Published online Mar 26, 2023. doi: 10.4330/wjc.v15.i3.76
Peer-review started: October 2, 2022
First decision: November 25, 2022
Revised: December 31, 2022
Accepted: February 22, 2023
Article in press: February 22, 2023
Published online: March 26, 2023
Processing time: 169 Days and 14.5 Hours
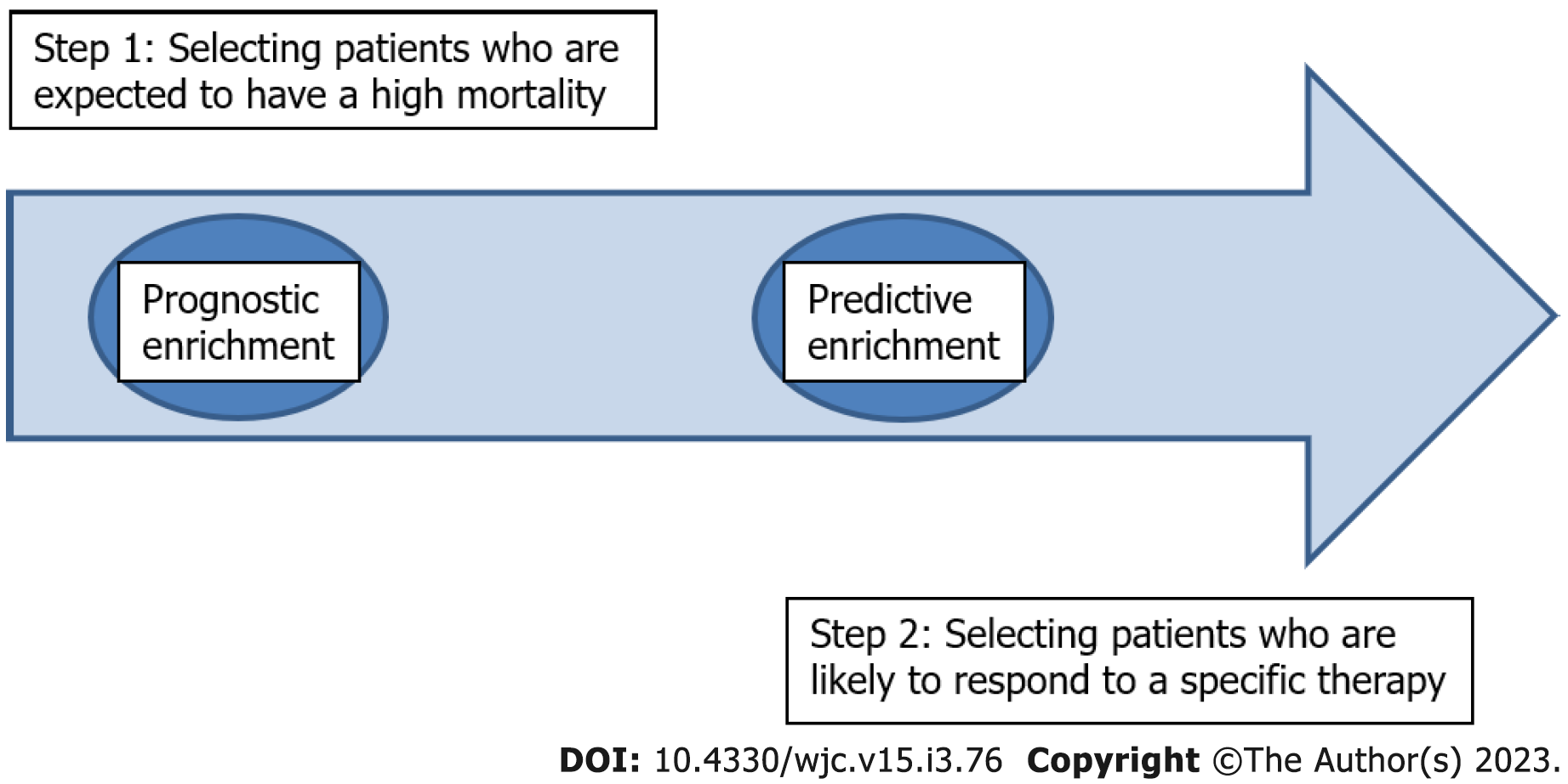
Chronic kidney disease (CKD) patients face an unacceptably high morbidity and mortality, mainly from cardiovascular diseases. Diabetes mellitus, arterial hypertension and dyslipidemia are highly prevalent in CKD patients. Established therapeutic protocols for the treatment of diabetes mellitus, arterial hypertension, and dyslipidemia are not as effective in CKD patients as in the general population. The role of non-traditional risk factors (RF) has gained interest in the last decades. These entail the deranged clinical spectrum of secondary hyperparathyroidism involving vascular and valvular calcification, under the term “CKD-mineral and bone disorder” (CKD-MBD), uremia per se, inflammation and oxidative stress. Each one of these non-traditional RF have been addressed in various study designs, but the results do not exhibit any applied clinical benefit for CKD-patients. The “crusade” against cardiorenal morbidity and mortality in CKD-patients is in some instances, derailed. We propose a therapeutic paradigm advancing from isolated treatment targets, as practiced today, to precision medicine involving patient phenotypes with distinct underlying pathophysiology. In this regard we propose two steps, based on current stratification management of corona virus disease-19 and sepsis. First, select patients who are expected to have a high mortality, i.e., a prognostic enrichment. Second, select patients who are likely to respond to a specific therapy, i.e., a predictive enrichment.
Core Tip: Stagnation in the Nephrology field has to be overcome with a new perspective. This new vision takes lessons from the past as personalized medicine, adapts precision medicine from today’s lessons from corona virus disease-19 and sepsis and looks into the future with the aid of the big data. Our proposal is that cardiorenal management should be stratified according to patient phenotypes and not as an assembly of individual targets.
- Citation: Bacharaki D, Petrakis I, Stylianou K. Redefying the therapeutic strategies against cardiorenal morbidity and mortality: Patient phenotypes. World J Cardiol 2023; 15(3): 76-83
- URL: https://www.wjgnet.com/1949-8462/full/v15/i3/76.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i3.76
Cardiovascular (CV) disease is a major contributor of mortality in chronic kidney disease (CKD) patients, especially in the late stage 5 on dialysis (5D), mounting up to 58% of causes[1,2]. Aside traditional CV risk factors (RF), as diabetes and arterial hypertension, non-traditional RF related to kidney disease per se seem to play a pivotal role in the complex interaction between the kidney and the heart[3]. Νon-traditional RF include secondary hyperparathyroidism resulting in vascular and valvular calcification, collectively termed as CKD-mineral and bone disorder (CKD-MBD)[4], uremia per se, inflammation, oxidative stress and dysbiotic gut microbiota[5].
CKD patients have long been excluded from cardiovascular clinical trials, for various reasons: (1) Inadequate surrogate outcomes and low event rate, especially in end stage renal disease, demand a prohibitory large sample size and an extensive follow up[6]; and (2) fear for negative results or adverse events, since the aforementioned non-traditional risk factors are recognized as potential disease modifiers[7]. Nephrology practice could be characterized as “low evidence” medicine, which pursues targeting traditional RF with data originating from the general, non-CKD-population[8].
Major clinical problems, such as the choice of treatment for non-valvular atrial fibrillation in dialysis patients, remain unsolved and clinical nephrologists “navigate through darkness” regarding therapeutic strategy[9]. In the case of hyperphosphatemia although there is numerous scientific evidence that “phosphate is a cardiovascular toxin”[10], there has been no randomized control trial (RCT) providing evidence that “correction” will translate into tangible cardiovascular benefit, set the optimal timing of intervention, the different means or the optimal serum phosphate target[11]. Yet the patients endure an overwhelming phosphate binder pills consumption[12] with enormous economic implications for healthcare[13].
Sodium-glucose cotransporter-2 inhibitors (SGLT2i), initially marketed as glucose lowering drugs in diabetes mellitus type2, are a game changer in the field of cardiorenal protection[14]. Their beneficial effects, regarding reduction in CV morbidity and mortality and renal function preservation, have been assessed by RCTs across CKD stages 1-3, notably with empagliflozin (EMPA REG OUTCOME)[15] and dapagliflozin (CAPA-CKD)[16]. The unprecedented success of this novel treatment stems from the pleiotropic effects of SGLT2i, targeting multiple intra-extrarenal pathways[17].
Another promising therapeutic tool is Mediterranean Diet (Med Diet) that has a pivotal role for cardiorenal protection[18]. Targeting all traditional and multiple non-traditional RF of cardiovascular morbidity and mortality along with exercise, Med Diet confers to an anti- inflammatory and anti- oxidative metabolic profile[19]. The level of adherence has been recently linked in an observational study with left ventricular geometry patterns in dialysis patients, a powerful independent risk factor of CV mortality in this particularly vulnerable population[20].
In clinical practice SGLT2i are currently tested as real world experience in advanced stages of CKD[21]. On the other hand nephrologists are still reluctant to prescribe vegetable based diets, as Med Diet, mostly for the fear or ignorance of handling potassium and/or provoking malnutrition[22].
The only positive RCT in the field of CV protection in CKD is the CANTOS trial (Canakinumab Anti-Inflammatory Thrombosis Outcome Study)[23], where the inhibition of the pro- inflammatory IL-1β was more beneficial in post myocardial infarction patients with glomerular filtration rate (GFR) < 60 mL/min/1.73 m2. The concept of “inflammaging”, introduced by Franceshi, unified all chronic degenerative conditions, in a common pathophysiology, which could be translated as a low grade sterile chronic inflammation resembling the natural course of ageing[24]. In observational studies IL-6 has been described as an independent predictor of outcome in pre-dialysis[25], hemodialysis[26] and peritoneal dialysis patients[27]. The association of inflammation and outcome seems so strong that a hypothesis proposal was made not to include inflamed and not inflamed patients in the same cohort in an RCT, since inflammation is powerful catalyst for other risk factors in CKD[28]. CKD patients are in a paradox state of both immune - paralysis (driving susceptibility to infections) and immune- activation (linked to CVD)[29].
Treatment failure, targeting “traditional” RF and nontraditional RF (hyperphosphatemia, CKD- MBD parameters) stems probably from the fact that there is no stratification management that would guide a precision or personalized medicine. Nephrology practice seems to be in a state of involuntary blindness as the crowd that pretends to see the clothes of the naked Emperor in Hans Christian Andersen’s tale[30]. In order to find a solution we propose the following 4 steps: (1) Gather the wisdom of the past in the form of personalized medicine; (2) adapt precision medicine from today’s lessons from corona virus disease 19 (COVID-19); (3) sepsis; and (4) look into the future with the aid of the big data.
Historically[31], the therapeutic practices have changed drastically from a “patient-centred” view to those of “evidence-based” medicine. “Germ theory of disease” in the 19th century, changed the “holistic view” perception of disease to a “specific cause for a specific disease”. The treatment approach shifted therefore, to a narrow approach that targeted a specific cause. The patient’s role diminished from an active contributor, through personal beliefs, adaptation and lifestyle choices, to a mere passive recipient of the treatment. Patients became “numbers” in any given trial, which will eventually provide the necessary information to form “therapeutic guidelines”[32]. Ironically and paradoxically, the contemporary nephrologist is called to manage CKD patients, who are at very high risk of cardiovascular morbidity and mortality, with guidelines based on weak evidence[6]. As mentioned Nephrology field lacks RCTs and the Cardiology field excludes CKD patients[7]. Furthermore, during the decision-making process the patient is a passive recipient of the diagnostic decision[32].
The pandemic of COVID-19 has taught us a great example of precision medicine. First it was discovered that patients respond differently to the “viral-intruder” and the host’s immune response, whether regulated or dysregulated leads to a favorable or unfavorable outcome respectively[33]. Later on, two distinct pathways were revealed[34] as well as an early biomarker for disease prediction (SUPAR-Soluble urokinase plasminogen activator receptor)[35]. This approach led to precision guided therapy with anakinra that showed remarkable benefit regarding respiratory failure and mortality[36].
In many aspects “sepsis” and “CKD” have many similarities. Both are heterogeneous syndromes with underlying “inflammation”. Sepsis is defined as “organ dysfunction caused by a dysregulated host response to infection”[37]. CKD is defined as kidney damage or GFR < 60 mL/min/1.73 m2 for 3 mo or more, irrespective of cause[38]. Based on this definition half of people over 75 are “labelled” as CKD, but there is debate if they can be regarded as “same risk” for renal deterioration or CV morbidity and mortality as younger people with the same stage of CKD[39]. At the same time CKD has a “systemic nature”[40] affecting multiple organ pathways, on a specific epigenetic background. In parallel sepsis, despite all the achievements in understanding its pathophysiology, is now regarded as “a multifaceted disruption of the finely tuned immunological balance of inflammation and anti-inflammation”[41]. There is a trend to identify patient phenotypes in order to stratify an accurate management[42].
The “big data” era of the last decade, a precious gift of the tremendous advances in computational technology has helped enormously diverse medical scientific fields, in terms of diagnosis, risk assessment and treatment, fueling precision medicine, but Nephrology field is lagging behind[43]. As an example, multi-omics data combined with clinical and demographic data helped to generate machine - learning models for prediction of preeclampsia[44]. Burning clinical issues regarding CKD patients, especially in advanced stages, as treatment of vascular disease, heart failure with reduced or preserved ejection fraction and prevention of sudden cardiac death do not have solid answers yet[8]. Big data science from electronic health records and longitudinal follow up could be a surrogate of RCTs assisting clinical decision[6].
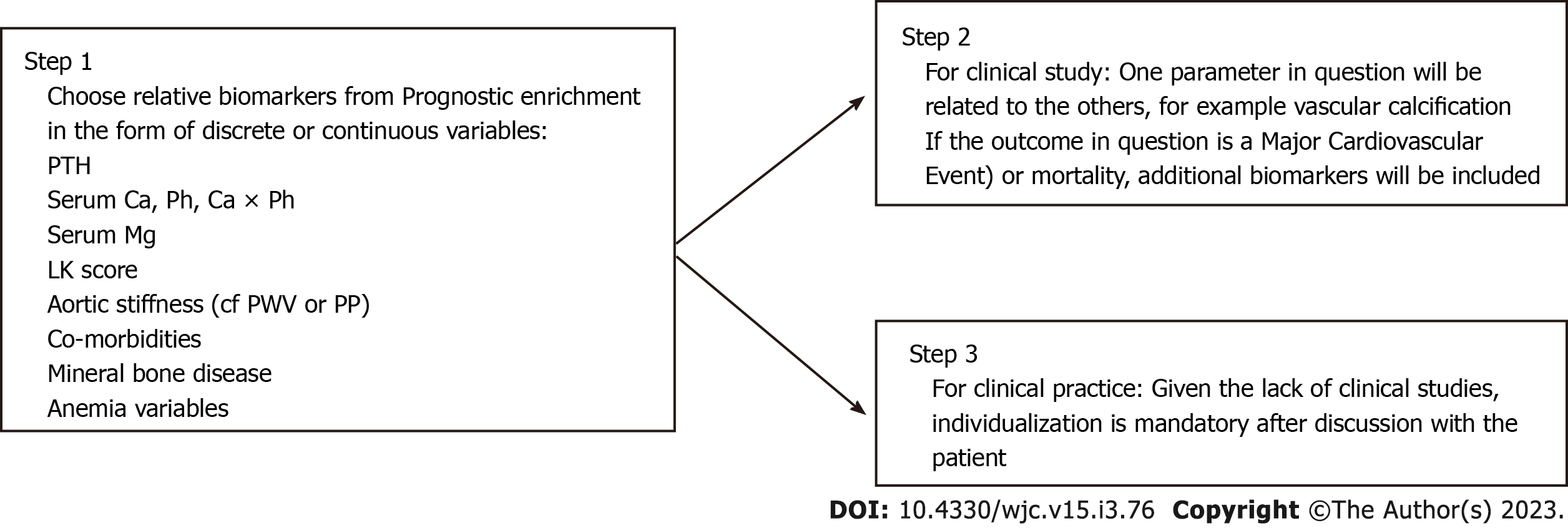
Our hypothesis is that there could be a paradigm shift in the field of nephrology regarding patient stratification and targeted management. In order to accomplish this transition the search for «biomarkers» could be helpful, as in sepsis[45]. The first step of a “prognostic enrichment”, i.e., select patients who are suspected to have high mortality, could be followed by “predictive enrichment”, i.e., patients who are likely to respond to a specific therapy (Figure 1). In this regard various biomarkers could be tested alone or in combination as: (1) Those already used in clinical practice (Table 1); and (2) the established biomarkers of cardiorenal syndrome[46] and those that could be found from multi- omics technology (blood and/or urine samples)[47].
| Category | Parameters | Evaluation method | |
| Continuous | Discrete based on trials | ||
| Laboratory | eGFR | CKD-EPI (mL/min/1.73 m2) | CKD stages 1,2,3a/b,4,5 |
| uACR | Mg albumin/g creatinine | Albuminuria stages A1,A2,A3 | |
| hs-CRP | |||
| Serum magnesium | |||
| PTH | Intact PTH (pg/mL) | KDIGO < 150, 150-500, > 500 pg/mL | |
| Anemia variables | Ht/Hb/TSAT/Ferritin/Hepcidin | ||
| Radiology | LVMI | LV mass indexed to body surface area (g/m2) | Geometry types |
| Lateral abdominal X-ray | Scale from 0-24 | Leena Kaupilla Score ≤ 4 vs > 4 | |
| Aortic stiffness | Pulse wave velocity carotid-femoral PWV (m/s) | CF-PWV < 8.8, 8.8-12, > 12 m/s | |
| Clinical status | Frailty | Nine-point clinical frailty scale | |
| Aortic stiffness | Pulse pressure (mmHg) | ||
| Physical activity | Handgrip strength | ||
| Diet | Mediterranean Diet Score Panagiotakos Scale 0-24 | ||
| Co-morbidities | DM, CAD, PAD, stroke, COPD | Charlson comorbidity index | |
| Bones | Mineral bone density (DEXA) | Values from DEXA (g/cm2) | Ostopenia ostoporosis |
One example of prognostic enrichment in nephrology involves the “heat map” based on GFR levels and albuminuria. It has been extensively validated and has a broad clinical application[48,49]. The CORD study in hemodialysis patients showed that vascular calcification (assessed from plain lateral abdominal X-ray), and arterial stiffness (measured by carotid-femoral pulse wave velocity) are independent prognostic markers of adverse outcome[50].
Regarding predictive enrichment one could utilize CORD study as an implementation paradigm (Figure 2). The authors showed that increased arterial stiffness -associated CV risk, is less pronounced at higher levels of calcification. Also an impressive number (19% of 993 pts “non - calcifiers” i.e., with no visible calcification deposits in lateral X-ray) was identified[51]. This implies the existence of genetic predisposition. This heterogeneity of the dialysis patient population could be contributing to the inconclusive results of the EVOLVE trial. In this study, lowering parathormone levels and targeting adverse CKD-MBD parameters as serum phosphorus and vascular calcification did not produce a statistically significant benefit in preventing CV events[52]. Another example is the interaction between two strong independent predictors of CV mortality, as serum magnesium (sMg)[53] in combination with abdominal aortic calcification (AAC). We have shown that in peritoneal dialysis patients with AAC in the higher tertile of the baseline distribution, sMg levels were not predictive of outcome[54].
Erythropoietin stimulating agents have revolutionized the treatment of CKD related anemia in the last decades. Hypoxia inducible factor polyl-hydroxylase inhibitors (HIF-PHIs) promote erythropoietin transcription and synthesis in the liver/kidney. INNO2VATE trials have proven the non-inferiority of vadadustat compared with darbopoetin - alfa concerning the cardiovascular safety[55]. However there are long-term safety concerns related to HIF pathway interactions involving tumor growth, diabetic retinopathy, and or CKD progression. Till now no HIF-PHI is licensed for the treatment of CKD-anemia within the European Union.
Considering erythropoietin use in CKD population a U-shaped effect exists[56]. The optimal erythropoietin dose to achieve the desired level of hemoglobin (10-11.5 g/dL) for the individual patient is not known and is almost always a matter of individual assessment. Furthermore, assays detecting markers of inflammation (e.g., hepcidin) which would predict clinical response in anemia lack in everyday clinical use.
In the stagnant era of effective treatment in the vulnerable population of CKD for CV morbidity and mortality, a paradigm shift seems mandatory. It is time to search for specific “biomarkers” to identify those at risk and even more those that would benefit from a targeted intervention. It is time to apply precision medicine through patient phenotypes.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ayar Y, Turkey; Gupta P, United States; Hasabo EA, Sudan; Li Z, China S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7995] [Cited by in RCA: 8524] [Article Influence: 405.9] [Reference Citation Analysis (0)] |
| 2. | Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1265] [Cited by in RCA: 1535] [Article Influence: 127.9] [Reference Citation Analysis (0)] |
| 3. | Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1271] [Cited by in RCA: 1419] [Article Influence: 83.5] [Reference Citation Analysis (0)] |
| 4. | Waziri B, Duarte R, Naicker S. Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD): Current Perspectives. Int J Nephrol Renovasc Dis. 2019;12:263-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 5. | Noels H, Jankowski J. Editorial on the Special Issue "Comorbidities in Chronic Kidney Disease". Toxins (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Nadkarni GN, Coca SG, Wyatt CM. Big data in nephrology: promises and pitfalls. Kidney Int. 2016;90:240-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Ishida JH, Johansen KL. Exclusion of Patients With Kidney Disease From Cardiovascular Trials. JAMA Intern Med. 2016;176:124-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation. 2021;143:1157-1172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 531] [Cited by in RCA: 1083] [Article Influence: 270.8] [Reference Citation Analysis (2)] |
| 9. | Wald R, Dorian P, Harel Z. Benefits and Risks of Anticoagulation in Dialysis Patients With Nonvalvular Atrial Fibrillation: Navigating Through Darkness. J Am Coll Cardiol. 2020;75:286-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Leifheit-Nestler M, Vogt I, Haffner D, Richter B. Phosphate Is a Cardiovascular Toxin. Adv Exp Med Biol. 2022;1362:107-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 11. | Vervloet M. Modifying Phosphate Toxicity in Chronic Kidney Disease. Toxins (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009;4:1089-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 431] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 13. | Chaiyakittisopon K, Pattanaprateep O, Ruenroengbun N, Sapankaew T, Ingsathit A, Mckay GJ, Attia J, Thakkinstian A. Evaluation of the cost-utility of phosphate binders as a treatment option for hyperphosphatemia in chronic kidney disease patients: a systematic review and meta-analysis of the economic evaluations. Eur J Health Econ. 2021;22:571-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Brown E, Wilding JPH, Alam U, Barber TM, Karalliedde J, Cuthbertson DJ. The expanding role of SGLT2 inhibitors beyond glucose-lowering to cardiorenal protection. Ann Med. 2021;53:2072-2089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 15. | Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7124] [Cited by in RCA: 8290] [Article Influence: 829.0] [Reference Citation Analysis (1)] |
| 16. | Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383:1436-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1586] [Cited by in RCA: 3130] [Article Influence: 626.0] [Reference Citation Analysis (1)] |
| 17. | Salvatore T, Galiero R, Caturano A, Rinaldi L, Di Martino A, Albanese G, Di Salvo J, Epifani R, Marfella R, Docimo G, Lettieri M, Sardu C, Sasso FC. An Overview of the Cardiorenal Protective Mechanisms of SGLT2 Inhibitors. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 133] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 18. | Chauveau P, Aparicio M, Bellizzi V, Campbell K, Hong X, Johansson L, Kolko A, Molina P, Sezer S, Wanner C, Ter Wee PM, Teta D, Fouque D, Carrero JJ; European Renal Nutrition (ERN) Working Group of the European Renal Association-European Dialysis Transplant Association (ERA-EDTA). Mediterranean diet as the diet of choice for patients with chronic kidney disease. Nephrol Dial Transplant. 2018;33:725-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 19. | Ikizler TA, Robinson-Cohen C, Ellis C, Headley SAE, Tuttle K, Wood RJ, Evans EE, Milch CM, Moody KA, Germain M, Limkunakul C, Bian A, Stewart TG, Himmelfarb J. Metabolic Effects of Diet and Exercise in Patients with Moderate to Severe CKD: A Randomized Clinical Trial. J Am Soc Nephrol. 2018;29:250-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 20. | Bacharaki D, Petrakis I, Kyriazis P, Markaki A, Pleros C, Tsirpanlis G, Theodoridis M, Balafa O, Georgoulidou A, Drosataki E, Stylianou K. Adherence to the Mediterranean Diet Is Associated with a More Favorable Left Ventricular Geometry in Patients with End-Stage Kidney Disease. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 21. | Yau K, Dharia A, Alrowiyti I, Cherney DZI. Prescribing SGLT2 Inhibitors in Patients With CKD: Expanding Indications and Practical Considerations. Kidney Int Rep. 2022;7:1463-1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 115] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 22. | Cases A, Cigarrán-Guldrís S, Mas S, Gonzalez-Parra E. Vegetable-Based Diets for Chronic Kidney Disease? Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 23. | Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4997] [Cited by in RCA: 6583] [Article Influence: 822.9] [Reference Citation Analysis (0)] |
| 24. | Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14:576-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1183] [Cited by in RCA: 1871] [Article Influence: 267.3] [Reference Citation Analysis (0)] |
| 25. | Barreto DV, Barreto FC, Liabeuf S, Temmar M, Lemke HD, Tribouilloy C, Choukroun G, Vanholder R, Massy ZA; European Uremic Toxin Work Group (EUTox). Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int. 2010;77:550-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 229] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 26. | Pecoits-Filho R, Bárány P, Lindholm B, Heimbürger O, Stenvinkel P. Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant. 2002;17:1684-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 292] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 27. | Cho Y, Johnson DW, Vesey DA, Hawley CM, Pascoe EM, Clarke M, Topley N; balANZ Trial Investigators. Baseline serum interleukin-6 predicts cardiovascular events in incident peritoneal dialysis patients. Perit Dial Int. 2015;35:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Carrero JJ, Stenvinkel P. Persistent inflammation as a catalyst for other risk factors in chronic kidney disease: a hypothesis proposal. Clin J Am Soc Nephrol. 2009;4 Suppl 1:S49-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 29. | Kato S, Chmielewski M, Honda H, Pecoits-Filho R, Matsuo S, Yuzawa Y, Tranaeus A, Stenvinkel P, Lindholm B. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3:1526-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 778] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 30. | Bacharaki D, Diamandopoulos A. Emperor's syndrome in the COVID-19 era: Time for patient-centered nephrology? World J Nephrol. 2021;10:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Hajar R. History of medicine timeline. Heart Views. 2015;16:43-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Weaver RR. Reconciling evidence-based medicine and patient-centred care: defining evidence-based inputs to patient-centred decisions. J Eval Clin Pract. 2015;21:1076-1080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3174] [Cited by in RCA: 2916] [Article Influence: 583.2] [Reference Citation Analysis (0)] |
