Peer-review started: August 28, 2022
First decision: November 21, 2022
Revised: December 4, 2022
Accepted: December 21, 2022
Article in press: December 21, 2022
Published online: January 26, 2023
Processing time: 135 Days and 23 Hours
Several reports show that two types of coronary vasospasm (diffuse and focal spasm) are associated with the severity or prognosis of coronary spasm in patients with vasospastic angina (VSA). It is unclear whether intracoronary pressure differs between the two spasm types.
To investigate such relationships using a pressure wire during the spasm provocation test (SPT) in patients with VSA.
Eighty-seven patients with VSA (average age: 67 years; 50 men, 37 women) underwent SPT. During the SPT, a pressure wire was advanced into the distal portion of the right coronary artery and left anterior descending coronary artery, and the ratio of the intracoronary pressure to the aortic pressure (Pd/Pa) was continuously monitored. An SPT was performed using acetylcholine (ACh), and the presence of coronary spasm was defined as the presence of > 90% arterial narrowing in response to an ACh infusion, with the usual chest symptoms and/or ischemic ECG changes. Focal spasm was defined as total or subtotal spasm within one segment of the AHA classification, while diffuse spasm was defined as > 90% spasm with two or more segments.
Among 87 patients, the frequencies of metabolic syndrome and having coronary atherosclerosis were higher in the focal group (n = 33) than in the diffuse spasm group (n = 54, P < 0.05). In the vessel analyses, in these 134 spastic segments, diffuse and focal spasms were detected in 100 and 34 vessels, respectively. The Pd/Pa at baseline was similar in both groups (diffuse: 0.96 ± 0.05, focal: 0.95 ± 0.05, P = 0.35); however, the Pd/Pa during coronary spasm was lower in focal spastic vessels (0.66 ± 0.20) than in diffuse spastic vessels (0.76 ± 0.11, P < 0.01), and the reduction in Pd/Pa during an SPT was also lower in focal spastic vessels (-0.29 ± 0.20) than in diffuse spastic vessels (-0.18 ± 0.11, P < 0.01). The presence of focal spasm was a significant factor responsible for reduction in Pd/Pa during SPT.
These findings suggest that focal spasm may be more severe than diffuse spasm, judging from the intracoronary pressure during coronary spasm.
Core Tip: Coronary spasm is classified into diffuse spasm and focal spasm based on its morphology; it is noted that focal spasm may have a worse prognosis. We compared the clinical backgrounds of patients with focal spasms. We also evaluated the intracoronary pressure in diffuse and focal spasms using a pressure wire. Patients in the focal spasm group were more likely to have metabolic syndrome and coronary atherosclerosis. The reduction in intracoronary pressure during coronary spasms was greater in focal spasms than in diffuse spasms. These findings suggest that the degree of ischemia may be greater in patients with focal spasms.
- Citation: Teragawa H, Oshita C, Uchimura Y. Does the intracoronary pressure differ according to two types (diffuse or focal) of coronary spasm? World J Cardiol 2023; 15(1): 1-12
- URL: https://www.wjgnet.com/1949-8462/full/v15/i1/1.htm
- DOI: https://dx.doi.org/10.4330/wjc.v15.i1.1
Coronary spasm is the transient vasoconstriction of epicardial coronary arteries that leads to the occurrence of myocardial ischemia[1] and is considered the cause of not only rest angina and effort angina but also acute coronary syndrome or ischemic cardiac arrest[1,2]. Guidelines and expert consensus documents on coronary spasms and vasospastic angina (VSA) have already been published, and more interest has been focused on coronary spasms since they have been identified as a cause of ischemia with nonobstructive coronary artery disease (INOCA) or myocardial infarction with nonobstructive coronary artery disease (MINOCA)[3].
However, several issues regarding coronary spasm are not fully understood or solved, including the protocol for the spasm provocation test (SPT), the sequence of SPT during comprehensive coronary angiography, the treatment of medically refractory coronary spasm, and the need for implantable cardioverter defibrillators in the event of cardiac arrest[2-6]. The endotype of coronary spasm as focal and diffuse spasm is one such unsolved problem regarding coronary spasm[5]. The clinical characteristics of these two types of coronary spasm have been reported to be different: focal spasm occurs more frequently in men than in women[7,8] and is more frequently recognized in atherosclerotic lesions than diffuse spasm[9]. Regarding prognosis, it has been demonstrated that the prognosis is worse in patients with VSA and focal spasm than in those with diffuse spasm[7,10,11]. The cause of the worse prognosis in focal spasm than in diffuse spasm may, in part, be the progression of atherosclerosis or destabilization of the atherosclerotic lesion with focal spasm[9,12-15]. The severity of myocardial ischemia due to focal or diffuse coronary spasm may be one of the possible mechanisms responsible for the difference in prognosis between the two types of coronary spasm; however, the method of assessing the severity of myocardial ischemia due to focal or diffuse spasm has been unclear.
Intracoronary pressure assessment was originally adopted in the clinical setting to assess the immediate coronary stenosis[16], and the distal pressure (Pd) divided by the aortic pressure (Pa) during hyperemia, which has been recognized as the fractional flow reserve (FFR), decreases according to myocardial ischemia[17]. Intracoronary pressure assessments during SPT have recently been used[18,19]. Furthermore, recently, in European countries and the United States, SPT has been performed after an assessment of coronary microvascular function using a pressure wire or Doppler wire[3], which means that there may be more opportunities to perform SPT using a pressure wire[20]. Thus, in the present study, we clarified the clinical characteristics of patients with VSA who manifested focal spasm during the SPT compared with those of patients with VSA who manifested diffuse spasm during the SPT and investigated whether the severity of myocardial ischemia (shown by the Pd/Pa during SPT) differed according to the type of spasm (focal or diffuse spasm).
This observational retrospective study included patients with VSA diagnosed with SPT using a pressure wire who visited our institution between January 2012 and March 2017 (n = 98). The exclusion criteria were as follows: Significant coronary stenosis (%stenosis > 50%, n = 7) or a previous medical history of percutaneous coronary intervention (n = 4). Ultimately, 87 patients were enrolled in the present study. The study protocol was approved by the ethics committee of our institution. Written informed consent was obtained from all participants.
SPT was performed as previously described[21]. During this period, SPT were performed on the right coronary artery (RCA) at our institution. After the initial Coronary angiography (CAG), a 0.014-inch pressure wire (PrimeWire Prestige Plus Guide Wire or Verrata Pressure Guide Wire; Phillips Volcano, Amsterdam, Holland) was advanced through a 5-Fr catheter into the distal segments of the RCA. Before this, we calibrated the pressure between the tips of the catheters and the pressure wire at the ostium of the coronary arteries. The ratios of distal intracoronary pressure (Pd), derived from the pressure wire, to Pa, derived from the catheter tips (Pd/Pa index), were continuously monitored. Subsequently, 20 μg and 50 μg doses of acetylcholine (ACh) were injected into the RCA. When coronary spasm was not induced by 50 μg ACh, a maximum dose of 80 μg ACh was infused into the RCA. The minimal Pd/Pa index in response to each ACh dose was recorded, and when the Pd/Pa index was reduced during the coronary spasm, the values just before the angiograms were adopted. CAG was performed immediately after coronary spasms were induced or the maximum ACh infusion was completed. If coronary spasm was induced but improved spontaneously, SPT of the left coronary artery (LCA) was performed without intracoronary injection of NTG into the RCA. In such cases, once the SPT for the LCA was completed, CAG was repeated following an NTG injection into the RCA. If the coronary spasm provoked by ACh infusion into the RCA was prolonged or severe enough to induce hemodynamic instability, an intracoronary injection of 0.3 mg) was administered to relieve spasms. Subsequently, SPT of the LCA was performed. In the LCA SPT, a pressure wire was advanced into the distal segments of the left anterior descending coronary artery (LAD) immediately after the second pressure calibration at the LCA ostium. SPT was performed by infusing 50 and 100 μg doses of ACh into the LCA using a similar method. If coronary spasm was not induced by 100 μg ACh, a maximum of 200 μg ACh was infused into the LCA. CAG was performed just after a coronary spasm was provoked or the maximum ACh infusion was completed. An intracoronary injection of 0.3 mg NTG was administered, followed by the final CAG for the LCA. In this study, low, moderate, and high doses of ACh were considered to be 20, 50, and 80 μg for RCA, respectively, and 50, 100, and 200 µg for LCA, respectively.
The minimal Pd/Pa index in response to each ACh dose was recorded, and when the Pd/Pa index was reduced during the coronary spasm, the values just before the angiograms were adopted. The difference in Pd/Pa (ΔPd/Pa) during the SPT was defined as the minimal Pd/Pa minus the baseline Pd/Pa. During the period when a PrimeWire Prestige Plus Guide Wire was available, the instantaneous free-wave ratio (iFR) was measured immediately before ACh administration in some patients. In addition, FFR was also measured in patients with coronary atherosclerosis using the conventional method of intravenous administration of adenosine triphosphate[22].
We used an autoinjector, as shown previously[21]. The coronary artery diameter was measured as previously described[21]. Lesions with > 20% stenosis were defined as atherosclerotic. We also investigated the possibility of myocardial bridging (MB), which is the presence of a > 20% systolic reduction in coronary artery diameter[23]. Complications of CAG and SPT include common severe complications, such as myocardial infarction, cerebral infarction, vascular trauma requiring surgery associated with CAG, induction of coronary spasm or coronary perforation associated with a pressure wire insertion, ventricular fibrillation (Vf), pulseless ventricular tachycardia (pVT) or hemodynamic compromise requiring catecholamine administration, and atrial fibrillation associated with SPT.
The activity of angina pectoris, when it occurs, was classified into three patterns: resting, exertion, and both resting and exertion. In addition, cold sweats and loss of consciousness were identified as serious signs of coronary spasms. VSA was defined as > 90% narrowing of coronary arteries on angiograms when provoked, accompanied by the presence of usual chest pain and/or the presence of an ST-segment deviation on ECG[2]. Focal spasm was defined as transient vessel narrowing of > 90% within the borders of one isolated coronary segment, as defined by the American Heart Association. Diffuse spasm was defined as a 90% diffuse vasoconstriction observed in ≥ 2 adjacent coronary segments of the coronary arteries[7]. In the present study, this assessment could be applied to each coronary artery; thus, in the assessment of each patient, the endotype of coronary spasm may differ between the RCA and LCA. In the present study, Group D consisted of patients with VSA and diffuse spasm that occurred in only one or both coronary arteries, and Group F consisted of patients with VSA and focal spasm that occurred in one coronary artery, both coronary arteries, or one of the coronary arteries. Multivessel spasms were defined as coronary spasms that occurred in ≥ 2 major coronary arteries. For multivessel spasms, we could not assess when the subsequent SPT was negative after the avoidable use of NTG[24].
In lesion analyses, for the site of coronary spasm, each coronary artery was divided into three parts (proximal, mid, and distal), and the central part of the coronary spasm was described as diffuse.
Regarding the medications taken before SPT, although coronary vasodilators were discontinued 48 h before SPT, we investigated the number of vasodilators.
Patients were asked about their current smoking status and any family history of coronary artery disease (FH-CAD) was recorded. Hypertension, dyslipidemia, diabetes mellitus, metabolic syndrome (MtS), and chronic kidney disease were defined based on the standard definitions and are described in previous papers[25,26]. The left ventricular ejection fraction was measured using cardiac ultrasonography. In the majority of study participants (n = 80), flow-mediated dilation (FMD), as an endothelium-dependent function, and NTG-induced dilation (NID), as an endothelium-independent function, were measured as previously described[27].
Data are presented as mean ± SD or median with interquartile ranges for non-normally distributed data and non-continuous variables. Baseline characteristics of the groups were compared using Student’s unpaired t-tests, Wilcoxon signed-rank tests, or χ2 analysis, as appropriate. Multivariate regression analysis was performed to determine the factors responsible for ΔPd/Pa during the SPT. Statistical analyses were performed using the JMP version 16 (SAS Institute Inc., United States). A P value of < 0.05 was considered statistically significant.
There were 54 patients (62%) in Group D and 33 (38%) in Group F. In Group D, diffuse spasm occurred in both the RCA and LAD in 32 patients, only in the LCA in 14 patients, and only in the RCA (the LCA was not assessed due to the unavoidable use of NTG) in eight patients. In Group F, focal spasm occurred both in the RCA and LCA in four patients, only in the LCA in 13 patients, in the LCA despite diffuse spasm in the RCA in seven patients, in the RCA despite diffuse spasm in the LCA in eight patients, and in the RCA (the LCA was not assessed due to an unavoidable use of NTG) in one patient. Patient characteristics are shown in Table 1. The frequency of MtS was higher in Group F than in Group F (P = 0.04). The frequency of hypertension tended to be higher in Group F than in Group D (P = 0.06). There were no significant differences in the blood chemical parameters displayed in Table 2. The NID tended to be lower in Group F than in Group D, but there was no significant difference in FMD between the two groups. Regarding the medications taken before admission, the frequency of calcium-channel blocker (CCB) consumption was significantly higher in Group F than in Group D (P = 0.01), and the frequency of long-acting nitrate consumption tended to be higher in Group F than in Group D (P = 0.05, Table 3). On CAG and SPT, the frequencies of coronary atherosclerosis (P = 0.03) and MB (P = 0.047, Table 3, P = 0.05) were significantly higher in Group F than in Group D, whereas the frequency of occurrence of multivessel spasm did not differ significantly between the two groups (Table 3). No serious complications occurred with CAG, and no coronary spasm was induced with pressure wire insertion or coronary perforation. In the SPT, no Vf or pVT occurred, but hemodynamic instability requiring catecholamine was observed in two patients (6%) in Group F and one patient (2%, P = 0.30) in Group D. Atrial fibrillation during SPT was observed in four patients (12%) in Group F and two patients (4%, P = 0.13) in Group D, but the difference was not statistically significant.
| Group D | Group F | P value | |
| No. (%) | 54 (62) | 33 (38) | |
| Age (yr) | 65 ± 11 | 69 ± 10 | 0.06 |
| Male/Female | 28/26 | 22/11 | 0.18 |
| BMI (kg/m2) | 24.4 ± 4.2 | 24.7 ± 3.4 | 0.77 |
| Coronary risk factors (%) | |||
| Current smoker | 11 (20) | 8 (24) | 0.67 |
| Hypertension | 34 (63) | 27 (82) | 0.06 |
| Dyslipidemia | 36 (67) | 19 (58) | 0.39 |
| Diabetes mellitus | 10 (19) | 9 (27) | 0.34 |
| Family history of CAD (%) | 12 (22) | 8 (24) | 0.83 |
| MtS (%) | 12 (22) | 14 (42) | 0.04 |
| CKD (%) | 16 (30) | 13 (39) | 0.35 |
| Group D | Group F | P value | |
| Blood chemical parameters | |||
| Total cholesterol (mg/dL) | 193 ± 33 | 202 ± 40 | 0.26 |
| Triglyceride (mg/dL) | 142 ± 76 | 143 ± 58 | 0.93 |
| HDL-cholesterol (mg/dL) | 57 ± 17 | 57 ± 17 | 0.99 |
| LDL-cholesterol (mg/dL) | 107 ± 25 | 116 ± 32 | 0.16 |
| Fasting blood sugar (mg/dL) | 103 ± 30 | 107 ± 15 | 0.53 |
| Hemoglobin A1c (%) | 5.8 ± 0.9 | 6.1 ± 0.8 | 0.18 |
| C-reactive protein (mg/dL) | 0.06 (0.04, 0.18) | 0.07 (0.02, 0.17) | 0.74 |
| Brain natriuretic peptide (pg/mL) | 16 (10, 35) | 20 (10, 30) | 0.92 |
| eGFR (mL/min/1.73 m2) | 70.7 ± 14.5 | 67.8 ± 14.8 | 0.37 |
| Echographic parameters | |||
| LVEF on UCG (%) | 66 ± 9 | 67 ± 6 | 0.61 |
| FMD on brachial ultrasonography (%) | 3.9 ± 2.8 (n = 47) | 2.8 ± 3.3 (n = 33) | 0.11 |
| NID on brachial ultrasonography (%) | 15.2 ± 7.5 (n = 47) | 12.3 ± 5.2 (n = 33) | 0.05 |
| Group D | Group F | P value | |
| Medications taken before CAG | |||
| Calcium-channel blocker (%) | 11 (20) | 16 (48) | 0.01 |
| Long-acting nitrate (%) | 6 (11) | 9 (27) | 0.05 |
| Statins (%) | 26 (48) | 9 (27) | 0.05 |
| Anti-platelet therapy (%) | 14 (26) | 9 (27) | 0.89 |
| VSA-related symptom | |||
| Cold sweating or syncope (%) | 5 (9) | 4 (12) | 0.67 |
| CAG.SPT | |||
| Coronary atherosclerosis (%) | 28 (52) | 25 (76) | 0.03 |
| MB (%) | 7 (13) | 10 (30) | 0.05 |
| Multi-vessel spasm (%, n) | 32 (74, 43) | 19 (66, 29) | 0.42 |
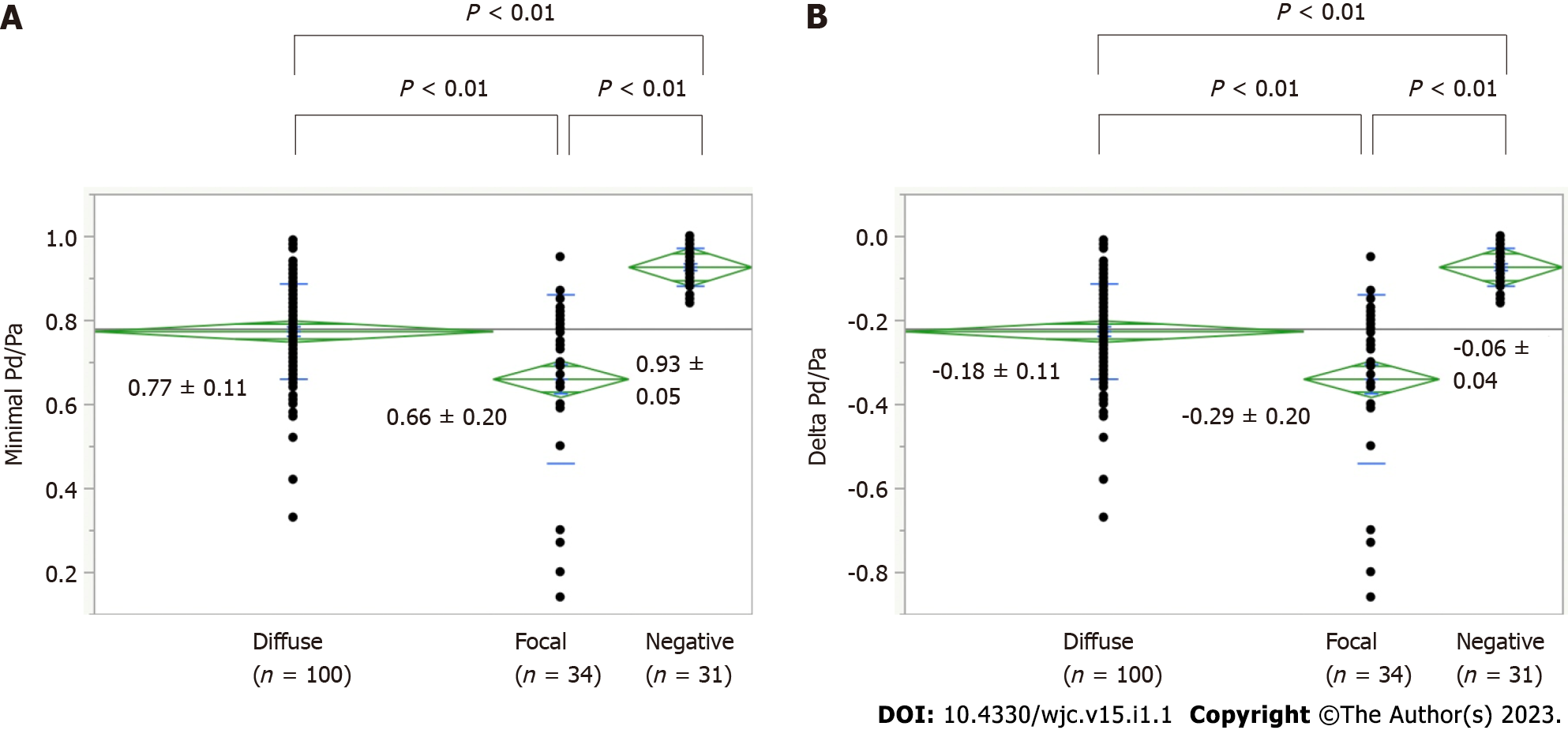
In 87 study participants, because of a small RCA (n = 6) and inability to advance a guidewire into the distal coronary artery (n = 1 in the RCA, n = 2 in the LAD, total n = 3), 165 coronary arteries were assessed for intracoronary pressure using a pressure wire, including 80 RCAs and 85 LADs). Among the 165 coronary arteries, there were 100 diffuse spasms, 34 focal spasms, and 31 negatives, including the case with unavoidable use of NTG. The lesion characteristics of diffuse and focal spasms are shown in Table 4. Coronary atherosclerosis was more frequently observed during focal spasms than during diffuse spasms (P = 0.01). The baseline Pd/Pa and iFR did not differ significantly between the two groups; however, the minimal Pd/Pa during the SPT and ΔPd/pa during the SPT were significantly lower in focal spasm than in diffuse spasm (P < 0.01, Figure 1). The ACh dose at spasm provocation differed significantly between the two groups (P = 0.03). The frequencies of subtotal/total occlusion on CAG and ST elevation on ECG were more frequently associated with focal spasms than with diffuse spasms. FFR after SPT did not differ significantly between the two groups, perhaps because the number of study participants was small. Multivariate regression analyses using the endotype of coronary spasm, presence of coronary atherosclerosis, and dose of ACh at spasm provocation showed that focal spasm was a significant factor responsible for the reduction of intracoronary pressure during ACh provocation (P < 0.01, Table 5).
| Diffuse spasm | Focal spasm | P value | |
| No. | 100 | 34 | |
| ST elevation on ECG during SPT | 6 (6) | 13 (35) | < 0.01 |
| Total or subtotal occlusion on CAG | 9 (9) | 9 (26) | 0.02 |
| Spastic vessels: RCA/LAD | 46/54 | 13/21 | 0.43 |
| Spasm site | |||
| Proximal/Mid/Distal | 12/57/31 | 5/16/13 | 0.58 |
| Dose of ACh at spasm provocation | |||
| Low (%)/Moderate (%)/High (%) | 32 (32)/65 (65)/3 (3) | 13 (38)/16 (47)/5 (15) | 0.03 |
| Intracoronary pressure indexes | |||
| Baseline Pd/Pa | 0.96 ± 0.05 | 0.95 ± 0.05 | 0.35 |
| Minimal Pd/Pa | 0.77 ± 0.11 | 0.66 ± 0.20 | < 0.01 |
| ΔPd/Pa | - 0.19 ± 0.11 | - 0.29 ± 0.20 | < 0.01 |
| iFR (n) | 0.96 ± 0.07 (n = 52) | 0.95 ± 0.08 (n = 17) | 0.83 |
| FFR (n) | 0.85 ± 0.08 (n = 26) | 0.80 ± 0.10 (n = 8) | 0.18 |
| Factors | F value | P value |
| Focal spasm | 14.14 | < 0.01 |
| Atherosclerosis | 1.38 | 0.24 |
| ACh dose at spasm provocation | 1.08 | 0.34 |
| R2 = 0.18 | ||
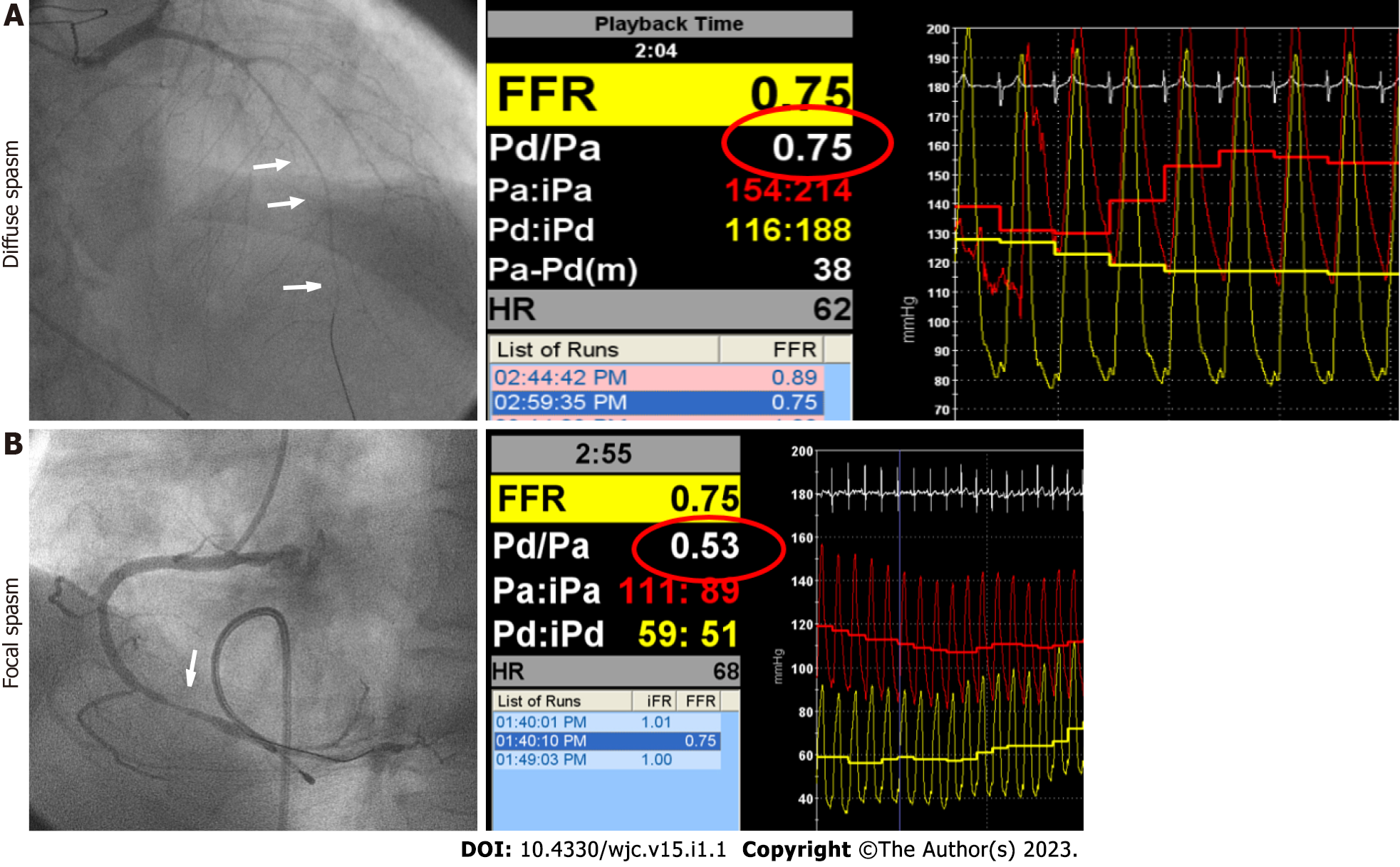
The representative cases of focal and diffuse spasms with intracoronary pressure are shown in Figure 2.
This study investigated the clinical characteristics of patients with VSA and focal spasm, and compared these characteristics with those of patients with diffuse spasm. Our data showed a higher frequency of MtS and coronary atherosclerosis in patients with VSA and focal spasms. Lesion analyses using a pressure wire showed that the reduction in Pd/Pa during SPT was significantly greater in focal spasm than in diffuse spasm, as well as a higher frequency of coronary atherosclerosis. These findings may indicate that focal spasm causes a greater degree of myocardial ischemia than diffuse spasm does.
According to several studies investigating the prognoses of focal and diffuse spasms[7,10,11], focal spasm has the worst prognosis. Sato et al[7] reported that the major cardiovascular events (MACE)-free rate over six years of follow-up was 92.5% in patients with focal spasm and 96.5% in those with diffuse spasm, showing that focal spasm is a factor indicating a worse prognosis. Kim et al[10] showed that the MACE-free rate over 2 years of follow-up was 91.8% in patients with focal spasm and 96.8% in those with diffuse spasm, and that the cause of MACE in patients with focal spasm was acute coronary syndrome, especially unstable angina (UAP). Nishimiya et al[11] demonstrated that the prognosis over 6 years of follow-up was worse in patients with focal spasm than in those with diffuse spasm, and that the causes of MACE included nonfatal myocardial infarction and readmission for UAP or heart failure.
Several studies have used intracoronary imaging modalities for the possible mechanisms of focal spasm-induced worse prognoses[9,12-15]. Several studies using intravascular ultrasonography have demonstrated the presence of atherosclerosis at focal spastic sites[12-14]. Using optical coherence tomography (OCT), Kitano et al[9] demonstrated that intima area, maximum intima thickness, and lipid content were higher in focal spasm sites than in diffuse spasm sites. In a recent study using OCT, intraplaque neovessels and macrophage infiltration were also more frequently observed in focal spastic segments than in diffuse spastic segments[11]. Using coronary angioscopy (CAS), Kitano et al[9] also showed that atherosclerotic yellow plaques and thrombi were more frequently observed in focal spastic sites than in diffuse spastic sites. Our previous report using CAS showed that intracoronary thrombi were recognized only in focal spastic sites[15]. Taken together, these findings suggest that unstable atherosclerotic lesions may be present at the focal spastic sites. Recently, it was reported that coronary microvascular dysfunction may contribute to INOCA[28], and such coronary microvascular dysfunction was more frequently observed in coronary arteries with focal spasm than in those with diffuse spasm[22]. Such coexistence of coronary microvascular dysfunction may, in part, contribute to readmission for UAP.
In the present study, we focused on the severity of myocardial ischemia caused by focal spasms. The possibility of higher severity of myocardial ischemia by focal spasm has been demonstrated not only by ECG findings (with ST elevation) but also by the reduction of intracoronary pressure during coronary spasm induced using a pressure wire, independent of the presence of atherosclerotic lesions, spasm vessels, and the spasm segment of the coronary artery. Recently, SPT has been recommended after the assessment of microvascular function using a pressure wire[20], which shows an increase in the opportunity for SPT using a pressure wire. We hope that further studies will confirm these findings.
In the present study, the dose of ACh at spasm provocation was different for focal and diffuse spasm, and Sueda et al[29] reported that focal spasm tends to cause coronary spasm at lower loading doses of ACh, which may be consistent with the findings of the present study. In contrast, higher doses of ACh during spasm provocation appeared to be more frequently observed in focal spasms. In the present study, the frequency of coronary dilators, such as CCB or long-acting nitrates, was high in Group F. Although we could not evaluate chest symptoms on admission in the present study, the high frequency of coronary dilator medications on admission may be due to the aim of improving chest symptoms due to the high degree of myocardial ischemia caused by focal spasm. At our institution, as per the guidelines[2], coronary dilators were discontinued 48 h prior to the SPT, but the long-acting CCB may have remained effective, resulting in higher ACh doses in the SPT in Group F.
Regarding the clinical characteristics of patients with focal spasm, it has been reported that more men than women have focal spasm[7,8]; however, our study did not confirm that more men had focal spasm. The backgrounds of the study participants may have differed slightly. Moreover, the finding of more patients with MtS and hypertension may also support the finding that patients with focal spasms had more atherosclerotic lesions. We observed a different trend of endothelium-dependent vasodilatory response in vascular endothelial dysfunction; however, it is unclear whether this is a result or a cause, and we would like to investigate this in a future study with more participants. It is still possible that there may have been a slight residual effect of the oral medication.
The implication of this study is that focal spasm may mean that the degree of ischemia is so great that effective and sufficient doses of coronary dilators should be administered. This could improve the prognosis of patients with focal spasm; however, future prospective studies are needed.
This study had several limitations. First, it was a single-center study with a small number of patients, and the results of the present examination may not be applicable to all patients with coronary spasm. Second, in classifying patients, those with both focal and diffuse spasms were assigned to the focal spasm group; however, it is unclear whether this classification is correct. Third, in this study, a pressure wire was inserted to apply a load, and it was unclear whether the insertion of the pressure wire affected the morphology of the coronary spasm. The morphology of the coronary spasm may have been affected by the insertion of a pressure wire. We also encountered a case in which it was difficult to distinguish the distal portion of a coronary spasm because of the pressure wire. Fourth, noninvasive tests such as ergonovine stress echocardiography[30] and coronary computed tomography[31,32] have been used to evaluate coronary spasm, and their usefulness has been reported; however, these tests are not routinely performed in our hospital and have not been evaluated. Fifth, we have experienced that troponin, a myocardial enzyme, is positive during severe myocardial ischemia in some VSA patients. We believe this is an important indicator for assessing the severity of myocardial ischemia, even in VSA patients. However, at our institution, it is not routinely measured in patients with worsening chest symptoms and a long duration of attacks. Finally, the doses of ACh that induced the focal/diffuse spasms were different. Although this factor was not significant in the multivariate analysis, it may have influenced the reduction in intracoronary pressure during ACh provocation.
In conclusion, patients with focal spasm may have more atherosclerotic lesions and a greater degree of myocardial ischemia than those with diffuse spasm. Since the prognosis of patients with focal spasm has been reported to be poor, it may be necessary to administer adequate doses of coronary dilators, especially in these patients. Further prospective studies and multicenter registries are needed to clarify the need for medication.
Coronary spasm can be divided into two types: Focal spasm and diffuse spasm, but the prognosis for focal spasm is reported to be worse than that for diffuse spasm.
The cause of the worse prognosis in focal spasm is unclear, and although the degree of myocardial ischemia may be more severe in focal spasm, no method has been established to evaluate the severity of coronary spasm.
The objective of the present study was to investigate such relationships using a pressure wire during the spasm provocation test (SPT) in patients with vasospastic angina (VSA).
Eighty-seven patients with VSA (average age: 67 years; 50 men, 37 women) underwent SPT. During SPT, a pressure wire was advanced into the distal portion of the right coronary artery and the left anterior descending coronary artery, and the ratio of intracoronary pressure to aortic pressure (Pd/Pa) was continuously monitored. An SPT was performed with acetylcholine (ACh), and the presence of coronary spasm was defined as the presence of > 90% arterial narrowing in response to an ACh infusion, with the usual chest symptoms and/or ischemic ECG changes. Focal spasm was defined as total or subtotal spasm within one segment of the AHA classification, while diffuse spasm was defined as > 90% spasm with two or more segments. The group with focal spasm in at least one major coronary artery was classified as the focal group, and the group without focal spasm as the diffuse group.
Among 87 patients, the frequencies of metabolic syndrome and coronary atherosclerosis were higher in the focal group (n = 33) than in the diffuse spasm group (n = 54, P < 0.05). In vessel analyzes, in these 134 spastic segments, diffuse and focal spasms were detected in 100 and 34 vessels, respectively. Pd/Pa at baseline was similar in both groups (diffuse: 0.96 ± 0.05, focal: 0.95 ± 0.05, P = 0.35); however, Pd/Pa during coronary spasm was lower in focal spastic vessels (0.66 ± 0.20) than in diffuse spastic vessels (0.76 ± 0.11, P < 0.01), and the reduction in Pd/Pa during an SPT was also lower in focal spastic vessels (-0.29 ± 0.20) than in diffuse spastic vessels (-0.18 ± 0.11, P < 0.01). The presence of focal spasm was a significant factor responsible for the reduction in Pd/Pa during SPT.
These findings suggest that focal spasm may be more severe than diffuse spasm, judging by intracoronary pressure during coronary spasm. This mechanism may be involved in the poor prognosis of focal spasm, and careful measures, such as intensified drug therapy, should be taken when focal spasm is detected.
In recent years, more patients have been evaluated for coronary microvascular dysfunction using pressure wires and then for coronary artery spasm induced by ACh, and it is possible that pressure wires will be used more frequently to induce coronary artery spasm. It will be important to confirm the findings of this study in more cases.
We thank Ms. Akemi Seno for secretarial assistance. We also thank the staff of the catheterization laboratory, cardiovascular ward, and cardiovascular outpatient clinic.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mzhavanadze ND, Russia; Zhang S, United States S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Yasue H, Nakagawa H, Itoh T, Harada E, Mizuno Y. Coronary artery spasm--clinical features, diagnosis, pathogenesis, and treatment. J Cardiol. 2008;51:2-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 378] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 2. | JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ J. 2014;78:2779-2801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 356] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 3. | Kunadian V, Chieffo A, Camici PG, Berry C, Escaned J, Maas AHEM, Prescott E, Karam N, Appelman Y, Fraccaro C, Louise Buchanan G, Manzo-Silberman S, Al-Lamee R, Regar E, Lansky A, Abbott JD, Badimon L, Duncker DJ, Mehran R, Capodanno D, Baumbach A. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J. 2020;41:3504-3520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 503] [Article Influence: 125.8] [Reference Citation Analysis (0)] |
| 4. | Takagi Y, Takahashi J, Yasuda S, Miyata S, Tsunoda R, Ogata Y, Seki A, Sumiyoshi T, Matsui M, Goto T, Tanabe Y, Sueda S, Sato T, Ogawa S, Kubo N, Momomura S, Ogawa H, Shimokawa H; Japanese Coronary Spasm Association. Prognostic stratification of patients with vasospastic angina: a comprehensive clinical risk score developed by the Japanese Coronary Spasm Association. J Am Coll Cardiol. 2013;62:1144-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 5. | Teragawa H, Oshita C, Ueda T. Coronary spasm: It's common, but it's still unsolved. World J Cardiol. 2018;10:201-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Sueda S, Shinohara T, Takahashi N, Shite J, Shoji T, Akao M, Kijima Y, Masuyama T, Miyaji T, Yamamoto K, Iwasaki Y, Yoshida R, Nakamura S, Ogino Y, Kimura K, Sasai M, Suzuki H, Wakatsuki T, Asajima H, Teragawa H, Ishikawa T, Kitamura K, Oda T, Nakayama T, Kobayashi Y, Sunada D, Yamaki M, Nishizaki F, Tomita H, Usuda K, Fujinaga H, Kuramitsu S, Ando K, Kiyooka T, Kadota K, Ishii Y, Ohtani H, Maekawa Y, Taguchi E, Nakao K, Kobayashi N, Seino Y, Nakagawa H, Saito Y, Komuro I, Sasaki Y, Ikeda S, Yamaguchi O, Kakutani A, Imanaka T, Ishihara M, Ishii M, Kaikita K, Tsujita K. Clinical Therapy in Patients with Aborted Sudden Cardiac Death due to Coronary Spasm. Journal of Coronary Artery Disease. 2020;26:91-99. [DOI] [Full Text] |
| 7. | Sato K, Kaikita K, Nakayama N, Horio E, Yoshimura H, Ono T, Ohba K, Tsujita K, Kojima S, Tayama S, Hokimoto S, Matsui K, Sugiyama S, Yamabe H, Ogawa H. Coronary vasomotor response to intracoronary acetylcholine injection, clinical features, and long-term prognosis in 873 consecutive patients with coronary spasm: analysis of a single-center study over 20 years. J Am Heart Assoc. 2013;2:e000227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 8. | Sueda S, Sakaue T. Sex-related Differences in Patients with Positive Coronary Spasm as Identified by Acetylcholine Testing. Intern Med. 2021;60:2357-2365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Kitano D, Takayama T, Sudo M, Kogo T, Kojima K, Akutsu N, Nishida T, Haruta H, Fukamachi D, Kawano T, Kanai T, Hiro T, Saito S, Hirayma A. Angioscopic differences of coronary intima between diffuse and focal coronary vasospasm: Comparison of optical coherence tomography findings. J Cardiol. 2018;72:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Kim DW, Her SH, Ahn Y, Shin DI, Han SH, Kim DS, Choi DJ, Kwon HM, Gwon HC, Jo SH, Rha SW, Baek SH. Clinical outcome according to spasm type of single coronary artery provoked by intracoronary ergonovine tests in patients without significant organic stenosis. Int J Cardiol. 2018;252:6-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Nishimiya K, Suda A, Fukui K, Hao K, Takahashi J, Matsumoto Y, Mitsuishi K, Watanabe T, Ohyama K, Sugisawa J, Tsuchiya S, Satoh K, Shindo T, Godo S, Kikuchi Y, Shiroto T, Yasuda S, Shimokawa H. Prognostic Links Between OCT-Delineated Coronary Morphologies and Coronary Functional Abnormalities in Patients With INOCA. JACC Cardiovasc Interv. 2021;14:606-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Yamagishi M, Miyatake K, Tamai J, Nakatani S, Koyama J, Nissen SE. Intravascular ultrasound detection of atherosclerosis at the site of focal vasospasm in angiographically normal or minimally narrowed coronary segments. J Am Coll Cardiol. 1994;23:352-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 161] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Koyama J, Yamagishi M, Tamai J, Kawano S, Daikoku S, Miyatake K. Comparison of vessel wall morphologic appearance at sites of focal and diffuse coronary vasospasm by intravascular ultrasound. Am Heart J. 1995;130:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Saito S, Yamagishi M, Takayama T, Chiku M, Koyama J, Ito K, Higashikata T, Seguchi O, Honye J, Kanmatsuse K. Plaque morphology at coronary sites with focal spasm in variant angina: study using intravascular ultrasound. Circ J. 2003;67:1041-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Teragawa H, Orita Y, Oshita C, Uchimura Y. Intracoronary Thrombogenicity in Patients with Vasospastic Angina: An Observation Using Coronary Angioscopy. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek J Koolen JJ, Koolen JJ. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334:1703-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1629] [Cited by in RCA: 1614] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 17. | Ahn JM, Park DW, Shin ES, Koo BK, Nam CW, Doh JH, Kim JH, Chae IH, Yoon JH, Her SH, Seung KB, Chung WY, Yoo SY, Lee JB, Choi SW, Park K, Hong TJ, Lee SY, Han M, Lee PH, Kang SJ, Lee SW, Kim YH, Lee CW, Park SW, Park SJ; IRIS-FFR Investigators†. Fractional Flow Reserve and Cardiac Events in Coronary Artery Disease: Data From a Prospective IRIS-FFR Registry (Interventional Cardiology Research Incooperation Society Fractional Flow Reserve). Circulation. 2017;135:2241-2251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 18. | Teragawa H, Fujii Y, Ueda T, Murata D, Nomura S. Case of angina pectoris at rest and during effort due to coronary spasm and myocardial bridging. World J Cardiol. 2015;7:367-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Teragawa H, Fujii Y, Uchimura Y, Ueda T. Importance of a second spasm provocation test: Four cases with an initial negative spasm provocation test. World J Cardiol. 2017;9:289-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Ford TJ, Ong P, Sechtem U, Beltrame J, Camici PG, Crea F, Kaski JC, Bairey Merz CN, Pepine CJ, Shimokawa H, Berry C; COVADIS Study Group. Assessment of Vascular Dysfunction in Patients Without Obstructive Coronary Artery Disease: Why, How, and When. JACC Cardiovasc Interv. 2020;13:1847-1864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 21. | Teragawa H, Oshita C, Orita Y. Clinical significance of prolonged chest pain in vasospastic angina. World J Cardiol. 2020;12:450-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Teragawa H, Oshita C, Uchimura Y, Akazawa R, Orita Y. Coronary Microvascular Vasodilatory Function: Related Clinical Features and Differences According to the Different Coronary Arteries and Types of Coronary Spasm. J Clin Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Teragawa H, Oshita C, Uchimura Y. The Impact of Myocardial Bridging on the Coronary Functional Test in Patients with Ischaemia with Non-Obstructive Coronary Artery Disease. Life (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Teragawa H, Oshita C, Uchimura Y. Clinical Characteristics and Prognosis of Patients with Multi-Vessel Coronary Spasm in Comparison with Those in Patients with Single-Vessel Coronary Spasm. J Cardiovasc Dev Dis. 2022;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 25. | Teragawa H, Oshita C, Ueda T. History of gastroesophageal reflux disease in patients with suspected coronary artery disease. Heart Vessels. 2019;34:1631-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Sueda S, Shinohara T, Takahashi N, Shite J, Shoji T, Akao M, Kijima Y, Masuyama T, Miyaji T, Yamamoto K, Iwasaki Y, Yoshida R, Nakamura S, Ogino Y, Kimura K, Sasai M, Suzuki H, Wakatsuki T, Asajima H, Teragawa H, Ishikawa T, Kitamura K, Oda T, Nakayama T, Kobayashi Y, Sunada D, Yamaki M, Nishizaki F, Tomita Y, Usuda K, Fujinaga H, Kuramitsu S, Andou K, Kiyooka T, Kadota K, Ishii Y, Ohtani H, Maekawa Y, Taguchi E, Nakao K, Kobayashi N, Seino Y, Nakagawa H, Saito Y, Komuro I, Sasaki Y, Ikeda S, Yamaguchi O, Kakutani A, Imanaka T, Ishihara M, Ishii M, Kaikita K, Tsujita K. Questionnaire in patients with aborted sudden cardiac death due to coronary spasm in Japan. Heart Vessels. 2020;35:1640-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Teragawa H, Kato M, Kurokawa J, Yamagata T, Matsuura H, Chayama K. Usefulness of flow-mediated dilation of the brachial artery and/or the intima-media thickness of the carotid artery in predicting coronary narrowing in patients suspected of having coronary artery disease. Am J Cardiol. 2001;88:1147-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, Hood S, McGeoch R, McDade R, Yii E, Sidik N, McCartney P, Corcoran D, Collison D, Rush C, McConnachie A, Touyz RM, Oldroyd KG, Berry C. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. J Am Coll Cardiol. 2018;72:2841-2855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 540] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 29. | Sueda S, Kohno H, Fukuda H, Inoue K, Suzuki J, Watanabe K, Ochi T, Uraoka T. Clinical and angiographical characteristics of acetylcholine- induced spasm: relationship to dose of intracoronary injection of acetylcholine. Coron Artery Dis. 2002;13:231-236. [PubMed] |
| 30. | Song JK, Park SW, Kang DH, Hong MK, Kim JJ, Lee CW, Park SJ. Safety and clinical impact of ergonovine stress echocardiography for diagnosis of coronary vasospasm. J Am Coll Cardiol. 2000;35:1850-1856. [PubMed] |
| 31. | Kang KM, Choi SI, Chun EJ, Kim JA, Youn TJ, Choi DJ. Coronary vasospastic angina: assessment by multidetector CT coronary angiography. Korean J Radiol. 2012;13:27-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Kang EJ, Kim MH, De Jin C, Seo J, Kim DW, Yoon SK, Park TH, Lee KN, Choi SI, Yoon YE. Noninvasive detection of coronary vasospastic angina using a double-acquisition coronary CT angiography protocol in the presence and absence of an intravenous nitrate: a pilot study. Eur Radiol. 2017;27:1136-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |