is unlikely to be the cause of syncope, with reflex and OH syncope being the most probable etiologies[3,107].
Patients with left ventricular disfunction are at high risk of cardiac and arrhythmic syncope[6]. In observational studies, unexplained syncope in this population has been associated with an increased risk of sudden death[1,9,79,109,110], although the evidence for the benefit of an ICD is limited. In general, the direct implantation of an ICD is indicated in those patients who fulfil the primary prevention criteria (NYHA class II-III heart failure, with LVEF < 35% on optimized pharmacological therapy). The evidence regarding the management of syncope in patients with mid-range LVEF is even more scant. Current ESC syncope clinical practice guidelines[1], which are similar to ACC/AHA/HRS[9] guidelines, suggest a work-up in line with general recommendations and state that the implantation of an ICD should be considered in patients with systolic dysfunction and unexplained syncope. The implantation of a cardiac monitor (ICM) is an alternative that may be considered in patients with recurrent episodes. Newly published ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of SCD[19] suggest a conservative strategy based on risk stratification and ICM implantation in patients with no other direct indication for an ICD.
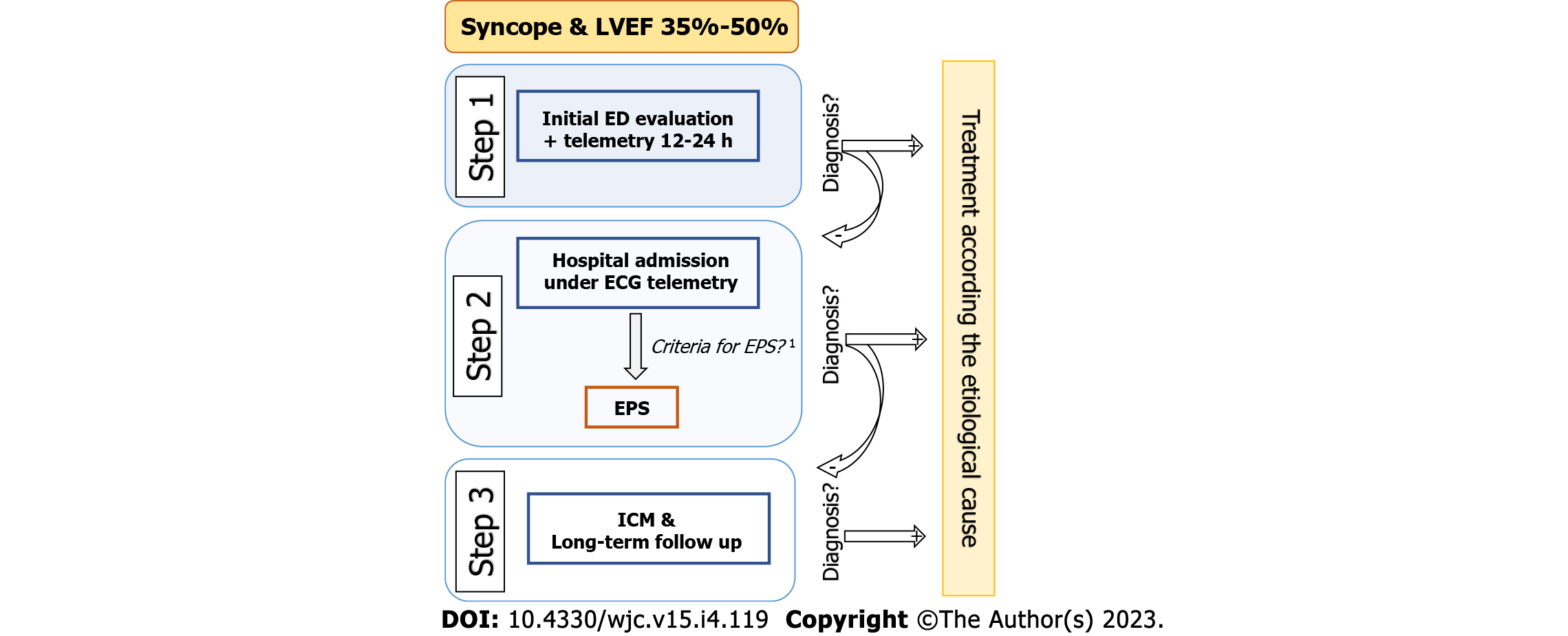
Our group has recently investigated a similar strategy based on a stepwise protocol[79]. In summary, the diagnostic work-up for syncope in this population is based on three steps. Step 1 consists of the initial assessment in the ED. In a systematic manner, a clinical history and physical examination are performed, including testing for OH and CSM (if not contraindicated), general bloodwork, chest x-ray, and 12-lead ECG, as well as 12-24-h telemetry monitoring and a transthoracic echocardiogram. In cases where no certain or highly probable diagnosis is reached, it is considered unexplained syncope, and the patient is admitted to the hospital. Step 2 involves the hospital admission with continuous ECG monitoring and carrying out an invasive EPS if the following criteria are fulfilled: (1) Presence of conduction disorder on baseline ECG [1st degree AV block, Mobitz type 1 s degree AV block, complete RBBB or left bundle branch block (LBBB), BFB, left anterior or posterior fascicular block]; (2) Clinical, electrocardiographic, and/or imaging evidence of myocardial scar (history of myocardial infarction, presence of Q waves on surface ECG, presence of late enhancement on cardiac magnetic resonance imaging, and/or presence of necrosis on myocardial perfusion single-photon emission computed tomography scan); and (3) History of palpitations prior to the syncopal episode. If these criteria are not fulfilled, the EPS is not carried out, and the patient moves on to Step 3. Step 3 involves implanting an implantable cardiac monitor with subsequent clinical monitoring (Figure 3).
In a recent published study that evaluated patients with unexplained syncope (excluding patients diagnosed in step 1), it was found that the application of this systematic protocol had a high diagnostic yield with a low rate of sudden death[79]. The overall diagnostic yield with both steps was 68.3%. Of note, the most common cause was arrhythmia. In 60 patients (57.7% of the total patients and 84.5% of the total diagnoses), a rhythm disorder was identified as the cause of the syncopal episode, with a high proportion of bradycardias, mostly due to AV block (47 patients, 45.2%). VT was the second most frequent cause, although it was significantly less common (9.6%). Most of the arrhythmias, be they AV block or VT, were able to be diagnosed in step 2. Another key finding of the study was that the diagnoses reached allowed treatment to be effectively guided. The sudden or unknown cause mortality rate of 0.9 per 100 person-years was comparable with general mortality rates published in the literature in patients with mid-range left ventricular dysfunction without syncope[106,111-113]. The findings of this study, in line with others on patients with structural heart disease[3,107,114], suggest that a stepwise diagnostic strategy and prolonged monitoring may be a safe and effective management alternative, reducing the number of patients requiring an ICD.
The hallmark of HCM is the abnormal increase of left ventricular wall thickness unrelated to abnormal loading conditions such as high BP, valvular heart diseases, or congenital heart disease. At the histopathological level, HCM is characterized by an increase in the size of myocardial cells and disordered myocardial cell organization with interstitial fibrosis that may predispose patients to suffer from ventricular arrhythmias. HCM carries a mortality rate that ranges from 1%-2%. New data from cohorts of patients with ICDs suggest that the mortality rate might be even lower (about 0.8%) and that it is related with several risk factors summarized in the HCM-SCD score[115]. The eight variables included in the risk score are: Age, LV wall thickness, left atrial size, left ventricular outflow tract (LVOT) gradient, nonsustained ventricular tachycardia, unexplained syncope, and family history of SCD.
HCM patients may have different syncope etiologies such as: Hypovolemia, conduction system disorders, sustained ventricular tachycardia, LVOT obstruction, and abnormal vascular reflexes, etc[116-118]. After ruling out non-cardiogenic and neural-mediated causes, arrhythmic syncope is one of the more worrisome causes for syncope in those patients. Patients with unexplained syncope should be tested with at least 24-hr Holter recording and exercise time-to-exhaustion to rule out LVOT obstruction on exertion. After an extensive evaluation of causes of syncope in those patients without clear diagnosis, an ILR should be implanted[1,117]. Routine tilt table testing in patients with HCM may be associated with an unacceptable number of false positives, and its use should be limited to selected cases[117].
Syncope of unknown origin is included in the risk score with an independent hazard ratio of 2.05 (1.48, 2.82; P < 0.001)[115]. Patients with intermediate-risk and low-risk clinical profiles should be evaluated for additional risk factors not included in the score, following 2022 ESC guidelines[19]. LV systolic dysfunction, apical aneurysm, > 15% of LV mass with late gadolinium enhancement on CMR, and several sarcomeric mutations have demonstrated a higher risk of ventricular arrhythmias in different studies and should be considered when evaluating the risk profile of a given patient[117,118]. The risk of ventricular arrhythmias is nonetheless dynamic and needs to be reassessed at every clinical visit. Those patients with syncope and a high-risk clinical profile (SCD HCM risk score > 6%) or intermediate risk and other risk factors should be considered for ICD implantation (IIa B level of recommendation)[116], and those with intermediate risk (SCD HCM risk score 4%-6%) may be considered for ICD implantation (IIb B level of recommendation)[19].
A hemodynamic origin of syncope should be suspected in patients with valvular heart disease. However, other causes are possible[1,5,9,119-123]. The valvular heart disease with the highest risk of syncope is AoS[5,124,125]. Syncope is more frequent in severe stages of AoS but can occur in patients with moderate severity when suffering from other hemodynamic disturbances. Pharmacologic hypotension and atrial arrhythmias are also a frequent cause of syncope in patients with moderate and severe AoS[5,72,121,126]. In a recent study performed by our group in a cohort of patients with severe AoS and syncope, we observed that in 65% of the patients, the stenosis per se was initially identified as the likely cause of syncope, but later only 17.5% of the total cohort of patients was confirmed as having AoS as their final diagnosis. Conduction system disease and vasovagal etiologies were a more frequent cause of syncope in this population[5]. Importantly, syncope in the setting of a severe AoS has been suggested as having prognostic implications.
In a study published in 2019, these patients had a greater risk of mortality after aortic valve replacement in both the short-term (hazard ratio = 2.27; 95%CI: 1.04-4.95) and the long-term (hazard ratio = 2.11; 95%CI: 1.39-3.21) compared with patients who did not have syncope[127]. Although patients with syncope had somewhat different characteristics on echocardiography (smaller aortic valve area, smaller cardiac chambers, and lower ejection volumes), we believe that this rise in mortality was also partially due to the presence of other causes for the syncope such as underdiagnosed arrhythmias. In the cohort studied by Francisco-Pascual et al[5], those patients in whom it was not possible to precisely determine the cause of the syncope had more than triple short-term and medium-term mortality.
Furthermore, several studies have observed a high incidence of syncope and SCD after transcatheter aortic valve replacement (TAVR)[66,67,72,126,128]. It is theorized that induced conduction system delays after TAVR may predispose patients to suffer from electrical re-entry within the His-Purkinje system favoring a rare type of cardiac arrhythmia called bundle-branch re-entry in which the electrical impulse circulates between both branches of the conduction system with a slight delay often happening in the left bundle in the retrograde arm of the tachycardia. This arrhythmia is very rapid and frequently compromises the patient hemodynamically producing syncope or sudden cardiac arrest. The real incidence of this problem is unknown, but it needs to be kept in mind when evaluating a patient after a TAVR with some degree of conduction system delay.
Another significant but infrequent cause of syncope in patients with valvular heart disease is the presence of VF in patients with mitral valve prolapse, which has been named “the malignant mitral valve prolapse syndrome”. In a recent meta-analysis carried out by Nalliah et al[129], they reported the population prevalence of mitral valve prolapse (MVP) of 1.2% and the prevalence of MVP in SCD autopsies of 11.7%. Nonetheless an incidence of 0.14 SCD events per 100 patient-years in the community MVP cohort, deserves an in-depth investigation of other risk factors for ventricular arrhythmias such as the presence of myocardial fibrosis or frequent complex ventricular ectopy, as has been proposed.
In patients with conduction disturbances and syncope, the presence of bradyarrhythmia is always a concern although other causes may also be present. For example, in a recent cohort of 503 patients with unexplained syncope and BBB, arrhythmic syncope was identified in 57.9% patients, mostly secondary to AV block (51.3%). However, 12% were due to reflex syncope or an OH mechanism, 1.4% were due to ventricular tachycardia, and 10% were secondary to other causes[108].
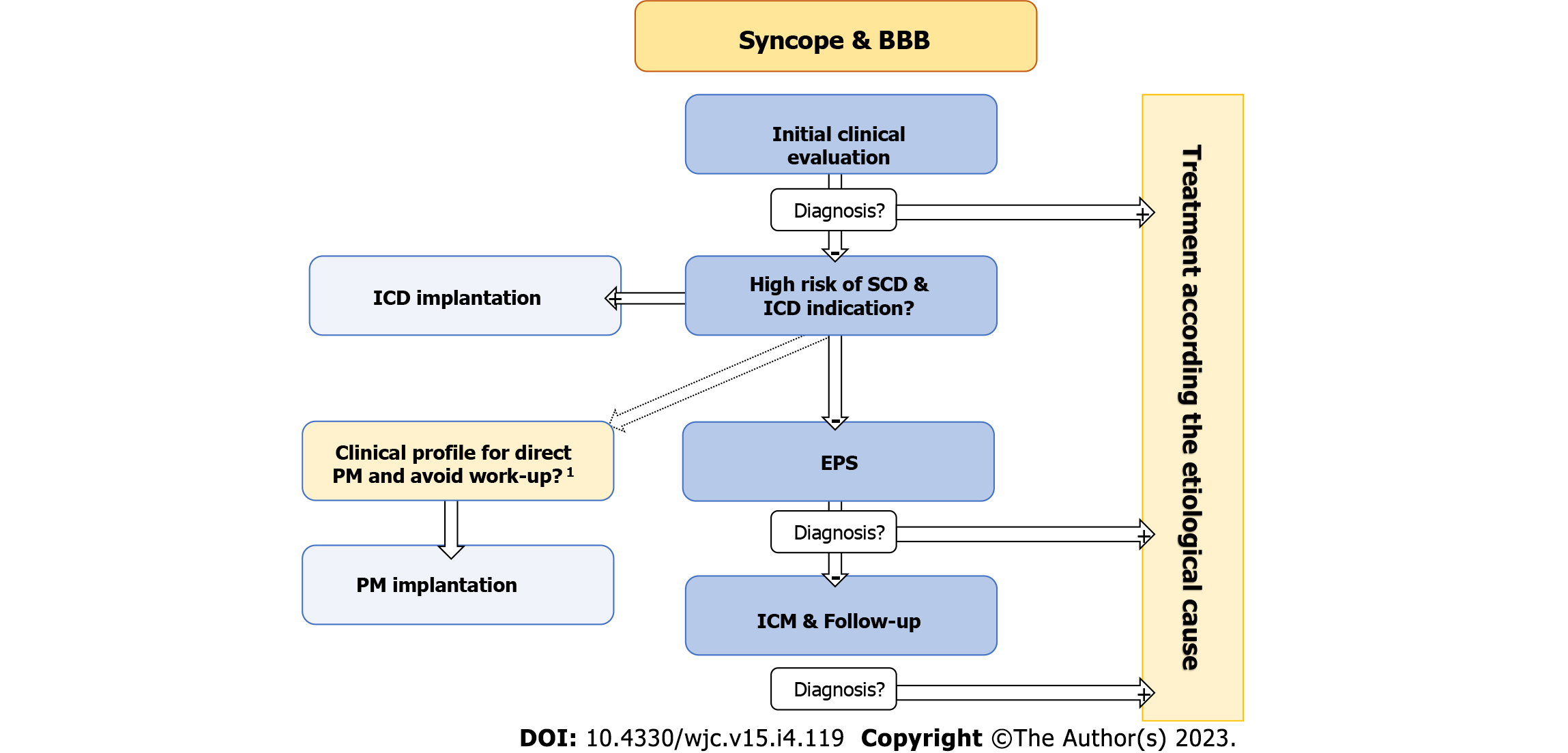
The optimal management of patients with unexplained syncope and BBB is still controversial[1,9,46-48,130,131]. In fact, the 2017 ACC/AHA Guidelines[9] suggest empirical direct pacemaker implantation after exclusion of other syncope etiologies, while ESC guidelines[1] recommend opting for a stepwise approach. The systematic stepwise approach (that includes an EPS and long-term follow-up with an ICM) was initially evaluated in the B4 study[47]. This study found that the diagnostic approach is safe and achieves a high rate of etiological diagnosis allowing for the selection of specific treatment and avoiding the implantation of unnecessary pacemakers. The results of the B4 study have been confirmed by several subsequent studies, some of them with a relatively high number of patients and long-term follow-up[80,108,132-134].
On the other hand, the strategy of direct pacemaker implantation was recently evaluated in the SPRITELY trial. This study randomized 105 patients older than 50 years with BFB (41 LBBB and 74 RBBB plus left fascicular block) and at least one syncope in the previous year to receive ICM or empirical pacemaker implantation. In the 33-mo follow-up period, the 57 patients randomized to the pacemaker arm showed a lower primary composite endpoint (cardiovascular death, syncope, bradycardia resulting in an intervention, and device complications) than the ILR arm; [20 (35%) vs 44 (76%); P < 0.0001]. However, the presence of syncope during follow-up was similar in both groups (29% vs 26%; P = 0.95)[135].
It must be highlighted that in the SPRITELY trial, EPS was not systematically carried out before ICM implantation, and therefore it cannot be considered as a direct comparison with the stepwise approach. Similar findings were previously found in the PRESS study[136], where patients were randomized to pacemaker in pacing mode (DDD at 60 bpm) or backup pacing mode (drug-drug interaction at 30 bpm). The primary endpoint of this study was a composite endpoint of syncope, presyncope with device intervention, or documented bradycardia and AVB, and patients allocated to active pacing had a significant reduction of this composite endpoint. However, when only syncope recurrences were analyzed separately, there were no differences between the two groups. Furthermore, there are some studies that have analyzed the recurrence rate in patients with syncope and BBB, in whom a pacemaker has been implanted, showing that syncope recurrence is higher in those patients in whom a pacemaker was implanted empirically than in those in whom a pacemaker was implanted after a positive EPS or a documented AVB[137,138].
With the available evidence, the authors of this review continue to support the stepwise approach to manage these patients. Nevertheless, direct pacemaker implantation should be considered in some patients, especially in elderly or frail patients after an individual risk/benefit assessment (Figure 4). According to the newest 2021 ESC guidelines for cardiac pacing and resynchronization[18], in patients with sinus bradycardia and syncope of unclear origin after a thorough work-up, an exercise test to evaluate chronotropic competence and an EPS to evaluate for sinus node overdrive suppression pathologic responses might be indicated. cSNRT (basal cycle length; normal value 525 ms) has demonstrated good predictive value in patients with sinus bradycardia despite the presence of symptoms (overall accuracy of cSNRT in predicting serious sinus node disease regardless of the presence of symptoms: 90%, 100% in the presence of symptoms; sensitivity of the test: 66%). Patients presenting with a ventricular rate below 40 bpm have a 70% probability of having an abnormal cSNRT. In patients with a basal HR of 50 to 55 bpm, the probability of finding an abnormal response in cSNRT test is 24%[139]. However, it should be noted that pacing patients with sinus node dysfunction has not demonstrated improved survival so far[18,131].
EPS diagnostic yield is higher in patients with sinus bradycardia or BFB and structural heart disease and is lower in patients with a normal ECG and no structural heart disease[46,50]. Thus, it is preferable to perform EPS in patients with higher pretest probability and implant a loop recorder in those with lower pretest probability. Patients with first degree AV block and second degree type I (Wenckebach) block presenting with syncope without a firm diagnosis after extensive study should be offered an EPS. The presence of second degree type II block or third degree AVB constitutes a clear indication for cardiac pacing. Patients with 2:1 AV block can be evaluated by increasing the sinus node rate (atropine 1 mg or exercise test). If the degree of block increases by increasing of the sinus rate, an infra-Hisian origin must be suspected, and pacemaker implantation should be considered. Patients with syncope and BFB represent a group whose risk of syncope is especially difficult to stratify. Therefore, in patients with BFB and syncope of unknown origin an EPS should be performed.
In the presence of an HV interval longer than 70 ms (basal) or > 100 ms after infusion of 2 mg/kg of flecainide (or other Vaughan Williams class I antiarrhythmic drugs), cardiac pacing should be considered[140]. The absence of high-risk characteristics in the EPS of patients with syncope and BBB or BFB does not preclude the development of paroxysmal AV block, and an ILR needs to be considered. Roca-Luque et al[50] demonstrated that the most predictive combination of conduction disorders were LBBB or RBBB + long PR interval + left fascicular block [odds ratio = 4.5 (1.06-20.01); P < 0.042], LBBB + prolonged PR interval [5.2 (1.52-17.74); P < 0.001], and RBBB + prolonged PR interval [3.8 (1.7-8.7); P < 0.001] in their 271 patient cohort in 2018.
Cardiac channelopathies are a group of diseases in which a mutation of different regulatory proteins of the action potential may predispose a patient to suffer from ventricular arrhythmias and SCD. Syncopal episodes in these patients might be due to non-sustained polymorphic VT or VF. In this section, we discussed the implications of the presence of syncope in patients with BrS, LQTS, and catecholaminergic PVT.
BrS was first described by the Brugada et al[141] in their elegant paper published in JACC in 1992. In their first publication of this syndrome, they described a cohort of 8 patients with RBBB and ST elevation in leads V1-V2-3 that suffered from aborted episodes of SCD[142].
Even though the mechanism of the electrical dysfunction leading to VF is not completely understood, it is believed that an increase in early repolarizing currents (Ito current) or a reduction in depolarizing currents (INaT) may lead to a phase II dispersion of repolarization and early post-depolarizations, which might generate phase II re-entries, possibly triggering VF. This electrical disorder seems to be more accentuated in the anterior part of the right ventricular outflow tract obstruction, where Ito current has been shown to be higher than in other heart sites. This latter observation might explain the isolated ST elevation in precordial leads and the effectiveness of ablation on the right ventricular outflow tract in patients with BrS and arrhythmic storm[143].
Patients with BrS pattern on ECG and syncope have a four-fold risk of sudden cardiac arrest, representing a 1.5% annual risk of sudden cardiac arrest. When the syncope cannot be classified as neuro-mediated or a cardiac origin is a possibility, ICD implantation should be considered[19]. Therefore, it is usually not necessary to perform an EPS to stratify the risk in the presence of unexplained syncope, as it is assumed to be high risk.
However, patients with sodium channel dysfunction may exhibit conduction system dysfunction as well. It is not infrequent for patients with those specific mutations to exhibit sinus bradycardia and/or BBB. Furthermore, reflex syncope is also frequent in young patients with BrS[144]. For these reasons, some authors have also suggested a more conservative approach, where implantation of a loop recorder can be considered in BrS patients with an unexplained syncope (not clearly cardiac) and without other indications for an ICD[19,145].
The hallmark of the LQTS is an inadequately prolonged corrected QT interval, measured from the beginning of the QRS complex to the point at which the descending limb of the T wave crosses the isoelectric baseline of the ECG. The measure is frequently performed in leads II or V5-6 where the T wave and the isoelectric baseline are often well demarcated. The diagnosis of LQTS is made in the presence of a cQT interval of ≥ 480 ms or a Schwartz score (including several clinical and electrocardiographic parameters) of > 3. In the presence of a cardiogenic syncope, the presence of a cQT ≥ 460 ms is sufficient to reach the diagnosis.
The mechanism of arrhythmogenicity in patients with LQTS seems to be related with dispersion of the repolarization. The prolongation of the repolarization is not homogeneous among the different layers of myocardium. Therefore, early post depolarization occurring over the T wave may generate functional re-entry patterns of conduction ultimately generating fibrillatory conduction.
Up to 17 different mutations leading to LQTS have been described. The majority of them are produced by three specific mutations. LQTS1 is produced by mutation in the α subunit of the delayed rectifier potassium channel with slow opening kinetics. This mutation comprises 40%-55% of cases. LQTS1 patients are prone to suffering from ventricular arrhythmias during sports or physical activity (especially during swimming). LQTS2 is caused by a mutation in the α subunit of the delayed rectifier potassium channel with rapid opening kinetics. This mutation is present in up to 30%-45% of cases, and ventricular arrhythmias are frequent during loud noises and in the postpartum period in females. The activating mutation in the α subunit of the sodium channel (INaT) keeps the channel opened beyond phase 0, increasing late sodium currents (INaL), thus prolonging repolarization and therefore the QT interval. This mutation is present in 5%-10% of patients and is related to fatal events during rest or sleep[146].
It has been observed that LQTS patients respond favorably to beta-blockers; thus every patient with a diagnosis of LQTS should be treated with beta-blockers. Apparently, non-specific beta-blockers, such as propranolol or nadolol, have shown better results with a lower incidence of ventricular arrhythmias. If patients suffer from syncope despite the use of beta-blockers, an ICD must be implanted for the prevention of SCD[147].
Catecholaminergic PVT (CPVT) is an inherited channelopathy in which several mutations may affect the intracellular handling of calcium release-uptake. The overload of cytoplasmatic calcium leads to cell membrane voltage instability leading to delayed depolarizations that lead to the characteristic arrhythmia of this disorder, bidirectional ventricular tachycardia (also seen in digitalis toxicity), or VF.
The mutation in the ryanodine receptor gene, inherited in an autosomal dominant manner, is the cause of 50%-55% of cases. A new mutation in the calsequestrin gene has been described and has an autosomal recessive inheritance pattern. The ryanodine receptor gene mutation generates an aberrant ryanodine channel that permeabilizes the channel to calcium release. The calsequestrin proteins work close to the ryanodine channel, regulating its function.
Patients with CPVT are prone to ventricular arrhythmias related to exercise. Ventricular arrhythmias usually occur with HR over 130 bpm. With increasing levels of exercise, patients may exhibit monomorphic ectopy, polymorphic ectopy, non-sustained VT, bidirectional VT, and finally, if the exercise continues, VF. CPVT is a highly arrhythmogenic condition with a cardiac event rate of up to 80% at 40 years. Therefore, a low threshold for ICD implantation is advised. The use of non-selective beta-blockers has been shown to reduce the incidence of ventricular arrhythmias from 25% to 11% at 8 years[148].
Probably due to small cohorts, no single risk factor has demonstrated sufficient prognostic value to be used routinely. The 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of SCD recommend implantation in patients with CPVT who have survived a cardiac arrest (class I C) and should be considered in patients with CPVT and either arrhythmic syncope or presence of polymorphic VT or bidirectional VT on maximal tolerated doses of beta-blockers (class IIa C)[19].
Syncope is a symptom that involves a heterogeneous group of pathologies ranging from trivial causes to diseases with a high risk of sudden death. The highest mortality and SCD risk occur when syncope is associated with underlying cardiac disease, in particular when the main cause of syncope is not well established and treated properly. Arrhythmia is the most common cause of cardiac syncope. Appropriate risk stratification and work-up to determine the main cause of the event is warranted to improve the prognosis of patients. This review provided an update on the important and novel data about arrhythmic syncope, the value of the different diagnostic tests, and the specific characteristics in some particular populations such as patients with cardiomyopathies or channelopathies. This review emphasized the importance of an appropriate stepwise approach work-up and interventions.
The authors would like to thank Mr. S Venegas for his help with the illustrations and language editing.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: European Society of Cardiology.
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Moussa BS, Egypt; Soe KK, United States S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Zhao S
| 1. | Brignole M, Moya A, de Lange FJ, Deharo JC, Elliott PM, Fanciulli A, Fedorowski A, Furlan R, Kenny RA, Martín A, Probst V, Reed MJ, Rice CP, Sutton R, Ungar A, van Dijk JG; ESC Scientific Document Group. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;39:1883-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1294] [Cited by in RCA: 1137] [Article Influence: 162.4] [Reference Citation Analysis (0)] |
| 2. | de Ruiter SC, Wold JFH, Germans T, Ruiter JH, Jansen RWMM. Multiple causes of syncope in the elderly: diagnostic outcomes of a Dutch multidisciplinary syncope pathway. Europace. 2018;20:867-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Shenthar J, Prabhu MA, Banavalikar B, Benditt DG, Padmanabhan D. Etiology and Outcomes of Syncope in Patients With Structural Heart Disease and Negative Electrophysiology Study. JACC Clin Electrophysiol. 2019;5:608-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Solano A, Menozzi C, Maggi R, Donateo P, Bottoni N, Lolli G, Tomasi C, Croci F, Oddone D, Puggioni E, Brignole M. Incidence, diagnostic yield and safety of the implantable loop-recorder to detect the mechanism of syncope in patients with and without structural heart disease. Eur Heart J. 2004;25:1116-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Francisco-Pascual J, Rodenas E, Belahnech Y, Rivas-Gándara N, Pérez-Rodon J, Santos-Ortega A, Benito B, Roca-Luque I, Cossio-Gil Y, Serra Garcia V, Llerena-Butron S, Rodríguez-García J, Moya-Mitjans A, García-Dorado D, Ferreira-González I. Syncope in Patients With Severe Aortic Stenosis: More Than Just an Obstruction Issue. Can J Cardiol. 2021;37:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 6. | Kapoor WN. Syncope. N Engl J Med. 2000;343:1856-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 221] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Malasana G, Brignole M, Daccarett M, Sherwood R, Hamdan MH. The prevalence and cost of the faint and fall problem in the state of Utah. Pacing Clin Electrophysiol. 2011;34:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS), Moya A, Sutton R, Ammirati F, Blanc JJ, Brignole M, Dahm JB, Deharo JC, Gajek J, Gjesdal K, Krahn A, Massin M, Pepi M, Pezawas T, Ruiz Granell R, Sarasin F, Ungar A, van Dijk JG, Walma EP, Wieling W. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30:2631-2671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1194] [Cited by in RCA: 1226] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 9. | Shen WK, Sheldon RS, Benditt DG, Cohen MI, Forman DE, Goldberger ZD, Grubb BP, Hamdan MH, Krahn AD, Link MS, Olshansky B, Raj SR, Sandhu RK, Sorajja D, Sun BC, Yancy CW. 2017 ACC/AHA/HRS Guideline for the Evaluation and Management of Patients With Syncope: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2017;70:e39-e110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 213] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 10. | Soteriades ES, Evans JC, Larson MG, Chen MH, Chen L, Benjamin EJ, Levy D. Incidence and prognosis of syncope. N Engl J Med. 2002;347:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 910] [Cited by in RCA: 787] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 11. | Parry SW, Tan MP. An approach to the evaluation and management of syncope in adults. BMJ. 2010;340:c880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Colman N, Nahm K, Ganzeboom KS, Shen WK, Reitsma J, Linzer M, Wieling W, Kaufmann H. Epidemiology of reflex syncope. Clin Auton Res. 2004;14 Suppl 1:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 163] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 13. | Kapoor WN, Karpf M, Wieand S, Peterson JR, Levey GS. A prospective evaluation and follow-up of patients with syncope. N Engl J Med. 1983;309:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 504] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 14. | Eagle KA, Black HR, Cook EF, Goldman L. Evaluation of prognostic classifications for patients with syncope. Am J Med. 1985;79:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Khoo C, Chakrabarti S, Arbour L, Krahn AD. Recognizing life-threatening causes of syncope. Cardiol Clin. 2013;31:51-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Ungar A, Del Rosso A, Giada F, Bartoletti A, Furlan R, Quartieri F, Lagi A, Morrione A, Mussi C, Lunati M, De Marchi G, De Santo T, Marchionni N, Brignole M; Evaluation of Guidelines in Syncope Study 2 Group. Early and late outcome of treated patients referred for syncope to emergency department: the EGSYS 2 follow-up study. Eur Heart J. 2010;31:2021-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR, Goldschlager NF, Hamilton RM, Joglar JA, Kim RJ, Lee R, Marine JE, McLeod CJ, Oken KR, Patton KK, Pellegrini CN, Selzman KA, Thompson A, Varosy PD. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:e51-e156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 245] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 18. | Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, Barrabés JA, Boriani G, Braunschweig F, Brignole M, Burri H, Coats AJS, Deharo JC, Delgado V, Diller GP, Israel CW, Keren A, Knops RE, Kotecha D, Leclercq C, Merkely B, Starck C, Thylén I, Tolosana JM, Leyva F, Linde C, Abdelhamid M, Aboyans V, Arbelo E, Asteggiano R, Barón-Esquivias G, Bauersachs J, Biffi M, Birgersdotter-Green U, Bongiorni MG, Borger MA, Čelutkienė J, Cikes M, Daubert JC, Drossart I, Ellenbogen K, Elliott PM, Fabritz L, Falk V, Fauchier L, Fernández-Avilés F, Foldager D, Gadler F, De Vinuesa PGG, Gorenek B, Guerra JM, Hermann Haugaa K, Hendriks J, Kahan T, Katus HA, Konradi A, Koskinas KC, Law H, Lewis BS, Linker NJ, Løchen ML, Lumens J, Mascherbauer J, Mullens W, Nagy KV, Prescott E, Raatikainen P, Rakisheva A, Reichlin T, Ricci RP, Shlyakhto E, Sitges M, Sousa-Uva M, Sutton R, Suwalski P, Svendsen JH, Touyz RM, Van Gelder IC, Vernooy K, Waltenberger J, Whinnett Z, Witte KK. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Europace. 2022;24:71-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 192] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 19. | Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, Charron P, Corrado D, Dagres N, de Chillou C, Eckardt L, Friede T, Haugaa KH, Hocini M, Lambiase PD, Marijon E, Merino JL, Peichl P, Priori SG, Reichlin T, Schulz-Menger J, Sticherling C, Tzeis S, Verstrael A, Volterrani M; ESC Scientific Document Group. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022;43:3997-4126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 1414] [Article Influence: 471.3] [Reference Citation Analysis (0)] |
| 20. | Calkins H, Shyr Y, Frumin H, Schork A, Morady F. The value of the clinical history in the differentiation of syncope due to ventricular tachycardia, atrioventricular block, and neurocardiogenic syncope. Am J Med. 1995;98:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 128] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Blanc JJ, L'Her C, Touiza A, Garo B, L'Her E, Mansourati J. Prospective evaluation and outcome of patients admitted for syncope over a 1 year period. Eur Heart J. 2002;23:815-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 190] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 22. | Costantino G, Perego F, Dipaola F, Borella M, Galli A, Cantoni G, Dell'Orto S, Dassi S, Filardo N, Duca PG, Montano N, Furlan R; STePS Investigators. Short- and long-term prognosis of syncope, risk factors, and role of hospital admission: results from the STePS (Short-Term Prognosis of Syncope) study. J Am Coll Cardiol. 2008;51:276-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 23. | Olde Nordkamp LR, van Dijk N, Ganzeboom KS, Reitsma JB, Luitse JS, Dekker LR, Shen WK, Wieling W. Syncope prevalence in the ED compared to general practice and population: a strong selection process. Am J Emerg Med. 2009;27:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Sheldon R. How to Differentiate Syncope from Seizure. Cardiol Clin. 2015;33:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Sheldon R, Rose S, Ritchie D, Connolly SJ, Koshman ML, Lee MA, Frenneaux M, Fisher M, Murphy W. Historical criteria that distinguish syncope from seizures. J Am Coll Cardiol. 2002;40:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 201] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 26. | Alciati A, Shiffer D, Dipaola F, Barbic F, Furlan R. Psychogenic Pseudosyncope: Clinical Features, Diagnosis and Management. J Atr Fibrillation. 2020;13:2399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Coleman DK, Long B, Koyfman A. Clinical Mimics: An Emergency Medicine-Focused Review of Syncope Mimics. J Emerg Med. 2018;54:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Runser LA, Gauer RL, Houser A. Syncope: Evaluation and Differential Diagnosis. Am Fam Physician. 2017;95:303-312. [PubMed] |
| 29. | Del Rosso A, Ungar A, Maggi R, Giada F, Petix NR, De Santo T, Menozzi C, Brignole M. Clinical predictors of cardiac syncope at initial evaluation in patients referred urgently to a general hospital: the EGSYS score. Heart. 2008;94:1620-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 210] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 30. | Ahmed N, Frontera A, Carpenter A, Cataldo S, Connolly GM, Fasiolo M, Cripps T, Thomas G, Diab I, Duncan ER. Clinical Predictors of Pacemaker Implantation in Patients with Syncope Receiving Implantable Loop Recorder with or without ECG Conduction Abnormalities. Pacing Clin Electrophysiol. 2015;38:934-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Tobías-Castillo PE, Jordán-Marchité P, Martínez-Martínez M, Francisco-Pascual J. Patrón electrocardiográfico catastrófico durante neumonía por COVID-19. REC: CardioClinics. 2022;57:139-140. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 32. | Francisco-Pascual J. ECG, December 2016. Rev Esp Cardiol. 2016;69:1217. [DOI] [Full Text] |
| 33. | Yokokawa M, Okamura H, Noda T, Satomi K, Suyama K, Kurita T, Aihara N, Kamakura S, Shimizu W. Neurally mediated syncope as a cause of syncope in patients with Brugada electrocardiogram. J Cardiovasc Electrophysiol. 2010;21:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Thiruganasambandamoorthy V, Kwong K, Wells GA, Sivilotti MLA, Mukarram M, Rowe BH, Lang E, Perry JJ, Sheldon R, Stiell IG, Taljaard M. Development of the Canadian Syncope Risk Score to predict serious adverse events after emergency department assessment of syncope. CMAJ. 2016;188:E289-E298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 35. | Krediet CT, Parry SW, Jardine DL, Benditt DG, Brignole M, Wieling W. The history of diagnosing carotid sinus hypersensitivity: why are the current criteria too sensitive? Europace. 2011;13:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Claesson JE, Kristensson BE, Edvardsson N, Währborg P. Less syncope and milder symptoms in patients treated with pacing for induced cardioinhibitory carotid sinus syndrome: a randomized study. Europace. 2007;9:932-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Maggi R, Menozzi C, Brignole M, Podoleanu C, Iori M, Sutton R, Moya A, Giada F, Orazi S, Grovale N. Cardioinhibitory carotid sinus hypersensitivity predicts an asystolic mechanism of spontaneous neurally mediated syncope. Europace. 2007;9:563-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Brignole M, Moya A, de Lange FJ, Deharo JC, Elliott PM, Fanciulli A, Fedorowski A, Furlan R, Kenny RA, Martín A, Probst V, Reed MJ, Rice CP, Sutton R, Ungar A, van Dijk JG; ESC Scientific Document Group. Practical Instructions for the 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;39:e43-e80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 39. | Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz I, Schondorff R, Stewart JM, van Dijk JG. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 946] [Cited by in RCA: 1122] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 40. | Ungar A, Mussi C, Del Rosso A, Noro G, Abete P, Ghirelli L, Cellai T, Landi A, Salvioli G, Rengo F, Marchionni N, Masotti G; Italian Group for the Study of Syncope in the Elderly. Diagnosis and characteristics of syncope in older patients referred to geriatric departments. J Am Geriatr Soc. 2006;54:1531-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | Bartoletti A, Alboni P, Ammirati F, Brignole M, Del Rosso A, Foglia Manzillo G, Menozzi C, Raviele A, Sutton R. 'The Italian Protocol': a simplified head-up tilt testing potentiated with oral nitroglycerin to assess patients with unexplained syncope. Europace. 2000;2:339-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 203] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 42. | Brignole M, Sutton R, Menozzi C, Garcia-Civera R, Moya A, Wieling W, Andresen D, Benditt DG, Grovale N, De Santo T, Vardas P; International Study on Syncope of Uncertain Etiology 2 (ISSUE 2) Group. Lack of correlation between the responses to tilt testing and adenosine triphosphate test and the mechanism of spontaneous neurally mediated syncope. Eur Heart J. 2006;27:2232-2239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Piotrowski R, Baran J, Sikorska A, Krynski T, Kulakowski P. Cardioneuroablation for Reflex Syncope: Efficacy and Effects on Autonomic Cardiac Regulation-A Prospective Randomized Trial. JACC Clin Electrophysiol. 2023;9:85-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 82] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 44. | Aksu T, Gupta D, D'Avila A, Morillo CA. Cardioneuroablation for vasovagal syncope and atrioventricular block: A step-by-step guide. J Cardiovasc Electrophysiol. 2022;33:2205-2212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Dhingra RC. Sinus node dysfunction. Pacing Clin Electrophysiol. 1983;6:1062-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 46. | Moya A, Rivas-Gandara N, Perez-Rodón J, Franciso-Pascual J, Santos-Ortega A, Fumero P, Roca-Luque I. Syncope and bundle branch block : Diagnostic approach. Herzschrittmacherther Elektrophysiol. 2018;29:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Moya A, García-Civera R, Croci F, Menozzi C, Brugada J, Ammirati F, Del Rosso A, Bellver-Navarro A, Garcia-Sacristán J, Bortnik M, Mont L, Ruiz-Granell R, Navarro X; Bradycardia detection in Bundle Branch Block (B4) study. Diagnosis, management, and outcomes of patients with syncope and bundle branch block. Eur Heart J. 2011;32:1535-1541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 48. | Roca-Luque I, Francisco-Pascual J, Oristrell G, Rodríguez-García J, Santos-Ortega A, Martin-Sanchez G, Rivas-Gandara N, Perez-Rodon J, Ferreira-Gonzalez I, García-Dorado D, Moya-Mitjans A. Syncope, conduction disturbance, and negative electrophysiological test: Predictive factors and risk score to predict pacemaker implantation during follow-up. Heart Rhythm. 2019;16:905-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 49. | Bergfeldt L, Edvardsson N, Rosenqvist M, Vallin H, Edhag O. Atrioventricular block progression in patients with bifascicular block assessed by repeated electrocardiography and a bradycardia-detecting pacemaker. Am J Cardiol. 1994;74:1129-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Roca-Luque I, Oristrell G, Francisco-Pasqual J, Rodríguez-García J, Santos-Ortega A, Martin-Sanchez G, Rivas-Gandara N, Perez-Rodon J, Ferreira-Gonzalez I, García-Dorado D, Moya-Mitjans A. Predictors of positive electrophysiological study in patients with syncope and bundle branch block: PR interval and type of conduction disturbance. Clin Cardiol. 2018;41:1537-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Olshansky B, Hahn EA, Hartz VL, Prater SP, Mason JW. Clinical significance of syncope in the electrophysiologic study versus electrocardiographic monitoring (ESVEM) trial. The ESVEM Investigators. Am Heart J. 1999;137:878-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Wellens HJ, Brugada P, Stevenson WG. Programmed electrical stimulation of the heart in patients with life-threatening ventricular arrhythmias: what is the significance of induced arrhythmias and what is the correct stimulation protocol? Circulation. 1985;72:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 199] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 53. | Mittal S, Hao SC, Iwai S, Stein KM, Markowitz SM, Slotwiner DJ, Lerman BB. Significance of inducible ventricular fibrillation in patients with coronary artery disease and unexplained syncope. J Am Coll Cardiol. 2001;38:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Rivas-Gándara N, Francisco-Pascual J, Pijuan-Domenech A, Ribera-Solé A, Dos-Subirá L, Benito B, Terricabras M, Pérez-Rodon J, Subirana MT, Santos-Ortega A, Roses-Noguer F, Miranda B, Moya-Mitjans À, Ferreira-González I. Risk stratification of ventricular arrhythmias in repaired tetralogy of Fallot. Rev Esp Cardiol (Engl Ed). 2021;74:935-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 55. | Rivas-Gándara N, Dos-Subirá L, Francisco-Pascual J, Rodríguez-García J, Pijuan-Domenech A, Benito B, Valente F, Pascual-González G, Santos-Ortega A, Miranda B, Pérez-Rodon J, Ribera-Solé A, Burcet-Rodriguez G, Roses-Noguer F, Gordon B, Rodríguez-Palomares J, Ferreira-González I. Substrate characterization of the right ventricle in repaired tetralogy of Fallot using late enhancement cardiac magnetic resonance. Heart Rhythm. 2021;18:1868-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Hernández-Madrid A, Paul T, Abrams D, Aziz PF, Blom NA, Chen J, Chessa M, Combes N, Dagres N, Diller G, Ernst S, Giamberti A, Hebe J, Janousek J, Kriebel T, Moltedo J, Moreno J, Peinado R, Pison L, Rosenthal E, Skinner JR, Zeppenfeld K; ESC Scientific Document Group. Arrhythmias in congenital heart disease: a position paper of the European Heart Rhythm Association (EHRA), Association for European Paediatric and Congenital Cardiology (AEPC), and the European Society of Cardiology (ESC) Working Group on Grown-up Congenital heart disease, endorsed by HRS, PACES, APHRS, and SOLAECE. Europace. 2018;20:1719-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 57. | Sroubek J, Probst V, Mazzanti A, Delise P, Hevia JC, Ohkubo K, Zorzi A, Champagne J, Kostopoulou A, Yin X, Napolitano C, Milan DJ, Wilde A, Sacher F, Borggrefe M, Ellinor PT, Theodorakis G, Nault I, Corrado D, Watanabe I, Antzelevitch C, Allocca G, Priori SG, Lubitz SA. Programmed Ventricular Stimulation for Risk Stratification in the Brugada Syndrome: A Pooled Analysis. Circulation. 2016;133:622-630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 187] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 58. | Khairy P, Landzberg MJ, Gatzoulis MA, Lucron H, Lambert J, Marçon F, Alexander ME, Walsh EP. Value of programmed ventricular stimulation after tetralogy of fallot repair: a multicenter study. Circulation. 2004;109:1994-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 268] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 59. | Francisco-Pascual J, Rivas-Gándara N, Santos-Ortega A, Pérez-Rodón J, Benito B, Belahnech Y, Ferreira-González I. Cardiac biometric variables and arrhythmic events during COVID-19 pandemic lockdown in patients with an implantable cardiac monitor for syncope work-up. Med Clin. 2021;156:496-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 60. | PérezRodon J, FranciscoPascual J, RivasGándara N, RocaLuque I, Bellera N, MoyaMitjans À. Cryptogenic Stroke And Role Of Loop Recorder. J Atr Fibrillation. 2014;7:1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 61. | Francisco-Pascual J, Santos-Ortega A, Roca-Luque I, Rivas-Gándara N, Pérez-Rodón J, Milà-Pascual L, García-Dorado D, Moya-Mitjans À. Diagnostic Yield and Economic Assessment of a Diagnostic Protocol With Systematic Use of an External Loop Recorder for Patients With Palpitations. Rev Esp Cardiol (Engl Ed). 2019;72:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 62. | Francisco-Pascual J, Olivella San Emeterio A, Rivas-Gándara N, Pérez-Rodón J, Benito B, Santos-Ortega A, Moya-Mitjans À, Rodríguez García J, Llerena Butrón SI, Cantalapiedra Romero J, Ferreira González I. High incidence of subclinical atrial fibrillation in patients with syncope monitored with implantable cardiac monitor. Int J Cardiol. 2020;316:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | Pagola J, Juega J, Francisco-Pascual J, Bustamante A, Penalba A, Pala E, Rodriguez M, De Lera Alfonso M, Arenillas JF, Cabezas JA, Moniche F, de Torres R, Montaner J, González-Alujas T, Alvarez-Sabin J, Molina CA; Crypto-AF study group. Large vessel occlusion is independently associated with atrial fibrillation detection. Eur J Neurol. 2020;27:1618-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 64. | Palà E, Pagola J, Juega J, Francisco-Pascual J, Bustamante A, Penalba A, Comas I, Rodriguez M, De Lera Alfonso M, Arenillas JF, de Torres R, Pérez-Sánchez S, Cabezas JA, Moniche F, González-Alujas T, Molina CA, Montaner J. B-type natriuretic peptide over N-terminal pro-brain natriuretic peptide to predict incident atrial fibrillation after cryptogenic stroke. Eur J Neurol. 2021;28:540-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 65. | Pagola J, Juega J, Francisco-Pascual J, Moya A, Sanchis M, Bustamante A, Penalba A, Usero M, Cortijo E, Arenillas JF, Calleja AI, Sandin-Fuentes M, Rubio J, Mancha F, Escudero-Martinez I, Moniche F, de Torres R, Pérez-Sánchez S, González-Matos CE, Vega Á, Pedrote AA, Arana-Rueda E, Montaner J, Molina CA; CryptoAF investigators. Yield of atrial fibrillation detection with Textile Wearable Holter from the acute phase of stroke: Pilot study of Crypto-AF registry. Int J Cardiol. 2018;251:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 66. | Rodés-Cabau J, Urena M, Nombela-Franco L, Amat-Santos I, Kleiman N, Munoz-Garcia A, Atienza F, Serra V, Deyell MW, Veiga-Fernandez G, Masson JB, Canadas-Godoy V, Himbert D, Castrodeza J, Elizaga J, Francisco Pascual J, Webb JG, de la Torre JM, Asmarats L, Pelletier-Beaumont E, Philippon F. Arrhythmic Burden as Determined by Ambulatory Continuous Cardiac Monitoring in Patients With New-Onset Persistent Left Bundle Branch Block Following Transcatheter Aortic Valve Replacement: The MARE Study. JACC Cardiovasc Interv. 2018;11:1495-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 67. | Muntané-Carol G, Urena M, Nombela-Franco L, Amat-Santos I, Kleiman N, Munoz-Garcia A, Atienza F, Serra V, Deyell MW, Veiga-Fernandez G, Masson JB, Canadas-Godoy V, Himbert D, Castrodeza J, Elizaga J, Francisco Pascual J, Webb JG, de la Torre Hernandez JM, Asmarats L, Pelletier-Beaumont E, Philippon F, Rodés-Cabau J. Arrhythmic burden in patients with new-onset persistent left bundle branch block after transcatheter aortic valve replacement: 2-year results of the MARE study. Europace. 2021;23:254-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 68. | Gorenek B Chair, Bax J, Boriani G, Chen SA, Dagres N, Glotzer TV, Healey JS, Israel CW, Kudaiberdieva G, Levin LÅ, Lip GYH, Martin D, Okumura K, Svendsen JH, Tse HF, Botto GL Co-Chair; ESC Scientific Document Group. Device-detected subclinical atrial tachyarrhythmias: definition, implications and management-an European Heart Rhythm Association (EHRA) consensus document, endorsed by Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE). Europace. 2017;19:1556-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 69. | Steinberg JS, Varma N, Cygankiewicz I, Aziz P, Balsam P, Baranchuk A, Cantillon DJ, Dilaveris P, Dubner SJ, El-Sherif N, Krol J, Kurpesa M, La Rovere MT, Lobodzinski SS, Locati ET, Mittal S, Olshansky B, Piotrowicz E, Saxon L, Stone PH, Tereshchenko L, Turitto G, Wimmer NJ, Verrier RL, Zareba W, Piotrowicz R. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm. 2017;14:e55-e96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 156] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 70. | Francisco-Pascual J, Cantalapiedra-Romero J, Pérez-Rodon J, Benito B, Santos-Ortega A, Maldonado J, Ferreira-Gonzalez I, Rivas-Gándara N. Cardiac monitoring for patients with palpitations. World J Cardiol. 2021;13:608-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (2)] |
| 71. | Task Force members, Brignole M, Vardas P, Hoffman E, Huikuri H, Moya A, Ricci R, Sulke N, Wieling W; EHRA Scientific Documents Committee, Auricchio A, Lip GY, Almendral J, Kirchhof P, Aliot E, Gasparini M, Braunschweig F; Document Reviewers, Lip GY, Almendral J, Kirchhof P, Botto GL; EHRA Scientific Documents Committee. Indications for the use of diagnostic implantable and external ECG loop recorders. Europace. 2009;11:671-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 229] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 72. | Muntané-Carol G, Nombela-Franco L, Serra V, Urena M, Amat-Santos I, Vilalta V, Chamandi C, Lhermusier T, Veiga-Fernandez G, Kleiman N, Canadas-Godoy V, Francisco-Pascual J, Himbert D, Castrodeza J, Fernandez-Nofrerias E, Baudinaud P, Mondoly P, Campelo-Parada F, De la Torre Hernandez JM, Pelletier-Beaumont E, Philippon F, Rodés-Cabau J. Late arrhythmias in patients with new-onset persistent left bundle branch block after transcatheter aortic valve replacement using a balloon-expandable valve. Heart Rhythm. 2021;18:1733-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 73. | Thiruganasambandamoorthy V, Rowe BH, Sivilotti MLA, McRae AD, Arcot K, Nemnom MJ, Huang L, Mukarram M, Krahn AD, Wells GA, Taljaard M. Duration of Electrocardiographic Monitoring of Emergency Department Patients With Syncope. Circulation. 2019;139:1396-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 74. | Croci F, Brignole M, Alboni P, Menozzi C, Raviele A, Del Rosso A, Dinelli M, Solano A, Bottoni N, Donateo P. The application of a standardized strategy of evaluation in patients with syncope referred to three syncope units. Europace. 2002;4:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 75. | Benezet-Mazuecos J, Ibanez B, Rubio JM, Navarro F, Martín E, Romero J, Farre J. Utility of in-hospital cardiac remote telemetry in patients with unexplained syncope. Europace. 2007;9:1196-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 76. | Pagola J, Juega J, Francisco-Pascual J, Rodriguez M, Dorado L, Martinez R, De Lera-Alfonso M, Arenillas JF, Cabezas JA, Moniche F, de Torres R, Montaner J, Muchada M, Boned S, Requena M, García-Tornel A, Rodríguez-Villatoro N, Rodríguez-Luna D, Deck M, Olivé M, Rubiera M, Ribó M, Alvarez-Sabin J, Molina CA. Intensive 90-day textile wearable Holter monitoring: an alternative to detect paroxysmal atrial fibrillation in selected patients with cryptogenic stroke. Heart Vessels. 2023;38:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 77. | Locati ET, Moya A, Oliveira M, Tanner H, Willems R, Lunati M, Brignole M. External prolonged electrocardiogram monitoring in unexplained syncope and palpitations: results of the SYNARR-Flash study. Europace. 2016;18:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 78. | Moya Mitjans A, Francisco Pascual J, Pérez-Rodón J, Rivas Gándara N, Roca-Luque I, Garcia-Dorado D. Nuevos avances en la monitorización electrocardiográfica prolongada: Reveal LINQ TM. Cuad Estimulación Cardíaca. 2014;7:15-23. |
| 79. | Francisco-Pascual J, Rodenas-Alesina E, Rivas-Gándara N, Belahnech Y, Olivella San Emeterio A, Pérez-Rodón J, Benito B, Santos-Ortega A, Moya-Mitjans À, Casas G, Cantalapiedra-Romero J, Maldonado J, Ferreira-González I. Etiology and prognosis of patients with unexplained syncope and mid-range left ventricular dysfunction. Heart Rhythm. 2021;18:597-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 80. | Francisco-Pascual J, Rivas-Gándara N, Bach-Oller M, Badia-Molins C, Maymi-Ballesteros M, Benito B, Pérez-Rodon J, Santos-Ortega A, Sambola-Ayala A, Roca-Luque I, Cantalapiedra-Romero J, Rodríguez-Silva J, Pascual-González G, Moya-Mitjans À, Ferreira-González I. Sex-Related Differences in Patients With Unexplained Syncope and Bundle Branch Block: Lower Risk of AV Block and Lesser Need for Cardiac Pacing in Women. Front Cardiovasc Med. 2022;9:838473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 81. | Moya A, Brignole M, Sutton R, Menozzi C, Garcia-Civera R, Wieling W, Andresen D, Benditt DG, Garcia-Sacristán JF, Beiras X, Grovale N, Vardas P; International Study on Syncope of Uncertain Etiology 2 (ISSUE 2) Group. Reproducibility of electrocardiographic findings in patients with suspected reflex neurally-mediated syncope. Am J Cardiol. 2008;102:1518-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 82. | Solbiati M, Casazza G, Dipaola F, Barbic F, Caldato M, Montano N, Furlan R, Sheldon RS, Costantino G. The diagnostic yield of implantable loop recorders in unexplained syncope: A systematic review and meta-analysis. Int J Cardiol. 2017;231:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 83. | Krahn AD, Klein GJ, Yee R, Skanes AC. Randomized assessment of syncope trial: conventional diagnostic testing versus a prolonged monitoring strategy. Circulation. 2001;104:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 229] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 84. | Da Costa A, Defaye P, Romeyer-Bouchard C, Roche F, Dauphinot V, Deharo JC, Jacon P, Lamaison D, Bathélemy JC, Isaaz K, Laurent G. Clinical impact of the implantable loop recorder in patients with isolated syncope, bundle branch block and negative workup: a randomized multicentre prospective study. Arch Cardiovasc Dis. 2013;106:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 85. | Farwell DJ, Freemantle N, Sulke N. The clinical impact of implantable loop recorders in patients with syncope. Eur Heart J. 2006;27:351-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 86. | Thiruganasambandamoorthy V, Ramaekers R, Rahman MO, Stiell IG, Sikora L, Kelly SL, Christ M, Claret PG, Reed MJ. Prognostic value of cardiac biomarkers in the risk stratification of syncope: a systematic review. Intern Emerg Med. 2015;10:1003-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 87. | du Fay de Lavallaz J, Badertscher P, Nestelberger T, Zimmermann T, Miró Ò, Salgado E, Christ M, Geigy N, Cullen L, Than M, Martin-Sanchez FJ, Di Somma S, Peacock WF, Morawiec B, Walter J, Twerenbold R, Puelacher C, Wussler D, Boeddinghaus J, Koechlin L, Strebel I, Keller DI, Lohrmann J, Michou E, Kühne M, Reichlin T, Mueller C. B-Type Natriuretic Peptides and Cardiac Troponins for Diagnosis and Risk-Stratification of Syncope. Circulation. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 88. | Stark CB, Smit V, Mitra B. Review article: Utility of troponin after syncope: A systematic review and meta-analysis. Emerg Med Australas. 2019;31:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 89. | Thiruganasambandamoorthy V, Sivilotti MLA, Le Sage N, Yan JW, Huang P, Hegdekar M, Mercier E, Mukarram M, Nemnom MJ, McRae AD, Rowe BH, Stiell IG, Wells GA, Krahn AD, Taljaard M. Multicenter Emergency Department Validation of the Canadian Syncope Risk Score. JAMA Intern Med. 2020;180:737-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 90. | Zimmermann T, du Fay de Lavallaz J, Nestelberger T, Gualandro DM, Lopez-Ayala P, Badertscher P, Widmer V, Shrestha S, Strebel I, Glarner N, Diebold M, Miró Ò, Christ M, Cullen L, Than M, Martin-Sanchez FJ, Di Somma S, Peacock WF, Keller DI, Bilici M, Costabel JP, Kühne M, Breidthardt T, Thiruganasambandamoorthy V, Mueller C; BASEL IX Investigators†, Belkin M, Leu K, Lohrmann J, Boeddinghaus J, Twerenbold R, Koechlin L, Walter JE, Amrein M, Wussler D, Freese M, Puelacher C, Kawecki D, Morawiec B, Salgado E, Martinez-Nadal G, Inostroza CIF, Mandrión JB, Poepping I, Rentsch K, von Eckardstein A, Buser A, Greenslade J, Reichlin T, Bürgler F. International Validation of the Canadian Syncope Risk Score : A Cohort Study. Ann Intern Med. 2022;175:783-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 91. | Quinn JV, Stiell IG, McDermott DA, Sellers KL, Kohn MA, Wells GA. Derivation of the San Francisco Syncope Rule to predict patients with short-term serious outcomes. Ann Emerg Med. 2004;43:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 262] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 92. | Costantino G, Casazza G, Reed M, Bossi I, Sun B, Del Rosso A, Ungar A, Grossman S, D'Ascenzo F, Quinn J, McDermott D, Sheldon R, Furlan R. Syncope risk stratification tools vs clinical judgment: an individual patient data meta-analysis. Am J Med. 2014;127:1126.e13-1126.e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 93. | du Fay de Lavallaz J, Badertscher P, Zimmermann T, Nestelberger T, Walter J, Strebel I, Coelho C, Miró Ò, Salgado E, Christ M, Geigy N, Cullen L, Than M, Javier Martin-Sanchez F, Di Somma S, Frank Peacock W, Morawiec B, Wussler D, Keller DI, Gualandro D, Michou E, Kühne M, Lohrmann J, Reichlin T, Mueller C; BASEL IX Investigators. Early standardized clinical judgement for syncope diagnosis in the emergency department. J Intern Med. 2021;290:728-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 94. | Sattler SM, Skibsbye L, Linz D, Lubberding AF, Tfelt-Hansen J, Jespersen T. Ventricular Arrhythmias in First Acute Myocardial Infarction: Epidemiology, Mechanisms, and Interventions in Large Animal Models. Front Cardiovasc Med. 2019;6:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 95. | Georgeson S, Linzer M, Griffith JL, Weld L, Selker HP. Acute cardiac ischemia in patients with syncope: importance of the initial electrocardiogram. J Gen Intern Med. 1992;7:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 96. | Brembilla-Perrot B, Suty-Selton C, Beurrier D, Houriez P, Nippert M, de la Chaise AT, Louis P, Claudon O, Andronache M, Abdelaal A, Sadoul N, Juillière Y. Differences in mechanisms and outcomes of syncope in patients with coronary disease or idiopathic left ventricular dysfunction as assessed by electrophysiologic testing. J Am Coll Cardiol. 2004;44:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 97. | Roca-Luque I, Rivas-Gándara N, Francisco-Pascual J, Rodriguez-Sanchez J, Cuellar-Calabria H, Rodriguez-Palomares J, García-Del Blanco B, Pérez-Rodon J, Santos-Ortega A, Rosés-Noguer F, Marsal R, Rubio B, García DG, Moya Mitjans A. Preprocedural imaging to guide transcoronary ethanol ablation for refractory septal ventricular tachycardia. J Cardiovasc Electrophysiol. 2019;30:448-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 98. | Pérez-Rodon J, Galve E, Pérez-Bocanegra C, Soriano-Sánchez T, Recio-Iglesias J, Domingo-Baldrich E, Alzola-Guevara M, Ferreira-González I, Marsal JR, Ribera-Solé A, Gutierrez García-Moreno L, Cruz-Carlos LM, Rivas-Gandara N, Roca-Luque I, Francisco-Pascual J, Evangelista-Masip A, Moya-Mitjans À, García-Dorado D. A risk score to predict the absence of left ventricular reverse remodeling: Implications for the timing of ICD implantation in primary prevention. J Cardiol. 2018;71:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 99. | Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4998] [Cited by in RCA: 4784] [Article Influence: 239.2] [Reference Citation Analysis (0)] |
| 100. | Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5067] [Cited by in RCA: 4833] [Article Influence: 210.1] [Reference Citation Analysis (0)] |
| 101. | McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599-3726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8225] [Cited by in RCA: 7261] [Article Influence: 1815.3] [Reference Citation Analysis (0)] |
| 102. | Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2791] [Cited by in RCA: 4530] [Article Influence: 906.0] [Reference Citation Analysis (0)] |
| 103. | Brugada P, Green M, Abdollah H, Wellens HJ. Significance of ventricular arrhythmias initiated by programmed ventricular stimulation: the importance of the type of ventricular arrhythmia induced and the number of premature stimuli required. Circulation. 1984;69:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 244] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 104. | Brodsky MA, Mitchell LB, Halperin BD, Raitt MH, Hallstrom AP; AVID Investigators. Prognostic value of baseline electrophysiology studies in patients with sustained ventricular tachyarrhythmia: the Antiarrhythmics Versus Implantable Defibrillators (AVID) trial. Am Heart J. 2002;144:478-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 105. | Link MS, Saeed M, Gupta N, Homoud MK, Wang PJ, Estes NA 3rd. Inducible ventricular flutter and fibrillation predict for arrhythmia occurrence in coronary artery disease patients presenting with syncope of unknown origin. J Cardiovasc Electrophysiol. 2002;13:1103-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 106. | Bhambhani V, Kizer JR, Lima JAC, van der Harst P, Bahrami H, Nayor M, de Filippi CR, Enserro D, Blaha MJ, Cushman M, Wang TJ, Gansevoort RT, Fox CS, Gaggin HK, Kop WJ, Liu K, Vasan RS, Psaty BM, Lee DS, Brouwers FP, Hillege HL, Bartz TM, Benjamin EJ, Chan C, Allison M, Gardin JM, Januzzi JL Jr, Levy D, Herrington DM, van Gilst WH, Bertoni AG, Larson MG, de Boer RA, Gottdiener JS, Shah SJ, Ho JE. Predictors and outcomes of heart failure with mid-range ejection fraction. Eur J Heart Fail. 2018;20:651-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 107. | Menozzi C, Brignole M, Garcia-Civera R, Moya A, Botto G, Tercedor L, Migliorini R, Navarro X; International Study on Syncope of Uncertain Etiology (ISSUE) Investigators. Mechanism of syncope in patients with heart disease and negative electrophysiologic test. Circulation. 2002;105:2741-2745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 109] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 108. | Francisco-Pascual J, Rivas-Gándara N, Maymi-Ballesteros M, Badia-Molins C, Bach-Oller M, Benito B, Pérez-Rodón J, Santos-Ortega A, Roca-Luque I, Rodríguez-Silva J, Jordán-Marchite P, Moya-Mitjans À, Ferreira-González I. Arrhythmic risk in single or recurrent episodes of unexplained syncope with complete bundle branch block. Rev Esp Cardiol (Engl Ed). 2022;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 109. | Ruwald MH, Okumura K, Kimura T, Aonuma K, Shoda M, Kutyifa V, Ruwald AC, McNitt S, Zareba W, Moss AJ. Syncope in high-risk cardiomyopathy patients with implantable defibrillators: frequency, risk factors, mechanisms, and association with mortality: results from the multicenter automatic defibrillator implantation trial-reduce inappropriate therapy (MADIT-RIT) study. Circulation. 2014;129:545-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 110. | Phang RS, Kang D, Tighiouart H, Estes NA 3rd, Link MS. High risk of ventricular arrhythmias in patients with nonischemic dilated cardiomyopathy presenting with syncope. Am J Cardiol. 2006;97:416-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 111. | Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo-Leiro MG, Harjola VP, Parissis J, Laroche C, Piepoli MF, Fonseca C, Mebazaa A, Lund L, Ambrosio GA, Coats AJ, Ferrari R, Ruschitzka F, Maggioni AP, Filippatos G. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail. 2017;19:1574-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 644] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 112. | Hsu JJ, Ziaeian B, Fonarow GC. Heart Failure With Mid-Range (Borderline) Ejection Fraction: Clinical Implications and Future Directions. JACC Heart Fail. 2017;5:763-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 113. | Avula HR, Leong TK, Lee KK, Sung SH, Go AS. Long-Term Outcomes of Adults With Heart Failure by Left Ventricular Systolic Function Status. Am J Cardiol. 2018;122:1008-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 114. | Pezawas T, Stix G, Kastner J, Wolzt M, Mayer C, Moertl D, Schmidinger H. Unexplained syncope in patients with structural heart disease and no documented ventricular arrhythmias: value of electrophysiologically guided implantable cardioverter defibrillator therapy. Europace. 2003;5:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 115. | O'Mahony C, Jichi F, Pavlou M, Monserrat L, Anastasakis A, Rapezzi C, Biagini E, Gimeno JR, Limongelli G, McKenna WJ, Omar RZ, Elliott PM; Hypertrophic Cardiomyopathy Outcomes Investigators. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur Heart J. 2014;35:2010-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 840] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 116. | Authors/Task Force members, Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733-2779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2292] [Cited by in RCA: 3068] [Article Influence: 278.9] [Reference Citation Analysis (0)] |
| 117. | Brignole M, Cecchi F, Anastasakis A, Crotti L, Deharo JC, Elliott PM, Fedorowski A, Kaski JP, Limongelli G, Maron MS, Olivotto I, Ommen SR, Parati G, Shen W, Ungar A, Wilde A. Syncope in hypertrophic cardiomyopathy (part II): An expert consensus statement on the diagnosis and management. Int J Cardiol. 2023;370:330-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 118. | Mascia G, Crotti L, Groppelli A, Canepa M, Merlo AC, Benenati S, Di Donna P, Della Bona R, Soranna D, Zambon A, Porto I, Olivotto I, Parati G, Brignole M, Cecchi F. Syncope in hypertrophic cardiomyopathy (part I): An updated systematic review and meta-analysis. Int J Cardiol. 2022;357:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 119. | Hammarsten JF. Syncope in aortic stenosis. AMA Arch Intern Med. 1951;87:274-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 120. | Dhingra RC, Amat-y-Leon F, Pietras RJ, Wyndham C, Deedwania PC, Wu D, Denes P, Rosen KM. Sites of conduction disease in aortic stenosis: significance of valve gradient and calcification. Ann Intern Med. 1977;87:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 35] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 121. | Kleczyński P, Dimitrow PP, Dziewierz A, Wiktorowicz A, Rakowski T, Surdacki A, Dudek D. Predictors of syncope in patients with severe aortic stenosis: The role of orthostatic unload test. Cardiol J. 2020;27:749-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 122. | Roca-Luque I, Rivas-Gándara N, Dos-Subirà L, Francisco-Pascual J, Pijuan-Domenech A, Pérez-Rodon J, Santos-Ortega A, Roses-Noguer F, Ferreira-Gonzalez I, García-Dorado García D, Moya Mitjans A. Predictors of Acute Failure Ablation of Intra-atrial Re-entrant Tachycardia in Patients With Congenital Heart Disease: Cardiac Disease, Atypical Flutter, and Previous Atrial Fibrillation. J Am Heart Assoc. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 123. | Roca-Luque I, Rivas Gándara N, Dos Subirà L, Francisco Pascual J, Pérez-Rodon J, Pijuan Domenech A, Subirana MT, Miranda B, Santos Ortega A, Casaldàliga Ferrer J, García-Dorado García D, Moya Mitjans A. Intra-atrial re-entrant tachycardia in patients with congenital heart disease: factors associated with disease severity. Europace. 2018;20:1343-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 124. | Richards AM, Nicholls MG, Ikram H, Hamilton EJ, Richards RD. Syncope in aortic valvular stenosis. Lancet. 1984;2:1113-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 125. | Omran H, Fehske W, Rabahieh R, Hagendorff A, Pizzulli L, Zirbes M, Lüderitz B. Valvular aortic stenosis: risk of syncope. J Heart Valve Dis. 1996;5:31-34. [PubMed] |
| 126. | Urena M, Hayek S, Cheema AN, Serra V, Amat-Santos IJ, Nombela-Franco L, Ribeiro HB, Allende R, Paradis JM, Dumont E, Thourani VH, Babaliaros V, Francisco Pascual J, Cortés C, Del Blanco BG, Philippon F, Lerakis S, Rodés-Cabau J. Arrhythmia burden in elderly patients with severe aortic stenosis as determined by continuous electrocardiographic recording: toward a better understanding of arrhythmic events after transcatheter aortic valve replacement. Circulation. 2015;131:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
| 127. | Goliasch G, Kammerlander AA, Nitsche C, Dona C, Schachner L, Öztürk B, Binder C, Duca F, Aschauer S, Laufer G, Hengstenberg C, Bonderman D, Mascherbauer J. Syncope: The Underestimated Threat in Severe Aortic Stenosis. JACC Cardiovasc Imaging. 2019;12:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 128. | Faroux L, Muntané-Carol G, Urena M, Nombela-Franco L, Amat-Santos I, Kleiman N, Munoz-Garcia A, Atienza F, Serra V, Deyell MW, Veiga-Fernandez G, Masson JB, Canadas-Godoy V, Himbert D, Fischer Q, Castrodeza J, Elizaga J, Pascual JF, Webb JG, de la Torre JM, Asmarats L, Pelletier-Beaumont E, Alméndarez M, Couture T, Philippon F, Rodes-Cabau J. Late Electrocardiographic Changes in Patients With New-Onset Left Bundle Branch Block Following Transcatheter Aortic Valve Implantation. Am J Cardiol. 2020;125:795-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 129. | Nalliah CJ, Mahajan R, Elliott AD, Haqqani H, Lau DH, Vohra JK, Morton JB, Semsarian C, Marwick T, Kalman JM, Sanders P. Mitral valve prolapse and sudden cardiac death: a systematic review and meta-analysis. Heart. 2019;105:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 130. | Sheldon RS, Lei LY, Solbiati M, Chew DS, Raj SR, Costantino G, Morillo C, Sandhu RK. Electrophysiology studies for predicting atrioventricular block in patients with syncope: A systematic review and meta-analysis. Heart Rhythm. 2021;18:1310-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 131. | Moya A, Roca-Luque I, Francisco-Pascual J, Perez-Rodón J, Rivas N. Pacemaker therapy in syncope. Cardiol Clin. 2013;31:131-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 132. | Marti-Almor J, Cladellas M, Bazan V, Altaba C, Guijo M, Delclos J, Bruguera-Cortada J. Long-term mortality predictors in patients with chronic bifascicular block. Europace. 2009;11:1201-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 133. | Martí-Almor J, Cladellas M, Bazán V, Delclós J, Altaba C, Guijo MA, Vila J, Mojal S, Bruguera J. [Novel predictors of progression of atrioventricular block in patients with chronic bifascicular block]. Rev Esp Cardiol. 2010;63:400-408. [PubMed] |
| 134. | Azocar D, Ruiz-Granell R, Ferrero A, Martínez-Brotons A, Izquierdo M, Domínguez E, Palau P, Morell S, García-Civera R. Syncope and bundle branch block. Diagnostic yield of a stepped use of electrophysiology study and implantable loop recorders. Rev Esp Cardiol. 2011;64:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 135. | Sheldon R, Talajic M, Tang A, Becker G, Essebag V, Sultan O, Baranchuk A, Ritchie D, Morillo C, Krahn A, Brignole M, Manns B, Maxey C, Raj SR; SPRITELY Investigators. Randomized Pragmatic Trial of Pacemaker Versus Implantable Cardiac Monitor in Syncope and Bifascicular Block. JACC Clin Electrophysiol. 2022;8:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 136. | Santini M, Castro A, Giada F, Ricci R, Inama G, Gaggioli G, Calò L, Orazi S, Viscusi M, Chiodi L, Bartoletti A, Foglia-Manzillo G, Ammirati F, Loricchio ML, Pedrinazzi C, Turreni F, Gasparini G, Accardi F, Raciti G, Raviele A. Prevention of syncope through permanent cardiac pacing in patients with bifascicular block and syncope of unexplained origin: the PRESS study. Circ Arrhythm Electrophysiol. 2013;6:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 137. | Kalscheur MM, Donateo P, Wenzke KE, Aste M, Oddone D, Solano A, Maggi R, Croci F, Page RL, Brignole M, Hamdan MH. Long-Term Outcome of Patients with Bifascicular Block and Unexplained Syncope Following Cardiac Pacing. Pacing Clin Electrophysiol. 2016;39:1126-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 138. | Aste M, Oddone D, Donateo P, Solano A, Maggi R, Croci F, Solari D, Brignole M. Syncope in patients paced for atrioventricular block. Europace. 2016;18:1735-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 139. | Gann D, Tolentino A, Samet P. Electrophysiologic evaluation of elderly patients with sinus bradycardia: a long-term follow-up study. Ann Intern Med. 1979;90:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 94] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 140. | Roca-Luque I, Francisco-Pasqual J, Oristrell G, Rodríguez-García J, Santos-Ortega A, Martin-Sanchez G, Rivas-Gandara N, Perez-Rodon J, Ferreira-Gonzalez I, García-Dorado D, Moya-Mitjans A. Flecainide Versus Procainamide in Electrophysiological Study in Patients With Syncope and Wide QRS Duration. JACC Clin Electrophysiol. 2019;5:212-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 141. | Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2298] [Cited by in RCA: 2126] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 142. | Benito B, Brugada J, Brugada R, Brugada P. Síndrome de Brugada. Rev Esp Cardiol. 2009;62:1297-1315. [RCA] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 143. | Brugada J, Pappone C, Berruezo A, Vicedomini G, Manguso F, Ciconte G, Giannelli L, Santinelli V. Brugada Syndrome Phenotype Elimination by Epicardial Substrate Ablation. Circ Arrhythm Electrophysiol. 2015;8:1373-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 176] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 144. | Hernandez-Ojeda J, Arbelo E, Jorda P, Borras R, Campuzano O, Sarquella-Brugada G, Iglesias A, Mont L, Brugada R, Brugada J. The role of clinical assessment and electrophysiology study in Brugada syndrome patients with syncope. Am Heart J. 2020;220:213-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 145. | Scrocco C, Ben-Haim Y, Devine B, Tome-Esteban M, Papadakis M, Sharma S, Macfarlane PW, Behr ER. Role of subcutaneous implantable loop recorder for the diagnosis of arrhythmias in Brugada syndrome: A United Kingdom single-center experience. Heart Rhythm. 2022;19:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 146. | Medeiros-Domingo A, Iturralde-Torres P, Ackerman MJ. [Clinical and genetic characteristics of long QT syndrome]. Rev Esp Cardiol. 2007;60:739-752. [PubMed] |
| 147. | Chockalingam P, Crotti L, Girardengo G, Johnson JN, Harris KM, van der Heijden JF, Hauer RN, Beckmann BM, Spazzolini C, Rordorf R, Rydberg A, Clur SA, Fischer M, van den Heuvel F, Kääb S, Blom NA, Ackerman MJ, Schwartz PJ, Wilde AA. Not all beta-blockers are equal in the management of long QT syndrome types 1 and 2: higher recurrence of events under metoprolol. J Am Coll Cardiol. 2012;60:2092-2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 201] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 148. | Hayashi M, Denjoy I, Extramiana F, Maltret A, Buisson NR, Lupoglazoff JM, Klug D, Hayashi M, Takatsuki S, Villain E, Kamblock J, Messali A, Guicheney P, Lunardi J, Leenhardt A. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation. 2009;119:2426-2434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 375] [Article Influence: 23.4] [Reference Citation Analysis (0)] |