Published online Jan 26, 2023. doi: 10.4330/wjc.v15.i1.23
Peer-review started: July 18, 2022
First decision: September 26, 2022
Revised: November 14, 2022
Accepted: December 13, 2022
Article in press: December 13, 2022
Published online: January 26, 2023
Processing time: 177 Days and 21.6 Hours
ST-elevation myocardial infarction (STEMI) refers to a clinical syndrome that features symptoms of myocardial ischemia with consequent ST-elevation on electrocardiography and an associated rise in cardiac biomarkers. Rapid restoration of brisk flow in the coronary vasculature is critical in reducing mortality and morbidity. In patients with STEMI who could not receive primary percutaneous coronary intervention (PCI) on time, pharmacoinvasive strategy (thrombolysis followed by timely PCI within 3-24 h of its initiation) is an effective option.
To analyze the role of delayed pharmacoinvasive strategy in the window period of 24-72 h after thrombolysis.
This was a physician-initiated, single-center prospective registry between January 2017 and July 2017 which enrolled 337 acute STEMI patients with partially occluded coronary arteries. Patients received routine pharmacoinvasive therapy (PCI within 3-24 h of thrombolysis) in one group and delayed pharmacoinvasive therapy (PCI within 24-72 h of thrombolysis) in another group. The primary endpoint was major adverse cardiac and cerebrovascular events (MACCE) within 30 d of the procedure. The secondary endpoints included major bleeding as defined by Bleeding Academic Research Consortium classification, angina, and dyspnea within 30 d.
The mean age in the two groups was comparable (55.1 ± 10.1 years vs 54.2 ± 10.5 years, P = 0.426). Diabetes was present among 20.2% and 22.1% of patients in the routine and delayed groups, respectively. Smoking rate was 54.6% and 55.8% in the routine and delayed groups, respectively. Thrombolysis was initiated within 6 h of onset of symptoms in both groups (P = 0.125). The mean time from thrombolysis to PCI in the routine and delayed groups was 16.9 ± 5.3 h and 44.1 ± 14.7 h, respectively. No significant difference was found for the occurrence of measured clinical outcomes in the two groups within 30 d (8.7% vs 12.9%, P = 0.152). Univariate analysis of demographic characteristics and risk factors for patients who reported MACCE in the two groups did not demonstrate any significant correlation. Secondary endpoints such as angina, dyspnea, and major bleeding were non-significantly different between the two groups.
Delayed PCI pharmacoinvasive strategy in a critical diseased but not completely occluded artery beyond 24 h in patients who have been timely thrombolyzed seems a reasonable strategy.
Core Tip: Pharmacoinvasive strategy with percutaneous coronary intervention (PCI) within 3 to 24 h after successful thrombolysis has been proven to be a viable alternative to primary PCI. In resource poor countries, patients often present to the interventionist beyond 24 h of thrombolysis due to logistic reasons. The results of this study demonstrate that the clinical outcomes of delayed pharmacoinvasive therapy (24-72 h of initiation of thrombolysis) are comparable to those of routine pharmacoinvasive (3-24 h of initiation of thrombolysis) in patients with acute ST-elevation myocardial infarction (STEMI). Hence, in critically diseased acute STEMI patients who have been timey thrombolysed, delayed PCI (24-72 h following thrombolysis) appears a reasonable strategy.
- Citation: Sethi R, Mohan L, Vishwakarma P, Singh A, Sharma S, Bhandari M, Shukla A, Sharma A, Chaudhary G, Pradhan A, Chandra S, Narain VS, Dwivedi SK. Feasibility and efficacy of delayed pharmacoinvasive therapy for ST-elevation myocardial infarction. World J Cardiol 2023; 15(1): 23-32
- URL: https://www.wjgnet.com/1949-8462/full/v15/i1/23.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i1.23
ST-elevation myocardial infarction (STEMI), a potentially lethal diagnosis, refers to a clinical syndrome that encompasses symptoms of myocardial ischemia with consequent ST-elevation on electrocardiography and an associated rise in cardiac biomarkers. Rapid restoration of brisk flow in the coronary vasculature is critical in reducing mortality and morbidity. Primary percutaneous coronary intervention (PCI) is the global standard of care for patients presenting with acute STEMI[1,2]. However, the practicality of all patients reaching the PCI-capable center within 1 h is a challenge. Thus, in patients with acute STEMI who cannot get primary PCI in a timely manner, pharmacoinvasive strategy is considered as an effective and viable option[3,4]. This is particularly true in developing nations where this delay often crosses the golden period of 24 h due to the exponentially increasing disease burden and limited availability of resources. The famous Occluded Artery Trial (OAT), failed to show any advantage of performing PCI (beyond 72 h) + optimal medical therapy compared to optimal medical therapy alone[5].
Therefore, in the present study we analyzed a novel concept of ‘delayed pharmacoinvasive therapy’ in acute STEMI patients with partially occluded coronary vasculature who had received thrombolysis within the first 12 h of symptoms onset and underwent PCI in a window period of 24-72 h, and compared both routine and delayed pharmacoinvasive strategies in such patients.
This was a physician-initiated, single-center prospective registry which enrolled STEMI patients who were thrombolyzed within 12 h of symptom onset and subsequently underwent PCI between January 2017 and July 2017. The study protocol was approved by the local Institutional Review Board and was performed in accordance with Declaration of Helsinki. Written informed consent was obtained from all patients or from their designees before enrollment.
The enrolled patients with STEMI were either admitted to peripheral hospitals, thrombolyzed, and referred to us; or else were directly admitted to our hospital but could not undergo primary PCI and thus received thrombolysis. For various nonspecific reasons, some of them could not undergo PCI within 3-24 h of initiation of thrombolytic therapy. The common reasons for this delay were financial constraints and imbalance between the service seekers and providers which does not support 24 h functioning of catheterization laboratory, even in tertiary care centers. The period of 24-72 h has remained a grey area for the decision of primary PCI in the literature but is one of the usually encountered strategy in low resource clinical setup and used in many centers with PCI, if vessels are still found to be occluded on angiography. We called this group as delayed pharmacoinvasive group. To evaluate the effectiveness of this strategy, we compared the results with those obtained in the cohort who underwent routine pharmacoinvasive therapy (thrombolyzed within 12 h of symptom onset followed by PCI within 3-24 h initiation of thrombolysis). The groups were not randomized. Stated simply, Group 1 (routine) represented those patients undergoing PCI < 24 h of symptom onset and Group 2 (delayed) consisted of those subjects undergoing PCI between 24-72 h of symptom onset.
Patients who underwent primary PCI were excluded from the study. Other exclusion criteria included contraindication for thrombolysis, patients presenting beyond the window period for thrombolysis, or patients with totally occluded arteries on angiogram within 24-72 h.
The primary endpoint was major adverse cardiac and cerebrovascular events (MACCE) within 30 d that included composite of death, rehospitalization due to reinfarction and congestive heart failure, target vessel revascularization, and stroke. The secondary endpoints included individual primary endpoints, major bleeding as defined by Bleeding Academic Research Consortium classification, and angina and dyspnea within 30 d. The impact of time of thrombolysis to PCI on the clinical outcome (< 24 h, 24-48 h, and 48-72 h) was also assessed.
The statistical review of this study was performed by a biomedical statistician from King George's Medical University. All the data were analyzed using the Statistical Package for the Social Sciences (SPSS for Windows version 20.0; Chicago, IL, United States). Categorical and continuous variables are summarized as frequency (percentage) and the mean ± SD, respectively. The difference between groups was verified using Chi-square test for categorical variables and independent sample t-test for continuous variables. P < 0.05 was considered statistically significant.
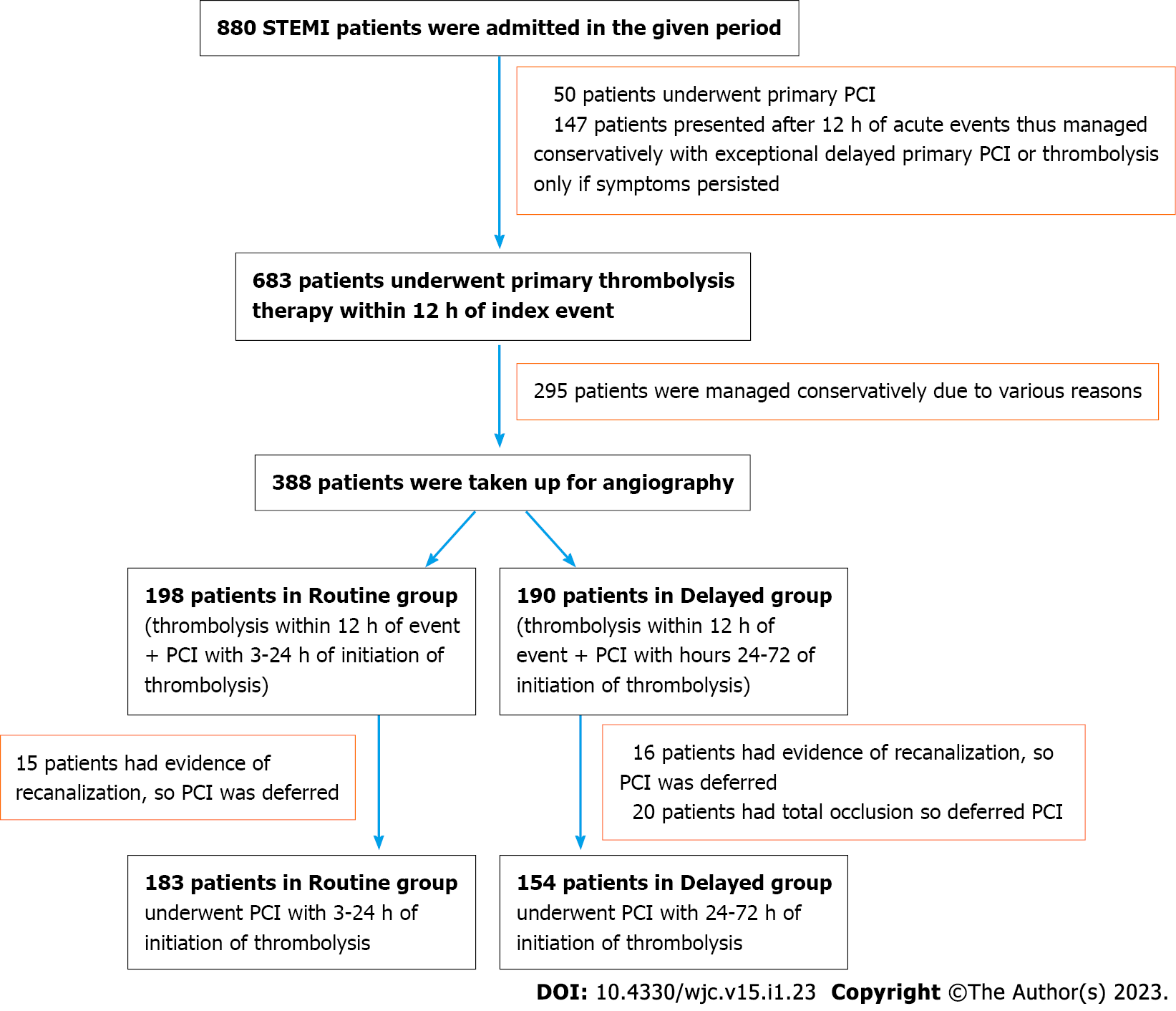
The flow chart of selection of study patients is demonstrated in Figure 1. Among 880 STEMI patients who presented at our tertiary care center in the given period, 337 were divided into two groups: (1) 183 patients in the routine group who underwent PCI within 3-24 h of initiation of thrombolysis; and (2) 154 patients in the delayed group who underwent PCI within 24-72 h of initiation of thrombolysis.
Demographic characteristics of the study cohorts (routine group vs delayed group) are compared in Table 1. Mean age in the two groups was comparable (55.1 ± 10.1 years vs 54.2 ± 10.5 years, P = 0.426), with a predominance of male patients in both groups (87.4% in routine group and 89.6% in delayed group). The occurrence of anterior wall STEMI and non-anterior STEMI was almost equally distributed in the routine and delayed groups (53.6% vs 57.1% and 46.4% vs 42.9%; P = 0.509, respectively). In both groups, around 58% of patients had single vessel disease while 42% had multiple vessel disease. A statistically significant difference was noted in mean left ventricular ejection fraction between the two groups (routine: 46.9 ± 4.7 and delayed: 45.8 ± 4.5; P = 0.034).
| Characteristic | Routine (n = 183) | Delayed (n = 154) | P value |
| Age, years | 55.1 ± 10.1 | 54.2 ± 10.5 | 0.426 |
| Age group, n (%) | |||
| 20–29 yr | 0 | 2 (1.3) | 0.197 |
| 30–39 yr | 13 (7.1) | 10 (6.5) | |
| 40–49 yr | 38 (20.8) | 34 (22.1) | |
| 50–59 yr | 47 (25.7) | 52 (33.8) | |
| 60–69 yr | 71 (38.8) | 43 (27.9) | |
| 70–79 yr | 14 (7.7) | 13 (8.4) | |
| Gender, n (%) | |||
| Male | 160 (87.4) | 138 (89.6) | 0.533 |
| Female | 23 (12.6) | 16 (10.4) | |
| Risk factors, n (%) | |||
| Diabetes | 37 (20.2) | 34 (22.1) | 0.677 |
| Hypertension | 49 (26.8) | 43 (27.9) | 0.814 |
| Smoking | 100 (54.6) | 86 (55.8) | 0.825 |
| Killip class, n (%) | |||
| Class 1 | 165 (90.2) | 129 (83.8) | 0.176 |
| Class 2 | 11 (6.0) | 10 (6.5) | |
| Class 3 | 5 (2.7) | 10 (6.5) | |
| Class 4 | 2 (1.1) | 5 (3.2) | |
| Left ventricular ejection fraction (%) | 46.9 ± 4.7 | 45.8 ± 4.5 | 0.034 |
| Type of myocardial infarction, n (%) | |||
| AWMI | 98 (53.6) | 88 (57.1) | 0.509 |
| Non-AWMI | 85 (46.4) | 66 (42.9) | |
| Type of coronary artery disease, n (%) | |||
| SVD | 105 (57.4) | 89 (57.8) | 0.939 |
| MVD | 78 (42.6) | 65 (42.2) | |
| Symptoms to needle time, h | 5.2 ± 3.4 | 5.8 ± 4.5 | 0.125 |
| Thrombolysis to PCI time, h | 16.9 ± 5.3 | 44.1 ± 14.7 | 0.001 |
In both the routine and delayed groups, thrombolysis was initiated within 6 h of onset of symptoms (5.2 ± 3.4 h vs 5.8 ± 4.5 h, P = 0.125). The mean time from thrombolysis to PCI was 16.9 ± 5.3 h in the routine group while it was 44.1 ± 14.7 h (an average 27 h late) in the delayed group.
The clinical outcomes within 30 d of the procedure in the two groups are depicted in Table 2. The primary endpoint. i.e., MACCE, was reported in 16 (8.7%) patients in the routine group and in 20 (12.9%) patients in the delayed group (P = 0.152). Angina occurred in 4 (2.2%) patients in the routine group and in 1 (0.6%) in the delayed group (P = 0.381). Dyspnea occurred in 6 (3.3%) and 5 (3.2%) in the routine and delayed groups, respectively (P = 0.99).
| Clinical outcome | Routine (n = 183) | Delayed (n = 154) | P value |
| Primary endpoints, n (%) | |||
| MACCE | 16 (8.7) | 20 (12.9) | 0.152 |
| Secondary endpoints, n (%) | |||
| Death | 5 (2.7) | 3 (1.9) | 0.732 |
| Myocardial infarction | 2 (1.1) | 7 (4.5) | 0.085 |
| Target vessel revascularization | 3 (1.6) | 3 (1.9) | 0.999 |
| Congestive heart failure | 6 (3.3) | 7 (4.5) | 0.547 |
| Stroke | 0 | 0 | 0.999 |
| Angina | 4 (2.2) | 1 (0.6) | 0.381 |
| Dyspnea | 6 (3.3) | 5 (3.2) | 0.999 |
| Major Bleeding1 | 1 (0.5) | 3 (1.9) | 0.335 |
To analyze the effect of time from thrombolysis to PCI on clinical outcomes, we further divided the delayed group into two subgroups based on the time from thrombolysis to PCI: (1) 24-≤ 48 h (n = 96); and (2) 48-72 h (n = 58). The two subgroups were both compared with the routine group (thrombolysis to PCI time < 24 h; n = 183). However, no statistically significant difference was observed in measured clinical outcomes among the three groups (Table 3).
| Routine | Delayed | P value | ||||
| Group A (≤ 24.0 h), n = 183 | Group B (24.0 ≤ 48.0 h), n = 96 | Group C (48.0–72.0 h), n = 58 | Group A vs Group B | Group A vs Group C | Group B vs Group C | |
| Primary outcomes, n (%) | ||||||
| MACCE | 16 (8.7) | 13 (13.5) | 7 (12.06) | 0.212 | 0.263 | 0.965 |
| Secondary outcomes, n (%) | ||||||
| Death | 5 (2.7) | 2 (2.1) | 1 (1.7) | 0.999 | 0.999 | 0.999 |
| Myocardial infarction | 2 (1.1) | 3 (3.1) | 4 (6.9) | 0.343 | 0.093 | 0.427 |
| Target vessel revascularization | 3 (1.6) | 2 (2.1) | 1 (1.7) | 0.999 | 0.999 | 0.999 |
| Congestive heart failure | 6 (3.3) | 6 (6.3) | 1 (1.7) | 0.245 | 0.999 | 0.256 |
| Stroke | 0 | 0 | 0 | - | - | - |
| Major bleeding1 | 1 (0.5) | 1 (1.0) | 2 (3.4) | 0.999 | 0.145 | 0.557 |
Univariate analysis of demographic characteristics and risk factors for patients who reported MACCE in the two groups are outlined in Table 4. A significant correlation was reported between Killip class II and the occurrence of primary outcomes in the routine group (odds ratio: 4.59; 95% confidence interval: 1.08-19.40).
| Variable | Events (16/183) | Routine (3-24 h) | Events (21/154) | Delayed (> 24 h) |
| OR (95%CI) | OR (95%CI) | |||
| Age (> 65 yr) | 1/16 | 0.68 (0.08-5.48) | 3/18 | 1.31 (0.34-4.98) |
| Age (55-65 yr) | 9/81 | 1.70 (0.62-4.77) | 8/47 | 1.48 (0.57-3.86) |
| Age (45-55 yr) | 1/41 | 0.21 (0.03-1.65) | 5/56 | 0.50 (0.17-1.46) |
| Age (35-45 yr) | 4/38 | 1.30 (0.40-4.30) | 4/26 | 1.19 (0.36-3.87) |
| Age (< 35 yr) | 1/7 | 1.79 (0.20-15.86) | 1/7 | 1.06 (0.12-9.26) |
| Male | 15/160 | 2.28 (0.29-18.10) | 17/138 | 0.42 (0.12-1.46) |
| KILLIP Class-1 | 13/165 | 0.43 (0.11-1.67) | 17/132 | 0.67 (0.20-2.20) |
| KILLIP Class-2 | 3/11 | 4.59 (1.08-19.40) | 1/9 | 0.78 (0.09-6.59) |
| AWMI | 9/99 | 1.10 (0.39-3.09) | 10/88 | 0.64(0.25-1.61) |
| Type 2 DM | 3/37 | 0.9 (0.24-3.35) | 2/34 | 0.33(0.07-1.50) |
| Smoking | 10/100 | 1.43 (0.50-4.10) | 14/86 | 1.69 (0.64-4.47) |
| HTN | 4/49 | 0.90 (0.28-2.95) | 4/43 | 0.57 (0.18-1.79) |
| LVEF (35-45) | 3/70 | 0.34 (0.09-1.25) | 7/73 | 0.51 (0.19-1.34) |
| LVEF (> 45) | 12/112 | 2.01 (0.62-6.50) | 14/78 | 2.16 (0.82-5.68) |
| MVD | 7/78 | 1.05 (0.37-2.96) | 11/65 | 1.61 (0.64-4.05) |
| SVD | 9/98 | 1.13 (0.40-3.17) | 10/87 | 0.66 (0.26-1.66) |
| CrCl (30-60) | 1/29 | 0.33 (0.04-2.61) | 5/30 | 1.35 (0.45-4.03) |
| CrCl (60-90) | 11/113 | 1.40 (0.47-4.22) | 11/78 | 1.08 (0.43-2.72) |
| CrCl (> 90) | 4/40 | 1.21 (0.37-3.99) | 5/42 | 0.81 (0.28-2.37) |
Primary PCI within 1 h of symptom onset is the standard of care strategy in acute STEMI[1,6]. However, the real world scenarios are not always ideal, thus decision making in such cases is a challenge for interventional cardiologists[7-10]. As per guidelines, pharmacoinvasive therapy (thrombolysis followed with PCI within 3-24 h) is recommended as an effective option in patients with acute STEMI who could not receive primary PCI within this golden hour[6]. Furthermore, there is a lacuna in the literature regarding the role of PCI, in patients who present in a window of 24-72 h of thrombolysis. This period is critical and the benefits of reperfusion of partially occluded artery must be balanced against the potential harm from procedure-related complications, myocardial injury because of distal embolization of athero-thrombotic debris, and loss of recruitable collateral flow to other coronary territories[11,12]. In our study, we compared the effectiveness of routine (PCI within 24 h of thrombolysis) and delayed (PCI within 24-72 h of thrombolysis) pharmacoinvasive therapies and the results revealed no statistically significant difference in the clinical outcome between two therapies within 30 d of the procedure.
Almost a decade ago, OAT-trial was published to test whether opening a totally occluded infarct related artery, 3-28 d following acute STEMI, will improve the clinical outcome or not. The results of that trial cautioned about a trend towards excess non-fatal re-infarction when PCI was performed in stable patients with a totally occluded infarct related artery, 3 to 28 d after STEMI, and did not show any reduction in major cardiovascular events during a mean follow-up of 3 years among these patients[5,13]. Furthermore, in an analysis from the Melbourne Interventional Group registry of 4307 patients with STEMI who underwent PCI, no mortality hazard was reported where PCI was delayed beyond the first 24 h but was performed within the index admission. However, they have not defined/specified the index admission in terms of time/hours[14].
A meta-analysis of ten randomized controlled trials on timing of PCI in non-STEMI patients showed no reduction in death or re-infarction rate in early vs delayed intervention. However, recurrent ischemia and length of stay were significantly reduced with an early invasive strategy[15]. In non-STEMI cases, a delayed invasive approach is recommended, with an early invasive strategy within 24 h in high-risk patients and a delayed invasive strategy within 72 h in intermediate risk patients[16]. As randomized controlled trials of these kinds are difficult to plan for STEMI patients, decisions must be based on observational studies or clinical registries. Recently, a randomized controlled trial was published for transient STEMI in which the outcomes of a STEMI-like approach (with an immediate invasive strategy) were compared with a non-STEMI like approach (with a delayed invasive strategy) and the results showed no difference in clinical outcomes[17].
PCI in any scenario after 72 h is not recommended as it can be more detrimental than beneficial to vascularize the myocardium which is already dead[18]. In the present study, the mean time from symptom onset to angiography was 22.0 ± 6.6 h and 49.4 ± 15.5 h in the routine and delayed groups, respectively. Contrary to this, these time windows are significantly less in the reported literature [3,19]. Notably, it is difficult to compare the triage and referral facilities between developed and developing countries. Delayed presentation was one of the most important factors in our study determining the poor primary outcomes as compared to Western data. The delay in reaching STEMI care hospital in our country is multifactorial: (1) Delay in recognition of chest symptom by patients themselves; (2) unavailability of electrocardiogram machine at peripheral health care centers; (3) incompetency in diagnosing and taking decision for referral to higher centers by the health care provider; and (4) poor transportation services. However, in our opinion these loopholes in our systems are not too difficult to handle. The lag time for patient presentation can be reduced by creating public awareness regarding symptoms of acute coronary syndrome, educating the grass root level health care providers, ensuring the availability of an electrocardiogram machine at peripheral health care centers, and strengthening the ambulance services. Increasing the number of catheterization laboratories and their working hours by increasing the number of work force will also prevent the procedural delays.
The primary outcome, i.e., MACCE within 30 d, was reported in 8.7% in the routine group and 13.6% in the delayed group (P = 0.152). The STEMI patients undergoing primary PCI have witnessed a wide range of MACCE (1.6% to 23.3%) with in 30 d, in various studies and variation depends on the baseline risk factors of the study population and pharmacological intervention prior to PCI[20-22]. Furthermore, in our cohort Killip class was the most important predictor of worse outcomes among all the clinical parameters analyzed by univariate analysis. Killip class II patients had larger infarct and poorer left ventricular function as compared to Killip class I and it is a well-recognized fact that the outcome of STEMI with high Killip class (≥ class II) is poor[23,24]. Male sex and left ventricular ejection fraction > 45% were the other two parameters which reported moderate significance in predicting the outcomes.
Our study is the first of its kind to clearly document the useful role of delayed pharmacoinvasive therapy (24-72 h of initiation of thrombolysis) in patients with acute STEMI, which is extremely important and practical in low resource high burden settings.
There were several limitations to our study. First, we enrolled a comparatively small number of patient population and had a shorter duration of the study. As randomized controlled trials are difficult to conduct in these subjects because of ethical and legal issues, keeping in mind our preliminary results which support delayed pharmacoinvasive therapy in a specified group of population, prospective registries must be encouraged to conclude further. Second, despite a prospective design, we did not use Cox proportional hazard model which has been shown to have more statistical power than logistic regression model in cross sectional studies[25]. However, when the follow-up period is short and event rates are low (as in our study), both methods may be comparable[26]. Third, we did not evaluate the psychological impact of a delayed PCI or the Post Traumatic Stress Disorder symptoms during the extra waiting period. Fourth, despite a high rate of smoking at baseline, data regarding persistent smoking at 30 d was not available. However, counselling for smoking cessation was provided to all smokers as a protocol.
The results of the present study specifically established that the clinical outcomes of delayed pharmacoinvasive therapy (24-72 h of initiation of thrombolysis) are comparable to those of routine pharmacoinvasive (3-24 h of initiation of thrombolysis) in patients with acute STEMI. Delayed PCI (24-72 h following thrombolysis) in critical diseased but not completely occluded arteries, which have been timely thrombolysed, seems a reasonable strategy in acute STEMI patients.
ST-elevation myocardial infarction (STEMI) when untreated is a potentially fatal condition and timely primary percutaneous coronary intervention (PCI) is the key to improving outcomes.
In developing countries, despite multiple guidelines and interventions, the primary PCI coverage in STEMI remains low in clinical practice. PCI within 24 h of thrombolysis (pharmacoinvasive approach) has emerged as a viable alternative to primary PCI. However, due to logistic and financial reasons, patients in developing world may undergo PCI late (> 24 h) after thrombolysis.
This study aimed to analyze the safety and feasibility of delayed pharmacoinvasive strategy in the window period of 24-72 h after thrombolysis. Group 1 (routine) represented those patients undergoing PCI < 24 h of symptom onset and Group 2 (delayed) consisted of those subjects undergoing PCI between 24-72 h of symptom onset.
This was a single center, prospective registry at a tertiary care center. The primary endpoint was major adverse cardiac and cerebrovascular events (MACCE) within 30 d of the procedure.
Among 337 patients with STEMI who underwent thrombolyis, there was no difference in measured clinical outcomes (MACCE) at 30 d between the routine pharmacoinvasive and delayed pharmacoinvasive groups (8.7% vs 12.9%, P = 0.152). The mean time from thrombolysis to PCI in the routine and delayed groups was 16.9 ± 5.3 h and 44.1 ± 14.7 h, respectively.
Delayed PCI pharmacoinvasive strategy in a critical diseased but not completely occluded artery beyond 24 h in patients who have been timely thrombolyzed seems a reasonable strategy.
Late PCI after thromobolysis in STEMI is common in developing world due to logistic and financial reasons. This study demonstrates the safety and feasibility of such delayed pharmacoinvasive PCI, lending credibility to this approach utilized in daily practice.
We thank Ms. Ekta Patel for her assistance in organizing and writing the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Heart Association; European Society of Cardiology.
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cang Y, China; Carlan SJ, United States S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2931] [Cited by in RCA: 2776] [Article Influence: 126.2] [Reference Citation Analysis (1)] |
| 2. | Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes (GUSTO IIb) Angioplasty Substudy Investigators. A clinical trial comparing primary coronary angioplasty with tissue plasminogen activator for acute myocardial infarction. N Engl J Med. 1997;336:1621-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 617] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 3. | Armstrong PW, Gershlick AH, Goldstein P, Wilcox R, Danays T, Lambert Y, Sulimov V, Rosell Ortiz F, Ostojic M, Welsh RC, Carvalho AC, Nanas J, Arntz HR, Halvorsen S, Huber K, Grajek S, Fresco C, Bluhmki E, Regelin A, Vandenberghe K, Bogaerts K, Van de Werf F; STREAM Investigative Team. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N Engl J Med. 2013;368:1379-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 479] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 4. | Armstrong PW, Boden WE. Reperfusion paradox in ST-segment elevation myocardial infarction. Ann Intern Med. 2011;155:389-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Hochman JS, Lamas GA, Buller CE, Dzavik V, Reynolds HR, Abramsky SJ, Forman S, Ruzyllo W, Maggioni AP, White H, Sadowski Z, Carvalho AC, Rankin JM, Renkin JP, Steg PG, Mascette AM, Sopko G, Pfisterer ME, Leor J, Fridrich V, Mark DB, Knatterud GL; Occluded Artery Trial Investigators. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med. 2006;355:2395-2407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 553] [Cited by in RCA: 459] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 6. | Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7073] [Cited by in RCA: 6651] [Article Influence: 950.1] [Reference Citation Analysis (0)] |
| 7. | Dai X, Kaul P, Smith SC Jr, Stouffer GA. Predictors, treatment, and outcomes of STEMI occurring in hospitalized patients. Nat Rev Cardiol. 2016;13:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Alexander T, Mullasari AS, Joseph G, Kannan K, Veerasekar G, Victor SM, Ayers C, Thomson VS, Subban V, Gnanaraj JP, Narula J, Kumbhani DJ, Nallamothu BK. A System of Care for Patients With ST-Segment Elevation Myocardial Infarction in India: The Tamil Nadu-ST-Segment Elevation Myocardial Infarction Program. JAMA Cardiol. 2017;2:498-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 9. | Krumholz HM, Herrin J, Miller LE, Drye EE, Ling SM, Han LF, Rapp MT, Bradley EH, Nallamothu BK, Nsa W, Bratzler DW, Curtis JP. Improvements in door-to-balloon time in the United States, 2005 to 2010. Circulation. 2011;124:1038-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 222] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 10. | Zheng W, Yu CM, Liu J, Xie WX, Wang M, Zhang YJ, Sun J, Nie SP, Zhao D. Patients with ST-segment elevation of myocardial infarction miss out on early reperfusion: when to undergo delayed revascularization. J Geriatr Cardiol. 2017;14:524-531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Singh M, Rihal CS, Lennon RJ, Garratt KN, Mathew V, Holmes DR Jr. Prediction of complications following nonemergency percutaneous coronary interventions. Am J Cardiol. 2005;96:907-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Porto I, Selvanayagam JB, Van Gaal WJ, Prati F, Cheng A, Channon K, Neubauer S, Banning AP. Plaque volume and occurrence and location of periprocedural myocardial necrosis after percutaneous coronary intervention: insights from delayed-enhancement magnetic resonance imaging, thrombolysis in myocardial infarction myocardial perfusion grade analysis, and intravascular ultrasound. Circulation. 2006;114:662-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Hochman JS, Lamas GA, Knatterud GL, Buller CE, Dzavik V, Mark DB, Reynolds HR, White HD; Occluded Artery Trial Research Group. Design and methodology of the Occluded Artery Trial (OAT). Am Heart J. 2005;150:627-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Yudi MB, Ajani AE, Andrianopoulos N, Duffy SJ, Farouque O, Ramchand J, Gurvitch R, Lefkovits J, Freeman M, Brennan A, Clark DJ, Reid C, Eccleston D; Melbourne Interventional Group. Early versus delayed percutaneous coronary intervention in patients with non-ST elevation acute coronary syndromes. Coron Artery Dis. 2016;27:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Bonello L, Laine M, Puymirat E, Lemesle G, Thuny F, Paganelli F, Michelet P, Roch A, Kerbaul F, Boyer L. Timing of Coronary Invasive Strategy in Non-ST-Segment Elevation Acute Coronary Syndromes and Clinical Outcomes: An Updated Meta-Analysis. JACC Cardiovasc Interv. 2016;9:2267-2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S; ESC Scientific Document Group. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4045] [Cited by in RCA: 4396] [Article Influence: 439.6] [Reference Citation Analysis (0)] |
| 17. | Lemkes JS, Janssens GN, van der Hoeven NW, van de Ven PM, Marques KMJ, Nap A, van Leeuwen MAH, Appelman YEA, Knaapen P, Verouden NJW, Allaart CP, Brinckman SL, Saraber CE, Plomp KJ, Timmer JR, Kedhi E, Hermanides RS, Meuwissen M, Schaap J, van der Weerdt AP, van Rossum AC, Nijveldt R, van Royen N. Timing of revascularization in patients with transient ST-segment elevation myocardial infarction: a randomized clinical trial. Eur Heart J. 2019;40:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Menon V, Ruzyllo W, Carvalho AC, Almeida de Sousa JM, Forman SA, Jaworska K, Lamas GA, Roik M, Thuaire C, Turgeman Y, Hochman JS. Infarct artery distribution and clinical outcomes in occluded artery trial subjects presenting with non-ST-segment elevation myocardial infarction (from the long-term follow-up of Occluded Artery Trial [OAT]). Am J Cardiol. 2013;111:930-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Bøhmer E, Hoffmann P, Abdelnoor M, Arnesen H, Halvorsen S. Efficacy and safety of immediate angioplasty versus ischemia-guided management after thrombolysis in acute myocardial infarction in areas with very long transfer distances results of the NORDISTEMI (NORwegian study on DIstrict treatment of ST-elevation myocardial infarction). J Am Coll Cardiol. 2010;55:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 20. | Vos NS, Amoroso G, Vink MA, Maarse M, Adams R, Herrman JR, Patterson MS, van der Schaaf RJ, Slagboom T, de Winter RJ. Prehospital Prasugrel Versus Ticagrelor in Real-World Patients With ST-Elevation Myocardial Infarction Referred for Primary PCI: Procedural and 30-Day Outcomes. J Invasive Cardiol. 2018;30:431-436. [PubMed] |
| 21. | Choudhary S. Association of syntax score with short-term outcomes among acute ST-elevation myocardial infarction patients undergoing primary PCI. Indian Heart J. 2017;69 Suppl 1:S20-S23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Berwanger O, Santucci EV, de Barros E Silva PGM, Jesuíno IA, Damiani LP, Barbosa LM, Santos RHN, Laranjeira LN, Egydio FM, Borges de Oliveira JA, Dall Orto FTC, Beraldo de Andrade P, Bienert IRC, Bosso CE, Mangione JA, Polanczyk CA, Sousa AGMR, Kalil RAK, Santos LM, Sposito AC, Rech RL, Sousa ACS, Baldissera F, Nascimento BR, Giraldez RRCV, Cavalcanti AB, Pereira SB, Mattos LA, Armaganijan LV, Guimarães HP, Sousa JEMR, Alexander JH, Granger CB, Lopes RD; SECURE-PCI Investigators. Effect of Loading Dose of Atorvastatin Prior to Planned Percutaneous Coronary Intervention on Major Adverse Cardiovascular Events in Acute Coronary Syndrome: The SECURE-PCI Randomized Clinical Trial. JAMA. 2018;319:1331-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 23. | Vicent L, Velásquez-Rodríguez J, Valero-Masa MJ, Díez-Delhoyo F, González-Saldívar H, Bruña V, Devesa C, Juárez M, Sousa-Casasnovas I, Fernández-Avilés F, Martínez-Sellés M. Predictors of high Killip class after ST segment elevation myocardial infarction in the era of primary reperfusion. Int J Cardiol. 2017;248:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Killip T 3rd, Kimball JT. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol. 1967;20:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1539] [Cited by in RCA: 1492] [Article Influence: 25.7] [Reference Citation Analysis (1)] |
| 25. | van der Net JB, Janssens AC, Eijkemans MJ, Kastelein JJ, Sijbrands EJ, Steyerberg EW. Cox proportional hazards models have more statistical power than logistic regression models in cross-sectional genetic association studies. Eur J Hum Genet. 2008;16:1111-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Annesi I, Moreau T, Lellouch J. Efficiency of the logistic regression and Cox proportional hazards models in longitudinal studies. Stat Med. 1989;8:1515-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 2.4] [Reference Citation Analysis (0)] |