| 34. | Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, Damoraki G, Gkavogianni T, Adami ME, Katsaounou P, Ntaganou M, Kyriakopoulou M, Dimopoulos G, Koutsodimitropoulos I, Velissaris D, Koufargyris P, Karageorgos A, Katrini K, Lekakis V, Lupse M, Kotsaki A, Renieris G, Theodoulou D, Panou V, Koukaki E, Koulouris N, Gogos C, Koutsoukou A. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe. 2020;27:992-1000.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1306] [Cited by in RCA: 1556] [Article Influence: 311.2] [Reference Citation Analysis (0)] |
| 35. | Rovina N, Akinosoglou K, Eugen-Olsen J, Hayek S, Reiser J, Giamarellos-Bourboulis EJ. Soluble urokinase plasminogen activator receptor (suPAR) as an early predictor of severe respiratory failure in patients with COVID-19 pneumonia. Crit Care. 2020;24:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 36. | Kyriazopoulou E, Poulakou G, Milionis H, Metallidis S, Adamis G, Tsiakos K, Fragkou A, Rapti A, Damoulari C, Fantoni M, Kalomenidis I, Chrysos G, Angheben A, Kainis I, Alexiou Z, Castelli F, Serino FS, Tsilika M, Bakakos P, Nicastri E, Tzavara V, Kostis E, Dagna L, Koufargyris P, Dimakou K, Savvanis S, Tzatzagou G, Chini M, Cavalli G, Bassetti M, Katrini K, Kotsis V, Tsoukalas G, Selmi C, Bliziotis I, Samarkos M, Doumas M, Ktena S, Masgala A, Papanikolaou I, Kosmidou M, Myrodia DM, Argyraki A, Cardellino CS, Koliakou K, Katsigianni EI, Rapti V, Giannitsioti E, Cingolani A, Micha S, Akinosoglou K, Liatsis-Douvitsas O, Symbardi S, Gatselis N, Mouktaroudi M, Ippolito G, Florou E, Kotsaki A, Netea MG, Eugen-Olsen J, Kyprianou M, Panagopoulos P, Dalekos GN, Giamarellos-Bourboulis EJ. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med. 2021;27:1752-1760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 380] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 37. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 17193] [Article Influence: 1910.3] [Reference Citation Analysis (2)] |
| 38. | Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67:2089-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2145] [Cited by in RCA: 2521] [Article Influence: 126.1] [Reference Citation Analysis (0)] |
| 39. | Ellam T, Twohig H, Khwaja A. Chronic kidney disease in elderly people: disease or disease label? BMJ. 2016;352:h6559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Zoccali C, Vanholder R, Massy ZA, Ortiz A, Sarafidis P, Dekker FW, Fliser D, Fouque D, Heine GH, Jager KJ, Kanbay M, Mallamaci F, Parati G, Rossignol P, Wiecek A, London G; European Renal and Cardiovascular Medicine (EURECA-m) Working Group of the European Renal Association - European Dialysis Transplantation Association (ERA-EDTA). The systemic nature of CKD. Nat Rev Nephrol. 2017;13:344-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 301] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 41. | Jarczak D, Kluge S, Nierhaus A. Sepsis-Pathophysiology and Therapeutic Concepts. Front Med (Lausanne). 2021;8:628302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 202] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 42. | Li H, Markal A, Balch JA, Loftus TJ, Efron PA, Ozrazgat-Baslanti T, Bihorac A. Methods for Phenotyping Adult Patients in Sepsis and Septic Shock: A Scoping Review. Crit Care Explor. 2022;4:e0672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Saez-Rodriguez J, Rinschen MM, Floege J, Kramann R. Big science and big data in nephrology. Kidney Int. 2019;95:1326-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 44. | Marić I, Contrepois K, Moufarrej MN, Stelzer IA, Feyaerts D, Han X, Tang A, Stanley N, Wong RJ, Traber GM, Ellenberger M, Chang AL, Fallahzadeh R, Nassar H, Becker M, Xenochristou M, Espinosa C, De Francesco D, Ghaemi MS, Costello EK, Culos A, Ling XB, Sylvester KG, Darmstadt GL, Winn VD, Shaw GM, Relman DA, Quake SR, Angst MS, Snyder MP, Stevenson DK, Gaudilliere B, Aghaeepour N. Early prediction and longitudinal modeling of preeclampsia from multiomics. Patterns (N Y). 2022;3:100655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 45. | Seymour CW, Kennedy JN, Wang S, Chang CH, Elliott CF, Xu Z, Berry S, Clermont G, Cooper G, Gomez H, Huang DT, Kellum JA, Mi Q, Opal SM, Talisa V, van der Poll T, Visweswaran S, Vodovotz Y, Weiss JC, Yealy DM, Yende S, Angus DC. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA. 2019;321:2003-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 832] [Article Influence: 138.7] [Reference Citation Analysis (0)] |
| 46. | Goffredo G, Barone R, Di Terlizzi V, Correale M, Brunetti ND, Iacoviello M. Biomarkers in Cardiorenal Syndrome. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 47. | Virzì GM, Clementi A, Battaglia GG, Ronco C. Multi-Omics Approach: New Potential Key Mechanisms Implicated in Cardiorenal Syndromes. Cardiorenal Med. 2019;9:201-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Menon V, Greene T, Wang X, Pereira AA, Marcovina SM, Beck GJ, Kusek JW, Collins AJ, Levey AS, Sarnak MJ. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68:766-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 285] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 49. | Matsushita K, Jassal SK, Sang Y, Ballew SH, Grams ME, Surapaneni A, Arnlov J, Bansal N, Bozic M, Brenner H, Brunskill NJ, Chang AR, Chinnadurai R, Cirillo M, Correa A, Ebert N, Eckardt KU, Gansevoort RT, Gutierrez O, Hadaegh F, He J, Hwang SJ, Jafar TH, Kayama T, Kovesdy CP, Landman GW, Levey AS, Lloyd-Jones DM, Major RW, Miura K, Muntner P, Nadkarni GN, Naimark DM, Nowak C, Ohkubo T, Pena MJ, Polkinghorne KR, Sabanayagam C, Sairenchi T, Schneider MP, Shalev V, Shlipak M, Solbu MD, Stempniewicz N, Tollitt J, Valdivielso JM, van der Leeuw J, Wang AY, Wen CP, Woodward M, Yamagishi K, Yatsuya H, Zhang L, Schaeffner E, Coresh J. Incorporating kidney disease measures into cardiovascular risk prediction: Development and validation in 9 million adults from 72 datasets. EClinicalMedicine. 2020;27:100552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 50. | Verbeke F, Van Biesen W, Honkanen E, Wikström B, Jensen PB, Krzesinski JM, Rasmussen M, Vanholder R, Rensma PL; CORD Study Investigators. Prognostic value of aortic stiffness and calcification for cardiovascular events and mortality in dialysis patients: outcome of the calcification outcome in renal disease (CORD) study. Clin J Am Soc Nephrol. 2011;6:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 51. | Honkanen E, Kauppila L, Wikström B, Rensma PL, Krzesinski JM, Aasarod K, Verbeke F, Jensen PB, Mattelaer P, Volck B; CORD study group. Abdominal aortic calcification in dialysis patients: results of the CORD study. Nephrol Dial Transplant. 2008;23:4009-4015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 52. | EVOLVE Trial Investigators, Chertow GM, Block GA, Correa-Rotter R, Drüeke TB, Floege J, Goodman WG, Herzog CA, Kubo Y, London GM, Mahaffey KW, Mix TC, Moe SM, Trotman ML, Wheeler DC, Parfrey PS. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482-2494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 633] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 53. | Xiong J, He T, Wang M, Nie L, Zhang Y, Wang Y, Huang Y, Feng B, Zhang J, Zhao J. Serum magnesium, mortality, and cardiovascular disease in chronic kidney disease and end-stage renal disease patients: a systematic review and meta-analysis. J Nephrol. 2019;32:791-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 54. | Giannakopoulos P, Fokas S, Drosataki E, Tsotsorou O, Duni A, Theodoridis M, Stylianou K, Ntounousi E, Passadakis P, Katsoudas S, Kyriazis P, Bacharaki D, Vlahakos D. Abdominal Aortic Calcification Score Modifies the Prognostic Value of Serum Magnesium Levels on All-Cause Mortality in Peritoneal Dialysis Patients. Nephrol Dial Transplant. 2020;35:1168. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 55. | Eckardt KU, Agarwal R, Aswad A, Awad A, Block GA, Bacci MR, Farag YMK, Fishbane S, Hubert H, Jardine A, Khawaja Z, Koury MJ, Maroni BJ, Matsushita K, McCullough PA, Lewis EF, Luo W, Parfrey PS, Pergola P, Sarnak MJ, Spinowitz B, Tumlin J, Vargo DL, Walters KA, Winkelmayer WC, Wittes J, Zwiech R, Chertow GM. Safety and Efficacy of Vadadustat for Anemia in Patients Undergoing Dialysis. N Engl J Med. 2021;384:1601-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 56. | Regidor DL, Kopple JD, Kovesdy CP, Kilpatrick RD, McAllister CJ, Aronovitz J, Greenland S, Kalantar-Zadeh K. Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J Am Soc Nephrol. 2006;17:1181-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 300] [Article Influence: 15.8] [Reference Citation Analysis (0)] |