Published online Nov 26, 2023. doi: 10.4330/wjc.v15.i11.615
Peer-review started: September 6, 2023
First decision: September 20, 2023
Revised: September 27, 2023
Accepted: October 27, 2023
Article in press: October 27, 2023
Published online: November 26, 2023
Processing time: 77 Days and 16.9 Hours
Down syndrome, also known as trisomy 21 syndrome, is commonly associated with congenital heart disease, and can often result in early formation of pulmonary hypertension. The development of pulmonary hypertension can result from factors such as intracardiac and macrovascular shunts, and upper airway obstruction or hypoplasia of lung tissue. Individuals with Down syndrome and congenital heart disease have a significantly lower average life expectancy, with surgical intervention being the most viable treatment option to improve longevity.
We report the case of a 13-year-old boy with Down syndrome presenting with atrial septal defect and patent ductus arteriosus along with severe pulmonary hypertension. The electrocardiogram shows sinus rhythm and right ventricular hypertrophy. The echocardiogram shows an atrial septal defect with interrupted echo in the interatrial septum, measuring 0.813 cm in length. The patient was initially refused to be offered surgical treatment by many hospitals due to the high surgical risk and pulmonary artery resistance. After discussing the patient’s diagnosis and treatment options, we ultimately recommended surgical treatment. However, the patient and their family declined this recommendation and chose to be discharged. During the follow-up period of 6 mo, there were no significant improvements or deteriorations in the patient’s condition.
In conclusion, this case highlights the challenges faced by individuals with Down syndrome and congenital heart disease complicated by severe pulmonary hypertension. Timely intervention and a multidisciplinary approach are crucial for improving prognosis and life expectancy. Further research is needed to enhance our understanding and develop effective interventions for this popula
Core Tip: This case study presents a 13-year-old boy diagnosed with Down syndrome alongside atrial septal defect, patent ductus arteriosus, and severe pulmonary hypertension. A complex condition initially met with surgical treatment denial due to high risks, highlights the significant challenges faced by individuals with Down syndrome and congenital heart disease. This case highlights the discussion and educational value surrounding the decision to undergo surgery in complex congenital heart diseases. The educational value lies in the diagnostic and therapeutic approaches demonstrated by our team. Due to the relatively common occurrence of this case in the field of cardiology, our decision-making process holds significant value and applicability.
- Citation: Kong MW, Li YJ, Li J, Pei ZY, Xie YY, He GX. Down syndrome child with multiple heart diseases: A case report. World J Cardiol 2023; 15(11): 615-622
- URL: https://www.wjgnet.com/1949-8462/full/v15/i11/615.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i11.615
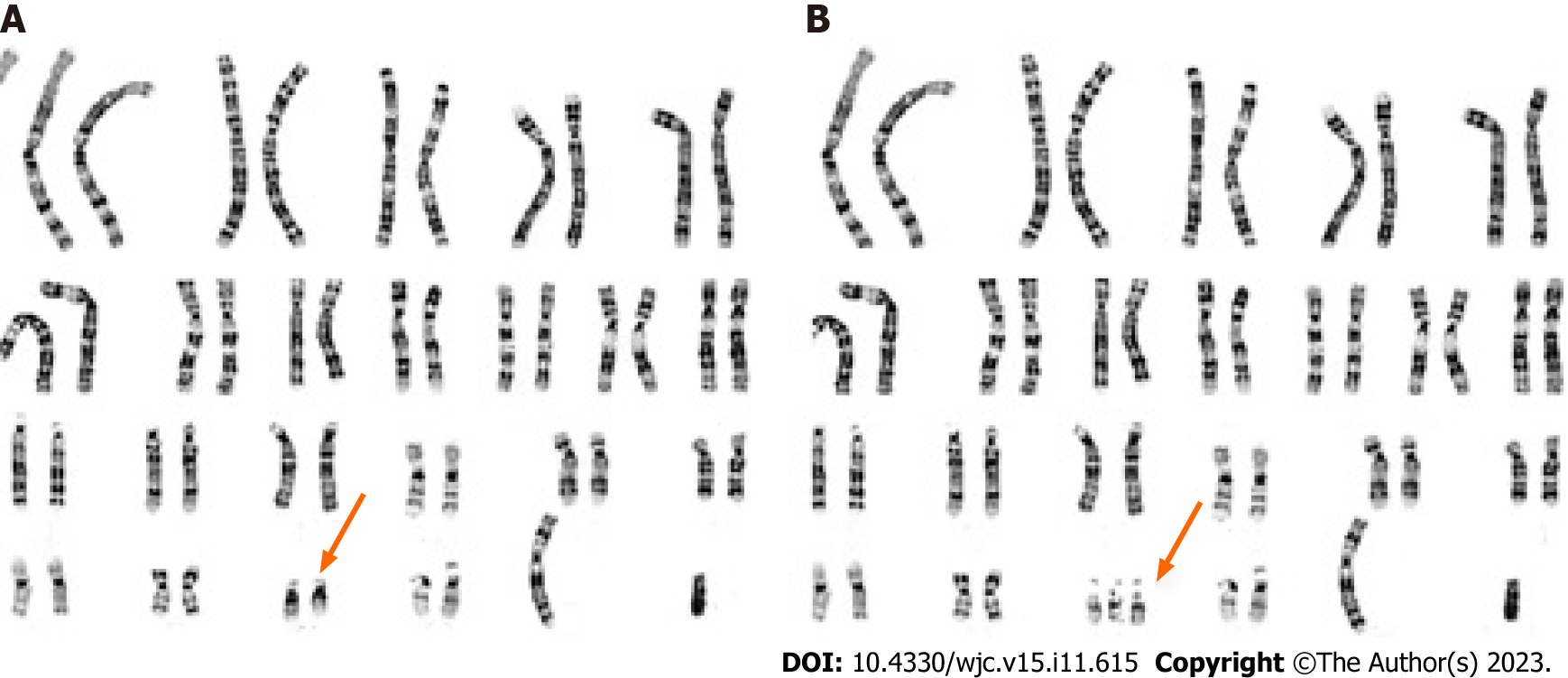
Down syndrome (DS), also known as trisomy 21 syndrome (Figure 1), is the most prevalent genetic disorder among Chinese children. The disease arises due to a mutation of the 21st pair of chromosomes, resulting in the duplication of genetic material. Common clinical manifestations include developmental abnormalities, intellectual disability, and an increased propensity for digestive tract and cardiovascular malformations[1]. Reports suggest that the incidence of congenital heart disease (CHD) in DS patients ranges from 40%-60% and can lead to heart failure, pulmonary vascular disease (PVD), and pneumonia[2,3]. Early diagnosis and surgical intervention are vital to prolong survival and enhance the quality of life of these children[4]. However, DS is a chromosomal disease impacting multiple systems, and opinions differ on the effectiveness of surgical intervention.
In the present case, the patient was refused to be offered surgical intervention by multiple hospitals due to the high risk of pulmonary arterial resistance. However, after extensive consultation, our hospital reassessed the patient’s situation and proposed a promising treatment plan that ultimately gave the child a chance at life. This case may serve as a valuable reference for the management and treatment of similar cases.
A 13-year-old boy presented to our hospital for further evaluation and treatment.
The patient was diagnosed as having DS with CHD. A patent ductus arteriosus (PDA) closure was successfully performed, but atrial septal defect (ASD) closure was withheld due to the patient’s complicated pulmonary arterial hypertension. After treatment, the patient’s activity tolerance improved. On July 29, 2020, the patient sought medical care at the Yan’an Hospital of Kunming City, where cardiac catheterization revealed a pulmonary artery pressure of 95/41/66 mmHg and a right ventricular pressure of 97/-10/38 mmHg. He was diagnosed with ASD complicated with severe pulmonary arterial hypertension, and continuation of oral Bosentan and Sildenafil for the management of pulmonary arterial hypertension was recommended.
The patient’s medical history revealed that at 2 mo of age, he was diagnosed at another hospital with CHD characterized by ASD and PDA. However, no treatment was provided. In 2018, the patient developed “cyanosis of the lips and shortness of breath after exercise” and was admitted to Fuwai Hospital, where he was diagnosed with ASD and PDA, along with an enlarged heart, severe pulmonary hypertension (PH), and cardiac function Grade II.
Physical examination revealed a pulse rate of 95 bpm, blood pressure of 113/55 mmHg, and pale purplish lips. Cardiac percussion examination revealed leftward expansion of the heart border. Auscultation revealed regular heart rhythm with mild hyperactivity of P2, but no discernible noise.
Frontal and lateral chest digital radiography (DR) revealed the presence of reticular shadows in the mediastinal area indicative of postoperative changes (Figure 2). A conventional 12-lead electrocardiogram (ECG) (Figure 3) was conducted during the outpatient visit, which revealed sinus rhythm and right chamber hypertrophy. An echocardiogram was also performed, which showed an ASD (central secondary foramen) with an interrupted middle echo in the atrial septum measuring 0.813 cm. The position and functionality of the PDA interventional closing device were normal, the right atrium and ventricle show enlarged diameters and increased wall thickness in the right ventricle (Figure 4). According to subsequent reports, the patient exhibits blood flow signals indicating left-to-right shunting that can be visualized in the atrium. Additionally, a small regurgitant jet can be observed at the tricuspid valve during atrial contraction, with peak flow velocity of 398cm/s and a peak pressure gradient of 63mmHg. Following these assessments, the patient was referred to our department for the management of “ASD and associated PH”.
The patient was finally diagnosed with: (1) ASD (central foramen secundum type); (2) moderate PH; (3) PDA (after interventional closure); and (4) DS.
For patients with inconsistent severe PH following the results of physical examination, ECG, chest DR, right cardiac catheterization, and cardiac ultrasound, it is necessary to consider the possibility of measurement instrument errors and judgment bias. We also considered the possibility of heart-lung combined transplantation. However, due to the high surgical trauma and shortage of donors, we took a conservative approach when discussing this option. Further surgical plans will be presented in the “DISCUSSION” section.
The patient ultimately did not accept our surgical recommendation and opted for conservative treatment (oral PH medication) before being discharged.
We conducted a 6-mo follow-up during which the patient did not show significant improvement or worsening of his condition. During the follow-up period, the patient sought medical care at two other hospitals and continued to decline surgical treatment.
After extensive discussion by our hospital’s expert group, the following findings in this case are summarized. The child had been diagnosed with DS associated with CHD. Despite a previous diagnosis by other medical facilities of severe PH (systolic pulmonary pressure > 70 mmHg), our physical examination only noted mild accentuated P2 heart sound, suggesting that the patient’s actual PH may be mild or nonexistent, or potentially present with a two-way shunt. And the child’s ECG demonstrated right ventricular high voltage without any signs of right bundle branch block, and with a low or upside-down ST-T wave, which suggests that the right ventricular hypertrophy may not have been significant. Chest DR indicated slightly expanded pulmonary artery sections without any sparse blood vessels indicative of the “no truncating phenomenon.” Therefore, further evaluation was necessary via right-heart catheterization to obtain critical indicators for assessing the patient’s pulmonary artery pressure. Further investigation was conducted to analyze the relationship between the patient’s PDA and ASD. In children with PDA, blood enters the pulmonary arteries through the ASD, leading to a significant increase in pulmonary blood flow (PBF) and left ventricular overload, resulting in an increase in long-term PBF and pulmonary vascular resistance (PVR), eventually leading to PH in later stages. As PH worsens, right-to-left shunting occurs at the atrial level, known as the Eisenmenger syndrome, indicated by clinical cyanosis. However, the child’s ultrasonic display did not show a significant increase in the left ventricle. This may be due to the merged ASD, which leads to an increase in the left heart load and the occurrence of left-to-right shunting at the atrial level. After interventional closure of the PDA, the left-to-right shunt in the atrium was reduced, and the systolic pulmonary pressure decreased compared to that previously, significantly improving the patient’s symptoms.
It is important to exercise caution and conduct proper communication and evaluation before considering the closure of an ASD in a child with DS combined with CHD, as the prognosis is poor, and the risks associated with anesthesia and surgery are substantial. It is also possible that, even after right heart catheterization, there still may not be any indication for interventional closure or surgery. If a patient’s parents fully understand their child’s medical condition, treatment plan, surgical risks and benefits, as well as prognosis, but still desire right heart catheterization and any necessary interventional therapy, it is advisable to arrange for general anesthesia appropriate for the cardiac catheterization procedure.
Unfortunately, the patient’s parents were unaware of the above situation and declined the surgical recommendation. Therefore, further cardiac catheterization for examination and treatment was not performed, and he continued to take pulmonary artery pressure-lowering drugs and was discharged from the hospital for follow-up.
We also performed a review of the related literature. CHD is the most common type of malformation associated with DS and is a significant cause of mortality in DS patients. The early onset of PH resulting from cardiac defects and respiratory tract hypoplasia can lead to PVD and heart failure, further worsening the timing of surgery and prognosis. Early diagnosis and timely intervention are crucial in prolonging the survival and improving the quality of life of DS patients.
DS is frequently associated with malformations across multiple organ systems, with CHD being the most significant factor affecting patient survival. Approximately 40%-60% of DS cases are associated with CHD[5]. The specific types of CHD associated with DS and their respective composition ratios vary across different countries and regions. In North East England, atrioventricular septal defect (AVSD) is the most common (42%) type of CHD associated with DS, followed by ventricular septal defect (VSD) at 31%. Other types such as ASD, tetralogy of Fallot (TOF), and PDA account for 15%, 5%, and 4%, respectively[5]. In Mexico, ASD is the most common type, accounting for approximately 38%, while VSD and PDA make up 30% and 21%, respectively[6]. In Germany, the most frequently observed type of CHD in DS cases is AVSD (51.2%), followed by VSD (25.1%), TOF (6.7%), and ASD (8.9%)[7]. In Asia, VSD is the most common type of CHD associated with DS, accounting for 43%, followed by AVSD (15.4%), ASD (13.4%), and TOF (13.4%)[8]. The reasons for these regional differences remain unclear.
The pathogenesis of PH in patients with DS and CHD is multifaceted and may be related to both anatomical and physiological changes in the lung circulation[9]. Abnormal shunting caused by heart malformations and other factors, such as upper respiratory tract obstruction and lung tissue development in DS children, contribute to the formation of PH. Deformities in the heart structure result in abnormal shunting, with shunts flowing from left to right causing an increase in PBF[10]. This creates additional shear force on the pulmonary vascular endothelium, leading to irreversible internal cellular damage. Forward-looking studies have found that more than half of DS children show signs of PH, with AVSD often being linked to its formation. Large internal defects cause a significant amount of blood to shunt from left to right after birth, resulting in pulmonary vascular bed damage and the formation of PVD[11,12].
Furthermore, the abnormal blood shunt in the heart increases the blood flow in small arteries, leading to early reflection spasm in the pulmonary arteries and increased pulmonary circulation resistance[13]. The prolonged high flow in the pulmonary circulation leads to the main pathological change of blood vessel remodeling, eventually resulting in irreversible PVD. Studies have demonstrated that, before the age of one year, DS children show lower average PBF and average PVR levels compared to non-DS children[14,15]. Approximately 10% of DS children are diagnosed with pulmonary vascular obstructive diseases (PVOD) before the age of one year, which is not observed in non-DS children. Furthermore, compared to non-DS children with CHDs, DS children with CHDs exhibit a faster increase in PVR and an equivalent trend towards PVOD in the first year after birth[16].
Endothelial progenitor cell dysfunction is also involved in the formation of PH in children with DS. Endothelial progenitor cells maintain the stability of blood vessels and participate in the production of vascular endothelial cells[17]. Their dysfunction is mainly reflected in a reduction of cell number and physiological function, leading to damage in maintaining the stability of blood vessels and resulting in severe or irreversible damage in the early stages of pulmonary vessel development[18]. Furthermore, several studies have indicated higher levels of various inflammatory mediators in DS children, including tumor necrosis factor-α, interleukin-6, and C-reactive protein, among others. This is also considered to impact the number of endothelial progenitor cells circulating in the blood[19].
Early onset, severe disease, and rapid progression of PH are observed in patients with DS and CHD. Therefore, early evaluation and diagnosis are critical to improving their prognosis. The assessment process involves echocardiography to determine the morphological changes in the heart and detect the presence of PH. Generally, evaluation of pulmonary arterial compression is conducted using the three-pointed reflux peak speed and Doppler ultrasonic images in combination with other ultrasonic indicators that could potentially indicate PH, such as increased pulmonary valve reflux speed, enlargement of the right heart cavity, and enlargement of the main pulmonary artery[20]. Evaluation also includes clinical manifestations, peripheral blood oxygen saturation, and other indicators to determine the level of PH.
In some cases, cardiac catheterization and acute pulmonary vascular dilation tests may be necessary to determine the level of PVD[21]. A pulmonary artery pressure of ≥ 25 mmHg under right cardiac catheterization in a static state is diagnostic for PH. Severe PH patients who cannot undergo surgery can lower their pulmonary artery pressure by taking oral diuretics and vasodilators, and partially benefit from targeted therapies[21].
Children with DS and CHD often experience repeated lung infections, weight loss, and advanced heart failure in the early stages before undergoing surgical treatment to correct cardiovascular malformations. Delayed treatment can result in an increased risk of mortality[16-18]. To investigate the status of DS with CHD over the past decade, we conducted a search of Chinese and international databases to identify cases who underwent surgical treatment. However, we found that some of these cases were deemed unsuitable for surgery (Table 1). It is important to recognize that delaying surgical treatment can increase the risk of severe complications and mortality. This highlights the need for early assessment and diagnosis to improve the prognosis of DS children with CHD.
| Ref. | Case | Not suitable for surgery (%) | Death without surgery (%) |
| Liu et al[22], 2015 | 77 | 17 (22.1) | 5 (6.5) |
| Gu et al[5], 2016 | 96 | 38 (39.6) | 4 (4.2) |
| Xu et al[23], 2019 | 30 | 0 (0) | 0 (0) |
| Guo et al[24], 2015 | 25 | 0 (0) | 0 (0) |
| Zahari et al[25], 2019 | 414 | 270 (65.1) | 37 (9.0) |
| Evans et al[26], 2014 | 4231 | 2200 (52.4) | 85 (1.9) |
| Baban et al[27], 2020 | 859 | 245 (28.5) | 34 (4.0) |
| Aziz et al[28], 2020 | 18 | 6 (33.3) | - |
| Santos et al[29], 2019 | 139 | 48 (34.5) | 10 (6.8) |
| Dias et al[30], 2016 | 102 | - | 3 (2.9) |
The data in Table 1 highlight a notable discrepancy between those deemed “unsuitable for surgery” and those who passed away without undergoing surgery in various studies. This difference may be due to the various types of combined heart diseases observed in different regions or sample size differences. Nevertheless, some children are unable to receive surgical treatment and may even pass away while waiting for surgery.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Surani S, United States; Akash Batta, India S-Editor: Lin C L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | Bull MJ. Down Syndrome. N Engl J Med. 2020;382:2344-2352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 332] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 2. | Antonarakis SE, Skotko BG, Rafii MS, Strydom A, Pape SE, Bianchi DW, Sherman SL, Reeves RH. Down syndrome. Nat Rev Dis Primers. 2020;6:9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 470] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 3. | Postolache L, De Jong C, Casimir G. Illustration of tessellation in Down syndrome. Ophthalmic Genet. 2020;41:135-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Jean AR. Down Syndrome: A Curative Prospect? AIMS Neuroscience. 2020;7:168-193. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Gu Y, Jin M, Zheng K, Liang YM, Wang XF, Xiao YY, Jiao M, Han L. 96 cases of Down syndrome with congenital heart disease. Zhonghua Shiyong Erke Zazhi. 2016;31:989-992. [DOI] [Full Text] |
| 6. | Espinola-Zavaleta N, Soto ME, Romero-Gonzalez A, Gómez-Puente Ldel C, Muñoz-Castellanos L, Gopal AS, Keirns C, Lupi-Herrera E. Prevalence of Congenital Heart Disease and Pulmonary Hypertension in Down's Syndrome: An Echocardiographic Study. J Cardiovasc Ultrasound. 2015;23:72-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Pfitzer C, Helm PC, Rosenthal LM, Berger F, Bauer UMM, Schmitt KR. Dynamics in prevalence of Down syndrome in children with congenital heart disease. Eur J Pediatr. 2018;177:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Al-Mendalawi MD. The spectrum of congenital heart diseases in down syndrome. A retrospective study from Northwest Saudi Arabia. Saudi Med J. 2016;37:1294-1295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 9. | Zhou J. Synemin promotes pulmonary artery smooth muscle cell phenotypic switch in shunt-induced pulmonary arterial hypertension. ESC Heart Fail. 2022;9:3221-3231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Molck MC, Simioni M, Vieira TP, Sgardioli IC, Gil-da-Silva-Lopes VL. Copy number variations (CNVs) in patients with congenital heart diseases and 22q11.2 DS clinical suspicion. In: 10th European Cytogenetics Conference 2015: 4-7 July 2015, Strasbourg - France. Chromosome Res. 2015;23 Suppl 1:1-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 11. | Hart SA, Nandi D, Backes CH, Cua CL. Impact of prenatal screening on congenital heart defects in neonates with Down syndrome in the US. Pediatr Res. 2021;90:1081-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Boussouf K, Zaidi Z, Amrane M, Hammoudi N, Mebarki M, Amalou SA. Study of congenital heart diseases in patients with Down syndrome in Algeria. East Mediterr Health J. 2017;23:632-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Al-Fahham MM, Ali YA. Pattern of congenital heart disease among Egyptian children: a 3-year retrospective study. Egypt Heart J. 2021;73:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Cheng AL, Takao CM, Wenby RB, Meiselman HJ, Wood JC, Detterich JA. Elevated Low-Shear Blood Viscosity is Associated with Decreased Pulmonary Blood Flow in Children with Univentricular Heart Defects. Pediatr Cardiol. 2016;37:789-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Kulik TJ. Pulmonary blood flow and pulmonary hypertension: Is the pulmonary circulation flowophobic or flowophilic? Pulm Circ. 2012;2:327-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Fukushima H, Kosaki K, Sato R, Yagihashi T, Gatayama R, Kodo K, Hayashi T, Nakazawa M, Tsuchihashi T, Maeda J, Kojima Y, Yamagishi H, Takahashi T. Mechanisms underlying early development of pulmonary vascular obstructive disease in Down syndrome: An imbalance in biosynthesis of thromboxane A2 and prostacyclin. Am J Med Genet A. 2010;152A:1919-1924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Wang Z, Li J, Wang X, Liu M, Liao M, Zhang C, Shang X. Evaluation of pulmonary arterial pressure in patients with connective tissue disease-associated pulmonary arterial hypertension by myocardial perfusion imaging. Ann Noninvasive Electrocardiol. 2022;27:e12927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 18. | Trujillo JT, Long J, Aboelnour E, Ogas J, Wisecaver JH. CHD Chromatin Remodeling Protein Diversification Yields Novel Clades and Domains Absent in Classic Model Organisms. Genome Biol Evol. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 19. | Rienzo M, Costa V, Scarpato M, Schiano C, Casamassimi A, Grimaldi V, Ciccodicola A, Napoli C. RNA-Seq for the identification of novel Mediator transcripts in endothelial progenitor cells. Gene. 2014;547:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Sun X, Kato H, Sato H, Han X, Hirofuji Y, Kato TA, Sakai Y, Ohga S, Fukumoto S, Masuda K. Dopamine-related oxidative stress and mitochondrial dysfunction in dopaminergic neurons differentiated from deciduous teeth-derived stem cells of children with Down syndrome. FASEB Bioadv. 2022;4:454-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Pennella CL, Cassina TM, Rossi JG, Baialardo EM, Rubio P, Deu MA, Peruzzo L, Guitter MR, Sanchez de La Rosa CG, Alfaro EM, Felice MS. Clonal Myeloproliferative Disorders in Patients with Down Syndrome-Treatment and Outcome Results from an Institution in Argentina. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Liu CJ, Mao SY, Fu YQ, Bai K, Xu F. A case-control study on postoperative complications and prognosis of congenital heart disease in children with Down syndrome. Zhongguo Xunzheng Erke Zazhi. 2015;10:182-186. |
| 23. | Xu YB, Zhou L, Wang SW, Hang YB, Cao JY, Li G. Surgical treatment of congenital heart disease pulmonary hypertension combined with Down syndrome in infants and young children. Zhongguo Xiongxinxueguan Waike Zazhi. 2019;26:461-464. |
| 24. | Guo SY, Cui CY, Li Q. Surgical treatment of congenital heart disease with pulmonary hypertension combined with Down syndrome. Zhongguo Shiyong Yixue Zazhi. 2015;6:111-112. |
| 25. | Zahari N, Mat Bah MN, A Razak H, Thong MK. Ten-year trend in prevalence and outcome of Down syndrome with congenital heart disease in a middle-income country. Eur J Pediatr. 2019;178:1267-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Evans JM, Dharmar M, Meierhenry E, Marcin JP, Raff GW. Association between Down syndrome and in-hospital death among children undergoing surgery for congenital heart disease: a US population-based study. Circ Cardiovasc Qual Outcomes. 2014;7:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Baban A, Olivini N, Cantarutti N, Calì F, Vitello C, Valentini D, Adorisio R, Calcagni G, Alesi V, Di Mambro C, Villani A, Dallapiccola B, Digilio MC, Marino B, Carotti A, Drago F. Differences in morbidity and mortality in Down syndrome are related to the type of congenital heart defect. Am J Med Genet A. 2020;182:1342-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Aziz S, Ayub M, Masood L, Amanullah M, Hameed R, Hashmi S, Ahmad W. Major Septal Defects: Comparative Study of Down Syndrome and Non-Down Syndrome Infants, Before and After Surgery. Pak J Med Sci. 2020;36:925-929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | Santos FCGB, Croti UA, Marchi CH, Murakami AN, Brachine JDP, Borim BC, Finoti RG, Godoy MF. Surgical Treatment for Congenital Heart Defects in Down Syndrome Patients. Braz J Cardiovasc Surg. 2019;34:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Dias FM, Cordeiro S, Menezes I, Nogueira G, Teixeira A, Marques M, Abecasis M, Anjos R. Congenital Heart Disease in Children with Down Syndrome: What Has Changed in the Last Three Decades? Acta Med Port. 2016;29:613-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |