Published online Oct 26, 2023. doi: 10.4330/wjc.v15.i10.508
- This article has been corrected.
- See: World J Cardiol. Apr 26, 2024; 16(4): 215-216
Peer-review started: July 23, 2023
First decision: September 4, 2023
Revised: September 17, 2023
Accepted: September 22, 2023
Article in press: September 22, 2023
Published online: October 26, 2023
Processing time: 92 Days and 23.4 Hours
Out-of-hospital cardiac arrest (OHCA) is a leading cause of death worldwide.
To explore factors influencing prehospital return of spontaneous circulation (P-ROSC) in patients with OHCA and develop a nomogram prediction model.
Clinical data of patients with OHCA in Shenzhen, China, from January 2012 to December 2019 were retrospectively analyzed. Least absolute shrinkage and selection operator (LASSO) regression and multivariate logistic regression were applied to select the optimal factors predicting P-ROSC in patients with OHCA. A nomogram prediction model was established based on these influencing factors. Discrimination and calibration were assessed using receiver operating characteristic (ROC) and calibration curves. Decision curve analysis (DCA) was used to evaluate the model’s clinical utility.
Among the included 2685 patients with OHCA, the P-ROSC incidence was 5.8%. LASSO and multivariate logistic regression analyses showed that age, bystander cardiopulmonary resuscitation (CPR), initial rhythm, CPR duration, ventilation mode, and pathogenesis were independent factors influencing P-ROSC in these patients. The area under the ROC was 0.963. The calibration plot demonstrated that the predicted P-ROSC model was concordant with the actual P-ROSC. The good clinical usability of the prediction model was confirmed using DCA.
The nomogram prediction model could effectively predict the probability of P-ROSC in patients with OHCA.
Core Tip: A large gap in the rate of prehospital return of spontaneous circulation remains between China and other countries and that the relative contributions of aid measures of the factors to prehospital return of spontaneous circulation vary across countries. There is still not such model, including pre-emergency medical service intervention factors and Prehospital emergency measures, developing for prehospital return of spontaneous circulation in China. Compared to similar models from other countries, the model proposed in the present study is interpretable, convenient to implement, easy to comprehend in busy prehospital processing, and comprehensive, including prehospital drug administration. Therefore, it could serve as a potentially assistive tool for clinical aid decision-making.
- Citation: Wang JJ, Zhou Q, Huang ZH, Han Y, Qin CZ, Chen ZQ, Xiao XY, Deng Z. Establishment of a prediction model for prehospital return of spontaneous circulation in out-of-hospital patients with cardiac arrest. World J Cardiol 2023; 15(10): 508-517
- URL: https://www.wjgnet.com/1949-8462/full/v15/i10/508.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i10.508
Out-of-hospital cardiac arrest (OHCA), a leading cause of death worldwide, has an average incidence of (30.0-97.1) cases per 100000 person-years[1], with a survival rate of only 8.8% at hospital discharge[2]. In China, 550000 people develop OHCA annually[2] with a survival rate of only 1.3% after discharge, making OHCA a major public health issue. The survival rate from hospital discharge of patients with OHCA who have achieved a return of spontaneous circulation (ROSC) to hospital handover is still approximately 10%[3]. Moreover, patients with prehospital ROSC (P-ROSC) have better neurological outcomes compared with those who do not. Some areas regions have reported high ROSC rates during hospital handovers, such as 25% in England[4] and 29.1% in Tasmania, Australia[5]. In China, however, the P-ROSC rate was only 6.26% in a recent survey in Beijing[6], demonstrating that a large gap still exists between countries.
Investigations have reported a range of pre-emergency medical service (EMS) intervention factors for patients with OHCA that are associated with P-ROSC, including age, prehospital drug administration, witnessed status, first rhythm, and response time[7]. The relative contribution of each of these factors to P-ROSC varies across countries. However, no model including pre-EMS intervention factors and prehospital emergency measures has been developed for ROSC during hospital handovers in China. Therefore, the development of an effective prediction model is required for ROSC during hospital handovers based on pre-EMS intervention factors and prehospital emergency measures.
This study aimed to identify independent factors associated with P-ROSC and develop and evaluate a nomogram model in China to predict whether ROSC can be achieved during the prehospital period in OHCA.
A multicenter retrospective study was conducted at the Shenzhen Center for Prehospital Care, which covered all hospitals in Shenzhen; that is, approximately 150 hospitals, from January 2012 to December 2019. All first-aid measures were performed according to the American Heart Association (AHA) Guidelines for cardiopulmonary resuscitation (CPR).
We retrospectively collected data from emergency medical technicians (EMTs). The inclusion criteria were patients with OHCA aged ≥ 18 years, The exclusion criteria were incomplete cases, dead patients (i.e., rigor mortis, lividity, decomposition, or decapitation) without CPR by the EMTs, and patients whose family members forwent all treatments.
Data were gathered from two sources: The Registration System of Pre-hospital First Aid Information and Statistics and the Patient Care Report Form. The extracted data included clinical features such as sex, age, time of arrest (i.e., 0-8, 8-16, or 16-24 min), season of arrest, bystander CPR, initial rhythm, CPR duration, ventilation mode, defibrillation, epinephrine dose, use of other drugs (atropine, lidocaine, and amiodarone), and outcomes (P-ROSC).
According to the 2015 AHA guidelines, ROSC was defined as the clinical indication of the presence of vital signs, including palpable pulses or blood pressure.
IBM SPSS Statistics for Windows, version 26.0, and R 3.1.2 were used to perform all analyses. The t-test and Mann–Whitney U test were used for numerical variables, while the chi-square test was applied to categorical variables. A model was established using least absolute shrinkage and selection operator (LASSO) regression and multivariable backward regression. For the LASSO regression, lambda.min (a minimum mean squared error) and lambda.1se (lambda.min with one standard error) were identified as the goodness penalty lambda based on the lambda-choosing path[8]. To screen for potential predictive factors, LASSO regression models with a lambda.1 se penalty were constructed. The variables selected via LASSO were included in a multivariate backward regression analysis to identify the independent influencing features of patients with OHCA. A nomogram prediction model was then constructed based on the variables with statistical significance. The discrimination and calibration of the model were assessed using receiver operating characteristic (ROC) and calibration curves, as well as the Hosmer–Lemeshow test. Internal verification was performed by strengthening the bootstrap method for 1000 repetitions, as shown in the calibration curves. The clinical practicability of the model was evaluated using decision curve analysis (DCA) according to the net benefit with different threshold probabilities.
A total of 2685 cases of patients with OHCA satisfied the inclusion and exclusion criteria. Table 1 presents the characteristics of all OHCA incidents in which first-aid treatment was implemented.
| Features | Patients | P value | ||
| Total (n = 2685) | ROSC failure (n = 2529) | ROSC (n = 156) | ||
| Gender | 0.730 | |||
| Female | 668 (24.9) | 631 (25.0) | 37 (23.7) | |
| Male | 2017 (75.1) | 1898 (75.0) | 119 (76.3) | |
| Age | 56.18 ± 17.98 | 56.63 ± 17.96 | 49.01 ± 16.82 | < 0.001 |
| Season | 0.570 | |||
| Spring | 636 (23.7) | 599 (23.7) | 37 (23.7) | |
| Summer | 625 (23.3) | 582 (23.0) | 43 (27.6) | |
| Autumn | 666 (24.8) | 632 (25.0) | 34 (21.8) | |
| Winter | 758 (28.2) | 716 (28.3) | 42 (26.9) | |
| Time (min) | 0.029 | |||
| 08-16 | 953 (35.5) | 888 (35.1) | 65 (41.7) | |
| 16-24 | 1049 (39.1) | 984 (38.9) | 65 (41.7) | |
| 00-08 | 683 (25.4) | 657 (26.0) | 26 (16.7) | |
| Bystander CPR | < 0.001 | |||
| No | 2246 (83.6) | 2135 (84.4) | 111 (71.2) | |
| Yes | 439 (16.4) | 394 (15.6) | 45 (28.8) | |
| Initial rhythm | < 0.001 | |||
| VF/VT | 293 (10.9) | 232 (9.2) | 61 (39.1) | |
| Asystole/PEA | 2358 (87.8) | 2278 (90.1) | 80 (51.3) | |
| Ag ECG (slow ventricular escape, bradycardia) | 34 (1.3) | 19 (0.8) | 15 (9.6) | |
| Duration of CPR | 35 (15.0) | 38 (18.0) | 10 (16.0) | < 0.001 |
| ETI | < 0.001 | |||
| No | 2194 (81.7) | 2104 (83.2) | 90 (57.7) | |
| Yes | 491 (18.3) | 425 (16.8) | 66 (42.3) | |
| DF | < 0.001 | |||
| No | 2000 (74.5) | 1914 (75.7) | 86 (55.1) | |
| Yes | 685 (25.5) | 615 (24.3) | 70 (44.9) | |
| Epinephrine dose | 3 (3.0) | 3 (3.0) | 3 (2.0) | < 0.001 |
| Atropine | 0.243 | |||
| No | 2081 (77.5) | 1966 (77.7) | 130 (83.3) | |
| Yes | 604 (22.5) | 563 (22.3) | 26 (16.7) | |
| Lidocaine or amiodarone | < 0.001 | |||
| No | 2556 (95.2) | 2426 (95.9) | 135 (86.5) | |
| Yes | 129 (4.8) | 103 (4.1) | 21 (13.5) | |
| Etiology | 0.019 | |||
| Cardiac | 1430 (53.3) | 1327 (52.5) | 103 (66.0) | |
| Trauma | 122 (4.5) | 116 (4.6) | 6 (3.8) | |
| Toxicosis or asphyxia | 45 (1.7) | 44 (1.7) | 1 (0.6) | |
| Brain and nervous | 64 (2.4) | 60 (2.4) | 4 (2.6) | |
| Unknow and other | 1024 (38.1) | 982 (38.8) | 42 (26.9) | |
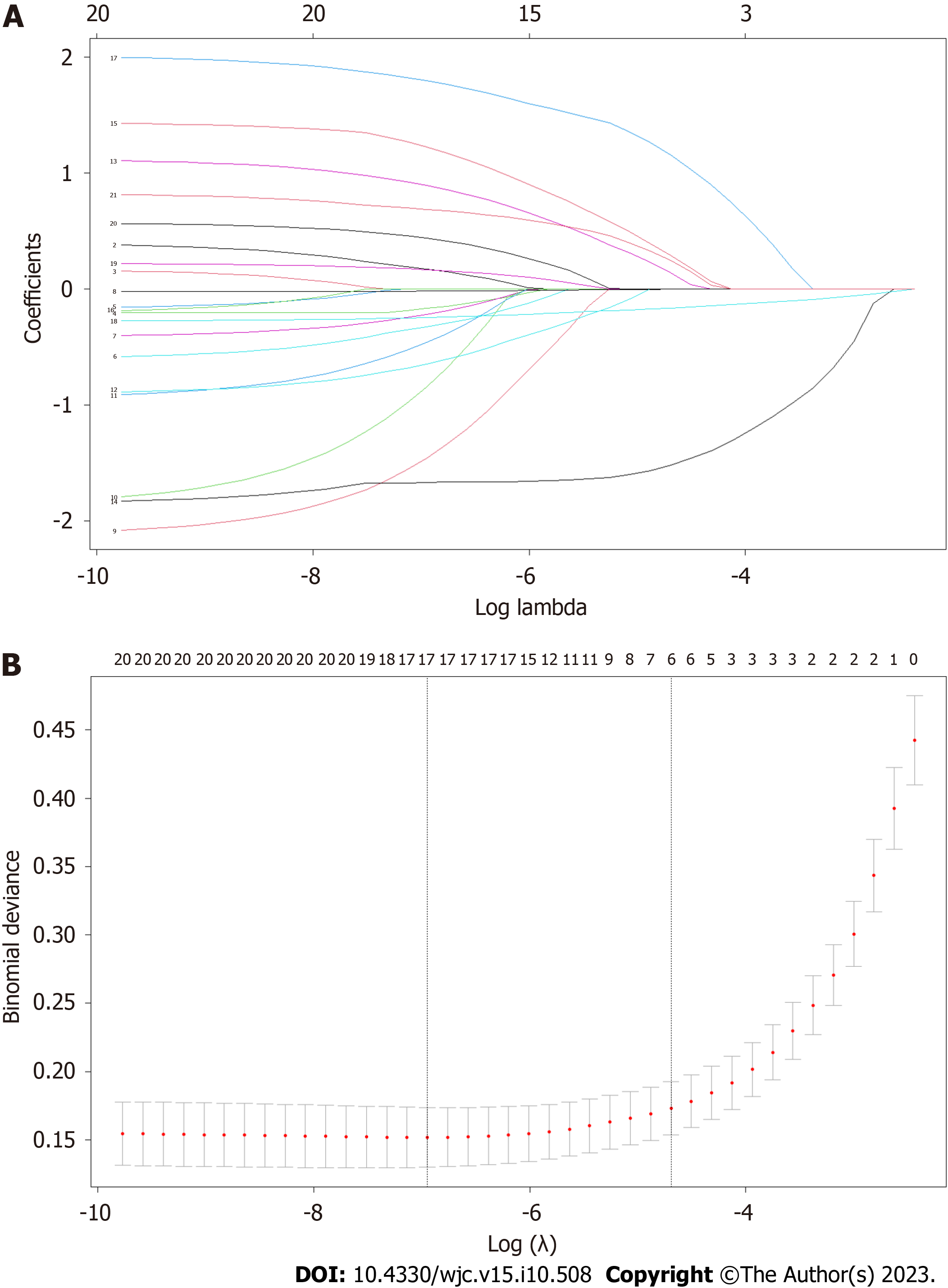
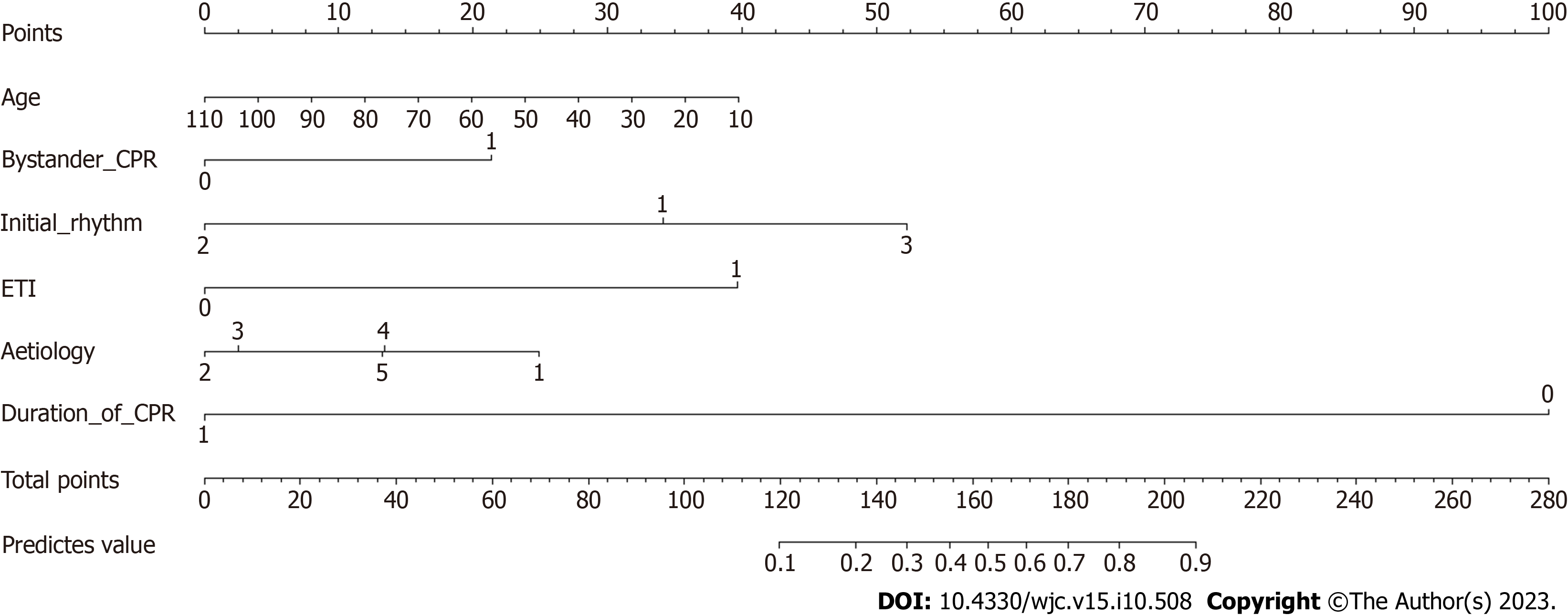
We applied LASSO regression to identify potential predictors and then employed multivariate backward logistic regression to establish the model. As shown in Figure 1, the LASSO regression identified seven features through the lambda.1se penalty: Age, bystander CPR, initial rhythm, CPR duration, ventilation mode, use of amiodarone and lidocaine, and etiology. A model was then formed based on the factors evaluated using multivariate logistic regression (Table 2, Figure 3), during which amiodarone was eliminated.
| Features | Multivariable analysis | Selected factors for model | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Age | 0.98 | 0.964-0.997 | 0.018 | 0.98 | 0.964-0.996 | 0.017 |
| Bystander CPR | 0.004 | 0.001 | ||||
| No | 1 | 1 | ||||
| Yes | 2.621 | 1.36-5.051 | 2.854 | 1.504-5.414 | ||
| Initial rhythm | < 0.001 | < 0.001 | ||||
| VF/VT | 1 | 1 | ||||
| Asystole/PEA | 0.174 | 0.087-0.349 | < 0.001 | 0.16 | 0.081-0.318 | < 0.001 |
| Ag ECG (slow ventricular escape, bradycardia) | 4.539 | 0.89-23.15 | 0.069 | 4.103 | 0.806-20.895 | 0.089 |
| CPR duration | 0.778 | 0.75-0.807 | < 0.001 | 0.776 | 0.749-0.805 | < 0.001 |
| ETI | < 0.001 | < 0.001 | ||||
| No | 1 | 1 | ||||
| Yes | 8.227 | 4.384-15.439 | 8.288 | 4.441-15.465 | ||
| Lidocaine or amiodarone | 0.113 | |||||
| No | 1 | |||||
| Yes | 2.271 | 0.823-6.271 | ||||
| Etiology | 0.008 | 0.007 | ||||
| Cardiac | 1 | 1 | ||||
| Trauma | 0.156 | 0.037-0.65 | 0.011 | 0.145 | 0.035-0.601 | 0.008 |
| Toxicosis or asphyxia | 0.171 | 0.007-4.026 | 0.275 | 0.163 | 0.007-3.907 | 0.263 |
| Brain and nervous | 0.393 | 0.079-1.95 | 0.253 | 0.371 | 0.075-1.844 | 0.226 |
| Unknow and other | 0.384 | 0.205-0.721 | 0.003 | 0.389 | 0.208-0.728 | 0.003 |
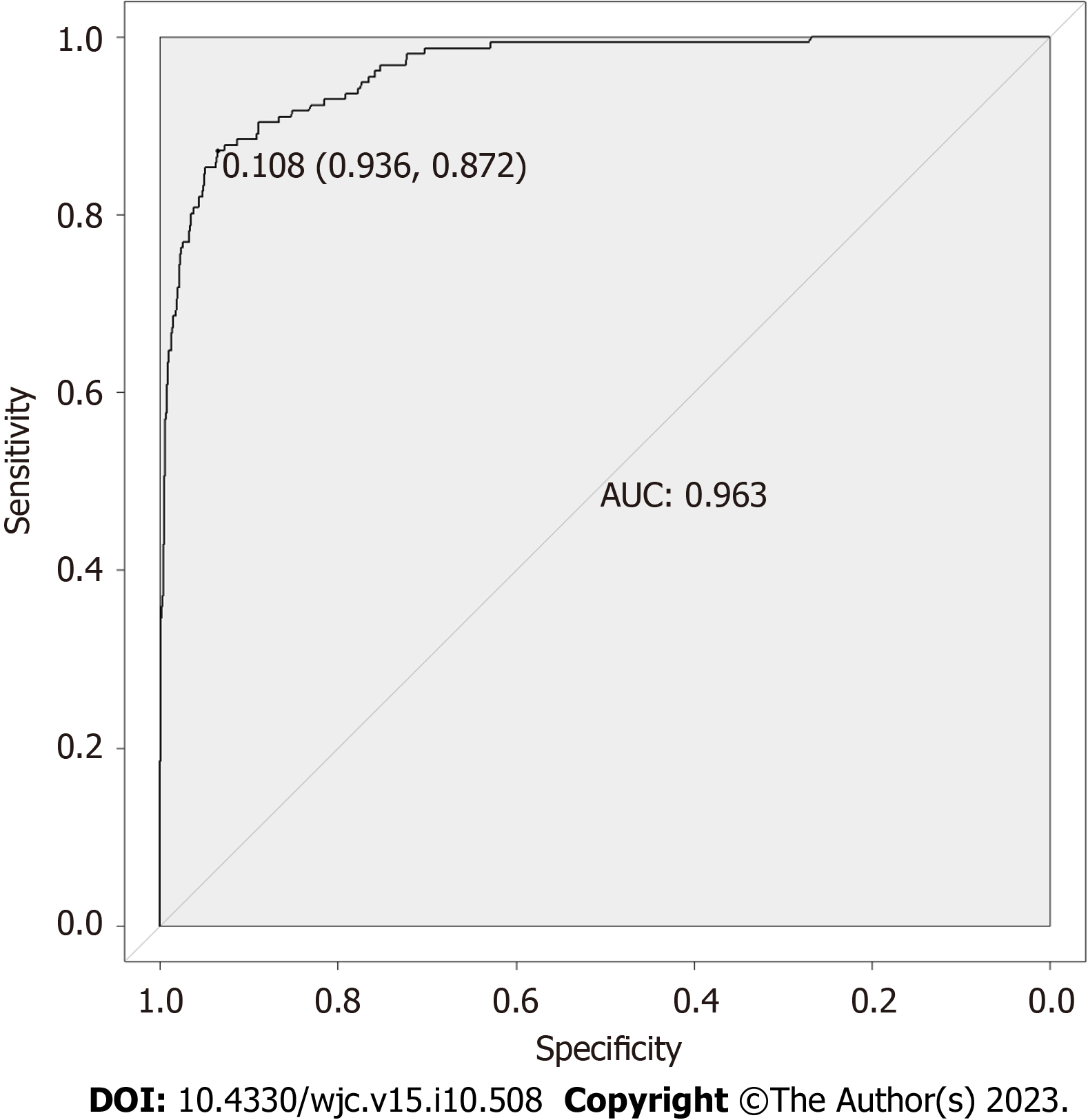
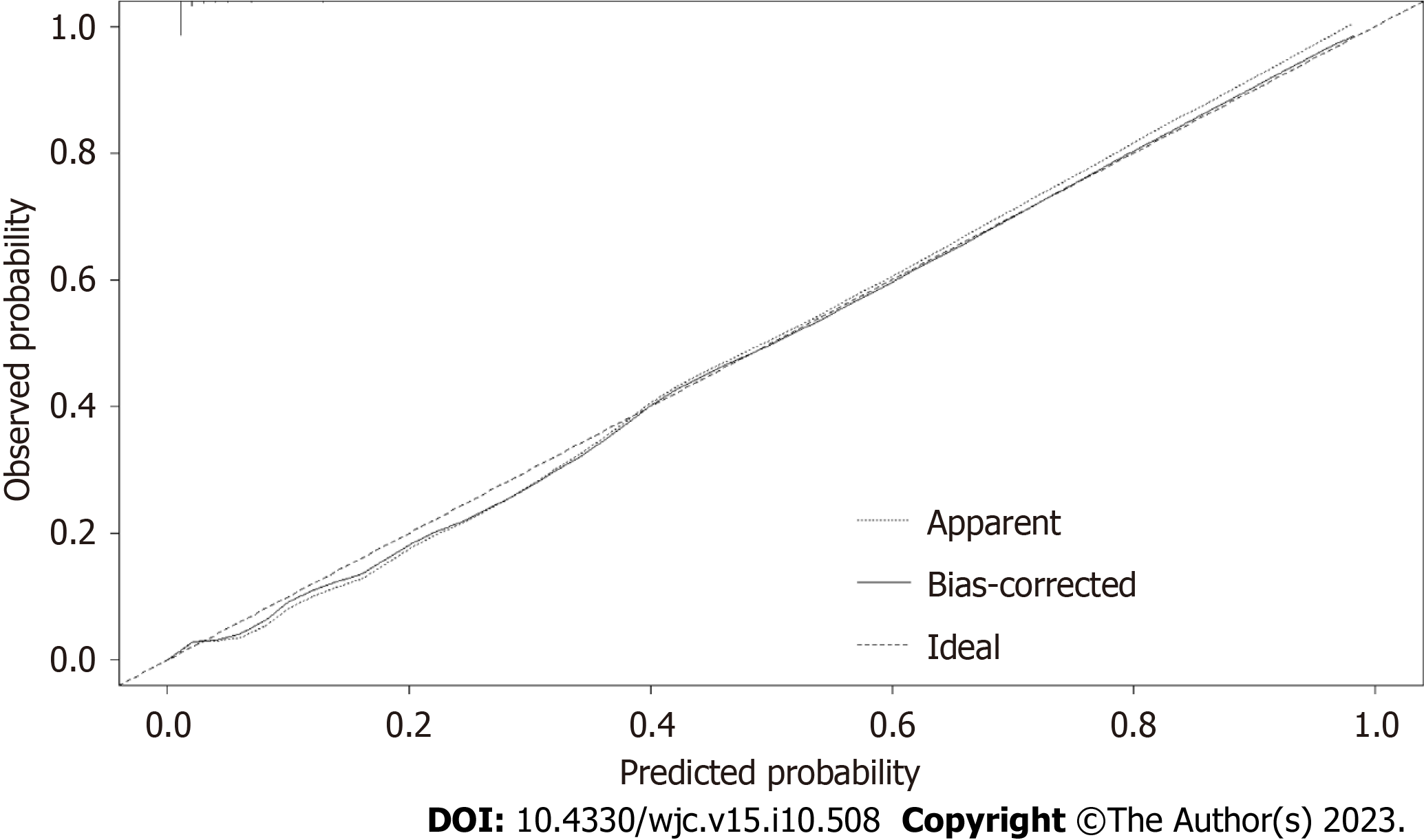
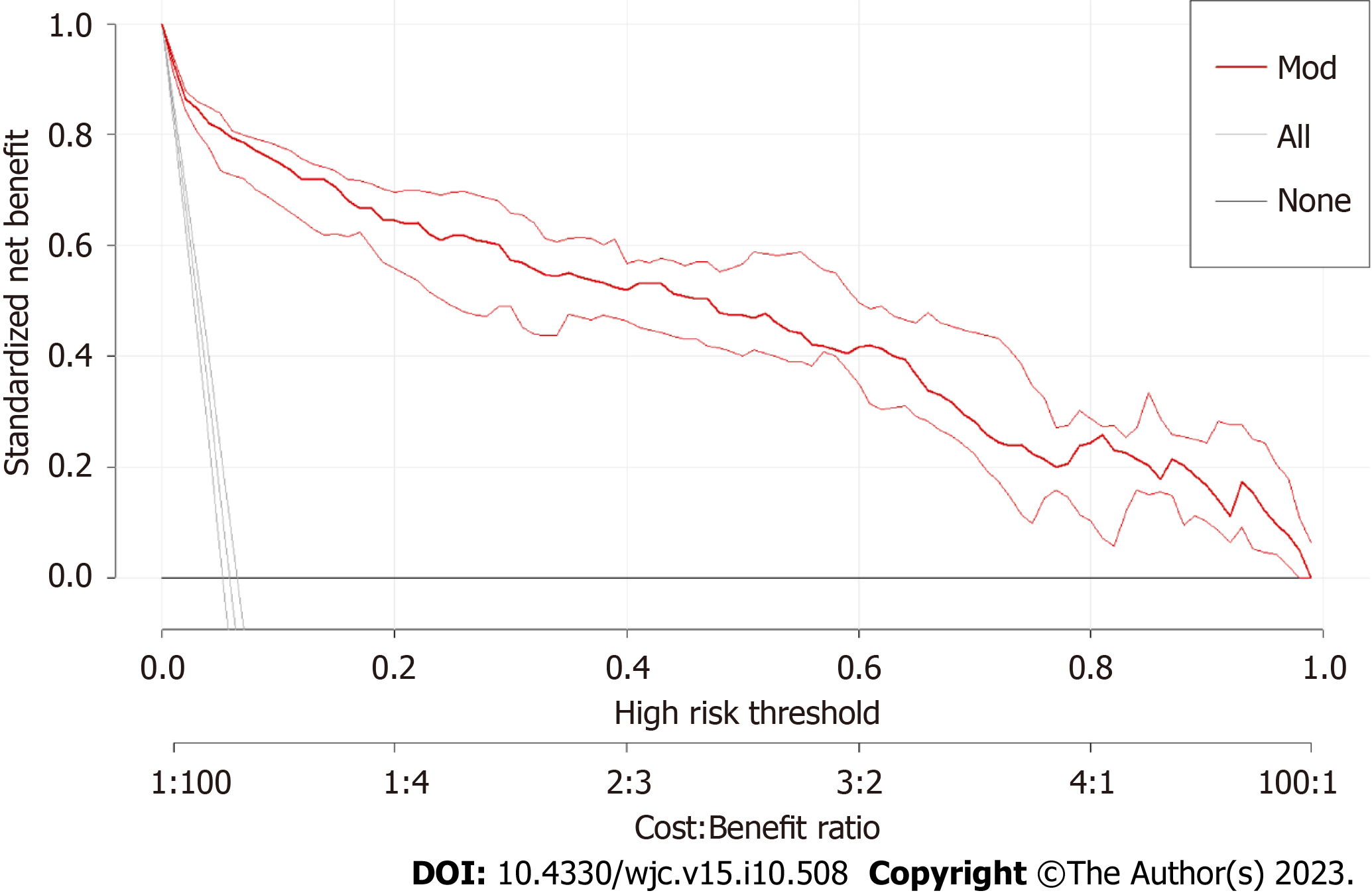
The area under the receiver operating characteristic curve (AUC) of the P-ROSC prediction model was 0.9627 (95%: 0.9485-0.9769), which demonstrated the good discrimination ability of the model (Figure 3). A strengthened bootstrap self-sampling method was used to verify the model internally. Furthermore, the calibration plots fitted well with the ideal curves (Figure 4), indicating that the predicted probability was consistent with the actual probability, as suggested by the results of the Hosmer–Lemeshow test (χ2 = 8.421, df = 8, P = 0.3935). DCA to evaluate the clinical utility showed a net benefit for the “treat-all” or “treat-none” strategy (Figure 5), which suggested that the model was clinically useful.
The survival to hospital discharge in patients with OHCA worldwide is only 4.5%[9]. P-ROSC is a short-term survival event. However, recent studies have shown that patients who achieve P-ROSC have better neurological outcomes than those who do not[10,11]. Therefore, pre-EMS intervention factors and prehospital emergency measures have been analyzed to evaluate whether EMS intervention measures, including drug treatments, are necessary to inform EMT decisions to terminate the rescue following an appropriate determination to terminate resuscitation.
Age is an influencing factor in OHCA outcomes[12], age is considered as an influencing factor of OHCA outcomes. Consistent with the results of previous studies[13], age was significantly associated with ROSC in the adjusted model (OR: 0.98, P < 0.001) in the present study. In terms of etiology, cardiovascular system diseases accounted for the largest proportion of patients with OHCA (53.3%) in this study, while OHCA caused by central nervous system diseases had the highest rate of P-ROSC failure, followed by patients with traumas.
In some regions, the number of CPR bystanders has reached 50%, particularly in advanced countries[14]. However, although it is increasing annually, the bystander CPR rate remained poor in the current study. Rajan et al[15] suggested that sustained bystander CPR could increase by more than two-fold. In our study, CPR by bystanders increased the chances of ROSC by 2.6-fold (P = 0.004) compared with no bystander CPR.
The initial rhythm is another critical factor because timely defibrillation can increase the ROSC rate when a shockable rhythm occurs[16]. In the present study, the initial rhythm was also an essential variable of P-ROSC after adjusting for other variables (P < 0.001). Moreover, patients with agonal electrocardiography (Ag ECG) characteristics, such as slow ventricular escape and bradycardia, who progressed to OHCA during treatment, had a higher P-ROSC rate, implying that a shorter time of no reflow time could improve prognosis.
Furthermore, CPR duration is a vital factor in predicting OHCA outcomes. Despite advances in CPR, no comprehensive agreement has been reached regarding the duration of CPR and the time for its termination in patients with OHCA. Funada et al[17] reported that CPR sustained in patients with OHCA > 26 min commonly caused ROSC failure. In the present study, the optimal cut-off time was 27.5 min and each additional minute of CPR was related to a 22% decrease in the probability of P-ROSC (OR: 0.78, P < 0.001) after adjustment for variable. Moreover, some studies suggested no possibility of ROSC in CPR lasting > 30 min, usually accompanied by irreversible damage to the brain[18]. Hence, uncertainties in the proper termination rules for CPR in basic and advanced life support care could increase pressure on ambulance transport, competition for medical resources, and risk of exposure to public accidents owing to high-speed transport[19]. However, the appropriate resuscitation termination for patients with OHCA remains controversial. Based on the model performance, we found that patients with OHCA and organ function as well as family abandonment of rescue do not require resuscitation times > 30 min. Conversely, in terms of OHCA of young adults (such as sudden cardiac deaths and sudden deaths of unknown causes), ECG manifestations of ventricular fibrillation or bradycardia, or a slow ventricular escape, the intensity of continuous resuscitation should be strengthened until the family approves that resuscitation can be terminated, even beyond 30 min of sustained CPR.
Finally, regarding prehospital advanced airway management (AAM) in patients with OHCA[20,21], some studies have shown that endotracheal intubation (ETI) can improve the probability of sustained ROSC, survival to hospital discharge, and neurologic outcomes[22]. In the present study, ETI was significantly associated with P-ROSC (OR: 8.28, P < 0.001). Moreover, Benoit et al[23] suggested that a delay in ETI was related to worse ROSC outcomes. In addition, Izawa et al[24] confirmed that AAM resulted in better survival in patients with non-shockable rhythms than in those with shockable rhythms, which might indicate the impact of shockable rhythms on the role of ETI in OHCA. Therefore, the effects of ETI in such situations warrant further investigation.
Ji et al[4], Morgan et al[5], and Navab et al[13] and others have established models in the United Kingdom, Australia, and Iran, respectively, to describe the influencing factors of clinical features and prehospital emergency measures on the ROSC rate as well as the survival rate of patients with OHCA. However, these researchers did not collect data on prehospital drug administration, including the use and dosage of epinephrine or other drugs. We developed a simple model applied to OHCA of all etiologies that covered pre-EMS intervention factors and prehospital emergency measures, including drugs and their dosage, although prehospital drug administration was not included in the model. Liu et al[7] developed a P-ROSC score for patients with OHCA that also collected prehospital drug administration data. However, the model was not appropriate for trauma-induced OHCA, which limited its wide application. Another prospective study that included individuals between 1998 and 2008 generated the RACA score, which is a widely applicable score for ROSC in OHCA. However, it is not a contemporary cohort[25].
Due to the retrospective design, the accuracy of data collection and potential confounders could not be assessed, and the identification of specific causalities was limited. Furthermore, incomplete data restricted the research. In addition, we did not collect no-reflow time data because the number of patients with OHCA witnessed by bystanders at the scene was too small.
We developed a simple and accessible model to predict the probability of achieving P-ROSC in China. The P-ROSC, with just six factors, is interpretable, convenient to implement, and comprehensive in busy prehospital processing; thus, it could serve as a possible assistive tool for clinical-aid decision-making.
Out-of-hospital cardiac arrest (OHCA) is a leading cause of death worldwide. In China, 550000 people develop OHCA annually with a survival rate of only 1.3% after discharge, making OHCA a major public health issue.
A large gap of prehospital return of spontaneous circulation (P-ROSC) rate remains between China and other countries and that the relative contributions of aid measures for each of these factors to P-ROSC vary across countries. There are still not such model, including pre-EMS intervention factors and Prehospital emergency measures, have currently been developed for P-ROSC in China.
To develop a nomogram prediction model which is interpretable, convenient to implement, easy to comprehend in busy prehospital processing, and comprehensive, including prehospital drug administration. Therefore, it could serve as a potentially assistive tool for clinical aid decision-making.
Clinical data of patients with OHCA were retrospectively analyzed A nomogram prediction model for P-ROSC in patients with OHCA was developed and validate.
Among the included 2685 patients with OHCA, the P-ROSC incidence was 5.8%. LASSO and multivariate logistic regression analyses showed that age, bystander cardiopulmonary resuscitation (CPR), initial rhythm, CPR duration, ventilation mode, and pathogenesis were independent factors influencing P-ROSC in these patients. The area under the ROC was 0.963. The calibration plot demonstrated that the predicted P-ROSC model was concordant with the actual P-ROSC. The good clinical usability of the prediction model was confirmed using decision curve analysis.
We developed a simple and accessible model to predict the probability of achieving P-ROSC in China. The P-ROSC, with just six factors, is interpretable, convenient to implement, and comprehensive in busy prehospital processing; thus, it could serve as a possible assistive tool for clinical-aid decision-making.
If we go one step further, we start to conduct prospective studies to identify the specific causalities and to improve the accuracy of data collection.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Emergency medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lakusic N, Croatia; Ong H, Malaysia S-Editor: Liu JH L-Editor: A P-Editor: Xu ZH
| 1. | Kiguchi T, Okubo M, Nishiyama C, Maconochie I, Ong MEH, Kern KB, Wyckoff MH, McNally B, Christensen EF, Tjelmeland I, Herlitz J, Perkins GD, Booth S, Finn J, Shahidah N, Shin SD, Bobrow BJ, Morrison LJ, Salo A, Baldi E, Burkart R, Lin CH, Jouven X, Soar J, Nolan JP, Iwami T. Out-of-hospital cardiac arrest across the World: First report from the International Liaison Committee on Resuscitation (ILCOR). Resuscitation. 2020;152:39-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 367] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 2. | Yan S, Gan Y, Jiang N, Wang R, Chen Y, Luo Z, Zong Q, Chen S, Lv C. The global survival rate among adult out-of-hospital cardiac arrest patients who received cardiopulmonary resuscitation: a systematic review and meta-analysis. Crit Care. 2020;24:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 529] [Article Influence: 105.8] [Reference Citation Analysis (0)] |
| 3. | Hawkes C, Booth S, Ji C, Brace-McDonnell SJ, Whittington A, Mapstone J, Cooke MW, Deakin CD, Gale CP, Fothergill R, Nolan JP, Rees N, Soar J, Siriwardena AN, Brown TP, Perkins GD; OHCAO collaborators. Epidemiology and outcomes from out-of-hospital cardiac arrests in England. Resuscitation. 2017;110:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 244] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 4. | Ji C, Brown TP, Booth SJ, Hawkes C, Nolan JP, Mapstone J, Fothergill RT, Spaight R, Black S, Perkins GD; OHCAO Collaborators. Risk prediction models for out-of-hospital cardiac arrest outcomes in England. Eur Heart J Qual Care Clin Outcomes. 2021;7:198-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Morgan DP, Muscatello D, Travaglia J, Hayen A. Retrospective observational cohort study of out-of-hospital cardiac arrest outcomes in Tasmania 2010-2014. Emerg Med Australas. 2020;32:631-637. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Xie X, Zheng J, Zheng W, Pan C, Ma Y, Zhu Y, Tan H, Han X, Yan S, Zhang G, Li C, Shao F, Wang C, Zhang J, Bian Y, Ma J, Cheng K, Liu R, Sang S, Zhang Y, McNally B, Ong MEH, Lv C, Chen Y, Xu F; BASIC-OHCA Coordinators and Investigators. Efforts to Improve Survival Outcomes of Out-of-Hospital Cardiac Arrest in China: BASIC-OHCA. Circ Cardiovasc Qual Outcomes. 2023;16:e008856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 7. | Liu N, Liu M, Chen X, Ning Y, Lee JW, Siddiqui FJ, Saffari SE, Ho AFW, Shin SD, Ma MH, Tanaka H, Ong MEH; PAROS Clinical Research Network Investigators. Development and validation of an interpretable prehospital return of spontaneous circulation (P-ROSC) score for patients with out-of-hospital cardiac arrest using machine learning: A retrospective study. EClinicalMedicine. 2022;48:101422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Waldmann P, Mészáros G, Gredler B, Fuerst C, Sölkner J. Evaluation of the lasso and the elastic net in genome-wide association studies. Front Genet. 2013;4:270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 9. | Myat A, Song KJ, Rea T. Out-of-hospital cardiac arrest: current concepts. Lancet. 2018;391:970-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 352] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 10. | Matsuyama T, Kitamura T, Kiyohara K, Nishiyama C, Nishiuchi T, Hayashi Y, Kawamura T, Ohta B, Iwami T. Impact of cardiopulmonary resuscitation duration on neurologically favourable outcome after out-of-hospital cardiac arrest: A population-based study in Japan. Resuscitation. 2017;113:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Park S, Lee SW, Han KS, Lee EJ, Jang DH, Lee SJ, Lee JS, Kim SJ; Korean Cardiac Arrest Research Consortium (KoCARC) Investigators. Optimal cardiopulmonary resuscitation duration for favorable neurological outcomes after out-of-hospital cardiac arrest. Scand J Trauma Resusc Emerg Med. 2022;30:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Lai CY, Lin FH, Chu H, Ku CH, Tsai SH, Chung CH, Chien WC, Wu CH, Chu CM, Chang CW. Survival factors of hospitalized out-of-hospital cardiac arrest patients in Taiwan: A retrospective study. PLoS One. 2018;13:e0191954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Navab E, Esmaeili M, Poorkhorshidi N, Salimi R, Khazaei A, Moghimbeigi A. Predictors of Out of Hospital Cardiac Arrest Outcomes in Pre-Hospital Settings; a Retrospective Cross-sectional Study. Arch Acad Emerg Med. 2019;7:36. [PubMed] |
| 14. | Christensen DM, Rajan S, Kragholm K, Søndergaard KB, Hansen OM, Gerds TA, Torp-Pedersen C, Gislason GH, Lippert FK, Barcella CA. Bystander cardiopulmonary resuscitation and survival in patients with out-of-hospital cardiac arrest of non-cardiac origin. Resuscitation. 2019;140:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Rajan S, Wissenberg M, Folke F, Hansen SM, Gerds TA, Kragholm K, Hansen CM, Karlsson L, Lippert FK, Køber L, Gislason GH, Torp-Pedersen C. Association of Bystander Cardiopulmonary Resuscitation and Survival According to Ambulance Response Times After Out-of-Hospital Cardiac Arrest. Circulation. 2016;134:2095-2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 16. | An HR, Han YR, Wang TH, Chi F, Meng Y, Zhang CY, Liang JQ, Li XL. Meta-Analysis of the Factors Influencing the Restoration of Spontaneous Circulation After Cardiopulmonary Resuscitation. Front Physiol. 2022;13:834352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 17. | Funada A, Goto Y, Tada H, Teramoto R, Shimojima M, Hayashi K, Kawashiri MA, Yamagishi M. Duration of cardiopulmonary resuscitation in patients without prehospital return of spontaneous circulation after out-of-hospital cardiac arrest: Results from a severity stratification analysis. Resuscitation. 2018;124:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Goto Y, Funada A, Goto Y. Relationship Between the Duration of Cardiopulmonary Resuscitation and Favorable Neurological Outcomes After Out-of-Hospital Cardiac Arrest: A Prospective, Nationwide, Population-Based Cohort Study. J Am Heart Assoc. 2016;5:e002819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 19. | Nas J, Kleinnibbelink G, Hannink G, Navarese EP, van Royen N, de Boer MJ, Wik L, Bonnes JL, Brouwer MA. Diagnostic performance of the basic and advanced life support termination of resuscitation rules: A systematic review and diagnostic meta-analysis. Resuscitation. 2020;148:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Chang H, Jeong D, Park JE, Kim T, Lee GT, Yoon H, Hwang SY, Cha WC, Shin TG, Sim MS, Jo IJ, Lee SH, Shin SD, Choi JH. Prehospital airway management for out-of-hospital cardiac arrest: A nationwide multicenter study from the KoCARC registry. Acad Emerg Med. 2022;29:581-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Onoe A, Kajino K, Daya MR, Nakamura F, Nakajima M, Kishimoto M, Sakuramoto K, Muroya T, Ikegawa H, Hock Ong ME, Kuwagata Y. Improved neurologically favorable survival after OHCA is associated with increased pre-hospital advanced airway management at the prefecture level in Japan. Sci Rep. 2022;12:20498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Chiang WC, Hsieh MJ, Chu HL, Chen AY, Wen SY, Yang WS, Chien YC, Wang YC, Lee BC, Wang HC, Huang EP, Yang CW, Sun JT, Chong KM, Lin HY, Hsu SH, Chen SY, Ma MH. The Effect of Successful Intubation on Patient Outcomes After Out-of-Hospital Cardiac Arrest in Taipei. Ann Emerg Med. 2018;71:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Benoit JL, McMullan JT, Wang HE, Xie C, Xu P, Hart KW, Stolz U, Lindsell CJ. Timing of Advanced Airway Placement after Witnessed Out-of-Hospital Cardiac Arrest. Prehosp Emerg Care. 2019;23:838-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Izawa J, Komukai S, Gibo K, Okubo M, Kiyohara K, Nishiyama C, Kiguchi T, Matsuyama T, Kawamura T, Iwami T, Callaway CW, Kitamura T. Pre-hospital advanced airway management for adults with out-of-hospital cardiac arrest: nationwide cohort study. BMJ. 2019;364:l430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 25. | Gräsner JT, Meybohm P, Lefering R, Wnent J, Bahr J, Messelken M, Jantzen T, Franz R, Scholz J, Schleppers A, Böttiger BW, Bein B, Fischer M; German Resuscitation Registry Study Group. ROSC after cardiac arrest--the RACA score to predict outcome after out-of-hospital cardiac arrest. Eur Heart J. 2011;32:1649-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |