Published online Mar 26, 2023. doi: 10.4330/wjc.v15.i3.84
Peer-review started: November 6, 2022
First decision: January 3, 2023
Revised: January 27, 2023
Accepted: March 3, 2023
Article in press: March 3, 2023
Published online: March 26, 2023
Processing time: 135 Days and 0.9 Hours
The use of biodegradable polymer drug-eluting stents (BP-DES) has been proven to minimize restenosis and stent thrombosis. The current post-marketing monitoring was observed at the 5-year clinical outcomes of individuals who had been treated with FlexyRap® DES in the real world.
To assess the safety and effectiveness of FlexyRap® DES at the 5-year follow-up in real-world settings.
Findings from a retrospective, multi-center, observational, post-market clinical follow-up study of patients treated with FlexyRap® DES for de novo coronary artery disease (CAD) were reported. During the 12-mo follow-up, the primary endpoint was target lesion failure, which was defined as the composite of cardiovascular death, target vessel myocardial infarction (TV-MI), and clinically driven target lesion revascularization.
The data of 500 patients received with FlexyRap® DES was obtained at the completion of the surveillance timeline of 5-year. After the implantation of FlexyRap® DES, the device success rate was 100%. Adverse events that led to major bleeding, permanent disability, or death were not experienced in the patients. The major adverse cardiac event rate at 12-mo, 3-year, and 5-year follow-up was 1 (0.2%), 0 (0%), and 1 (0.2%) respectively with 0 (0%) cardiovascular death, 2 (0.4%) TV-MI, and 0 (0%) TLR compositely. Furthermore, late stent thrombosis was found in 2 (0.4%) patients at the follow-up of 12-mo, very late stent thrombosis was observed in 2 patients (0.4%) at 3-year follow-up.
FlexyRap® DES was proved to be safe and efficacious in real-world patients with de novo CAD, indicating a lowered rate of cardiac events and stent thrombosis at 5-year follow-up.
Core Tip: Biodegradable polymer drug-eluting stents (BP-DES) have been proven to minimize restenosis and stent thrombosis. Our study evaluates the safety and effectiveness of FlexyRap® DES at the 5-year clinical response in real-world settings. The study proved the feasibility, safety, and efficacy of the FlexyRap® rapamycin-eluting stent for the treatment of de novo coronary artery disease, indicating low rates of events and stent thrombosis at 5-year follow-up.
- Citation: Garg N, Chawla R, Tandon V, Garg D, Parshottam N, Vani P, Neuss M. Real-world five-year outcomes of FlexyRap® cobalt-chromium rapamycin-eluting stents with biodegradable polymer in patients with de-novo coronary artery disease. World J Cardiol 2023; 15(3): 84-94
- URL: https://www.wjgnet.com/1949-8462/full/v15/i3/84.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i3.84
Percutaneous coronary intervention (PCI) is a frequently conducted cardiac procedure aimed at enhancing the quality of life and reducing symptoms for individuals suffering from coronary artery disease (CAD)[1]. CAD is the leading cause of mortality across the globe[2]. Drug eluting stents, commonly referred to as DES, are considered as the primary method of percutaneous coronary revascularization for patients experiencing acute coronary syndromes and stable ischemic heart disease[3]. Recent advancements in the design of newer generation DES have centered on enhancing tissue biocompatibility and facilitating arterial healing. This has been achieved by incorporating innovative stent platform materials with thinner struts, utilizing biocompatible or biodegradable polymers with improved coatings, and implementing novel antiproliferative agents by lowering the content of drug and precisely controlling the rate of elution[4]. The advent of DES has decreased the rates of restenosis and become the preferred method of choice for most of the patients undergoing the procedure of PCI[1]. These stents have become widely used across a range of anatomic and clinical aspects due to their reduced rates of restenosis and the requirement for the repetition of the revascularization procedure[2]. The utilization of a polymer that is biodegradable has the possibility of lowering the chronic inflammatory response of the wall of blood vessels, facilitating the process of re-endothelialization and reducing the likelihood of blood clots and late restenosis[5]. Biodegradable polymers are being considered and analyzed to acquire and carry drugs. Polymers like poly lactic acid, polyglycolic acid, and their copolymer, poly lactic-co-glycolic acid, are most prevalent as they sight characteristics to get completely degraded and metabolized in the body[6]. It would have improved safety and performance of DES as they deliver controlled release of anti-restenosis agent and gradual degradation of coating[7].
FlexyRap® is one such novel biodegradable rapamycin-eluting coronary stent that has been developed by using a unique patented design of radial star, semi-opened, hybrid FlexyStar® platform, with a lower 60 μm thickness of strut and flexible link made of L605 cobalt-chromium metal. This design ensures the optimal delivery of the drug, radio-opacity, radial strength, biocompatibility, and vessel conformability. The evidence supporting the effectiveness and safety of indigenously produced drug-eluting stents in patients with newly diagnosed coronary artery disease is limited[8]. This study aimed to assess post-market clinical follow-up of real-world safety and efficacy of the rapamycin-eluting FlexyRap® coronary stent system, made of biodegradable polymer, in patients with obstructive native coronary arteries over a 5-year period.
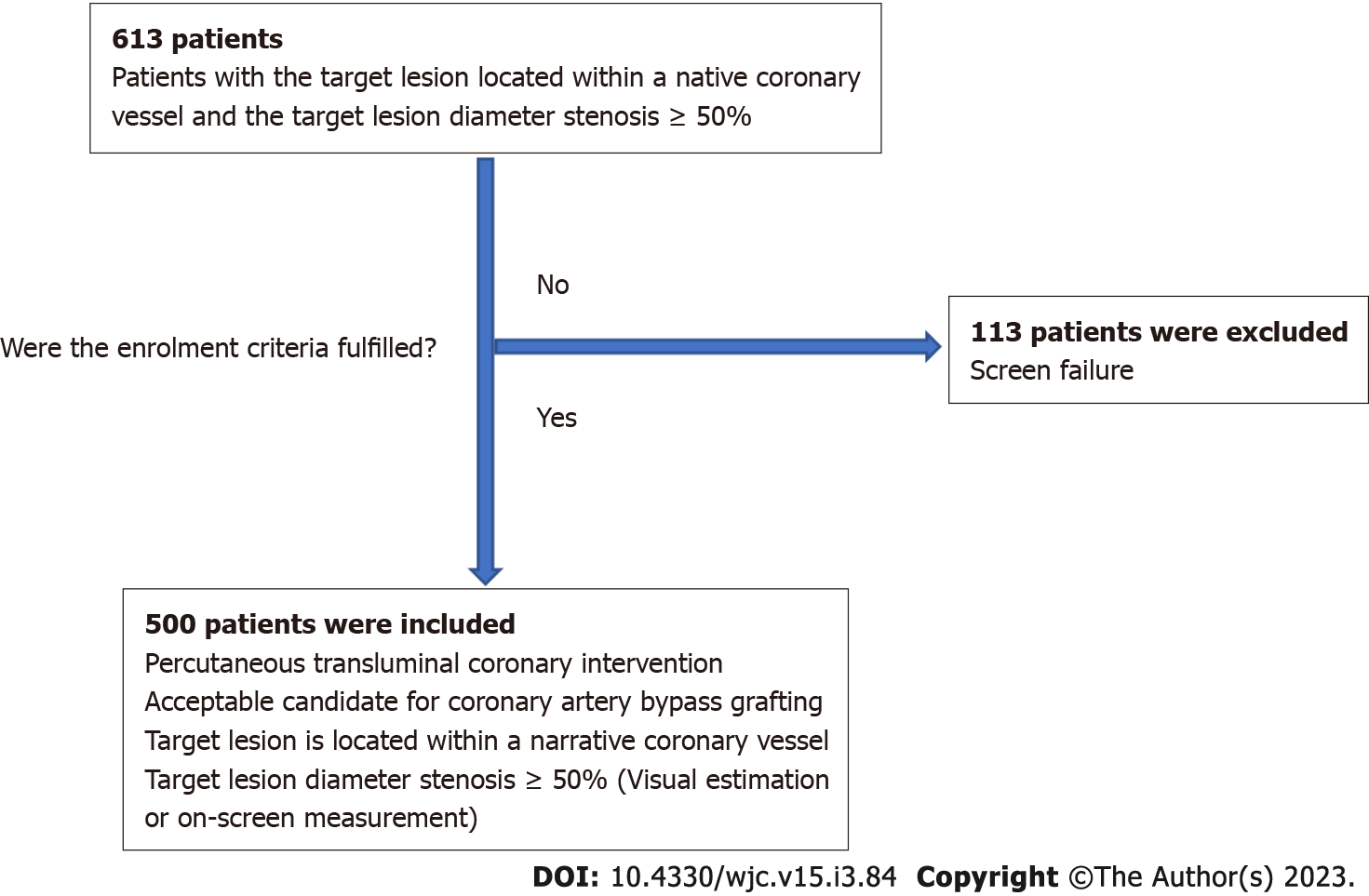
The FlexyRap® DES study was conducted at 5 centers with the total of 500 patients included in this study. The study was a retrospective, single-arm, multi-center, observational, post-market clinical follow-up conducted in 500 patients who were eligible for PCI and coronary artery bypass grafting (CABG). The patients in whom the target lesion located within a native coronary vessel and the target lesion diameter stenosis ≥ 50% were included in the study. Out of a total of 613 patients assessed for eligibility, 113 patients were excluded due to screen failure, and 500 patients were ultimately included in the study after meeting the predefined inclusion criteria as shown in Figure 1. The study was conducted in accordance with the declaration of Helsinki and IS0 14155:2020 GCP standards, ICH-GCP, MEDDEV 2.7.1 Appendix 1, MDR 2017/745 and applicable local regulatory requirements. The study was performed with the approval of an independent ethics committee. The PCI procedures were performed according to current standard guidelines. Clinical and angiographic data from all the patients who were treated with FlexyRap® DES were observed in this study. The clinical follow-up was performed at the time point of 12-mo, 3-year and 5-year after the discharge.
FlexyRap® cobalt chromium rapamycin-eluting coronary stent system consisting of a drug/polymer coated balloon expandable stent premounted on rapid exchange percutaneous transluminal coronary angioplasty (PTCA) balloon catheter. The stent is made from L605 cobalt chromium alloy (Co-Cr) which consists of cobalt, chromium, tungsten, iron and nickel with its strut thickness 60 μm. The stent is laser cut from the seamless tubing in hybrid design pattern and electro polished for ultra-smooth stent surface. The coating is comprised of biodegradable polymer matrix that contains an active pharmaceutical ingredient rapamycin (sirolimus). A conformal coating of a polymer carrier with approximately 1.0 µg/mm2 of rapamycin of total stent surface area with minimal nominal drug content of 32 µg on the smallest stent (7 mm) to maximum nominal drug content of 213 µg on the largest stent (45 mm). Stent of 48 mm in length approved by the Central Drug Standard Control Organization was also implanted in the desired population. The stent delivery balloon catheter system is a semi-compliant polyamide balloon, which is nominally 0.5 mm longer than the stent. The two opaque platinum-iridium markers are nominally placed beyond the stent at each end which defines the stent location in length. Two proximal delivery system shaft markers (90 cm and 100 cm proximal to distal tip) indicate the relative position of delivery system to the end of appropriate guiding catheter. FlexyRap® DES is available in various lengths (7, 10, 13, 15, 17, 20, 24, 28, 33, 38, 42, 45 and 48 mm) and diameters (2.25, 2.5, 2.75, 3.0, 3.5, 4.0 and 4.5 mm).
Procedural anticoagulation was achieved using unfractionated heparin (at least 5000 IU or 70-100 IU/kg to maintain an activated clotting time of > 250s during the procedure). Aspirin (≥ 100 mg) and clopidogrel (300-600 mg) or prasugrel (60 mg) were administered before or during the procedure at the investigator’s discretion. Patients continued to take aspirin (100 mg QD) indefinitely clopidogrel (75 mg QD) or prasugrel (60 mg) was administered for at least 6-mo after stent implantation in all patients and for at least 12-mo in those who did not have a high risk of bleeding. In addition, glycoprotein IIB/IIIA inhibitors were administered in certain patients at the investigator’s discretion. Biomarkers and electrocardiograms were recorded at different time points to assure the safety and well-being of patients.
Target lesion failure (TLF) is defined as a composite of cardiovascular death, target- vessel myocardial infarction (TV-MI), and clinically driven target lesion revascularization (CD-TLR)[9]. In the following study the primary endpoint was the TLF where the follow-up was taken at the interval of 12-mo and the secondary endpoints were cardiovascular death, TV-MI, clinically driven TLR, stent thrombosis (ST), target vessel failure, target vessel revascularization where the follow-up was taken at 12-mo, 3-year, and 5-year. The composite of cardiac death, target lesion-revascularization and myocardial infarction is defined as major adverse cardiac event (MACE). ST was also evaluated in this study which was classified according to the definitions of the academic research consortium[10]. Device success was defined as the successful delivery and deployment of the study stent at the intended target lesion, as well as the successful withdrawal of the delivery system, with final in-stent residual diameter stenosis of < 30% of all treated lesions, as determined by visual inspection or quantitative coronary angiography. Procedural success was defined as the delivery and deployment of the study stent at the intended target lesion, as well as the withdrawal of the delivery system, with a residual diameter stenosis of less than 30% as determined by visual inspection or quantitative coronary angiography, and no in-hospital major adverse cardiac event (death, MI, or repeat coronary revascularization of the target lesion)[11,12].
A sample size of 500 subjects was calculated based on the primary endpoint of the study. Categorical variables were summarized by frequency distribution for each categorical component (relative frequencies and percentage). All the analysis were done by using statistical package for the social sciences (SPSS) v.20. Results were reported as mean ± standard deviation for continuous variables and as number (%) for nominal variables. For changes in pre–post differences, Wilcoxon-test was used for ordinal variables and paired t-test for continuous variables. Other variables frequency was compared using the chi-square test or fisher’s exact test. Result was significant at P < 0.05. For time-to-event variables, survival curves were represented using Kaplan Meier estimates.
Baseline demographics and clinical characteristics are summarized in Table 1. The data for 500 patients were collected retrospectively at 12-mo, 3-year, and 5-year. The average age of the study patients was 59.30 ± 11.27 years, with the majority being male (70.2%). The most frequently occurring comorbidities were hypertension (43.4%), smoking (40.6%), diabetes mellitus (14%), alcoholic (9.6%), and dyslipidemia (3.4%). History of myocardial infarction was found in 54.8% followed by CAD (4.8%), PCI (4.2%) and stroke (1.8%). Out of 500 patients, 299 (59.8%) were having stable angina and 201 (40.2%) patients with unstable angina.
| Characteristics | FlexyRap® cobalt chromium rapamycin eluting coronary stent system; Number of patients, (n = 500) |
| Patient demographics | |
| Age, yr (mean ± SD) | 59.30 ± 11.27 |
| Male, n (%) | 351 (70.2) |
| Female, n (%) | 149 (29.8) |
| Heart rate (mean ± SD) | 86.36 ± 11.34 |
| Systolic blood pressure (mean ± SD) | 133.57 ± 20.88 |
| Diastolic blood pressure (mean ± SD) | 83.36 ± 9.70 |
| Haemoglobin (g/dL) (mean ± SD) | 12.64 ± 2.45 |
| Platelet count (mean ± SD) | 205.85 ± 52.19 |
| Baseline medical history, n (%) | |
| Hypertension | 217 (43.4) |
| Smoking current | 203 (40.6) |
| Diabetes mellitus | 70 (14) |
| Alcohol current | 48 (9.6) |
| Dyslipidemia | 17 (3.4) |
| Previous MI | 274 (54.8) |
| History of CAD | 24 (4.8) |
| Previous PCI | 21 (4.2) |
| Previous Stroke | 9 (1.8) |
| Baseline cardiac history, n (%) | |
| Stable angina | 299 (59.8) |
| Unstable angina | 201 (40.2) |
| Angina class, n (%) | |
| Class I | 12 (2.4) |
| Class II | 30 (6) |
| Class IIA | 1 (0.2) |
| Class IIB | 12 (2.4) |
| Class IIC | 6 (1.2) |
| Class III | 198 (39.6) |
| Class IIIA | 15 (3) |
| Class IIIB | 51 (10.2) |
| Class IIIC | 37 (7.4) |
| Class IV | 138 (27.6) |
| Disease vessel, n (%) | |
| Single vessel | 314 (62.8) |
| Double vessel | 150 (30) |
| Triple vessel | 27 (5.4) |
| Quadra vessel | 9 (1.8) |
| LVEF (mean ± SD) | 52.88 ± 15.46 |
| Serum creatinine (mean ± SD) | 1.47 ± 0.47 |
Lesion details are mentioned in the Table 2. Total 729 lesions were identified and 730 stents were deployed to treat the lesion. The average stent length and diameter was 26.03 ± 10.86 mm and 3.06 ± 0.41 mm. The device success rate were observed to be 100%.
| Procedural characteristics (n = 500) | |
| Lesion details | |
| Total number of lesions treated with FlexyRap® (n) | 730 |
| Total number of stents deployed (n) | 730 |
| Total stent per lesion (n = 500 patients) (Total no. stent deployed (730)/Total lesion locations (729) | 1.001 |
| Total lesion per vessel (n = 500 patients); Total lesion locations (729)/(Sum of total No of diseased vessel (731) | 0.997 |
| Lesion locations (729) n (%) | |
| D1 | 6 (0.82) |
| Distal LAD | 17 (2.33) |
| Distal LCx | 4 (0.54) |
| Distal RCA | 16 (2.19) |
| LAD | 279 (38.27) |
| LCx | 98 (13.44) |
| LM | 1 (0.13) |
| MID LAD | 19 (2.6) |
| MID LCx | 9 (1.23) |
| MID RCA | 15 (2.05) |
| O Mid | 7 (0.96) |
| OM | 4 (0.54) |
| OM1 | 8 (1.09) |
| OM2 | 6 (0.82) |
| OM3 | 1 (0.13) |
| OMI | 1 (0.13) |
| Osteoproximal LAD | 2 (0.27) |
| Osteoproximal RCA | 4 (0.54) |
| PDA | 7 (0.96) |
| PLV | 3 (0.41) |
| Proximal LAD | 23 (3.15) |
| Proximal RCA | 15 (2.05) |
| Proximal LCx | 7 (0.96) |
| PTCA | 8 (1.09) |
| Ramus intermedius | 8 (1.09) |
| RCA | 156 (21.40) |
| RCX | 2 (0.27) |
| PLB | 2 (0.27) |
| POM | 1 (0.13) |
| Stent length (mean ± SD) | 26.03 ± 10.86 |
| Stent diameter (mean ± SD) | 3.06 ± 0.41 |
| Type of stenosis, n (%) | |
| de novo | 500 (100) |
| Thrombus load (n = 731), n (%) | |
| None | 519 (71) |
| Mild | 90 (12.31) |
| Moderate | 59 (8.07) |
| Severe | 63 (8.62) |
| Lesion type [ACC/AHA classification] (n = 731), n (%) | |
| Type A | 20 (2.73) |
| Type B1 | 193 (26.40) |
| Type B2 | 302 (41.31) |
| Type C | 216 (29.55) |
| Stent balloon inflation pressure (atm) (mean ± SD) (n = 500) | 12.52 ± 1.75 |
| TIMI FLOW n (%) | |
| II | 9 (1.8) |
| III | 491 (98.2) |
| % of occlusion (mean ± SD) (n = 500) | 88.60 ± 8.79 |
| All values are presented in n (%) or mean ± SD | |
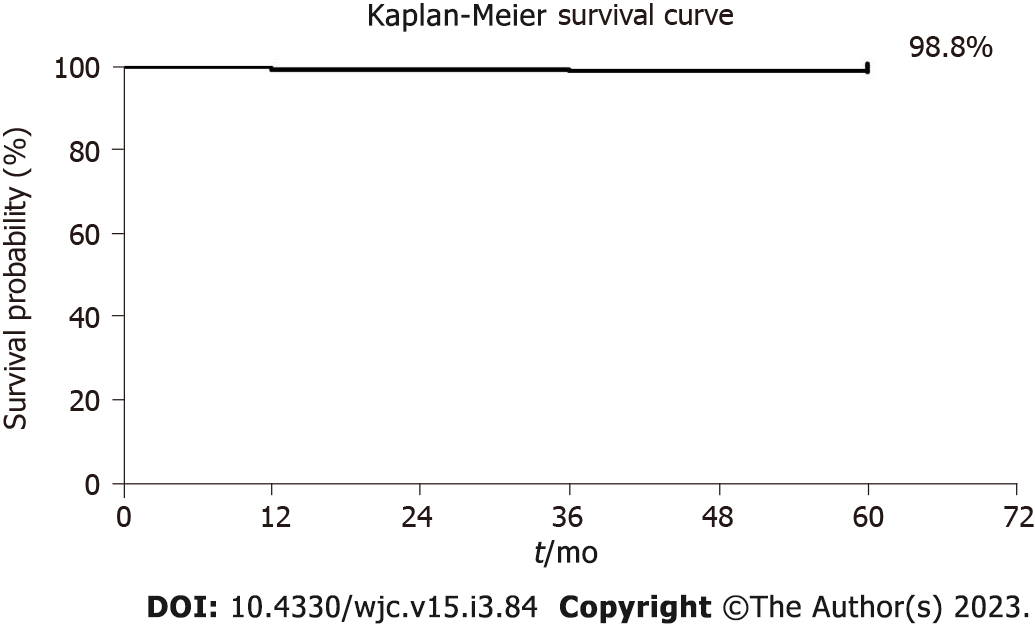
The cardiac event rate associated with the use of FlexyRap® DES at the follow-up of 12-mo, 3-year, and 5-year is presented in Table 3. Total 2 (0.4%) patients experienced MACE during 5-year. The MACE rate at 12-mo, 3-year, and 5-year follow-up was 1 (0.2%), 0 (0%), and 1 (0.2%) respectively with 0 (0%) cardiovascular death, 2 (0.4%) TV-MI and 0 (0%) TLR compositely. Furthermore, late stent thrombosis was found in 2 (0.4%) patients at 12-mo follow-up, very late stent thrombosis was observed in 2 patients (0.4%) at 3-year follow-up. The Kaplan-Meier method was used to conduct a time-to-event analysis, which showed a 98.8% result (Figure 2).
| Clinical event | 12-mo (n = 500) | 3-yr (n = 500) | 5-yr (n = 500) |
| TLF | 0 (0) | 0 (0) | 0 (0) |
| Cardiovascular Death | 0 (0) | 0 (0) | 0 (0) |
| TV-MI | 1 (0.2) | 0 (0) | 1 (0.2) |
| Clinically-driven TLR | 0 (0) | 0 (0) | 0 (0) |
| Late ST | 2 (0.4) | 0 (0) | 0 (0) |
| TVF | 0 (0) | 0 (0) | 0 (0) |
| TVR | 0 (0) | 0 (0) | 0 (0) |
| Very late ST | 0 (0) | 2 (0.4) | 0 (0) |
| Total MACE | 1 (0.2) | 0 (0) | 1 (0.2) |
In the proposed retrospective study, the FlexyRap® DES has showed exceptional positive results in the patients with de novo obstructive native CAD including high procedural success and clinical performance. The patient population had hypertension (43.4%), smoking (40.6%), diabetes mellitus (14%), previous myocardial infarction (54.8%), alcoholism (9.6%), and dyslipidemia (3.4%).
As per the observed study, FlexyRap® cobalt-chromium rapamycin-eluting coronary stent system consisting of drug/polymer coated balloon expandable stent is premounted on rapid exchange PTCA balloon catheter. The polymers are biodegradable, biocompatible, and bioresorbable. Degradation of these materials has been thoroughly studied and has been shown to be safely resorbed by the body after implantation. Rapamycin belongs to a class of therapeutic agents known as macrocyclic lactone or macrolide. It’s a cytostatic drug and an immunosuppressant. Rapamycin inhibits T cell activation and growth in response to antigenic stimuli and cytokines such as IL-3, IL-4, and IL-15 are inhibited through a unique mechanism that differs from other immunosuppressive agents. It has been noted that a variety of elements, including the design of the stent, thickness of its struts, antiproliferative agent used, release dynamics of the drug, the duration of drug release, and the type of polymer, can have an impact on the safety and effectiveness of coronary stent system[13]. The first-generation stent were constructed with bulky stent frameworks, making their delivery quite difficult[14]. However, the newest generation boasts thin struts and has demonstrated an 8% increase in its nominal pressure to rated burst pressure. These latest-generation FlexyRap® DES offer improved ease of delivery and vessel conformability, resulting in full deployment and proper placement against the vessel wall. Its design minimizes balloon overhang, reducing the likelihood of edge dissection or injury - a typical procedural issue in PCI. The results of the study, where procedural success was accomplished in all patients, support these claims. Compared to bare metal stent, the first-generation DES featuring a long-lasting polymer have been successful in lowering the rate of re-narrowing, but they have a higher incidence of late ST[15].
Also, the FlexyRap® has the advantage of not cracking, webbing, clumping, or adhering to the balloon surface, making it a promising option for coronary applications. The finding of 100% procedural success in this study can be attributed to these favorable product features.
Iglesias et al[16], compared the safety and effectiveness of ultrathin strut biodegradable polymer sirolimus-eluting stents (BP-SES) with thin strut durable polymer everolimus-eluting stents in patients experiencing acute ST-segment elevation myocardial infarction (STEMI). The results showed that 25 (4%) out of 649 patients who received (BP-SES) biodegradable polymer sirolimus-eluting stents and 36 (6%) out of 651 patients who received durable polymer everolimus-eluting stents (DP-EES) experienced TLF. Shetty et al[17], conducted a study illustrating the late-term clinical outcomes among patients treated with ultrathin strut BP-SES and thin-strut DP-EES where significant differences in target vessel MI and target lesion revascularization was observed. Out of 884 patients with BP-SES, target lesion failure was observed in 8.2% of patients, and 13.6% of patients shown up with TLF for DP-EES out of 450 patients[17]. Dani et al[8], assessed the comparative performance of a BP-SES compared with a DP-EES in the treatment of calcified or narrow vessel blockages. A total of 1553 patients were implanted with BP-SES and 784 patients with DP-EES with the validation of 12-mo follow-up. TLF and TV-MI were significantly lower in BP-SES than in DP-EES in non-small vessel lesions. In the patients with TLF, calcified lesions and cardiac death were numerically higher in DP-EES than in BP-SES. Similarly, the outcomes of the proposed study are comparable with the other studies where TLR was not observed in the patients and the TV-MI in 0.4% of the patients at the cumulative follow-up of 5-year demonstrating the successful clinical outcomes of the study device.
At the end of the 5-year analysis period, cumulative cardiac events presented with 0.4% of MACE where 0 (0%) cardiovascular death, 2 (0.4%) TV-MI, and 0 (0%) TLR was observed compositely, with 0.4% of late ST and 0.4% of very late ST. The unique configuration of the radial star segments and the minimal thickness of the struts ensure exceptional radial stability, facilitating the smooth navigational progress of the device through the circulatory system. Additionally, the decline in the occurrence of cardiac incidents is likely due to the biodegradable polymer's non-inflammatory properties and optimal drug release kinetics[17]. A decreased thickness of stent struts has been linked to a lower frequency of ST[8]. The main benefit of the study was that it was a 5-year follow-up thus the results were sustained in well- designed with longer follow-up duration. The positive outcomes seen in this study could be attributed to the unique design features of the product, such as the advanced stent design utilizing a biodegradable polymer that offers strong radial strength, reduced overhang from the balloon, low recoil, and consistent support. The device and procedural success rate were 100% for the patients implanted with FlexyRap® DES. The survival probability of 98.8% was observed.
One significant drawback of this study was its observational design and examination of retrospective data. However, this approach provides a more accurate representation of a diverse patient population, unlike randomized trials with strict criteria for enrollment.
In conclusion, the present PMCF study offers evidence regarding the safety, and effectiveness of the FlexyRap® rapamycin-eluting stent for treatment of de novo CAD. In the present study, FlexyRap® DES was found to have clinical benefits in treating patients with CAD in a real-world setting.
Drug-eluting stents manufactured with biodegradable polymers (BP-DES) effectively reduce restenosis and the risk of stent thrombosis.
The motivation of the present study is focused on the safety and effectiveness from the stent eluting rapamycin for treating the de novo coronary artery disease (CAD).
Our study evaluates the safety and effectiveness of FlexyRap® DES at the 5-year clinical response in real-world settings. The outcome of the study proved to be viable, safe, and efficacious results of the FlexyRap®, rapamycin-eluting stent for treating de novo CAD, indicating low rates of events and ST at 5-year follow-up.
Findings from a retrospective, multi-center, observational, post-market clinical follow-up study of individuals treated with FlexyRap® DES for de novo CAD. During the 12-mo follow-up, the primary endpoint was to determine the rate of target lesion failure (TLF). TLF was established as the culmination of three events: Death caused by cardiovascular issues, a myocardial infarction in the target vessel, and the requirement for revascularization of the target lesion due to clinical findings.
The major adverse cardiac event rate at 12-mo, 3-year, and 5-year follow-up was 1 (0.2%), 0 (0%) and 1 (0.2%) respectively with 0 (0%) cardiovascular death, 2 (0.4%) TV-MI and 0 (0%) TLR compositely. Furthermore, late stent thrombosis was found in 2 (0.4%) patients at the follow-up of 12-mo, very late stent thrombosis was observed in 2 patients (0.4%) at 3-year follow-up.
In conclusion, this PMCF study investigated the preliminary indications of the feasibility, safety, and effectiveness of using the FlexyRap® rapamycin-eluting stent for treating de novo lesion in CAD. In the present study, FlexyRap® DES was found to have clinical benefits in treating patients with CAD in a real-world setting.
To improve the inner luminal diameter and decrease the likelihood of repeat blockages in the treatment of de novo lesions in the native coronary arteries.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lakusic N, Croatia; Wang DW, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Jambunathan R, Basavanna D, Vani P, Neuss M, Janbandhu P. One-year outcomes of a NeoHexa sirolimus-eluting coronary stent system with a biodegradable polymer in all-comers coronary artery disease patients: Results from NeoRegistry in India. World J Cardiol. 2019;11:200-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Jain RK, Chakravarthi P, Shetty R, Ramchandra P, Polavarapu RS, Wander GS, Mohan B, Banker DN, Dharmadhikari A, Bansal SS, Jain N, Solanki D, Dhakaan J, Sharma VP, Mohanan PP, Ashokan PK, Manjunath BV, Hiregoudar N, Patil C, Balakrishnan N. One-year outcomes of a BioMime™ Sirolimus-Eluting Coronary Stent System with a biodegradable polymer in all-comers coronary artery disease patients: The meriT-3 study. Indian Heart J. 2016;68:599-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Mauri L, Doros G, Rao SV, Cohen DJ, Yakubov S, Lasala J, Wong SC, Zidar J, Kereiakes DJ. The OPTIMIZE randomized trial to assess safety and efficacy of the Svelte IDS and RX Sirolimus-eluting coronary stent Systems for the Treatment of atherosclerotic lesions: Trial design and rationale. Am Heart J. 2019;216:82-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Iglesias JF, Roffi M, Degrauwe S, Secco GG, Aminian A, Windecker S, Pilgrim T. Orsiro cobalt-chromium sirolimus-eluting stent: present and future perspectives. Expert Rev Med Devices. 2017;14:773-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Hamon M, Niculescu R, Deleanu D, Dorobantu M, Weissman NJ, Waksman R. Clinical and angiographic experience with a third-generation drug-eluting Orsiro stent in the treatment of single de novo coronary artery lesions (BIOFLOW-I): a prospective, first-in-man study. EuroIntervention. 2013;8:1006-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | BaoLin G, Ma PX. Synthetic biodegradable functional polymers for tissue engineering: a brief review. Sci China Chem. 2014;57:490-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 330] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 7. | Parsa E, Saroukhani S, Majlessi F, Poorhosseini H, Lofti-Tokaldany M, Jalali A, Salarifar M, Nematipour E, Alidoosti M, Aghajani H, Amirzadegan A, Kassaian SE. Biodegradable-Polymer Biolimus-Eluting Stents versus Durable-Polymer Everolimus-Eluting Stents at One-Year Follow-Up: A Registry-Based Cohort Study. Tex Heart Inst J. 2016;43:126-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Dani S, Gupta M, Pandya R, Goyal K, Vani P, Neuss M, Janbandhu P. Safety and Effectiveness of FlexyRap® Cobalt-Chromium Rapamycin- Eluting Stents with Biodegradable Polymer in Coronary Artery Disease: Results from a 2-year, Multicenter Postmarketing Study in India. J Interv Gen Cardiol 2019; 3. Available from: https://www.semanticscholar.org/paper/Safety-and-Effectiveness-of-FlexyRap%C2%AE-Stents-with-a-Dani-Gupta/dc06afa76563bbce1276ffb975421f244bb41938. |
| 9. | Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, Onuma Y, Morel MA, van Es GA, Zuckerman B, Fearon WF, Taggart D, Kappetein AP, Krucoff MW, Vranckx P, Windecker S, Cutlip D, Serruys PW; Academic Research Consortium. Standardized End Point Definitions for Coronary Intervention Trials: The Academic Research Consortium-2 Consensus Document. Eur Heart J. 2018;39:2192-2207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 10. | Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW; Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4265] [Cited by in RCA: 4698] [Article Influence: 261.0] [Reference Citation Analysis (0)] |
| 11. | Chang CC, Kogame N, Onuma Y, Byrne RA, Capodanno D, Windecker S, Morel MA, Cutlip DE, Krucoff MW, Stone GW, Lansky AJ, Mehran R, Spitzer E, Fraser AG, Baumbach A, Serruys PW. Defining device success for percutaneous coronary intervention trials: a position statement from the European Association of Percutaneous Cardiovascular Interventions of the European Society of Cardiology. EuroIntervention. 2020;15:1190-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, Fonarow GC, Jacobs JP, Jaff MR, Lichtman JH, Limacher MC, Mahaffey KW, Mehran R, Nissen SE, Smith EE, Targum SL; ACC/AHA TASK FORCE ON CLINICAL DATA STANDARDS MEMBERS. 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Nucl Cardiol. 2015;22:1041-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Navarese EP, Tandjung K, Claessen B, Andreotti F, Kowalewski M, Kandzari DE, Kereiakes DJ, Waksman R, Mauri L, Meredith IT, Finn AV, Kim HS, Kubica J, Suryapranata H, Aprami TM, Di Pasquale G, von Birgelen C, Kedhi E. Safety and efficacy outcomes of first and second generation durable polymer drug eluting stents and biodegradable polymer biolimus eluting stents in clinical practice: comprehensive network meta-analysis. BMJ. 2013;347:f6530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 14. | Navarese EP, Kowalewski M, Kandzari D, Lansky A, Górny B, Kołtowski L, Waksman R, Berti S, Musumeci G, Limbruno U, van der Schaaf RJ, Kelm M, Kubica J, Suryapranata H. First-generation versus second-generation drug-eluting stents in current clinical practice: updated evidence from a comprehensive meta-analysis of randomised clinical trials comprising 31 379 patients. Open Heart. 2014;1:e000064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 15. | Stefanini GG, Byrne RA, Serruys PW, de Waha A, Meier B, Massberg S, Jüni P, Schömig A, Windecker S, Kastrati A. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J. 2012;33:1214-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 313] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 16. | Iglesias JF, Muller O, Heg D, Roffi M, Kurz DJ, Moarof I, Weilenmann D, Kaiser C, Tapponnier M, Stortecky S, Losdat S, Eeckhout E, Valgimigli M, Odutayo A, Zwahlen M, Jüni P, Windecker S, Pilgrim T. Biodegradable polymer sirolimus-eluting stents versus durable polymer everolimus-eluting stents in patients with ST-segment elevation myocardial infarction (BIOSTEMI): a single-blind, prospective, randomised superiority trial. Lancet. 2019;394:1243-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 17. | Shetty R, Prajapati J, Pai U, Shetty K. Preliminary Evaluation of Clinical and Angiographic Outcomes with Biodegradable Polymer Coated Sirolimus-Eluting Stent in De Novo Coronary Artery Disease: Results of the MANIPAL-FLEX Study. Scientifica (Cairo). 2016;2016:9324279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |