Published online Feb 26, 2023. doi: 10.4330/wjc.v15.i2.64
Peer-review started: September 19, 2022
First decision: November 2, 2022
Revised: November 17, 2022
Accepted: February 8, 2023
Article in press: Feburary 8, 2023
Published online: February 26, 2023
Processing time: 155 Days and 3.5 Hours
Pulmonary vein stenosis (PVS) is an uncommon but known cause of morbidity and mortality in adults and children and can be managed with percutaneous re-vascularization strategies of pulmonary vein balloon angioplasty (PBA) or pulmonary vein stent implantation (PSI).
To study the safety and efficacy outcomes of PBA vs PSI in all patient categories with PVS.
We performed a literature search of all studies comparing outcomes of patients evaluated by PBA vs PSI for PVS. We selected all published studies comparing PBA vs PSI for PVS with reported outcomes of restenosis and procedure-related complications in all patient categories. In adults, PVS following atrial fibrillation ablation and in children PVS related to congenital etiology or post-procedural PVS following total or partial anomalous pulmonary venous return repair were included. The patient-centered outcomes were risk of restenosis requiring re-intervention and procedural-related complications. The meta-analysis was performed by computing odds ratios (ORs) using the random effects model based on underlying statistical heterogeneity.
Eight observational studies treating 768 severe PVS in 487 patients met our inclusion criteria. The age range of patients was 6 months to 70 years and 67% were males. The primary outcome of the re-stenosis requiring re-intervention occurred in 196 of 325 veins in the PBA group and 111 of 443 veins in the PSI group. Compared to PSI, PBA was associated with a significantly increased risk of re-stenosis (OR 2.91, 95%CI: 1.15-7.37, P = 0.025, I2 = 79.2%). Secondary outcomes of the procedure-related complications occurred in 7 of 122 patients in the PBA group and 6 of 69 in the PSI group. There were no statistically significant differences in the safety outcomes between the two groups (OR: 0.94, 95%CI: 0.23-3.76, P = 0.929), I2 = 0.0%).
Across all patient categories with PVS, PSI is associated with reduced risk of re-intervention and is as safe as PBA and should be considered first-line therapy for PVS.
Core Tip: 81.5% of patients with pulmonary vein stenosis undergoing a transcatheter intervention reported symptom of dyspnea. Pulmonary vein stent implantation (PSI) was superior to pulmonary vein balloon angioplasty (PBA) in preventing restenosis of the pulmonary vein. No difference in procedural related complications was noted between PSI and PBA. Differences in peri-procedural anticoagulation strategies between studies could have affected the outcome.
- Citation: Agasthi P, Sridhara S, Rattanawong P, Venepally N, Chao CJ, Ashraf H, Pujari SH, Allam M, Almader-Douglas D, Alla Y, Kumar A, Mookadam F, Packer DL, Holmes DR Jr, Hagler DJ, Fortuin FD, Arsanjani R. Safety and efficacy of balloon angioplasty compared to stent-based-strategies with pulmonary vein stenosis: A systematic review and meta-analysis. World J Cardiol 2023; 15(2): 64-75
- URL: https://www.wjgnet.com/1949-8462/full/v15/i2/64.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i2.64
Catheter ablation for atrial fibrillation in adults involves the use of radiofrequency energy to electrically isolate the pulmonary vein[1]. As injured tissue heals scar tissue extends deeper into vein from the ostium leading to pulmonary vein stenosis (PVS). Cryoballoon ablation therapy for atrial fibrillation can have similar consequences[2]. With increased utilization of techniques aimed to reduce PVS such as antral isolation, 3-dimensional mapping and use of intra-cardiac ultrasound, the incidence of PVS has declined substantially from 20%-40% to 1%-1.5% currently[3]. In children, PVS can be primary (idiopathic) or secondary (post-surgical) following repair of total or partial anomalous pulmonary venous return[4], post pulmonary vein isolation and in Fibrosing Mediastinitis, where the patients develop severe pulmonary vein stenosis which is challenging to treat. Patients with severe PVS report symptoms of pleuritic chest pain, cough, hemoptysis and dyspnea on exertion. Untreated severe PVS can be progressive leading to irreversible lung parenchymal damage, pulmonary hypertension, heart failure and death[5].
Percutaneous intervention with balloon angioplasty (PBA) or pulmonary vein stent implantation (PSI) is the current treatment modality in adults. Re-stenosis risk after percutaneous interventions is higher in all patient categories and there is increasing adoption of stent-based strategies[6]. Available literature on this topic reports risk of restenosis with balloon angioplasty in the range of 44%-73%[6-8] and risk of re-stenosis of stent-based strategies over 16 years is 18%[8]. PVS confers poor prognosis in children and is conventionally treated with catheter intervention including PBA/PSI and/or surgery. The former has been considered as a palliative approach. The mortality rate is as high as 47% at a median follow-up of 2 mo and re-intervention appeared to improve survival[5] and children with bare metal stents had better survival compared to drug-eluting stents (DES) and biliary atresia (BA)[9]. This may be dependent on the vessel size and on adjunctive therapy. The aim of this study is to perform a comprehensive analysis of safety and efficacy outcomes of percutaneous re-vascularization strategies of BA vs stent-based strategies for PVS in all patient categories.
The protocol detailing the methods of the systematic review and meta-analysis was registered on the International Prospective Register of Systematic Reviews. The current meta-analysis was performed using the guidelines set by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)[10]. Ethical review and approval were waived for this study, as our study is a meta-analysis and involves no interaction with human subjects and access to any subject identifiers.
We performed a comprehensive search for studies comparing PBA vs PSI in patient with PVSs using scientific databases (PubMed, EMBASE, Cochrane, Web of science, Scopus) from inception to December 2019. The search terms were pulmonary vein stenosis, balloon angioplasty, pulmonary balloon angioplasty, stents. The last search was run on December 31st, 2019. The authors (PA and SS) developed the search strategy along with a clinical information specialist (DA–D). The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist. Details of the search strategy are provided in the Supplementary Table 1-PRISMA checklist.
| Ref. | Patients | Mean age (yr) | Males (%) | Frequency of clinical symptoms; Dyspnea (%) | Hemoptysis (%) | Asymptomatic (%) | Severe PVS treated (n) |
| Qureshi et al[19] | 19 | 51 ± 13 | NA | 95 | 63 | 5 | 37 |
| Prieto et al[7] | 44 | 53 ± 11 | 70 | 88 | 23 | 7 | 68 |
| Neumann et al[6] | 12 | 58 | 70 | 77 | 8 | 17 | 15 |
| Fender et al[20] | 113 | 50 | 77 | 67 | 27 | 0 | 178 |
| Cory et al[5] | 30 | Median age-6.4 m | 50 | NA | NA | NA | 58 |
| Schoene et al[15] | 39 | 62.1 ± 9.0 | 60 | 79 | 26 | NA | 61 |
| Kurita et al[9] | 31 | 7 mo | 65 | NA | NA | NA | 53 |
| Suntharos et al[8] | 199 | 55 ± 12 | 78 | 83 | 13 | 13 | 319 |
Initial screening of the search results was performed by two reviewers (PA and SS). Title and abstract screening were first performed followed by comprehensive review of the entire manuscripts. When inconsistencies in screening were found and no consensus was reached a third reviewer (RA) casted the deciding vote.
We selected all published studies comparing PBA vs PSI for PVS with reported outcomes of re-stenosis and procedure-related complications in all patient categories. In adults, PVS following atrial fibrillation ablation and in children PVS related to congenital etiology or post-procedural PVS following total or partial anomalous pulmonary venous return repair are included. All types of stents are included. No restrictions on study selection based on outcomes were used. Studies which assessed stent-based strategies without PBA group, abstracts which are published without full text publications and studies lacking endpoint measures were excluded.
For all the studies included, we extracted: (1) Study participants characteristics including age, gender, imaging modality after ablation, frequency of clinical symptoms related to PVS, study's inclusion criteria; (2) types of intervention- PBA vs PSI, stent size, post-intervention antiplatelet therapy and follow up imaging; and (3) outcome measures including re-stenosis requiring re-intervention and procedure-related complications. Cochrane Consumers and Communication Review Group's data extraction template was used to develop a standardized data extraction sheet for screening studies. The two authors independently collected the data and kappa values were used to report agreement measures. The primary outcome was re-stenosis requiring re-intervention and the secondary outcome was major complications related to procedures including death, major adverse cardiac and cerebrovascular events, major in-hospital complications requiring prolonged hospitalization or additional therapy (i.e. major bleeding or vascular complication, cardiac tamponade)
The study quality of included studies was assessed using the Newcastle-Ottawa Quality Assessment Scale for Cohort Studies as shown in Supplementary Table 2 (http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm). Briefly, studies were quoted using prespecified items on patients' selection (representativeness and selection of patients, ascertainment of exposure, demonstration that outcome of interest was not present at the start of the study), comparability of cohorts based on the design or analysis, and assessment of outcomes (recording, adequacy of follow-up including length of follow up). Ratings for each item were added to provide a study quality score (maximal score, 9). Two independent reviewers (PA and SS) performed the Newcastle-Ottawa Scale grading. Discrepancies were resolved by consensus.
| Ref. | Study type | Enrolment Period | Main inclusion criteria | Imaging after ablation | Mean time between PVI and clinical symptoms | Revascularization approach | Stent size | Acute angiographic success | Primary outcome at follow-up | Follow-up |
| Qureshi et al[19], 2003 | Observational retrospective study | 2000-2002 | Severe PVS with clinical symptoms | CT-scans in symptomatic patients | 4 mo | Stepwise | 4-10 mm | NA | Freedom of reintervention | 10 ± 9 mo |
| Prieto et al[7], 2008 | Observational retrospective study | 2000-2007 | Severe PVS with clinical symptoms | CT-scans, lung perfusion scans in symptomatic patients | 11.5 mo | Stepwise/primary stenting | 8-10 mm | Residual stenosis ≤ 30% | Recurrence of symptoms requiring reintervention | 25 ± 21 mo |
| Neumann et al[6], 2009 | Observational prospective study | 2003-2005 | Severe PVS (> 70%) with clinical symptoms and/or significant perfusion defect | Surveillance imaging with MRI, lung perfusion scans, CT scans, TTE every 3 mo | NA | Stepwise (if rebound stenosis was observed after balloon dilatation)/primary stenting | 8-12 mm | NA | Clinically symptomatic restenosis | 48 mo |
| Fender et al[20], 2016 | Observational prospective study | 2000-2014 | Severe PVS (> 75%) with clinical symptoms | Surveillance imaging with CT-scans at 3 mo + CT-scans and lung perfusion scans in symptomatic patients | 4.0 ± 3.0 mo | Stepwise | 6-10 mm + DES 4 mm | Residual stenosis < 20% | Clinically symptomatic restenosis | 48 mo |
| Cory et al[5], 2017 | Observational retrospective study | 2005-2016 | Catheter intervention for PVS for patients < 18 yr | NA | NA | Stepwise/primary stenting | Median-DES 4 mm, BMS 5 mm | NA | Mortality following transcatheter PV intervention | Median of 30.6 mo |
| Schoene et al[15], 2018 | Observational retrospective study | 2004-2017 | Symptomatic PVS with > 70% in a single stenosis or > 60% in multiple ipsilateral stenosis | Initial screening process from 2004-2007- TEE 6-12 mo after PVI or when symptomatic, subsequent CT or MRI. Screening terminated in 2008, symptomatic patients underwent CT, MRI and/or PV angiography | 10.2 ± 8.0 mo | Stepwise/primary stenting | Median stent- 7 mm × 20 mm, DES 5 mm | Residual stenosis < 10%-20% | Restenosis rate following transcatheter intervention | Median of 6 mo |
| Kurita et al[9], 2019 | Observational retrospective study | 2001-2017 | PVS associated with total anomalous pulmonary venous connection and isolated congenital PVS | Combination of ultrasound, CT and angiography | Median 7 from birth | Stepwise/primary stenting-PCI/hybrid surgery | 3-8 mm | NA | In-stent restenosis following stent placement using CT or angiography ≥ 50% higher stenosis of stent size | 19 mo |
| Suntharos et al[8], 2019 | Observational retrospective study | 2000-2016 | PVS after PVI undergoing PCI | CT-scan pulmonary vein protocol, quantitative lung perfusion scan | NA | Stepwise/primary stenting | 3-16 mm | NA | Freedom of reinrevention | Median follow up-17 mo |
The meta-analysis was performed by computing odds ratios (ORs) using the random effects model based on underlying statistical heterogeneity. A biomedical statistician performed the statistical review of the study. We calculated the OR and 95% confidence intervals (CIs) for each treatment effect for each study and pooled the point estimates of OR from each study using the generic inverse-variance method of Der Simonian and Laird[10,11]. Stata SE Statistical Software: Release 14.1, College Station, TX: StataCorp LP, StataCorp 2015. I2 statistics were used to test statistical heterogeneity. The I2 statistics describes the percentage of variation across studies that is because of heterogeneity rather than those expected by random chance [I2 = 100% × (Q-df)/Q].
A CI for I2 was constructed using either (1) noncentral chi-squared distribution method of Hedges and Piggott (2001) or (2) test-based method of Higgins and Thompson. The heterogeneity of effect size estimates across these studies was quantified using the I2 statistic. The I2 statistic ranges in value from 0 to 100% (I2< 25%, low heterogeneity; I2= 25%–50%, moderate heterogeneity; and I2> 50%, substantial heterogeneity)[12]. Publication bias was assessed using a funnel plot and Egger’s regression test[13] (P < 0.05 was considered significant). A summary of evidence table was created to summarize the main results (patient-centered outcomes) using the GRADE Pro tool [Guideline Development Tool (Software), McMaster University, 2015 (developed by Evidence Prime, Inc)][14]. Sensitivity analysis was performed for primary analysis through an influence analysis by omitting one study at a time.
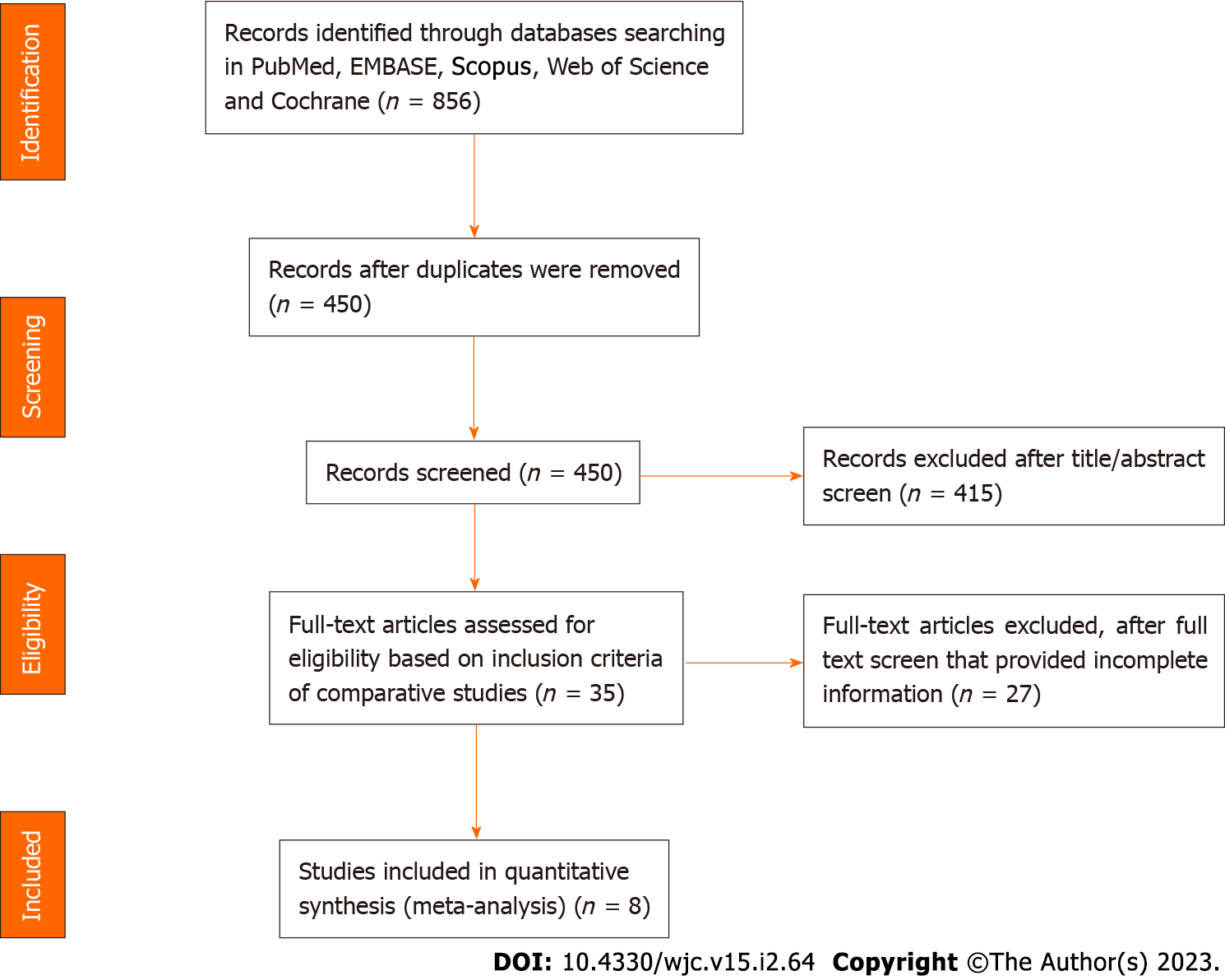
A total of 856 Citations were identified using Pubmed, EMBASE, Scopus, Web of Science, and Cochrane databases. We excluded 415 studies based on the title and abstracts. After these exclusions and screening rest of the studies in detail we found eight studies that met the inclusion criteria mentioned above. The PRISMA diagram was created for the systematic review Figure 1. Kappa for agreement on full text, and abstract inclusion was 0.89 (95%CI: 0.86-0.94).
Table 1 and 2 summarizes the study characteristics. The trials that were included were published between 2003 and 2019. Studies were observational prospective and retrospective cohort studies and had a follow-up duration of 6 mo to 48 mo. A total of 487 patients were included in this meta-analysis. Study population included children and adults; the age range of patients was 6 mo to 70 years. 67% of the study population were males, 81.5% of the study population reported symptoms of dyspnea and 8.4% of patients were asymptomatic. 768 severe PVS lesions were included from all studies. Severe pulmonary vein was defined as > 70% luminal stenosis of the pulmonary vein based on computed tomography (CT) imaging. For adults with PVS, the time between atrial fibrillation ablation/pulmonary vein isolation to the development of clinical symptoms ranged from 1 mo to 18 mo. The imaging protocols used to diagnose PVS were contrast-enhanced spiral CT scans, magnetic resonance imaging, lung perfusion scans. PVS was confirmed by invasive angiography. Procedural aspects consisted of right heart hemodynamic monitoring, selective pulmonary angiography, and access of left atrium by transseptal puncture. Interventions performed were predilation, gradual balloon dilation, stenting in a stepwise manner or primary stenting. Pulmonary vein surgery was required in 5 children in reintervention group with pericardial well procedure[5] and hybrid stenting was performed after cardiac arrest in the operating room in some children with precluding anatomic factors, difficult vascular access, multiple closely spaced ostium[9]. Post-procedural antiplatelet and anticoagulant therapy was employed to ensure vessel patency. CT imaging and other imaging modalities were employed to follow up patients (Table 3).
| Ref. | Antiplatelet therapy | Imaging modalities | Restenosis definition |
| Qureshi et al[19], 2003 | NR | CT-scans every 3 mo | PV narrowing > 70% of the original PV lumina |
| Prieto et al[7], 2008 | NR | CT-scans, lung perfusion scans at 3-12-24 mo | NR |
| Neumann et al[8], 2009 | ASA+Clopidogrel+Coumadin for 3 mo | CT-scans, lung perfusion scans every 3 mo | PV narrowing > 70%of the original PV lumina before PVI |
| Fender et al[20], 2016 | Coumadin+Clopidogrel | CT-scans, lung perfusion scans at 3-12-24 mo | PV narrowing > 75% in the previously treated PV |
| Cory et al[5], 2017 | NA | Angiography | Vein loss defined as PV atresia or PVs of uncertain status in deceased patients |
| Schoene et al[15], 2018 | ASA 4 weeks+Clopidogrel 6 mo+Coumadin or DOACs | CT-scans, MR imaging | PV narrowing > 70% in the previously treated PV |
| Kurita et al[9], 2019 | ASA, Ticlopidin, Warfarin | CT or angiography | In stent restenosis: ≥ 50% luminal narrowing |
| Suntharos et al[8], 2019 | Anticoagulation followed by low-dose aspirin | CT-scans, lung perfusion scans, angiography based on intervention-3 mo, 6 mo, 1yr | Severe restenosis/concern for progression to total occlusion |
The study compared PBA with PSI for patients with PVS. Bare metal stents, DES and hybrid stents placed surgically in children were included in this meta-analysis.
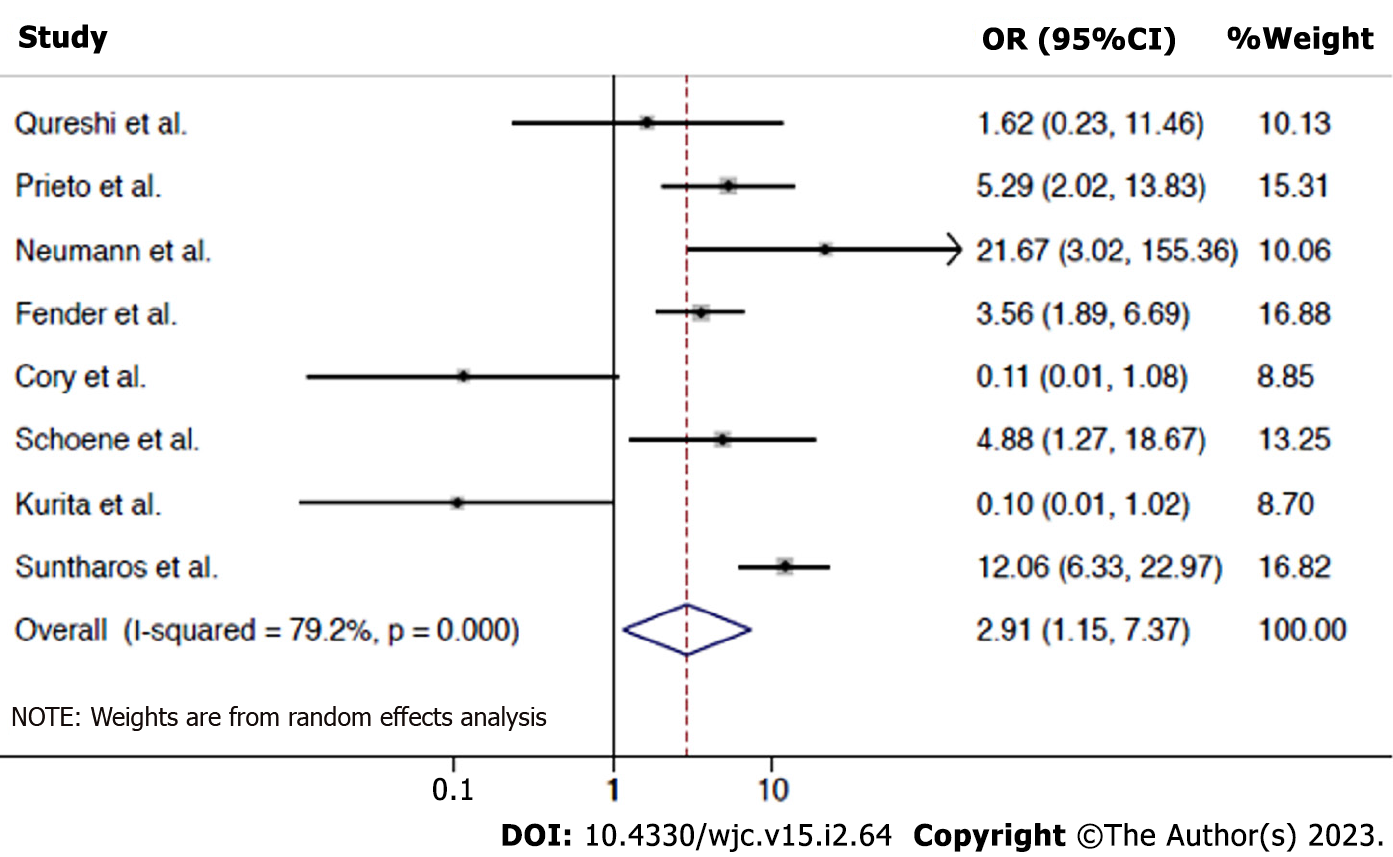
Risk of re-stenosis requiring re-intervention: The data were available for all the 8 studies including 487 patients. 196 events occurred in 325 PBA interventions and 111 events occurred in 443 PSI interventions. Results show that PBA is associated with a significantly higher risk of re-stenosis compared to PSI (OR 2.91, 95%CI: 1.15-7.37, P = 0.025). A high degree of heterogeneity was noted (I2 = 79.2%). Figure 2 shows the forest plots analysis for this outcome.
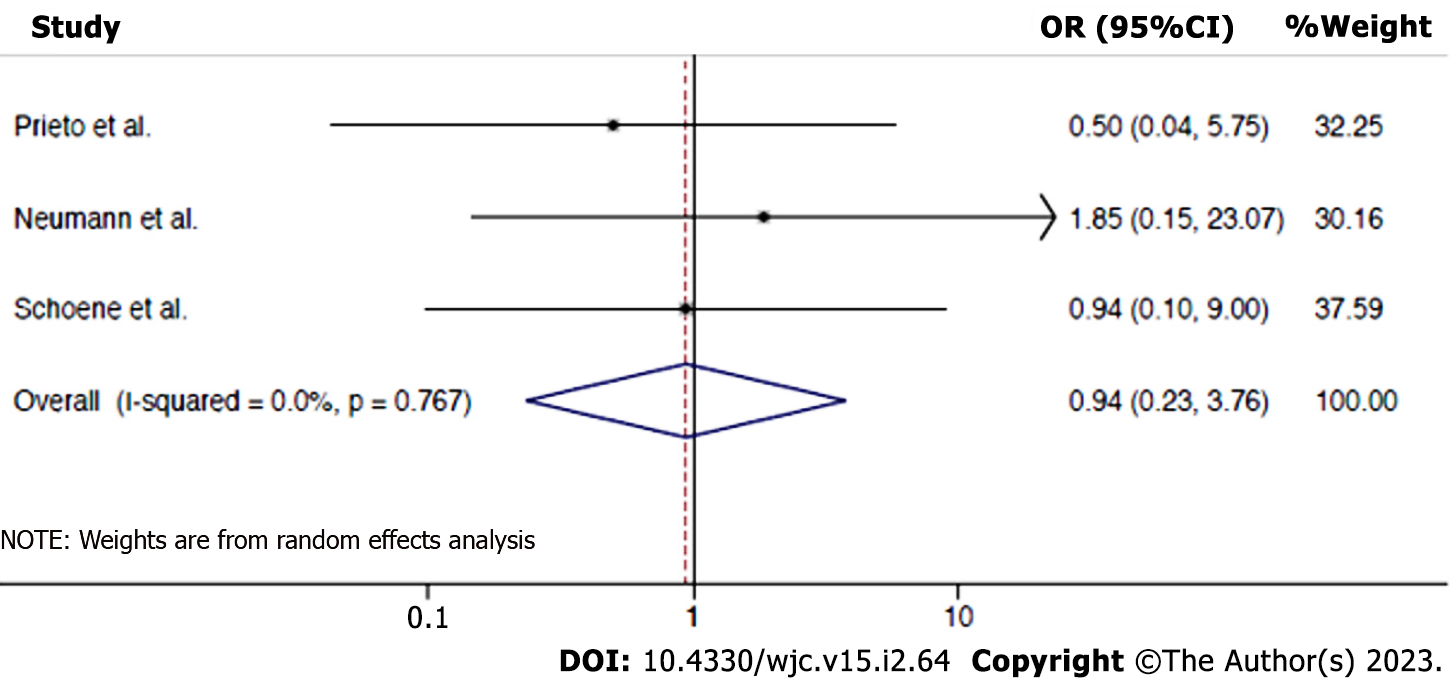
Risk of procedure-related complications: The data was available for 3 studies, 7 events occurred in 122 PBA interventions and 6 in 69 PSI interventions. Overall results show that there is no difference in procedure-related complications between PBA vs PSI for PVS (OR: 0.94, 95%CI: 0.23-3.76, P = 0.929), without heterogeneity (I2 = 0.0%). The forest plot analysis for this outcome is shown in Figure 3. In a study by Prieto et al[7], one patient in PBA group while undergoing pulmonary vein (PV) dilation developed an intimal flap needing stenting and had a transient ischemic event without permanent debility. Two patients in the stenting group developed tamponade requiring evacuation of pericardial space but there was no mortality. In a study by Neumann et al[6], there were 3 adverse events- one patient developed hemoptysis immediately after dilation of the left upper PV which stopped 10 min after protamine administration, one patient developed small dissection of the left upper PV during dilation before stenting distally with clinical hemoptysis which resolved by additional stenting of the vein distal to the original stenosis and allergic reaction to the contrast agent used was seen in one patient. In a study by Schoene et al[15], major events in PBA group were 2 wire-induced PV perforations with tamponade managed by pericardiocentesis and 2 balloon-induced PV ruptures with tamponade managed by urgent surgical repair in one and emergency stenting and pericardiocentesis in another. In the stent group, an acute stent thrombosis resulting in a stroke occurred which was complicated by intracerebral bleeding with thrombolytic therapy use but there was no mortality. Supplementary Tablesprovide further information regarding outcomes in the included studies (Supplementary Tables 3 and
| Outcomes | Anticipated absolute effectsa (95%CI) | Relative effect (95%CI) | No. of participants (studies) | Certainty of the evidence (GRADE)b | |
| Risk with PSI | Risk with PBA | ||||
| Restenosis | 251 per 1000 | 493 per 1000 (278 to 711) | OR 2.91 (1.15 to 7.37) | 487 (8 observational studies) | ⊕⊕⊕◯ MODERATEc |
| Procedure related complications | 87 per 1000 | 82 per 1000 (21 to 264) | OR 0.94 (0.23 to 3.76) | 191 (3 observational studies) | ⊕⊕⊕◯ MODERATEc |
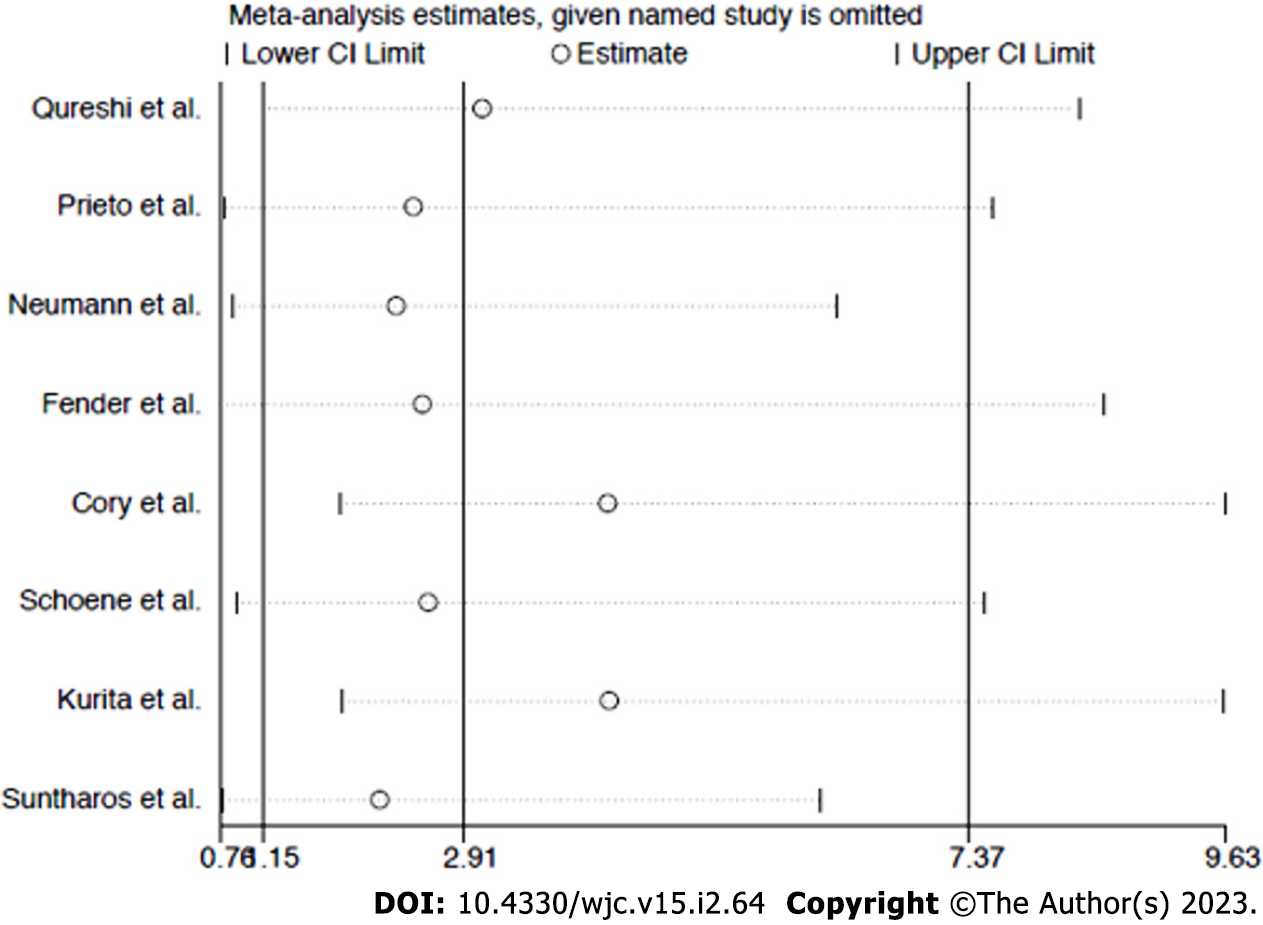
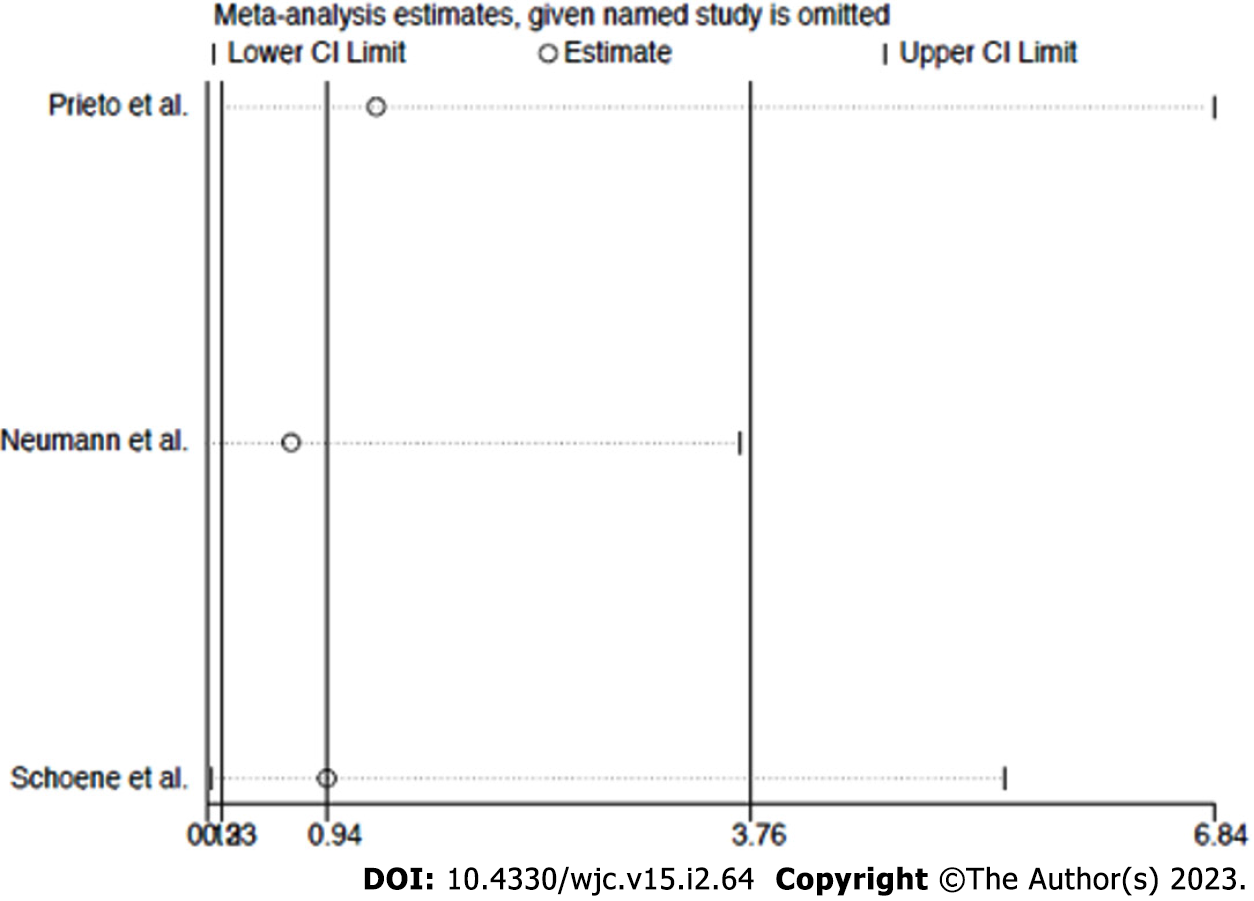
Sensitivity analysis: The funnel plot distribution of outcomes was derived from the standard error of the logarithm OR plotted against the OR of re-stenosis and procedure-related complications, respectively (Supplementary Figures 1 and 2). Influence analysis demonstrated that no single study significantly altered the summary ORs for the primary or secondary outcome, because the exclusion of each study did not alter the point estimate outside the 95%CI (Figure 4 and 5).
The analysis examines the safety and efficacy of intervention with PBA vs PSI in patients with PVS. The principal findings of our study include (1) Similar safety profile of PBA vs PSI in the management of PVS; and (2) A higher risk of re-stenosis with PBA in comparison to PSI in patients with PVS. The PSI demonstrated a lower risk of re-stenosis can be attributed to the use of stents in patients with higher risk and the use of devices not particularly designed for PVS intervention. A follow-up with cardiac imaging every 3-6 mo is usually done in patients with asymptomatic PVS with about 50%-70% stenosis, particularly with ipsilateral PVS, and revascularization is considered when the PVS progress to severe grade defined as luminal stenosis > 70% by CT imaging[16]. Intervention needs to be performed urgently in patients with concomitant ipsilateral PVS in order to prevent potential progressive vascular fibrosis, occlusion, atrophy, and congestion with consequent lung infarction[17].
In the advent of suboptimal results of angiography and the occurrence of complications post-dilation, an acute mechanical benefit is provided well by stents compared to PBA. In addition, it is suggested that there is a time-dependent reduction in patency of the vessel post-PBA, making stenting favored in terms of long-term advantages[18]. This can be ascribed to the pathophysiological mechanisms of the venous system as well as the histological features. This ensues from post-thrombotic fibrosis inside and around the vein, with extravenous compressive bands and accompanying perivenous fibrosis leading to the obstructive processes in intima at ablation sites, which also involves the distal sites to PV ostia. Stenting may be able to provide an advantage against these pathophysiological mechanisms.
Studies show a high success rate and low re-stenosis rates of PSI compared to PBA, with longer freedom from re-stenosis[6]. Hence, stenting can be considered a first-line strategy. Studies have also consistently shown that large stent sizes, have excellent clinical outcomes and long-term patencies. Meta-analyses showed that long-term patency is better with large stent sizes of 9-10 mm[6,7]. The incidence of in-stent re-stenosis is shown to be less in large stents, as opposed to small stents[17,19,20]. Although stenting is widely used in the pulmonary vein, the operator needs to be careful due to the risk of protruding into the LA, jailing PV side branches, and crossing a low-flow distal side branch[18]. Other frequent complications, such as hemoptysis and self-limiting hemorrhages, have been found to be similar between the two groups. Revascularization is indicated in the advent of elevated PA pressure levels or the presence of typical symptoms. There is a chance of missing the diagnosis as the progression is unpredictable, and clinical symptoms may be atypical and can appear late. But early diagnosis and intervention are essential to prevent irreversible pulmonary damage.
Limitations of the study need to be acknowledged. The present study analyzes data comparing PBA and PSI from observational studies, but not randomized controlled trials. The analysis tends to be challenging to interpret when patients are treated with stenting after trial and failure of BA, as observed in some studies. In addition, procedural success and severe PVS definitions differ widely in studies, subsequently causing substantial heterogeneity. Also, the follow-up imaging techniques and protocols vary widely in the studies, which come into play when diagnosing post-procedural re-stenosis. Lastly, the antiplatelet/anticoagulation regimens post-procedure varies considerably in studies which might have possibly modified the treatment effect. The regimens were not consistently reported among different studies (Cory et al[5], Prieto et al[7], and Qureshi et al[19] didn’t mention their regimens). The reported antiplatelet/anticoagulation regimens were also various, including 3 mo of dual-antiplatelet therapy[6], warfarin and aspirin/ticlopidine[9], and at least 6 mo of anticoagulation followed by long term aspirin[8]. Interestingly, the re-stenosis rates varied between the two studies included an anticoagulation agent (70% at 5 years, and 27% at 5 years), whereas the dual-antiplatelet regimen was associated with a 23% restenosis at about 4 years. This observation implies the post-procedural antiplatelet/anticoagulation regimens may have a minor role for restenosis.
The current analysis updates the summary of evidence by incorporating two recent observational studies. Overall, we found sufficient evidence evaluating the comparative efficacy of pulmonary vein isolation (PVI) and PBA in treating patients with PVS. The outcomes with a moderate grade of certainty of evidence include pulmonary restenosis and procedure-related complications (Table 4).
Percutaneous re-vascularization with stents appears to be superior to PBA, in regard to re-stenosis and the need for re-intervention. Hence, stenting should be considered as the first line of choice over BA. A further follow-up to ascertain the real success of the intervention and the re-stenosis patterns is crucial.
Pulmonary vein balloon angioplasty (PBA) and pulmonary vein stent implantation (PSI) are the two re-vascularization strategies used to manage pulmonary vein stenosis.
Both these strategies are widely used to treat pulmonary vein stenosis. Our study tends to explore outcomes and complications with each of these strategies
Our study tried to explore the safety and efficacy outcomes of two re-vascularization strategies Pulmonary vein balloon angioplasty vs pulmonary vein stent implantation in the management of pulmonary vein stenosis.
The meta-analysis was performed by computing odds ratios using the random effects model based on underlying statistical heterogeneity.
The primary outcome of the re-stenosis requiring re-intervention occurred in 196 of 325 veins in the PBA group and 111 of 443 veins in the PSI group. Compared to PSI, PBA was associated with a significantly increased risk of restenosis (OR 2.91, 95%CI: 1.15-7.37, P = 0.025, I2 = 79.2%).
Percutaneous re-vascularization with stents appears to be superior to PBA, in regard to re-stenosis and the need for re-intervention. Hence, stenting should be considered as the first line of choice over balloon angioplasty.
A further follow-up to ascertain the real success of the intervention and the re-stenosis patterns is crucial.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hu H, China; Ueda H, Japan S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Packer D, Skanes A. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1056] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 2. | Kuck KH, Fürnkranz A. Cryoballoon ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:1427-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Marrouche NF, Martin DO, Wazni O, Gillinov AM, Klein A, Bhargava M, Saad E, Bash D, Yamada H, Jaber W, Schweikert R, Tchou P, Abdul-Karim A, Saliba W, Natale A. Phased-array intracardiac echocardiography monitoring during pulmonary vein isolation in patients with atrial fibrillation: impact on outcome and complications. Circulation. 2003;107:2710-2716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 355] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 4. | Seale AN, Webber SA, Uemura H, Partridge J, Roughton M, Ho SY, McCarthy KP, Jones S, Shaughnessy L, Sunnegardh J, Hanseus K, Rigby ML, Keeton BR, Daubeney PE; British Congenital Cardiac Association. Pulmonary vein stenosis: the UK, Ireland and Sweden collaborative study. Heart. 2009;95:1944-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Cory MJ, Ooi YK, Kelleman MS, Vincent RN, Kim DW, Petit CJ. Reintervention Is Associated With Improved Survival in Pediatric Patients With Pulmonary Vein Stenosis. JACC Cardiovasc Interv. 2017;10:1788-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Neumann T, Kuniss M, Conradi G, Sperzel J, Berkowitsch A, Zaltsberg S, Wojcik M, Erkapic D, Dill T, Hamm CW, Pitschner HF. Pulmonary vein stenting for the treatment of acquired severe pulmonary vein stenosis after pulmonary vein isolation: clinical implications after long-term follow-up of 4 years. J Cardiovasc Electrophysiol. 2009;20:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Prieto LR, Schoenhagen P, Arruda MJ, Natale A, Worley SE. Comparison of stent versus balloon angioplasty for pulmonary vein stenosis complicating pulmonary vein isolation. J Cardiovasc Electrophysiol. 2008;19:673-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Suntharos P, Worley SE, Liu W, Siperstein M, Prieto LR. Long-term outcome of percutaneous intervention for pulmonary vein stenosis after pulmonary vein isolation procedure. Catheter Cardiovasc Interv. 2020;95:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Kurita Y, Baba K, Kondo M, Eitoku T, Kasahara S, Iwasaki T, Ohtsuki S, Tsukahara H. Clinical outcomes after the endovascular treatments of pulmonary vein stenosis in patients with congenital heart disease. Cardiol Young. 2019;29:1057-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26739] [Cited by in RCA: 30408] [Article Influence: 779.7] [Reference Citation Analysis (0)] |
| 11. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47158] [Article Influence: 2947.4] [Reference Citation Analysis (0)] |
| 12. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46497] [Article Influence: 2113.5] [Reference Citation Analysis (3)] |
| 13. | Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2638] [Article Influence: 109.9] [Reference Citation Analysis (0)] |
| 14. | GS HJ. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ: John Wiley & Sons; 2011. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Schoene K, Arya A, Jahnke C, Paetsch I, Nedios S, Hilbert S, Bollmann A, Hindricks G, Sommer P. Acquired Pulmonary Vein Stenosis After Radiofrequency Ablation for Atrial Fibrillation: Single-Center Experience in Catheter Interventional Treatment. JACC Cardiovasc Interv. 2018;11:1626-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Rostamian A, Narayan SM, Thomson L, Fishbein M, Siegel RJ. The incidence, diagnosis, and management of pulmonary vein stenosis as a complication of atrial fibrillation ablation. J Interv Card Electrophysiol. 2014;40:63-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Di Biase L, Fahmy TS, Wazni OM, Bai R, Patel D, Lakkireddy D, Cummings JE, Schweikert RA, Burkhardt JD, Elayi CS, Kanj M, Popova L, Prasad S, Martin DO, Prieto L, Saliba W, Tchou P, Arruda M, Natale A. Pulmonary vein total occlusion following catheter ablation for atrial fibrillation: clinical implications after long-term follow-up. J Am Coll Cardiol. 2006;48:2493-2499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Buiatti A, von Olshausen G, Martens E, Schinke K, Laugwitz KL, Hoppmann P, Ibrahim T. Balloon angioplasty versus stenting for pulmonary vein stenosis after pulmonary vein isolation for atrial fibrillation: A meta-analysis. Int J Cardiol. 2018;254:146-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Qureshi AM, Prieto LR, Latson LA, Lane GK, Mesia CI, Radvansky P, White RD, Marrouche NF, Saad EB, Bash DL, Natale A, Rhodes JF. Transcatheter angioplasty for acquired pulmonary vein stenosis after radiofrequency ablation. Circulation. 2003;108:1336-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Fender EA, Packer DL, Holmes DR Jr. Pulmonary vein stenosis after atrial fibrillation ablation. EuroIntervention. 2016;12 Suppl X:X31-X34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |