Published online Oct 26, 2023. doi: 10.4330/wjc.v15.i10.542
Peer-review started: June 17, 2023
First decision: August 10, 2023
Revised: August 23, 2023
Accepted: September 27, 2023
Article in press: September 27, 2023
Published online: October 26, 2023
Processing time: 129 Days and 1.8 Hours
Congenitally corrected levo-transposition of the great arteries (L-TGA) is a congenital heart disease in which the ventricles and great arteries are transposed from their typical anatomy. In L-TGA, the double discordance, atrioventricular and ventriculoarterial, create an acyanotic milieu which allows patients to survive their early decades, however, progressive systemic right ventricle (sRV) dys
A 40-year-old male with L-TGA presented with symptoms of acute decom
Anticoagulation with VKA is the mainstay of treatment in the absence of conclusive data supporting direct oral anticoagulant use in ICT in patients with congenital heart disease. This case illustrates the natural history of L-TGA and highlights the importance of surveillance and monitoring with dedicated cardiac imaging to identify complications.
Core Tip: Patients with congenital heart disease such as levo-transposition of the great arteries experience progressive cardiac dysfunction and remodeling which manifests as heart failure. This predisposes patients to the formation of intracardiac thrombus (ICT). We present a case of progressive systemic right ventricle (sRV) dysfunction resulting in an apical thrombus. Review of literature identified no cases of sRV thrombus making this one of the first reports. Guidelines do not exist for anticoagulation in patients with congenital heart disease and ICT. Therefore, clinical decisions are extrapolated from anticoagulation principles in patients without congenital heart disease. Considerations for direct oral anticoagulants in this population should be individualized and involve shared decision making.
- Citation: Almajed MR, Almajed A, Khan N, Obri MS, Ananthasubramaniam K. Systemic right ventricle complications in levo-transposition of the great arteries: A case report and review of literature. World J Cardiol 2023; 15(10): 542-552
- URL: https://www.wjgnet.com/1949-8462/full/v15/i10/542.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i10.542
Congenitally corrected transposition, or levo-transposition of the great arteries (L-TGA) is a rare congenital heart disease with an estimated prevalence of 0.4%-1.0% among patients with congenital heart disease[1,2]. The double discordance, atrioventricular and ventriculoarterial, creates an acyanotic milieu in which both pulmonary and systemic circulations exist and freely communicate[3]. This phenomenon, which is alluded to as “congenitally corrected”, allows patients to survive their early decades with minimal cardiac complications. A large prospective survival study found that 95.5% of patients survive the first month of life; 72.7% survive to the age of 3 and this percentage of patients continues to live to the age of 15[1]. Survival data among adults is variable due to the presence of different associated cardiac lesions and the heterogenous surgical interventions these patients undergo[4]. Among all patients with L-TGA, a minority survive beyond the fifth decade of life due to cardiac complications[5]. In those who undergo surgical correction as children, the 10-year survival rate ranges from 60%-70%[6,7].
The natural history of L-TGA involves a wide spectrum of cardiac complications that manifest during early adulthood. The atypical anatomy and pathophysiologic circulation with the systemic right ventricle (sRV) in L-TGA predisposes patients to progressive sRV dysfunction, systemic tricuspid valve (sTV) regurgitation, and conduction defects including heart block[8]. Patients commonly present with clinical manifestations of heart failure as the lifetime prevalence is 34% in L-TGA as opposed to 1%-2% across the general population[9,10].
A large multicenter study found that by the age of 45, heart failure develops in 67% of patients with L-TGA and associated cardiac lesions whereas it develops in 25% of patients with L-TGA and no associated cardiac lesions[9]. Anatomical surgical repair, which aims to correct the double discordance by making the morphologic left ventricle the systemic ventricle and the morphologic right ventricle the pulmonary ventricle, is associated with higher survival rates and lower morbidity[11-13]. Physiologic surgical repair, which targets associated cardiac lesions without correcting the double discordance, is associated with higher rates of sRV dysfunction and mortality in adulthood[14].
We report a case of sRV thrombus in a patient with L-TGA who presented with acute decompensated heart failure (ADHF) and discuss the state of current literature regarding anticoagulation management.
A 40-year-old male with L-TGA presented to the hospital with ADHF. Upon review of systems, he had no chest pain, palpitations, lightheadedness, or syncope; he also denied cough, sputum production, or fever. He was not recently ill and had no sick contacts.
Symptoms started three-weeks prior to presentation with progressive shortness of breath on exertion, orthopnea, paroxysmal nocturnal dyspnea, and bilateral lower limb swelling. His symptoms were severe enough to make conversation difficult and limited his activities of daily living.
His medical history was remarkable for L-TGA with an associated ventricular septal defect, he underwent physiologic surgical closure of the septal defect at three years of age. He was monitored by a pediatric cardiologist in childhood and early adulthood during; he remained free of cardiac symptoms during this time and was eventually lost to follow-up. At the age of thirty-five, he was hospitalized for ADHF and was found to have sRV dysfunction with severely reduced global systolic function and severe sTV regurgitation. He was also noted to have a progressive conduction disease as his previously known first degree atrioventricular block was replaced by a newly identified left-bundle branch block. He was medically managed for heart failure and underwent cardiac resynchronization therapy with biventricular pacemaker implantation. He followed with the advanced heart failure and transplant service but was then lost to follow-up for several years until this latest presentation to the hospital.
The patient did not have a family history of congenital heart disease, heart failure, or respiratory illness.
During this presentation, the patient was tachycardic (108 beats per minute) and tachypneic (25-40 breaths per minute); blood pressure was 104/68 mmHg and he was not hypoxic on room air. Height was 168 cm and weight was 92 kg. Physical exam was positive for decreased bilateral breath sounds with mild crepitation. Jugular venous distension was present and pitting edema was noted in the bilateral lower limbs.
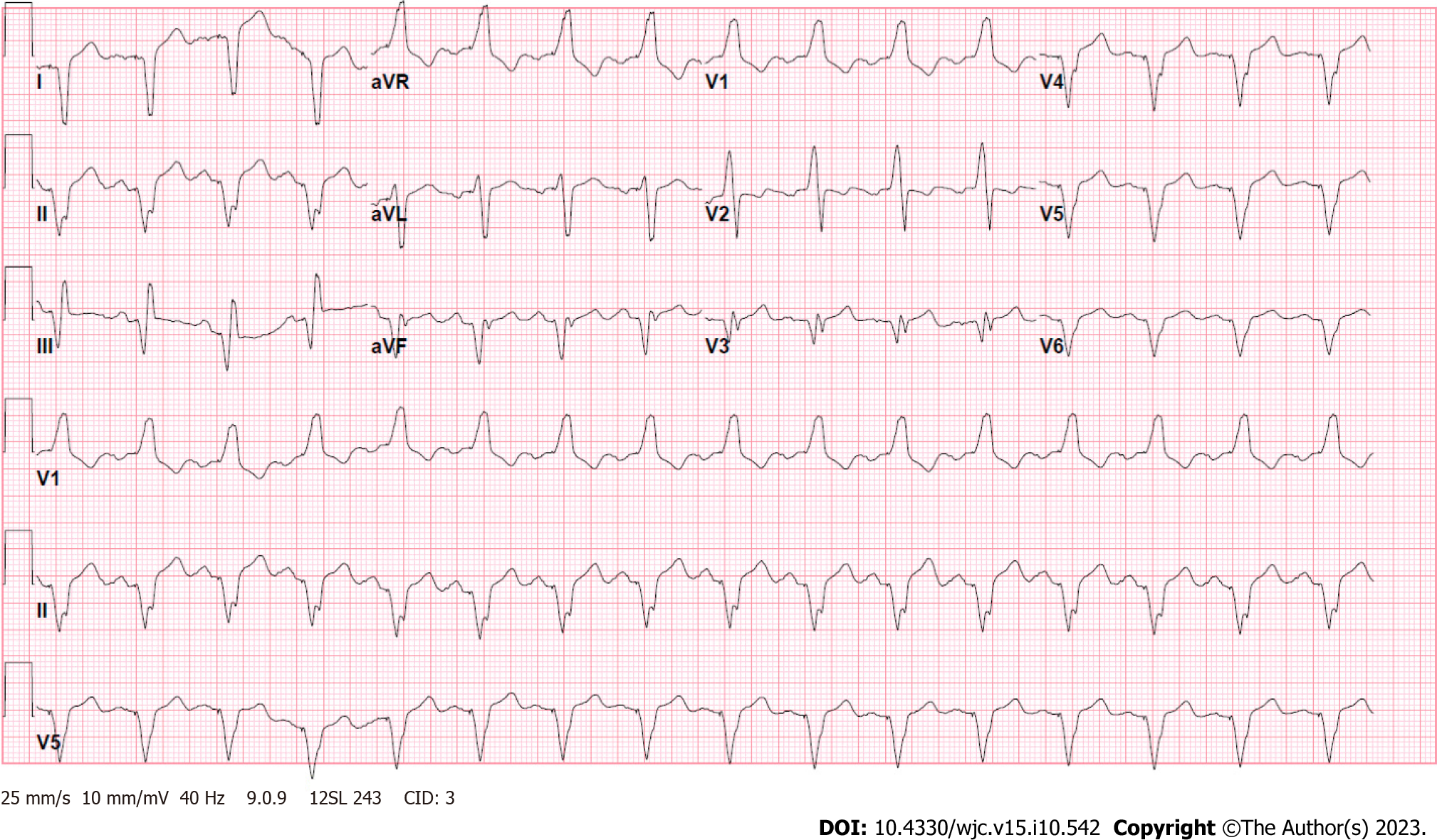
Laboratory tests were notable for a brain natriuretic peptide of 472 pg/mL, high-sensitivity troponin of 16 ng/L, venous lactate of 1.2 mmol/L, and creatinine of 0.80 mg/dL (Table 1). Electrocardiogram showed an atrial-sensed ventricularly-paced rhythm without acute abnormalities (Figure 1).
| Investigation | Patient result | Reference range |
| Sodium (mmol/L) | 138 | 135-145 |
| Potassium (mmol/L) | 4.1 | 3.5-5.0 |
| Chloride (mmol/L) | 104 | 98-111 |
| Carbon dioxide (mmol/L) | 22 | 21-35 |
| Blood urea nitrogen (mg/dL) | 19 | 10-25 |
| Creatinine (mg/dL) | 0.80 | < 1.28 |
| Venous lactate (mmol/L) | 1.2 | < 2.1 |
| High-sensitivity troponin (ng/L) | 16 | < 18 |
| Brain natriuretic peptide (pg/mL) | 472 | < 50 |
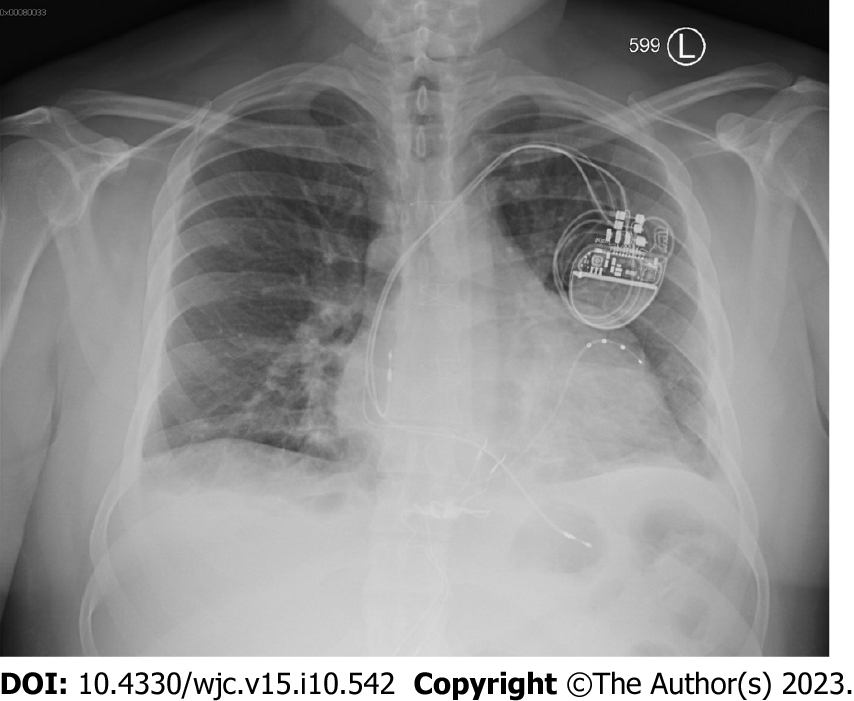
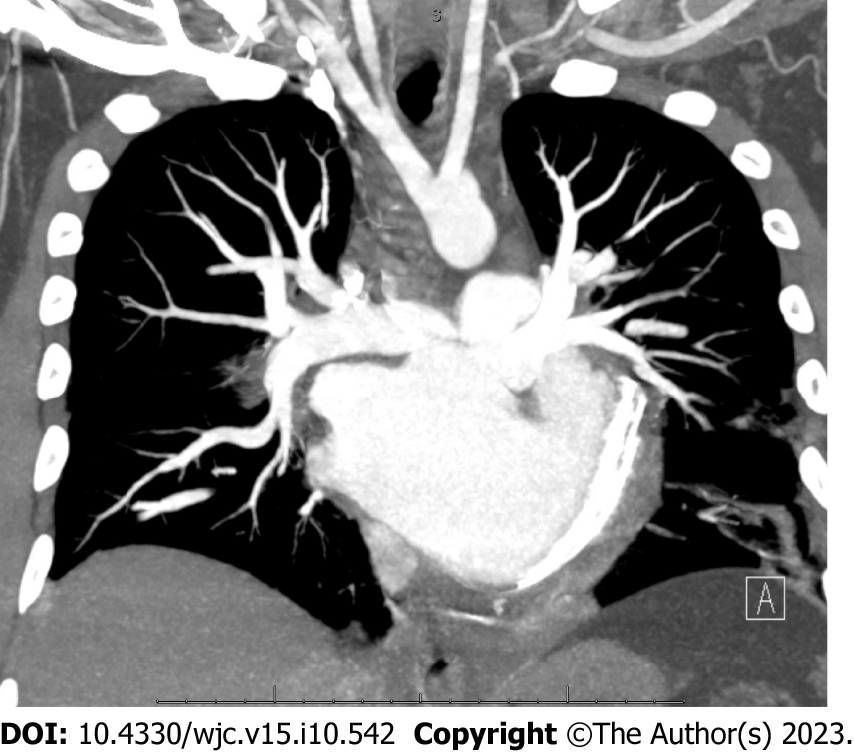
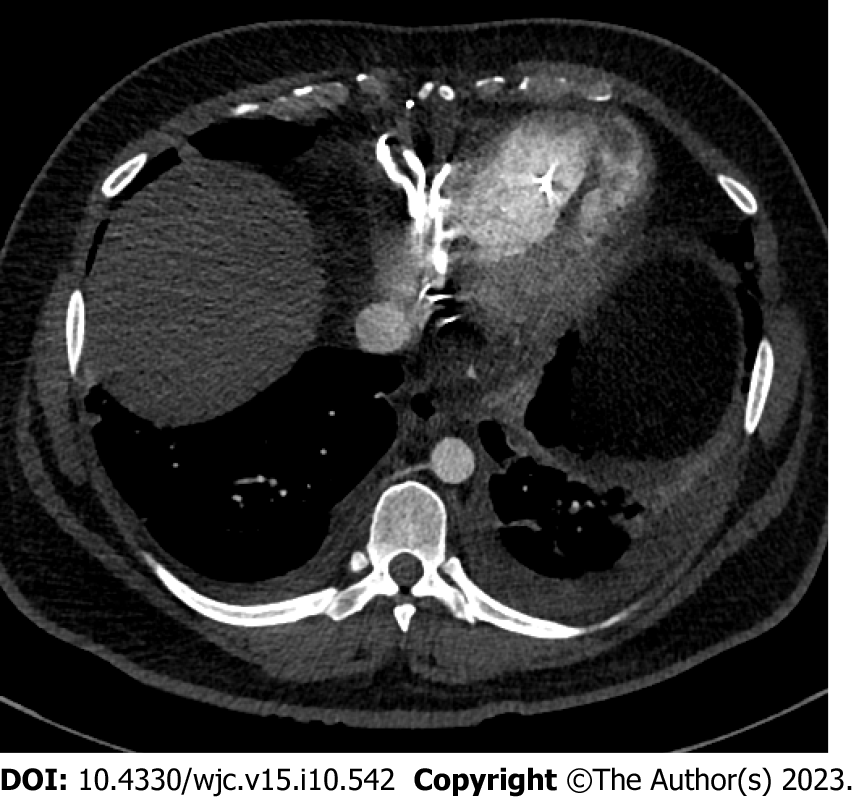
Chest X-ray was remarkable for cardiomegaly with small bilateral pleural effusions (Figure 2). Chest pulmonary angiography was negative for pulmonary embolism, pericardial effusion, or pneumothorax (Figure 3). His cardiac anatomy of L-TGA is visualized on chest computed tomography (Figure 4).
Our patient was managed for ADHF with guideline-directed medical therapy including diuresis with intravenous furosemide 40 mg once daily, oral metoprolol succinate 50 mg once daily, oral losartan 50 mg once daily, and oral dapagliflozin 10 mg daily. He had rapid clinical improvement with resolution of his symptoms and was transitioned to oral diuretic as needed.
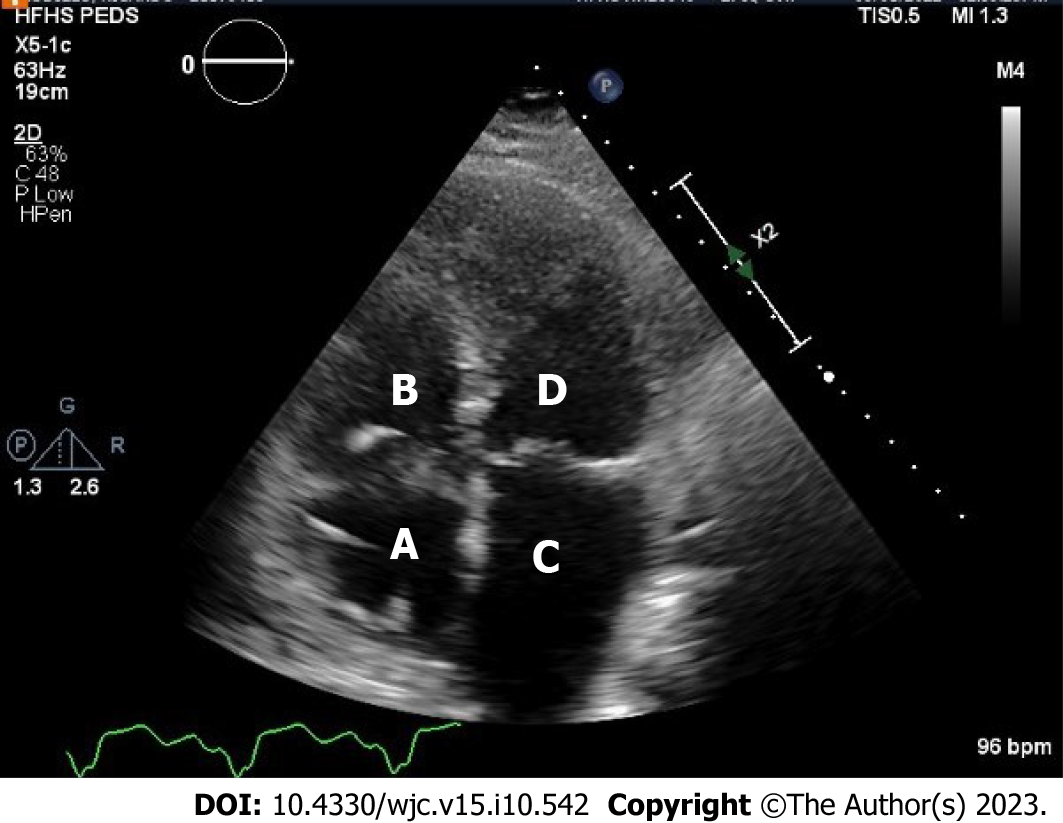
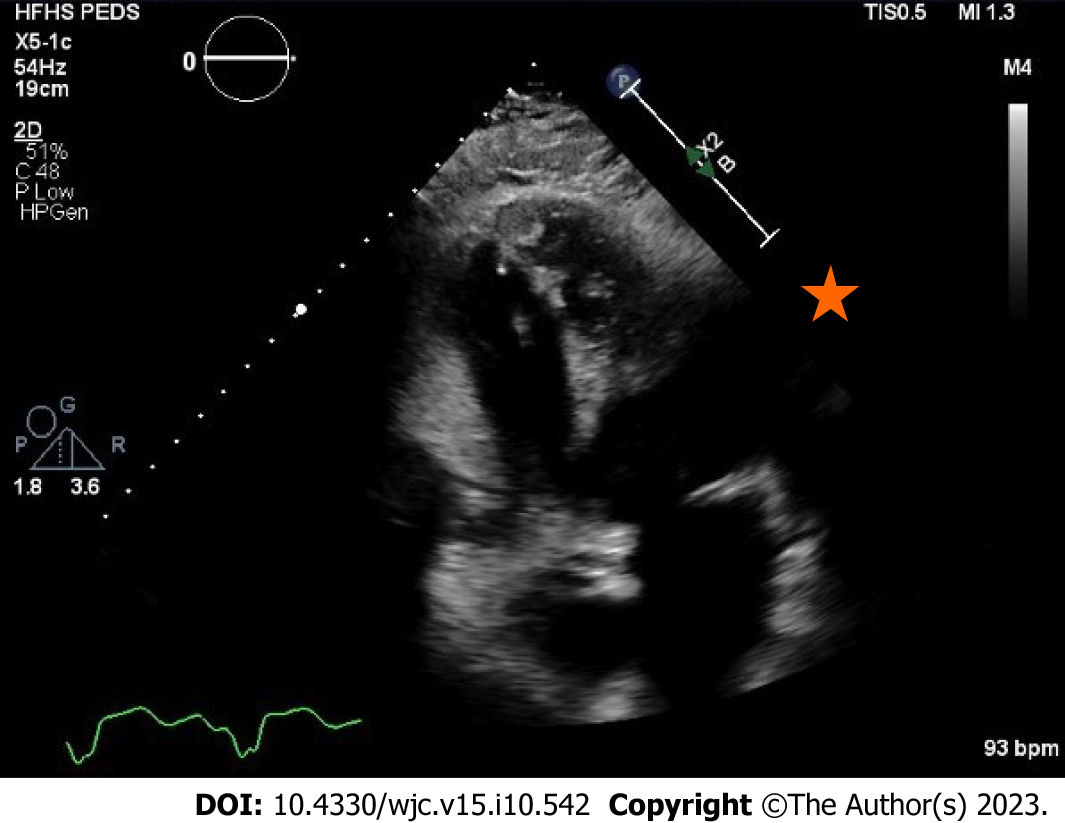
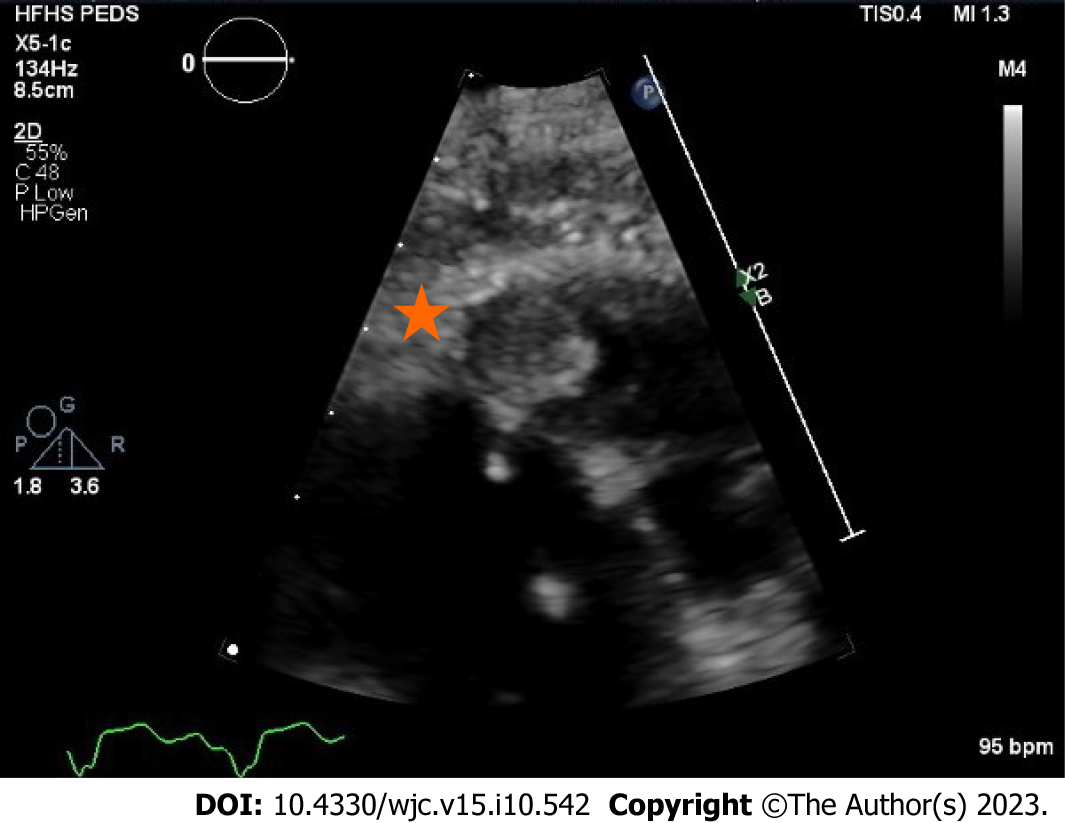
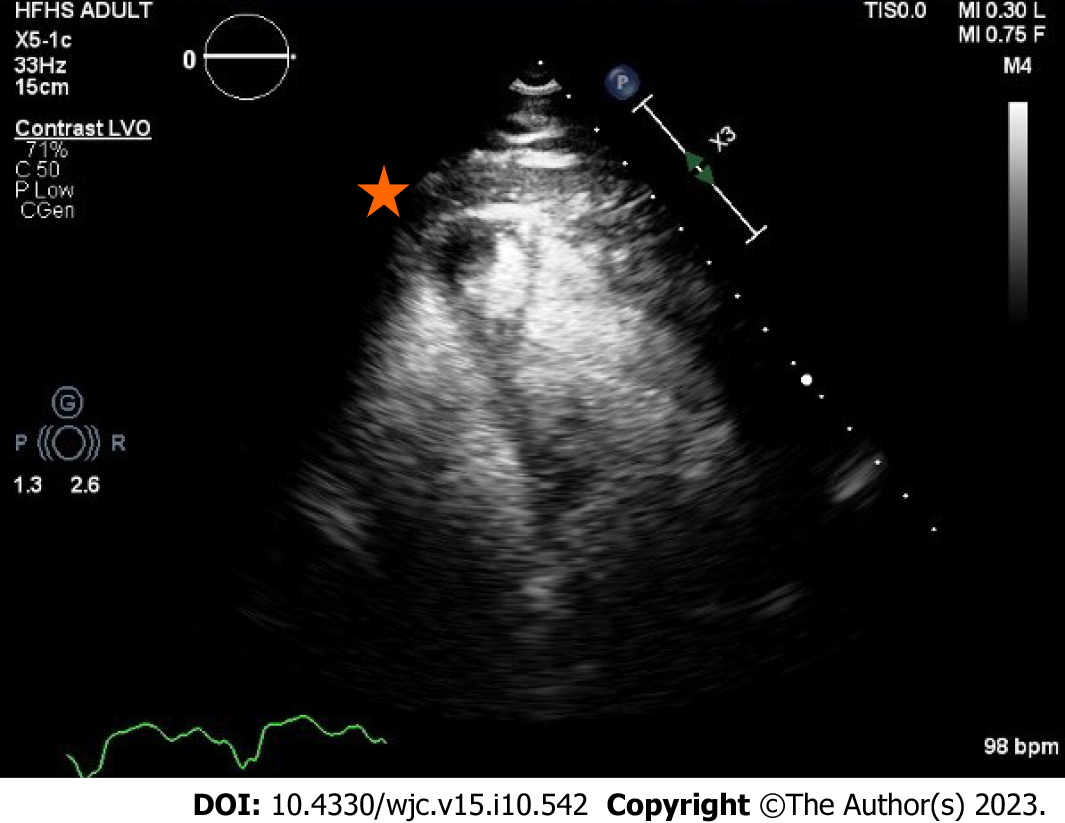
Transthoracic echocardiogram obtained prior to discharge showed a mildly reduced global sRV ejection fraction of 40%, severe sAV regurgitation, and a newly identified apical thrombus in the sRV (Figures 5-8). Transesophageal echocardiogram confirmed this finding with visualization of a 2.07 cm by 1.43 cm well-circumscribed mass.
The final diagnosis was ADHF complicated by a systemic right ventricular thrombus in the setting of L-TGA.
The presence of a sRV thrombus posed a dilemma given the limited literature on this topic. Our patient was anticoagulated with a vitamin K antagonist (VKA) and later referred for evaluation by advanced heart failure and heart transplant services.
On subsequent follow-up visits, our patient’s symptoms of heart failure continued to improve with medical optimization and cardiac rehabilitation. Transthoracic echocardiogram was performed 5 mo after the index echocardiogram that identified the sRV thrombus; it demonstrated interim resolution of the sRV thrombus with improvement in sAV regurgitation and estimated pulmonary artery systolic pressure (Table 2). Cardiopulmonary exercise testing (CPX) provides objective measures of cardiovascular fitness and allows them to be tracked over time; our patient’s peak oxygen uptake (peak VO2), a strong prognostic indicator in heart failure, showed improvement (Table 3). Tables 2 and 3 allow readers to understand the natural history and progression of L-TGA in adults by demonstrating findings from CPX and echocardiography from the patient’s initial visit in 2016 to the last known follow-up in 2022.
| Date of CPX | Peak HR (bpm) | Duration (min) | Peak VO2 (ml/kig/min) | VO2 at anerobic threshold (ml/kig/min) | Peak VO2 (% of age predicted) | Peak RER | Actual METS achieved | VE- | Peak double product | O2 pulse rest | O2 pulse peak |
| June 16, 2016 | 85 (46% predicted max) | 12.0 | 18.2 | 13.5 | 47 | 1.28 | 5.2 | 28.0 | 11900 | 4 | 16 |
| February 16, 2017 | 145 (78% predicted max) | 13.5 | 22.4 | 16.4 | 59 | 1.23 | 6.4 | 25.6 | 22040 | 4 | 12 |
| September 6, 2018 | 173 (94% predicted max) | 10.5 | 20.1 | - | 58 | 1.20 | 5.7 | 28.8 | 25258 | 3 | 11 |
| June 13, 2022 | 173 (96% predicted max) | 9.5 | 19.5 | - | 56 | 1.17 | 5.6 | 22.8 | 21106 | 3 | 11 |
| Date of study | Type of study | sRV parameters | sAV valve parameters | vLV parameters | vAV valve parameters | LA parameters | RA parameters | PA pressure (mmHg) | Other details |
| April 13, 2016 | Transthoracic | Severely reduced global RV SF. Moderately enlarged RV | Severe Reg (sAV Reg Vmax 4.17 m/s) | Mildly reduced LV SF (41%). Restrictive pattern of diastolic filling (G3) | Moderate-Severe Reg (vAV Reg Vmax 346.72 cm/s) | Mildly dilated | Normal | 51 | Side by side great arteries, anterior aorta consistent with L Trasnpostion of the Great Arteries (congenitally corrected). S/P VSD repair with intact patch in basal septum |
| November 10, 2016 | Transthoracic | Normal size and global systolic function (RV % FAC, A4C: 54.5%) | Moderate-Severe Reg (sAV Reg Vmax 5.28 m/s) | Mildly reduced LV SF (41%). Moderate hypokinesis of entire septal wall. Normal pattern of LV diastolic filling | Moderate Reg (vAV Reg Vmax 246.26 cm/s). Mildly thickened | Mildly dilated | Dilated | N/A | S/P BiV PPM |
| February 16, 2017 | Transthoracic | Mildly reduced global RV SF (RV % FAC, A4C: 44.7%). RV wall thickness is moderately increased | Severe Reg (sAV Reg Vmax 5.00 m/s) | Mildly reduced LV SF (48%). Normal size | Mild-moderate Reg (vAV Reg Vmax 332.84 cm/s) | Moderately dilated | Normal | 52 | Interim mild improvement in systemic RV function but persistent systemic AV valve severe regurgitation with moderate pulmonary hypertension |
| February 27, 2017 | Transthoracic | EF 54% (biplane) | EF 51% (A4C) | Limited study to quantify ventricular function | |||||
| September 25, 2018 | Transthoracic | Mild dysfunction. RV wall thickness is moderately increased | Moderate-Severe Reg (sAV Reg Vmax 4.74 m/s) | Mildly reduced LV SF (47%). Normal size | Mild Reg (vAV Reg Vmax 243.67 cm/s) | Mildly dilated | Normal | 32 | Overall no major changes noted compared to prior studies eccept for mild fluctuations in systemic RV function. |
| May 1, 2022 | Transthoracic | Mildly reduced global RV SF (40%) | Moderate-Severe Reg (sAV Reg Vmax 4.71 m/s) | EF 45% | Moderate Reg (vAV Reg Vmax 328.85 cm/s) | Moderately dilated | Normal | 46 | There is a 1.3 cm × 1.3 cm, well circumscribed mass with echolucent center, seen apically, and likely represents a thrombus. Saline contrast bubble study -ve |
| May 4, 2022 | Transesophageal | N/A | Moderate-Severe Reg (sAV Reg Vmax 2.46 m/s) | Moderately reduced LV SF (35%). Normal thickness | Moderate-Severe Reg (vAV Reg Vmax 383.31 cm/s) | N/A | N/A | N/A | Well-circumscribed mass measuring 2.07 cm × 1.43 cm in the morphologic RV/Systemic ventricle with central echolucency. Saline contrast bubble study was negative |
| September 30, 2022 | Transthoracic | Mildly reduced global RV SF (40-45%). Mildly enlarged sRV | Mild-Mod Reg | Low-normal LV SF function | Moderate-Severe Reg (vAV Reg Vmax 228.43 cm/s) | Upper normal | Normal | 24 | Interim resolution of small systemic RV apical thrombus. Extensive trabeculation related to systemic RV hypertrophy noted |
Intracardiac thrombus (ICT) involves the formation of a blood clot within the heart chambers. It typically occurs in the setting of acute myocardial infarction, left ventricular aneurysm, and cardiomyopathy with dilated chambers[15]. Pathophysiology of thrombus formation involves an interplay of prothrombotic state, tissue endothelial injury, and blood stasis as described by Virchow’s triad[16-18]. Systemic embolization of left-sided ICT results in clinical manifestations of stroke and transient ischemic attack, mesenteric ischemia and infarction, renal infarction, and acute limb ischemia. Pulmonary embolization of right-sided ICT results in pulmonary embolism. Diagnostic gold standard is identification of a thrombus on cardiac magnetic resonance imaging, although echocardiography with the use of echocardiographic contrast agents is a widely used initial modality[19].
ICT of the sRV is sparsely covered in the literature with limited data and guidelines available regarding the approach to management. Clinicians resort to extrapolating from practice standards for systemic left ventricular (sLV) thrombus. A review of literature identified no published case reports of sRV thrombus.
Current American and European guidelines for ICT in patients with structurally typical hearts are covered by class IIa, LOE C recommendations. Standard of care consists of anticoagulation with a VKA for 3-6 mo with an international normalized ratio (INR) target range of 2.0-3.0 followed by repeat imaging to assess for thrombus resolution[20,21]. Anticoagulation in this population has been shown to decrease major cardiovascular events including cerebral and systemic sequala of thrombus embolization[22]. Patients who have interval resolution of ICT on repeat imaging typically have anticoagulation discontinued, although some experts continue anticoagulation as a preventative measure in select patients with persistent and significant sLV wall hypokinesis due to the higher risk of recurrence[23]. In the absence of data on this population, patients with sRV dysfunction who develop right ventricular thrombus, such as our patient, can be similarly managed with anticoagulation using a VKA followed by repeat imaging.
Oral anticoagulant (OAC) agent of choice for the treatment of ICT has classically been a VKA as opposed to a direct OAC (DOAC), as early available literature demonstrated clinically significant difference in outcomes between the two agents. The largest study to date, a multicenter cohort study compared 514 patients with sLV thrombus and demon
Clinical consensus for the management of sRV thrombus in patients with congenital heart disease has been derived from the management principles of sLV thrombus in patients without congenital heart disease; it consists of OAC, interval repeat imaging, and case-by-case evaluation of anticoagulation duration. The advent of DOAC therapy has seen it become the OAC agent of choice for most anticoagulation indications which has raised questions regarding its applicability in the treatment of sRV thrombus in the setting of the limited data. The international NOTE registry evaluated 530 adults with congenital heart disease treated with OAC for various indications and concluded non-inferior efficacy and safety of DOAC use compared to VKA; subgroup analysis of 76 patients with sRV found high efficacy and safety rates[33,34]. Another similar study of 215 patients with different congenital heart diseases quoted high efficacy rates but non-negligible bleeding risks[35]. However, a retrospective cohort review of a German nationwide registry of 6504 adults with congenital heart disease on OAC determined that DOAC use was associated with higher rates of thromboembolism, bleeding, major adverse cardiac events, and all-cause mortality compared to VKA[36]. The discrepancy of results between the former and latter studies raises concerns can be explained by the significant heterogeneity including differences in age and complexity of lesions in the latter’s study population. In the absence of conclusive data supporting DOAC use in sRV thrombus in patients with congenital heart disease, VKA remains the OAC of choice.
Patients with sRV thrombus are typically younger than those with sLV thrombus given the earlier development of heart failure in the setting of congenital heart disease. The structural cardiac anomalies and abnormal hemodynamics are likely contributors to abnormal flow and blood stasis, this predisposes patients to thrombus formation. Younger patients are more likely to have educational and workplace commitments that cause time constraints. VKA therapy is particularly challenging in this population as dietary restrictions and frequent laboratory testing results in difficulty achieving and maintaining therapeutic INR levels; lower time in therapeutic range is associated with higher risk of stroke in patients with sLV[37]. Further studies evaluating the safety and efficacy of DOAC agents in sRV thrombus are necessary.
Systemic right ventricular thrombus is a rare complication of congenital heart disease. We describe the first reported case of sRV thrombus in a patient with L-TGA who presented with ADHF. Management of this condition is driven by expert opinion and extrapolation of treatment principles from ICT in patients with structurally normal hearts.
In the absence of conclusive data supporting DOAC use in sRV thrombus in patients with congenital heart disease, VKA bridged with intravenous heparin or subcutaneous low-molecular weight heparin and remains the time-honored approach particularly in patients with recent large thrombi and in the setting of dysfunctional ventricles or slow flow states. Transitioning to a DOAC in these cases should be individualized to a patient characteristics and imaging features; it should involve shared decision making regarding limited and conflicting literature. This case illustrates the natural history of L-TGA and highlights the importance of surveillance and monitoring in this patient population to prevent and treat complications.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ong H, Malaysia; Teragawa H, Japan S-Editor: Lin C L-Editor: A P-Editor: Yuan YY
| 1. | Samánek M, Vorísková M. Congenital heart disease among 815,569 children born between 1980 and 1990 and their 15-year survival: a prospective Bohemia survival study. Pediatr Cardiol. 1999;20:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 199] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 2. | Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, Gatzoulis MA, Gohlke-Baerwolf C, Kaemmerer H, Kilner P, Meijboom F, Mulder BJ, Oechslin E, Oliver JM, Serraf A, Szatmari A, Thaulow E, Vouhe PR, Walma E; Task Force on the Management of Grown-up Congenital Heart Disease of the European Society of Cardiology (ESC); Association for European Paediatric Cardiology (AEPC); ESC Committee for Practice Guidelines (CPG). ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J. 2010;31:2915-2957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1602] [Cited by in RCA: 1555] [Article Influence: 103.7] [Reference Citation Analysis (1)] |
| 3. | Wallis GA, Debich-Spicer D, Anderson RH. Congenitally corrected transposition. Orphanet J Rare Dis. 2011;6:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Komarlu R, Morell VO, Munoz RA, Tsifansky MD. Congenitally Corrected Transposition of the Great Arteries (ccTGA) or Levo-Transposition of the Great Arteries (l-TGA). In: Munoz R, Morell V, da Cruz E, Vetterly C, da Silva J, editor. Critical Care of Children with Heart Disease. Cham: Springer 2020: 369-77. [DOI] [Full Text] |
| 5. | Ikeda U, Furuse M, Suzuki O, Kimura K, Sekiguchi H, Shimada K. Long-term survival in aged patients with corrected transposition of the great arteries. Chest. 1992;101:1382-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Hraska V, Duncan BW, Mayer JE Jr, Freed M, del Nido PJ, Jonas RA. Long-term outcome of surgically treated patients with corrected transposition of the great arteries. J Thorac Cardiovasc Surg. 2005;129:182-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | van Son JA, Danielson GK, Huhta JC, Warnes CA, Edwards WD, Schaff HV, Puga FJ, Ilstrup DM. Late results of systemic atrioventricular valve replacement in corrected transposition. J Thorac Cardiovasc Surg. 1995;109:642-52; discussion 652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 115] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Filippov AA, Del Nido PJ, Vasilyev NV. Management of Systemic Right Ventricular Failure in Patients With Congenitally Corrected Transposition of the Great Arteries. Circulation. 2016;134:1293-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 9. | Graham TP Jr, Bernard YD, Mellen BG, Celermajer D, Baumgartner H, Cetta F, Connolly HM, Davidson WR, Dellborg M, Foster E, Gersony WM, Gessner IH, Hurwitz RA, Kaemmerer H, Kugler JD, Murphy DJ, Noonan JA, Morris C, Perloff JK, Sanders SP, Sutherland JL. Long-term outcome in congenitally corrected transposition of the great arteries: a multi-institutional study. J Am Coll Cardiol. 2000;36:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 424] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 10. | Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. 2020;22:1342-1356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 374] [Cited by in RCA: 1180] [Article Influence: 236.0] [Reference Citation Analysis (0)] |
| 11. | Spigel Z, Binsalamah ZM, Caldarone C. Congenitally Corrected Transposition of the Great Arteries: Anatomic, Physiologic Repair, and Palliation. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2019;22:32-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Bautista-Hernandez V, Marx GR, Gauvreau K, Mayer JE Jr, Cecchin F, del Nido PJ. Determinants of left ventricular dysfunction after anatomic repair of congenitally corrected transposition of the great arteries. Ann Thorac Surg. 2006;82:2059-65; discussion 2065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Hiramatsu T, Matsumura G, Konuma T, Yamazaki K, Kurosawa H, Imai Y. Long-term prognosis of double-switch operation for congenitally corrected transposition of the great arteries. Eur J Cardiothorac Surg. 2012;42:1004-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Mongeon FP, Connolly HM, Dearani JA, Li Z, Warnes CA. Congenitally corrected transposition of the great arteries ventricular function at the time of systemic atrioventricular valve replacement predicts long-term ventricular function. J Am Coll Cardiol. 2011;57:2008-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Meltzer RS, Visser CA, Fuster V. Intracardiac thrombi and systemic embolization. Ann Intern Med. 1986;104:689-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 142] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Massussi M, Scotti A, Lip GYH, Proietti R. Left ventricular thrombosis: new perspectives on an old problem. Eur Heart J Cardiovasc Pharmacother. 2021;7:158-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 17. | Garg P, van der Geest RJ, Swoboda PP, Crandon S, Fent GJ, Foley JRJ, Dobson LE, Al Musa T, Onciul S, Vijayan S, Chew PG, Brown LAE, Bissell M, Hassell MECJ, Nijveldt R, Elbaz MSM, Westenberg JJM, Dall'Armellina E, Greenwood JP, Plein S. Left ventricular thrombus formation in myocardial infarction is associated with altered left ventricular blood flow energetics. Eur Heart J Cardiovasc Imaging. 2019;20:108-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 18. | Yokota Y, Kawanishi H, Hayakawa M, Kumaki T, Takarada A, Nakanishi O, Fukuzaki H. Cardiac thrombus in dilated cardiomyopathy. Relationship between left ventricular pathophysiology and left ventricular thrombus. Jpn Heart J. 1989;30:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | McCarthy CP, Vaduganathan M, McCarthy KJ, Januzzi JL Jr, Bhatt DL, McEvoy JW. Left Ventricular Thrombus After Acute Myocardial Infarction: Screening, Prevention, and Treatment. JAMA Cardiol. 2018;3:642-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 20. | O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78-e140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1953] [Cited by in RCA: 2275] [Article Influence: 175.0] [Reference Citation Analysis (0)] |
| 21. | Ibánez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Rev Esp Cardiol (Engl Ed). 2017;70:1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 22. | Lattuca B, Bouziri N, Kerneis M, Portal JJ, Zhou J, Hauguel-Moreau M, Mameri A, Zeitouni M, Guedeney P, Hammoudi N, Isnard R, Pousset F, Collet JP, Vicaut E, Montalescot G, Silvain J; ACTION Study Group. Antithrombotic Therapy for Patients With Left Ventricular Mural Thrombus. J Am Coll Cardiol. 2020;75:1676-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 23. | Cruz Rodriguez JB, Okajima K, Greenberg BH. Management of left ventricular thrombus: a narrative review. Ann Transl Med. 2021;9:520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 24. | Robinson AA, Trankle CR, Eubanks G, Schumann C, Thompson P, Wallace RL, Gottiparthi S, Ruth B, Kramer CM, Salerno M, Bilchick KC, Deen C, Kontos MC, Dent J. Off-label Use of Direct Oral Anticoagulants Compared With Warfarin for Left Ventricular Thrombi. JAMA Cardiol. 2020;5:685-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 177] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 25. | Robinson AA, Ruth B, Dent J. Direct oral anticoagulants compared to warfarin for left ventricular thrombi: a single center experience. J Am Coll Cardiol. 2018;71:A981. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Daher J, Da Costa A, Hilaire C, Ferreira T, Pierrard R, Guichard JB, Romeyer C, Isaaz K. Management of Left Ventricular Thrombi with Direct Oral Anticoagulants: Retrospective Comparative Study with Vitamin K Antagonists. Clin Drug Investig. 2020;40:343-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 27. | Fleddermann AM, Hayes CH, Magalski A, Main ML. Efficacy of Direct Acting Oral Anticoagulants in Treatment of Left Ventricular Thrombus. Am J Cardiol. 2019;124:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 28. | Cochran JM, Jia X, Hamzeh I, Birnbaum Y. Direct oral anticoagulant use for left ventricular thrombus: a single center experience. Circulation. 2018;138:A16411. [DOI] [Full Text] |
| 29. | Isa WY, Hwong N, Yusof AK, Yusof Z, Loong N, Wan-Arfah N, Naing N. Apixaban vs warfarin in patients with left ventricular thrombus: A pilot prospective randomized outcome blinded study investigating size reduction or resolution of left ventricular thrombus. Journal of Clinical and Preventive Cardiology. 2020;9:150-154. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Abdelnabi M, Saleh Y, Fareed A, Nossikof A, Wang L, Morsi M, Eshak N, Abdelkarim O, Badran H, Almaghraby A. Comparative Study of Oral Anticoagulation in Left Ventricular Thrombi (No-LVT Trial). J Am Coll Cardiol. 2021;77:1590-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 31. | Alcalai R, Butnaru A, Moravsky G, Yagel O, Rashad R, Ibrahimli M, Planer D, Amir O, Elbaz-Greener G, Leibowitz D. Apixaban vs. warfarin in patients with left ventricular thrombus: a prospective multicentre randomized clinical trial‡. Eur Heart J Cardiovasc Pharmacother. 2022;8:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 32. | Levine GN, McEvoy JW, Fang JC, Ibeh C, McCarthy CP, Misra A, Shah ZI, Shenoy C, Spinler SA, Vallurupalli S, Lip GYH; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and Stroke Council. Management of Patients at Risk for and With Left Ventricular Thrombus: A Scientific Statement From the American Heart Association. Circulation. 2022;146:e205-e223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 148] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 33. | Yang H, Bouma BJ, Dimopoulos K, Khairy P, Ladouceur M, Niwa K, Greutmann M, Schwerzmann M, Egbe A, Scognamiglio G, Budts W, Veldtman G, Opotowsky AR, Broberg CS, Gumbiene L, Meijboom FJ, Rutz T, Post MC, Moe T, Lipczyńska M, Tsai SF, Chakrabarti S, Tobler D, Davidson W, Morissens M, van Dijk A, Buber J, Bouchardy J, Skoglund K, Christersson C, Kronvall T, Konings TC, Alonso-Gonzalez R, Mizuno A, Webb G, Laukyte M, Sieswerda GTJ, Shafer K, Aboulhosn J, Mulder BJM. Non-vitamin K antagonist oral anticoagulants (NOACs) for thromboembolic prevention, are they safe in congenital heart disease? Results of a worldwide study. Int J Cardiol. 2020;299:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 34. | Scognamiglio G, Fusco F, Hankel TC, Bouma BJ, Greutmann M, Khairy P, Ladouceur M, Dimopoulos K, Niwa K, Broberg CS, Miranda B, Budts W, Bouchardy J, Schwerzmann M, Lipczyńska M, Tobler D, Tsai SF, Egbe AC, Aboulhosn J, Fernandes SM, Garr B, Rutz T, Mizuno A, Proietti A, Alonso-Gonzalez R, Mulder BJM, Sarubbi B; non-vitamin K antagonist oral anticoagulants for thromboembolic prevention in adult congenital heart disease (NOTE) registry Investigators. Safety and efficacy of non-vitamin K antagonist oral anticoagulants for prevention of thromboembolism in adults with systemic right ventricle: Results from the NOTE international registry. Int J Cardiol. 2021;322:129-134. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 35. | Pujol C, Müssigmann M, Schiele S, Nagdyman N, Niesert AC, Kaemmerer H, Ewert P, Tutarel O. Direct oral anticoagulants in adults with congenital heart disease - a single centre study. Int J Cardiol. 2020;300:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Freisinger E, Gerß J, Makowski L, Marschall U, Reinecke H, Baumgartner H, Koeppe J, Diller GP. Current use and safety of novel oral anticoagulants in adults with congenital heart disease: results of a nationwide analysis including more than 44 000 patients. Eur Heart J. 2020;41:4168-4177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 37. | Camaj A, Fuster V, Giustino G, Bienstock SW, Sternheim D, Mehran R, Dangas GD, Kini A, Sharma SK, Halperin J, Dweck MR, Goldman ME. Left Ventricular Thrombus Following Acute Myocardial Infarction: JACC State-of-the-Art Review. J Am Coll Cardiol. 2022;79:1010-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 101] [Article Influence: 33.7] [Reference Citation Analysis (0)] |