Published online Jun 26, 2022. doi: 10.4330/wjc.v14.i6.343
Peer-review started: January 15, 2022
First decision: March 16, 2022
Revised: March 27, 2022
Accepted: May 17, 2022
Article in press: May 17, 2022
Published online: June 26, 2022
Processing time: 156 Days and 10.6 Hours
The coronavirus disease 2019 (COVID-19) mRNA vaccine against severe acute respiratory syndrome coronavirus 2 infections has reduced the number of symptomatic patients globally. A case series of vaccine-related myocarditis or pericarditis has been published with extensive vaccination, most notably in teenagers and young adults. Men seem to be impacted more often, and symptoms commonly occur within 1 wk after immunization. The clinical course is mild in the majority of cases. Based on the evidence, a clinical framework to guide physicians to examine, analyze, identify, and report suspected and confirmed cardiac dysfunction cases is needed. A standardized workup for every patient with strongly suspicious symptoms associated with the COVID-19 mRNA vaccine comprises serum cardiac troponin measurement and a 12-lead electrocardiogram (ECG). For patients with unexplained elevation of cardiac troponin and path
Core Tip: A possible hypersensitivity myocarditis with a consistent relationship to administering an mRNA coronavirus disease 2019 (COVID-19) vaccination was reported. While the actual prevalence of this adverse event is unclear at this time, the clinical manifestation and pathological findings point to a link with an inflammatory reaction to a COVID-19 immunization. However, acute myocarditis following mRNA COVID-19 vaccination was very low and mostly self-limited. Moreover, the high efficacy of mRNA COVID-19 vaccines in preventing further pandemic conditions, reducing disease severity, and the occurrence of a very low incidence of myocarditis following immunization should be a strength of an mRNA COVID-19 vaccine for public trust.
- Citation: Leowattana W, Leowattana T. COVID-19 vaccination and cardiac dysfunction. World J Cardiol 2022; 14(6): 343-354
- URL: https://www.wjgnet.com/1949-8462/full/v14/i6/343.htm
- DOI: https://dx.doi.org/10.4330/wjc.v14.i6.343
Myocarditis or pericarditis is an inflammatory heart condition caused by infections, drug exposure, or immune system activation[1,2]. It has a wide range of clinical manifestations and courses, with most cases resolving spontaneously. However, it is also a cause of sudden cardiac death in young people. Furthermore, in some instances, inflammation can induce significant scarring, which causes left ventricular (LV) remodeling and finally leads to dilated cardiomyopathy or a predominant hypokinetic nondilated phenotype of cardiomyopathy[3,4]. Myocarditis has previously been reported following non-mRNA vaccine immunization. The incidence of myocarditis after a live smallpox vaccination was 2.2-7.8 per 100000, which occurred within 30 d after immunization[5,6]. The majority of adverse events that occur after vaccination in these people resolve without long-term consequences. Measles-mumps-rubella, chickenpox, yellow fever, and oral polio vaccine, have a 0.24 per 100000 immunization risk of pericarditis or myocarditis in the 42 d following vaccination[7].
During post-marketing pharmacovigilance surveillance, a considerably higher incidence of myocarditis or pericarditis than predicted was detected after coronavirus disease 2019 (COVID-19) immunization using mRNA vaccines[8]. As a result, the United States Food and Drug Administration, followed by the European Medicines Agency, listed myocarditis and pericarditis as extremely uncommon adverse drug reactions in the product information for Moderna and Pfizer-BioNTech[9,10]. Myocarditis or pericarditis is becoming more common as an unusual consequence of the COVID-19 mRNA vaccines, particularly in young adults and adolescent men. This article delves deeper into this phenomenon and its possible pathophysiology. We also examine the trade-off between the danger of myocarditis or pericarditis from mRNA vaccination and the cardiac and other hazards associated with COVID-19.
Witberg et al[11] analyzed Clalit Health Services, Israel's biggest health care organization, for the myocarditis patients who had been immunized with one dose of the mRNA vaccine BNT162b2 (Pfizer–BioNTech). Clalit Health Services provided health care for 4.7 million patients (52% of the total population) regarding socioeconomic status and prevalence of co-existing diseases. They explored 2558421 members who were vaccinated with one dose of the mRNA vaccine; along with 2401605 members who were vaccinated with 2 doses. They discovered 54 patients who satisfied the research criteria for myocarditis. There were 41 mild cases, 12 moderate cases, and 1 fatal case. The median age of these patients was 27 (21-35) years, with 94% of them being males. Within 42 d of the first dosage, the incidence of myocarditis per 100000 vaccinated people was 2.13 (1.56-2.70) (mild = 1.62, intermediate = 0.47, and severe = 0.04). There was a higher incidence among male patients than female patients, with 4.12 (2.99-5.26) and 0.23 (0-0.49) per 100000 vaccinations, respectively (Table 1). Among all patients aged 16 years to 29 years, the incidence was 5.49 (3.59-7.39) per 100000 vaccinations. The incidence was reduced to 1.13 (0.66-1.60) per 100000 vaccinations among the patients who were 30 years of age or older. Male patients between the ages of 16 and 29 had the greatest incidence, with 10.69 (6.93-14.46) occurrences per 100000 immunizations. The most common clinical presentation was chest pain in 82% of the patients. Only 1 patient presented with hemodynamic instability. The electrocardiogram (ECG) showed 53% ST-segment elevation, 24% minor abnormalities, and a 21% normal pattern at presentation. All available data for cardiac troponin T (41 cases) showed a median peak of troponin T of 680 ng/L (275-2075). One patient's cardiac biopsy revealed perivascular lymphocyte and eosinophil infiltration. On admission, ECG findings (48 cases) showed 71% normal LV function. Fourteen patients (29%) showed some degree of LV dysfunction. Seventeen percent presented with mild symptom, four percent had mild symptom, four percent had moderate symptom, two percent had moderate-to-severe symptom, and two percent had severe symptom. Fifteen patients underwent cardiac magnetic resonance imaging (MRI) and showed normal LV function (Table 2). They concluded that the highest incidence of myocarditis after BNT162b2 mRNA vaccination was among male patients aged 16-29 years. The majority of myocarditis cases were mild to moderate in severity.
| Ref. | Type of vaccine | Study population | Incidence | Cardiac assessment methods | Main findings and clinical outcomes |
| Witberg et al[11], Israel | Pfizer-BioNTech | 54/2558421 (21-63 yr) | 2.13/100000 | Clinical presentation, ECG, ECHO, MRI, Troponin T | Myocarditis = 10.69/100000 in male ages 16-29 yr, 25.92% had LV dysfunction, 76% = mild, 22% = intermediate, 1 case had cardiogenic shock, 1 case died of unknown cause, 0.51/100000 after 1st dose and 2.15/100000 after 2nd dose |
| Mevorach et al[12], Israel | Pfizer-BioNTech | 136/9289765 (≥ 16 yr) | 1.46/100000 | Clinical presentation, ECHO, MRI, Troponin T, Endomyocardial biopsy | Myocarditis = 15.07/100000 in male ages 16-19 yr, 0.35/100000 after 1st dose, 2.28/100000 after 2nd dose, 94.85% = mild, 4.41% = intermediate, 1 case was fatal, endo-interstitial edema with neutrophils and mononuclear-cells infiltrates with no giant cells |
| Montgomery et al[13], United States | Pfizer-BioNTech/Moderna | 23/2810000 (20-51 yr) | 0.82/100000 | Clinical presentation, ECG, ECHO, MRI, Troponin T | Myocarditis = 1.88/100000 after 1st dose, 3.49/100000 after 2nd dose, and 4.36/100000 in male after 2nd dose |
| Perez et al[14], United States | Pfizer-BioNTech/Moderna/Johnson and Johnson | 7/175472 (12-106 yr) | 55.35/100000, Person-yr | Clinical presentation, ECG, ECHO, MRI, Troponin T | The overall incidence rate was 55.35 (22.25–114.00) per 100000 person-yr during the 2 wk after a dose of vaccine. The IRR for myocarditis following COVID-19 mRNA vaccination was increased for males at 6.69 (2.35–15.52), but it was not statistically significant for females at 1.41 (0.03–8.45) |
| Das et al[15], United States | Pfizer-BioNTech | 25/7735071 (12-17 yr) | 0.32/100000 | Clinical presentation, ECG, ECHO, MRI, Troponin T, CRP | Myocarditis = 0.04/100000 after 1st dose, 0.28/100000 after 2nd dose, and 0.26/100000 in male after 2nd dose |
| Li et al[16], United States | Pfizer-BioNTech/Moderna/Janssen | Age ≥ 12 yr | 0.598/100000 | VAERS | Pfizer–BioNTech had a higher incidence rate of 0.670/100000 than the rate of 0.498/100000 found for Moderna. The incidence rate following the 2nd dose was twice that of the 1st dose and was the highest in adolescents aged 12-17 yr, at 2.094/100000. The Janssen vaccine was not associated with myocarditis or pericarditis |
| Patone et al[17], United Kingdom | Pfizer-BioNTech/Moderna/AstraZeneca | 1615/38615491 (Age ≥ 16 yr) | 4.18/100000 | NIMS | The IRR of myocarditis = 1.76, 1.45, 8.38 after 1st dose of AstraZeneca, Pfizer-BioNTech, Moderna. IRR of myocarditis = 1.75, 23.10 after 2nd dose of Pfizer-BioNTech, Moderna. There was an increase in the risk of myocarditis within 1 wk after 1st dose of adenovirus and mRNA vaccines and a higher increased risk after 2nd dose of both mRNA vaccines, especially in under 40 yr |
| Simone et al[18], United States | Pfizer-BioNTech/Moderna | 15/2392924 (Age ≥ 18 yr) | 0.63/100000 | KPSC members with clinical presentation, ECG, ECHO, Troponin I | Myocarditis = 0.08/100000 after 1st dose, 0.58/100000 after 2nd dose over a 10-d period, all were men aged 20-32 yr. The IRR of myocarditis = 0.38 after 1st dose and 2.7 after 2nd dose |
| Nygaard et al[19], Denmark | Pfizer-BioNTech | 15/261334 (12-17 yr) | 5.74/100000 | Clinical presentation, ECG, ECHO, MRI, Troponin | Myocarditis = 3.06/100000 after 1st dose, 2.68/100000 after 2nd dose mostly in male (M:F = 6:1) |
| Husby et al[20], Denmark | Pfizer-BioNTech/Moderna | 269/4931775 (Age ≥ 12 yr) | 5.45/100000 | Danish Vaccination Register | HR of myocarditis/pericarditis = 1.34, 3.92 within 28 d from the vaccination of Pfizer-BioNTech, Moderna respectively. Myocarditis or pericarditis occurred at 1.4/100000 for Pfizer-BioNTech and 4.2/100000 for Moderna. Vaccination with Moderna vaccine was associated with an increased risk of myocarditis or pericarditis, especially in aged 12-39 yr |
| Diaz et al[21], United States | Pfizer-BioNTech/Moderna/Janssen | 57/2000287 (26-70 yr) | 2.85/100000 | Clinical presentation, ECG, ECHO, Troponin | Myocarditis = 1.0/100000 and pericarditis = 1.8/100000. Myocarditis and pericarditis were observed after the COVID-19 vaccination. Myocarditis developed rapidly in younger patients, mostly after the 2nd dose. Pericarditis affected older patients later, after either the 1st or 2nd dose |
| Chouchana et al[22], WHO | Pfizer-BioNTech/Moderna | 2277/716576 reports | NA | VigiBase | Over all myocarditis = 3.57/100000 with 12–17 yr = 3.69/100000, 18–29 yr = 1.97/100000, and ≥ 30 yr = 0.21/100000. Younger male aged 12–17 yr were more prone to report myocarditis or pericarditis with 22.3/100000. The median time to onset for myocarditis was 3 d after vaccine injection |
| Barda et al[23], Israel | Pfizer-BioNTech | 21/938812 | 2.23/100000 | Clinical presentation, ECG, ECHO, Troponin | Vaccination was most strongly associated with an elevated risk of myocarditis [risk ratio, 3.24 (1.55-12.44)]. Alternatively, SARS-CoV-2 infection was associated with a substantially increased risk of myocarditis [risk ratio, 18.28 (3.95-25.12)]. The BNT162b2-mRNA vaccine increased the incidence of a few adverse events over a 42-d follow-up period |
| Ref. | Type of vaccine | No. of myocarditiscases | Male/Female (%) | Median age in yr (IQR) | Myocarditis after 1st dose (%) | Myocarditis after 2nd dose (%) | Clinical severity F/I/M |
| Witberg et al[11], Israel | Pfizer-BioNTech | 54 | 51/3 (94/6) | 27 (21–35) | 17 (31.48) | 37 (68.52) | 1/12/41 |
| Mevorach et al[12], Israel | Pfizer-BioNTech | 136 | 118/18 (87/13) | - (16-> 30) | 19 (13.97) | 117 (86.03) | 1/6/129 |
| Montgomery et al[13], United States | Pfizer-BioNTech/Moderna | 23 | 23/0 (100/0) | 25 (20-51) | 3 (13.04) | 20 (86.96) | 0/7/16 |
| Perez et al[14], United States | Pfizer-BioNTech/Moderna | 7 | 6/1 (86/14) | 44 (22-71) | 1 (14.29) | 6 (85.71) | 0/6/1 |
| Das et al[15], United States | Pfizer-BioNTech | 25 | 22/3 (88/12) | 15 (12-17) | 3 (12.00) | 22 (88.00) | 0/22/3 |
| Simone et al[18], United States | Pfizer-BioNTech/Moderna | 15 | 15/0 (100/0) | 25 (20-32) | 2 (13.33) | 13 (86.67) | 0/15/0 |
| Nygaard et al[19], Denmark | Pfizer-BioNTech | 15 | 13/2 (87/12) | 17 (13-17) | 8 (53.33) | 7 (46.67) | 0/1/14 |
| Diaz et al[21], United States | Pfizer-BioNTech/Moderna | 20 | 15/5 (75/25) | 36 (26-48) | 4 (20.00) | 16 (80.00) | 2/17/1 |
Mevorach et al[12] retrospectively reviewed data regarding myocarditis cases. During the surveillance period, 9289765 Israeli residents were included. Of those, 5442696 individuals received the 1st dose and 5125635 received two BNT162b2 mRNA vaccines. They found that 136 cases were confirmed as diagnoses of myocarditis. In most cases, the clinical presentations in 129 cases were mild with a reso
Myocarditis symptoms usually appear within a few days of receiving the 2nd dose of the vaccine. After the 2nd dose of vaccine, they estimated that definite cases of myocarditis occurred at a rate of 1 in 26000 males and 1 in 218000 females in the general Israeli population. The majority of the patients had normal or mildly reduced ejection fraction (EF), but the EF in 4 patients was severely reduced. MRIs were performed on 48 people who had a positive result from a T2-based sequence or a T1-based sequence that indicated myocarditis. They concluded that the myocarditis incidence that occurred after 2 doses of the mRNA vaccine was very low, although the incidence was higher than in unvaccinated people. Myocarditis was mostly discovered in young male recipients after the second dose of the vaccine.
Montgomery et al[13] retrospectively studied a United States Military Health System case series that experienced myocarditis after COVID-19 vaccination (BNT162b2-mRNA and mRNA-1273 vaccines). The authors found that 23 male patients aged 20-51 years were evaluated for acute-onset chest pain following 2810000 mRNA COVID-19 vaccinations and confirmed acute myocarditis. The overall incidence was 0.82 per 100000 doses of vaccination. Myocarditis was reported at 1.88 per 100000 vaccinations after the 1st dose, at 3.49 per 100000 vaccinations after the 2nd dose, and at 4.36 per 100000 vaccinations in males after the 2nd dose. T-wave inversions, ST-segment elevations, and nonspecific ST changes were reported in 83% of patients as abnormal ECG findings. In 17% of the recipients, echocardiography (ECHO) demonstrated reduced LVEFs (40% to 50%). They concluded that the number of cases of myocarditis observed in the Military Health System exceeded some estimates of expected numbers, particularly when considering the subset of the population who received the 2nd dose of the mRNA vaccine. It's time to pay more attention to myocarditis as a potential side effect of the mRNA COVID-19 vaccination.
Perez et al[14] used the Mayo Clinic COVID-19 Vaccine Registry to conduct a retrospective case series study to assess post-immunization myocarditis. They discovered that a total of 7 people were diagnosed with myocarditis after receiving at least one dose of a COVID-19 mRNA vaccine (BNT162b2-mRNA and mRNA-1273 vaccines). The COVID-19 registry contained 175472 individuals (aged 12 years to 106 years), of whom 718 (0.4%) were adolescents aged 12 years to 15 years. Myocarditis was found to be 55.35 per 100000 person-years in the two weeks following a dose of COVID-19 mRNA vaccination. The incidence rate ratio (IRR) of myocarditis after mRNA vaccinations in males was 6.69 (2.35–15.52) which was higher than the Rochester Epidemiology Project during the years 2016–2020. However, the myocarditis IRR in females was 1.41 (0.03–8.45) which was not statistically significant. The most common presenting symptoms were chest pain, dyspnea, and fatigue, with a baseline troponin T of 486.5 ng/L (222.5–965.0). Seventy one percent of the patients had ECG abnormalities, including ST-segment changes. On ECHO examination, 3 patients (60%) were noted to have a reduced LVEF. Cardiac MRI was performed in 6 patients (86%) and showed myocardial delayed enhancement. Pericardial involvement was seen in 3 (50%) patients. Myocarditis is a rare side effect of COVID-19 mRNA vaccines, according to the authors. It affects adult males at a significantly higher rate than the general population.
Das et al[15] performed a study of 25 cases aged between 12 and 18 years who developed myopericarditis following the mRNA vaccine in 7735017 children and adolescents. The incidence of myopericarditis after COVID-19 mRNA vaccination was 0.32 per 100000 people. Myopericarditis has occurred in 0.04 per 100000 people after the 1st dose, 0.28 per 100000 persons following the 2nd dose, and 0.26 per 100000 males following the 2nd dose. The most common presenting symptom was chest pain. Sixty percent and seventy-two percent of patients reported the development of symptoms within 2 and 3 d of getting the second dosage of the vaccine, respectively. All 25 patients had elevated plasma troponin concentrations. Abnormal ECG was found in 84% of the patients, including 60% of the elevation of ST-segment, 24% of the isolation of nonspecific ST-segment changes, 4% of the depression of ST-segment, and 4% of the depression of PR interval. An ECHO was performed in all patients and showed normal LV function in 92% of the individuals. Cardiac MRI was done on 16 of 25 patients (64%) and revealed late gadolinium enhancement in 94% of cases. Myocardial edema on T2 mapping was found in 37.5% of the patients, and a small pericardial effusion was found in 19% of the patients. They concluded that the complication of mRNA vaccine-related myopericarditis has been observed primarily in men. It has not been thoroughly investigated if hormonal or other variables have a role in the manifestation of this disease.
Li et al[16] employed the Vaccination Adverse Event Reporting System, a nationwide early warning system that monitors potential vaccine safety issues, as the major data source. The COVID-19 mRNA vaccines and viral vector vaccinations were the vaccines studied in this study. They found that the incidence rate of myocarditis or pericarditis following COVID-19 vaccination was 0.598 (0.573–0.624) per 100000 doses of vaccinations. The incidence rate was highest in adolescents aged 12-17 years, with an incidence rate of 2.09 (1.90–2.30) per 100000 vaccinations. It decreased with increasing age to 0.59 (0.56–0.62) per 100000 in adults aged 18-64, and 0.19 (0.16–0.22) per 100000 aged 65 years and over. The incidence rate was higher for different vaccine types in the mRNA vaccines, at 0.59 (0.57–0.62) per 100000, than for a viral vector vaccine, at 0.56 (0.44–0.70) per 100000 vaccinations. Among mRNA vaccines, BNT162b2-mRNA vaccines had a higher incidence rate of 0.67 (0.63–0.70) cases per 100000 than the rate of 0.49 (0.46–0.53) cases per 100000 of the mRNA-1273 vaccine. Moreover, the incidence rate after the 2nd dose was twice that of the 1st dose of mRNA vaccinations. Fifty percent of myocarditis or pericarditis cases were reported 2 to 7 d after vaccinations, with 58.67% of cases having chest pain symptoms. They also found that reporting odds ratios (RORs) of the mRNA-1273 vaccine were 2.91 (2.21–3.83) compared with the RORs of the BNT162b2-mRNA vaccine at 5.37 (4.10–7.04). They concluded that the BNT162b2-mRNA vaccine was associated with significant risks of myocarditis or pericarditis in adolescents aged 12 to 17, with RORs ranging from 8.19 (4.37–15.36). A viral vector vaccine may be an alternative for consideration for individuals with myocardial injuries after mRNA vaccination.
Patone et al[17] conducted the study to find out the incidence of myocarditis or pericarditis after COVID-19 vaccinations in the United Kingdom using the English National Immunisation Database of COVID-19 vaccinations. During that period, 38615491 individuals received one dose of a viral vector vaccine ChAdOx1 (n = 20615911), an mRNA vaccine BNT162b2 (n = 16993389), or an mRNA vaccine 1273 (n = 1006191). Results showed an increased myocarditis risk at 1–7 d after the 1st dose of ChAdOx1-viral vector vaccine as an IRR of 1.76 (1.29-2.42), of BNT162b2-mRNA vaccine as an IRR of 1.45 (0.97-2.12), and of mRNA-1273 vaccine as an IRR of 8.38 (3.53-19.91). For the 2nd dose of the BNT162b2-mRNA vaccine, the IRR was 1.75 (1.13-2.70), the IRR of the mRNA-1273 vaccine was 23.10 (6.46-82.56). Over the 1–28 d post-vaccination, they observed an association with the 1st dose of ChAdOx1-viral vector vaccine, whose IRR was 1.29 (1.05-1.58), with BNT162b2-mRNA vaccine, whose IRR was 1.31 (1.03-1.66) and with mRNA-1273 vaccine, whose IRR was 2.97 (1.34-6.58). After a 2nd dose, the mRNA vaccine 1273 had higher risk [IRR = 9.84 (2.69–36.03)] compared with the BNT162b2-mRNA vaccine [IRR = 1.30 (0.98–1.72)]. They observed that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) immunization in adults was linked with a low myocarditis risk after 1 wk of getting the 1st immu
Simone et al[18] performed a study using Kaiser Permanente Southern California (KPSC) as a case study. The KPSC members' database (2392924) was used to evaluate acute myocarditis incidence and clinical outcomes among adults following mRNA vaccinations. In the vaccinated group, the authors discovered 15 cases of confirmed myocarditis (2 after the 1st dose and 13 after the 2nd dose). Over a 10-d observation period, the observed incidence after the 1st dosage was 0.08 cases per 100000 and 0.58 cases per 100000 after the 2nd dose. The IRR for myocarditis was 0.38 (0.05–1.40) after the 1st dose and 2.7 (1.4–4.8) after the 2nd dose. The most common clinical manifestation was chest pain (93%), which occurred between 1 and 5 d after vaccination. They determined that acute myocarditis following COVID-19 immunization was uncommon, with a prevalence of 5.8 occurrences in 1000000 people following the 2nd immunization (1 occurrence in 172414 fully immunized people). They proposed that the higher risk of myocarditis in adolescence males needs more research.
Nygaard et al[19] performed a prospective population-based cohort study to evaluate the incidence of myopericarditis after COVID-19 vaccination in Danish adolescents. They discovered that among 133477 males and 127857 females aged 12–17 years who got the first dose of the BNT162b2-mRNA vaccination, the incidence of myopericarditis was 97 and 16, respectively, per 1000000, corresponding to 1 in 10000 males and 1 in 63000 females. In contrast, 16900 males and 16044 females aged 12–17 years old infected with SARS-CoV-2 showed multisystem inflammatory syndrome in children, including myocarditis at an incidence of 1 in 2800 men and 1 in 5300 women, which was substantially more than the prevalence of myopericarditis following the immunization of the COVID-19 vaccine in both men and women. They determined that the frequency of myopericarditis in male adolescents following BNT162b2-mRNA immunization is greater than previously reported, and that more severe cases are more common.
Husby et al[20] conducted a study using the Danish Vaccination Register to evaluate the incidence of myocarditis or myopericarditis after BNT162b2-mRNA and mRNA-1273 vaccinations in Denmark. Among the cohort participants, 3482295 were vaccinated with BNT162b2-mRNA vaccines, whereas 498814 were immunized with mRNA-1273. Individuals who received the BNT162b2-mRNA vaccine exhibited a non-substantially higher risk of myocarditis in the 28-d following immunization compared to unvaccinated follow-up [hazard ratio (HR) = 1.34 (0.90–2.00)]. Individuals aged 12–39 years had a non-significantly higher rate in the 28-d following vaccination compared non-immunization people [HR = 1.48 (0.74–2.98)]. Individuals vaccinated with mRNA-1273, on the other hand, exhibited a substantially higher risk of myocarditis or myopericarditis compared to unvaccinated follow-up [HR = 3.92 (2.30–6.68)]. Furthermore, people aged 12–39 years had substantially higher rates of myocarditis or myopericarditis when compared to unvaccinated controls [HR = 5.24 (2.47–11.12)]. Myocarditis occurred at a rate of 1.7 (1.3-2.2) per 100000 vaccinated people. Within 28 d, the incidence rate of myocarditis following BNT162b2-mRNA vaccination or mRNA-1273 immunization was 1.4 (1.0–1.8) and 4.2 (2.6–6.4), respectively. Within 28 d of immunization, the equivalent rates of myocarditis from BNT162b2 vaccination was 1.6 (1.0-2.6) per 100000 people compared with mRNA-1273 vaccination was 5.7 (3.3-9.3) per 100000 people. The authors found that mRNA-1273 immunization was linked with a higher risk of myocarditis or myopericarditis compared to uninfected people, whereas BNT162b2-mRNA vaccination was related to a higher rate of myocarditis or myopericarditis among females. Furthermore, clinical outcomes following myocarditis or myopericarditis episodes were mostly modest, lending credence to the general safety of COVID-19 mRNA vaccinations.
Diaz et al[21] conducted a retrospective study to evaluate the incidence of myocarditis and pericarditis after COVID-19 vaccinations among 2000287 individuals who received at least one COVID-19 vaccination (52.6% received the BNT162b2-mRNA vaccine, 44.1% were given the mRNA-1273 vaccine, while 3.1% were given the Ad26.COV2.S vaccine). They found that 20 individuals had vaccine-related myocarditis [1.0 (0.61-1.54)] per 100000 vaccinations, and 37 individuals had pericarditis [1.8 (1.30-2.55)] per 100000 vaccinations, which occurred 3.5 d after being vaccinated. They concluded that mRNA vaccination was linked to myocarditis, particularly in adolescence males, within 3 d following the 2nd dose of immunization. Additionally, pericarditis may be more common than myocarditis among older patients.
Chouchana et al[22] conducted an observational retrospective analysis on inflammatory cardiac events that were reported regarding mRNA immunizations in the global safety database of the World Health Organization using a case–non-case methodology (VigiBase). Among the 26258646 reports in the VigiBase, 716576 were associated with the mRNA COVID-19 vaccination as a suspected medication, with 2277 cases of inflammatory heart responses identified as 1241 (54.5%) myocarditis, 851 (37.3%) pericarditis, and 167 (7.3%) myopericarditis. The majority of myocarditis cases have been observed in male patients aged between 12 and 29. Overall, the median duration from the beginning of myocarditis following immunization was 3 (2–14) d. When compared to myocarditis, a median onset of pericarditis was delayed to be 8 (3–21) d. Most of the myocarditis (81.8%) and pericarditis (57.8%) reported cases that required hospitalization, and 21.5% of myocarditis and 20.5% of pericarditis were life threatening. The overall incidence rate of myocarditis or pericarditis was 0.61 (0.57–0.65) per 100000 fully vaccinated individuals. In 2 doses of immunization, they found a significant increase in incidence rates in 12–17–year–old patients [3.69 (3.25–4.18) per 100000 people] and 18–29–year–old patients [1.97 (1.80–2.16) per 100000 people], while patients older than 30 years were 0.21 (0.19–0.24) per 100000 patients. Male patients had an elevated chance of reporting myocarditis with RORs = 9.4 (8.3–10.6) and diagnosed pericarditis with RORs = 3.7 (3.2–4.2). Age category analysis revealed a significant decrease in myocarditis reporting after mRNA immunizations in 12- to 17-year-olds [RORs = 22.3 (19.2–25.9)] and 18–to–29-year-olds [RORs = 6.6 (5.9–7.5)] individuals when compared to individuals older than 30 years. Across all age groups, this increased reporting was more significant in male patients than in female patients. While there was a considerable disproportionate reporting of myocarditis in teens and young adults, particularly in male patients, and it was an uncommon event, they discovered that it did not appear to compromise the vaccines' favorable benefit-risk balance.
Barda et al[23] evaluated the safety of the BNT162b2-mRNA COVID-19 vaccine using the largest health care organization in Israel. They discovered that the vaccinated and control groups each had an average of 884828 people. Vaccination was the strongest predictor of an increased risk of myocarditis [risk ratio, 3.24 (1.55–12.44)]. By contrast, infection with SARS-CoV-2 was linked to a significantly higher myocarditis rate [risk ratio, 18.28 (3.95–25.12)]. They concluded that the BNT162b2-mRNA vaccine increased the occurrence of a few side effects over a 42-d period of follow-up. Whereas the majority of these incidents were minor, myocarditis has the potential to be life-threatening. According to the findings, SARS-CoV-2 infection is a significant risk factor for myocarditis, as well as a number of other adverse outcomes.
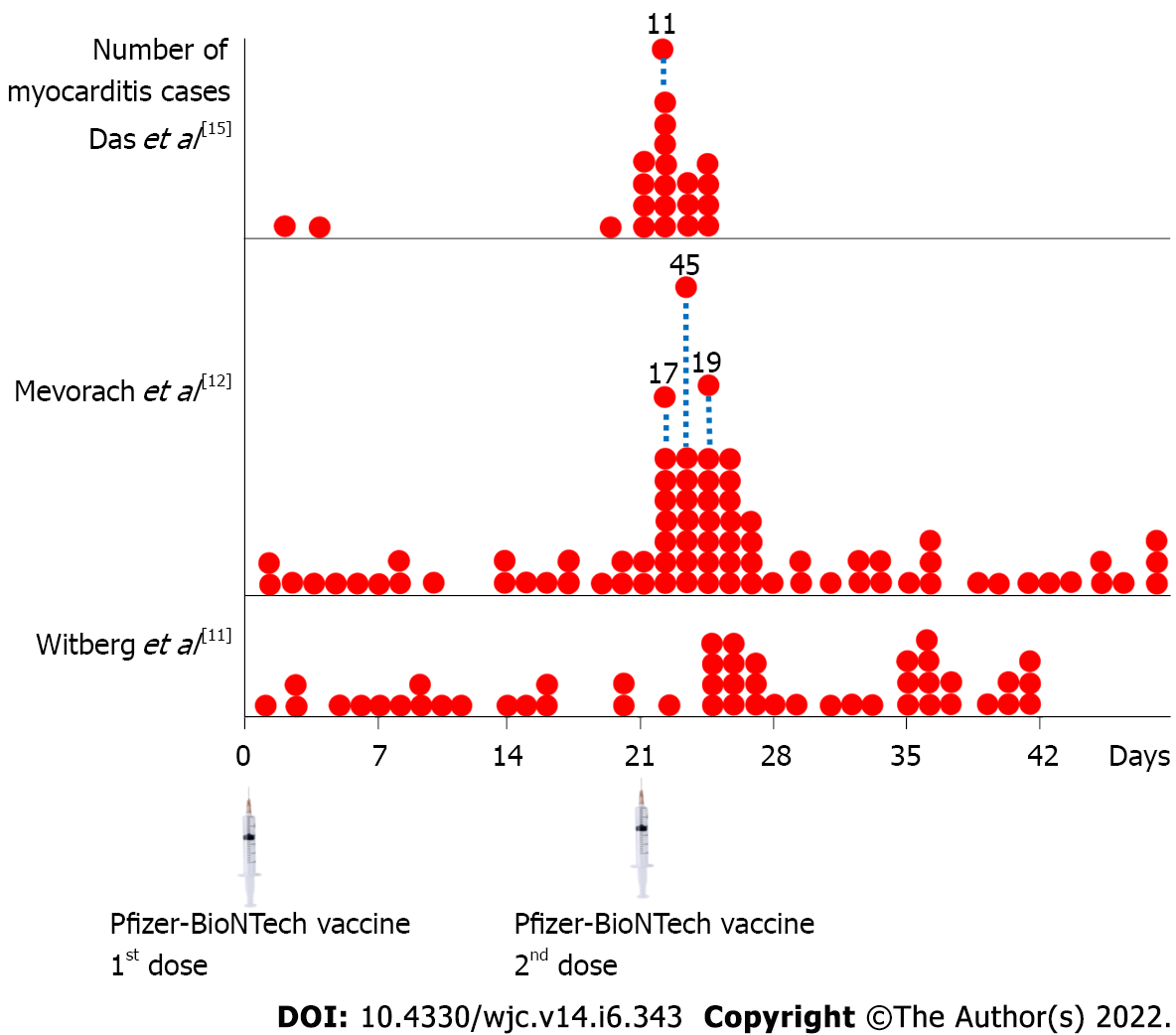
Table 2 summarizes the characteristics of acute myocarditis patients, such as sex, age, occurrence after the 1st or 2nd dose of mRNA COVID-19 vaccination, and clinical severity. Many investigators' data indicated the same result: Acute myocarditis was more prevalent in younger males, and over 90% occurred after the 2nd dose of immunization. Furthermore, practically all patients had mild to moderate clinical presentation and prognosis. Figure 1 shows the number of acute myocarditis cases after the 1st and 2nd doses of the Pfizer-BioNTech mRNA COVID-19 vaccine. According to these data, the majority of the cases occurred shortly after the 2nd dose of immunization, generally within 5 d. Close observation and monitoring of vaccination recipients are critical during this period.
Table 3 shows the clinical presentation of the patients with acute myocarditis after mRNA COVID-19 vaccinations, which usually included chest pain, myalgia, fatigue, and fever. The common ECG abnormalities were ST-elevation, non-specific ST/T changes, PR depression, T-wave inversion, and ventricular fibrillation. ECHO showed the reduction of LVEF. Laboratory findings demonstrated the elevation of cardiac troponin and C-reactive protein. The imaging studies of acute myocarditis patients after mRNA COVID-19 vaccination showed myocardial inflammation, myocardial edema, and delayed iodine enhancement.
| Clinical presentation of acute myocarditis | |
| 1 | Chest pain, Myalgia, Fatigue, Fever |
| 2 | Abnormal ECG: ST-elevation, Non-specific ST/T changes, PR depression, T-wave inversion, Ventricular fibrillation |
| 3 | Elevation of cardiac troponin |
| 4 | Elevation of CRP |
| 5 | Abnormal ECHO: LVEF reduction |
| 6 | Abnormal cardiac MRI: Myocardial inflammation, Myocardial edema |
| 7 | Abnormal cardiac spectral CT: Delayed iodine enhancement |
Despite the fact that the majority of the findings of various studies demonstrated the occurrence of acute myocarditis following mRNA COVID-19 immunization, the incidence was quite low. Furthermore, there were significant disparities across these trials in terms of ethnicity, age, underlying disorders, inherent immunity to the SARS-CoV-2 virus, and other genetic variables. More research should be conducted to understand this problem.
Ameratunga et al[24] reported a 57-year-old woman who died of fulminant necrotizing myocarditis after receiving the first dose of the BNT162b2 mRNA vaccine. The clinical manifestations were lethargy, fatigue, breathlessness, stiff neck, and upper limb pain. After that, she complained of back pain, a sore throat, and hematuria without palpitation or chest pain. The autopsy findings showed that the heart was normal without pericardial effusion and intra-cardiac thrombosis. There was a large thymoma mass (710 g) in the left pleural cavity. The histopathology demonstrated fulminant necrotizing eosinophilic myocarditis. Multifocal aggregates of lymphoid cells, histiocytes, and many eosinophils with isolated myocyte necrosis were seen in the free walls of both ventricles, the interventricular septum, and throughout the conduction system (sinoatrial and atrioventricular nodes) (Table 4). They concluded that the danger of myocarditis and other deadly consequences of COVID-19 infection outweighed the risk of these uncommon vaccine-related side effects. The advantages of immunization substantially outweigh the hazards of COVID-19 infection. Choi et al[25] published the autopsy results of a 22-year-old man who had chest discomfort 5 d after receiving the 1st dose of the BNT162b2-mRNA vaccination and died 7 h later. He developed ventricular fibrillation on ECG before cardiopulmonary resuscitation in the emergency department. The autopsy findings showed the heart weighed 470 g with multiple petechial hemorrhages on its surface.
| Ref. | No. of cases (age, sex)/vaccine | Gross findings | Histopathological findings |
| Ameratunga et al[24] | 1 (57, F)/Pfizer-BioNTech | The heart was normal without pericardial effusion and intra-cardiac thrombosis. There was a large thymoma mass (710 g) in the left pleural cavity | The heart sections showed fulminant necrotizing eosinophilic myocarditis. There were multifocal aggregates of lymphoid cells, histiocytes, and abundant eosinophils with focal myocyte necrosis in the free walls of both ventricles, interventricular septum, and around the conduction system (sino-atrial and atrioventricular nodes) |
| Choi et al[25] | 1 (22, M)/Pfizer-BioNTech | The heart weighed 470 g with multiple petechial hemorrhages on its surface. The pericardium was smooth with no fibrin deposition or exudate. The coronary arteries were patent, and the heart valves were unremarkable | The myocardial sections showed a diffuse inflammatory infiltration with neutrophils and histiocytes predominance. The inflammatory infiltrates dominant in the atria and around the sinoatrial and atrioventricular nodes with no inflammatory cells in the ventricular muscles |
| Schneider et al[26] | 1 (65, M)/Pfizer-BioNTech | The heart showed severe coronary sclerosis, massive cardiac hypertrophy, myocardial infarction scars | The myocardial sections showed myocarditis with lymphocytic and plasmacytoid infiltration of the perivascular space and the myocardium |
There was no fibrin buildup or exudate in the pericardium. The coronary arteries were patent, and the cardiac valves were in good condition. Within the heart, histology revealed a diffuse inflammatory infiltrate with neutrophils and histiocyte predominance. Inflammatory infiltrates were more common in the atria and around the sinoatrial and atrioventricular nodes, but there were few or no inflammatory cells in the ventricular area. They concluded that the major cause of mortality was myocarditis, which was linked to the BNT162b2-mRNA vaccination. Schneider et al[26] conducted an autopsy of 18 deceased people after COVID-19 vaccinations and found that only 1 case was related to the BNT162b2-mRNA vaccine. The autopsy findings showed severe coronary sclerosis, massive cardiac hypertrophy, and myocardial infarction scars. The histopathology showed myocarditis with lymphocytic and plasmacytoid infiltration of the perivascular space and the myocardium.
The mRNA COVID-19 vaccines are made up of the mRNA which was modified with nucleosides that codes for the spike protein of SARS-CoV-2 and is enclosed in nanoparticles of lipid that do not have live virus particles or DNA of virus. When the spike protein of a virus is created on the cell surface during the entry of mRNA-vaccine, it activates an adaptive immune response that detects and kills viruses expressing the spike protein. SARS-CoV-2 attachment to the host cell is blocked by vaccine-induced spike-protein IgG antibodies, which bind to the angiotensin-converting enzyme 2 receptor and destroy the virus[27].
Myocarditis caused by enterovirus or human herpesvirus infection is often severe in young adolescents and adult males. Genetic polymorphisms in genes producing HLA factors and, in a minority of individuals, genetic variants in genes encoding cytoskeletal, desmosomal, or sarcomeric proteins are related to this kind of myocarditis, which raises the chance of developing acute myocarditis following viral infection[28]. Myocarditis and pericarditis have previously been documented following immunization, particularly with smallpox and influenza vaccinations with shallow incidence rates[5]. There are sex-specific hormone alterations in COVID-19 mRNA-vaccination-related myocarditis and non-COVID-19 viral myocarditis[29].
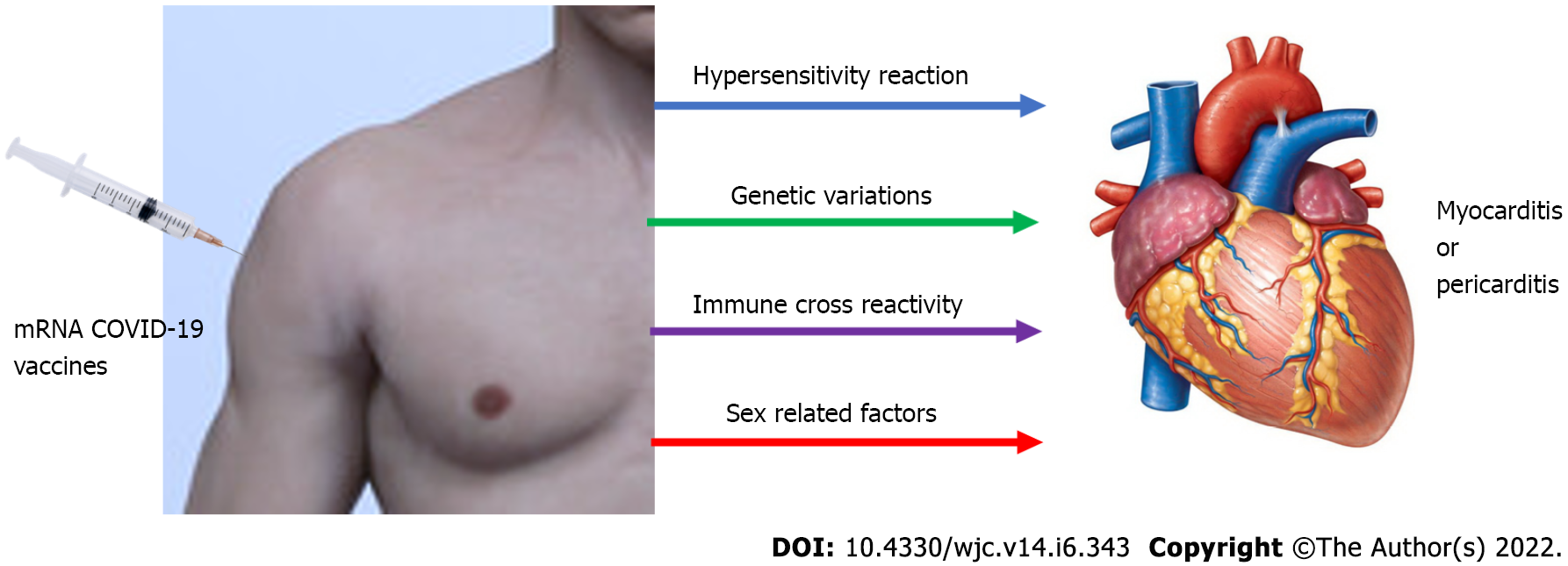
Although potential processes are unclear, various ideas may be possible. mRNA immune reactivity, cross reactivity with cardiac proteins, SARS-CoV-2 spike protein antibodies, and hormonal alterations are the four main mechanisms by which COVID-19 mRNA vaccines might induce hyper-immunity. Immune–genetic background, age, and sex can all impact these processes. The immune system may detect the vaccine's mRNA as an antigen, triggering immunological pathways and pro-inflammatory cascades in the heart. Despite the fact that nucleotide changes to mRNA diminish its innate immunogenicity, the mRNA immune response could induce an abnormal innate and acquired immunity, explaining why mRNA vaccinations induce a stronger immune response than other COVID-19 immunization approaches[30]. Another proposed mechanism is molecular mimicry between the SARS-CoV-2 spike protein and cardiac self-antigen presentation. Antibodies against SARS-CoV-2 spike proteins may cross-react with myocardial-myosin heavy chain. These autoantibodies might be inadvertent bystanders that cause myocardial inflammation and damage (Figure 2).
Moreover, they could represent a specific immune–genetic background that predisposes to hyper-immunity and myocarditis in response to any stimulus[31,32]. Furthermore, while COVID-19 mRNA-vaccination-related myocarditis is more common in men, changes in hormone signaling may play a role in its etiology. Testosterone has the capacity to reduce anti-inflammatory immune cells while increasing the aggressiveness of T helper cell-type immune responses. On the other hand, estrogen suppresses pro-inflammatory T cells, leading to a reduction in cell-mediated immune response. As a result, testosterone may still promote an abnormal acquired and innate immune response, which may explain why mRNA immunizations produce a larger immunological response than other methods of COVID-19 immunization in males than females[33-36].
Post-mRNA vaccination myocarditis is most common in young boys under 21 years old. The majority of instances appear within a few days of receiving the 2nd immunization dose. The most common symptom is chest pain, followed by fever or myalgia. The majority of patients have an abnormal ECG. Elevated cardiac troponin and inflammatory markers are mainly laboratory abnormalities. On cardiac MRI, most individuals have evidence of myocardial edema, or inflammation of the cardiac muscles. Possible causes of myocarditis or pericarditis following mRNA COVID-19 vaccines include antibodies to SARS-CoV-2 spike proteins, immune response to mRNA, cross reaction with cardiac proteins, and alteration in hormones. A more rigorous investigation was required to resolve this issue. Despite a few incidences of self-limited myocarditis, the risk-benefit evaluation for COVID-19 vaccination shows a positive balance for all age and gender categories; hence, COVID-19 vaccination is now recommended for all people aged 12 and above.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shamseldeen AA, Egypt; Watanabe A, Japan A-Editor: Yao QG S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636-2648, 2648a. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 2267] [Article Influence: 188.9] [Reference Citation Analysis (0)] |
| 2. | Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379:738-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 551] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 3. | Lynge TH, Nielsen TS, Gregers Winkel B, Tfelt-Hansen J, Banner J. Sudden cardiac death caused by myocarditis in persons aged 1-49 years: a nationwide study of 14 294 deaths in Denmark. Forensic Sci Res. 2019;4:247-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 4. | Maron BJ, Haas TS, Ahluwalia A, Murphy CJ, Garberich RF. Demographics and Epidemiology of Sudden Deaths in Young Competitive Athletes: From the United States National Registry. Am J Med. 2016;129:1170-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 200] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 5. | Su JR, McNeil MM, Welsh KJ, Marquez PL, Ng C, Yan M, Cano MV. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990-2018. Vaccine. 2021;39:839-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 117] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 6. | Morgan J, Roper MH, Sperling L, Schieber RA, Heffelfinger JD, Casey CG, Miller JW, Santibanez S, Herwaldt B, Hightower P, Moro PL, Hibbs BF, Levine NH, Chapman LE, Iskander J, Lane JM, Wharton M, Mootrey GT, Swerdlow DL. Myocarditis, pericarditis, and dilated cardiomyopathy after smallpox vaccination among civilians in the United States, January-October 2003. Clin Infect Dis. 2008;46 Suppl 3:S242-S250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Kuntz J, Crane B, Weinmann S, Naleway AL; Vaccine Safety Datalink Investigator Team. Myocarditis and pericarditis are rare following live viral vaccinations in adults. Vaccine. 2018;36:1524-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME, Broder KR, Gee J, Weintraub E, Shimabukuro T, Scobie HM, Moulia D, Markowitz LE, Wharton M, McNally VV, Romero JR, Talbot HK, Lee GM, Daley MF, Oliver SE. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices - United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977-982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 389] [Cited by in RCA: 400] [Article Influence: 100.0] [Reference Citation Analysis (0)] |
| 9. | Oster M. mRNA COVID-19 Vaccine-Associated Myocarditis. [cited 10 January 2022]. Available from: https://www.fda.gov/media/153514/download. |
| 10. | EMA. Comirnaty and Spikevax: possible link to very rare cases of myocarditis and pericarditis. [cited 10 January 2022]. Available from: https://www.ema.europa.eu/en/news/comirnaty-spikevax-possible-link-very-rare-cases-myocarditis-pericarditis. |
| 11. | Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, Grinberg T, Auster O, Dagan N, Balicer RD, Kornowski R. Myocarditis after Covid-19 Vaccination in a Large Health Care Organization. N Engl J Med. 2021;385:2132-2139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 352] [Cited by in RCA: 444] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 12. | Mevorach D, Anis E, Cedar N, Bromberg M, Haas EJ, Nadir E, Olsha-Castell S, Arad D, Hasin T, Levi N, Asleh R, Amir O, Meir K, Cohen D, Dichtiar R, Novick D, Hershkovitz Y, Dagan R, Leitersdorf I, Ben-Ami R, Miskin I, Saliba W, Muhsen K, Levi Y, Green MS, Keinan-Boker L, Alroy-Preis S. Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel. N Engl J Med. 2021;385:2140-2149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 396] [Cited by in RCA: 426] [Article Influence: 106.5] [Reference Citation Analysis (0)] |
| 13. | Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, Loran D, Hrncir D, Herring K, Platzer M, Adams N, Sanou A, Cooper LT Jr. Myocarditis Following Immunization With mRNA COVID-19 Vaccines in Members of the US Military. JAMA Cardiol. 2021;6:1202-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 397] [Article Influence: 99.3] [Reference Citation Analysis (0)] |
| 14. | Perez Y, Levy ER, Joshi AY, Virk A, Rodriguez-Porcel M, Johnson M, Roellinger D, Vanichkachorn G, Huskins WC, Swift MD. Myocarditis Following COVID-19 mRNA Vaccine: A Case Series and Incidence Rate Determination. Clin Infect Dis. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 15. | Das BB, Kohli U, Ramachandran P, Nguyen HH, Greil G, Hussain T, Tandon A, Kane C, Avula S, Duru C, Hede S, Sharma K, Chowdhury D, Patel S, Mercer C, Chaudhuri NR, Patel B, Ang JY, Asmar B, Sanchez J, Khan D. Myopericarditis after messenger RNA Coronavirus Disease 2019 Vaccination in Adolescents 12 to 18 Years of Age. J Pediatr. 2021;238:26-32.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 16. | Li M, Yuan J, Lv G, Brown J, Jiang X, Lu ZK. Myocarditis and Pericarditis following COVID-19 Vaccination: Inequalities in Age and Vaccine Types. J Pers Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Patone M, Mei XW, Handunnetthi L, Dixon S, Zaccardi F, Shankar-Hari M, Watkinson P, Khunti K, Harnden A, Coupland CAC, Channon KM, Mills NL, Sheikh A, Hippisley-Cox J. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28:410-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 409] [Cited by in RCA: 404] [Article Influence: 134.7] [Reference Citation Analysis (0)] |
| 18. | Simone A, Herald J, Chen A, Gulati N, Shen AY, Lewin B, Lee MS. Acute Myocarditis Following COVID-19 mRNA Vaccination in Adults Aged 18 Years or Older. JAMA Intern Med. 2021;181:1668-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 19. | Nygaard U, Holm M, Bohnstedt C, Chai Q, Schmidt LS, Hartling UB, Petersen JJH, Thaarup J, Bjerre J, Vejlstrup NG, Juul K, Stensballe LG. Population-based Incidence of Myopericarditis After COVID-19 Vaccination in Danish Adolescents. Pediatr Infect Dis J. 2022;41:e25-e28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 20. | Husby A, Hansen JV, Fosbøl E, Thiesson EM, Madsen M, Thomsen RW, Sørensen HT, Andersen M, Wohlfahrt J, Gislason G, Torp-Pedersen C, Køber L, Hviid A. SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ. 2021;375:e068665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 159] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 21. | Diaz GA, Parsons GT, Gering SK, Meier AR, Hutchinson IV, Robicsek A. Myocarditis and Pericarditis After Vaccination for COVID-19. JAMA. 2021;326:1210-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 291] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 22. | Chouchana L, Blet A, Al-Khalaf M, Kafil TS, Nair G, Robblee J, Drici MD, Valnet-Rabier MB, Micallef J, Salvo F, Treluyer JM, Liu PP. Features of Inflammatory Heart Reactions Following mRNA COVID-19 Vaccination at a Global Level. Clin Pharmacol Ther. 2022;111:605-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R, Hernán MA, Lipsitch M, Kohane I, Netzer D, Reis BY, Balicer RD. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N Engl J Med. 2021;385:1078-1090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 669] [Cited by in RCA: 721] [Article Influence: 180.3] [Reference Citation Analysis (0)] |
| 24. | Ameratunga R, Woon ST, Sheppard MN, Garland J, Ondruschka B, Wong CX, Stewart RAH, Tatley M, Stables SR, Tse RD. First Identified Case of Fatal Fulminant Necrotizing Eosinophilic Myocarditis Following the Initial Dose of the Pfizer-BioNTech mRNA COVID-19 Vaccine (BNT162b2, Comirnaty): an Extremely Rare Idiosyncratic Hypersensitivity Reaction. J Clin Immunol. 2022;42:441-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 25. | Choi S, Lee S, Seo JW, Kim MJ, Jeon YH, Park JH, Lee JK, Yeo NS. Myocarditis-induced Sudden Death after BNT162b2 mRNA COVID-19 Vaccination in Korea: Case Report Focusing on Histopathological Findings. J Korean Med Sci. 2021;36:e286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 26. | Schneider J, Sottmann L, Greinacher A, Hagen M, Kasper HU, Kuhnen C, Schlepper S, Schmidt S, Schulz R, Thiele T, Thomas C, Schmeling A. Postmortem investigation of fatalities following vaccination with COVID-19 vaccines. Int J Legal Med. 2021;135:2335-2345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 27. | Vitiello A, Ferrara F. Brief review of the mRNA vaccines COVID-19. Inflammopharmacology. 2021;29:645-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 28. | Heymans S, Eriksson U, Lehtonen J, Cooper LT Jr. The Quest for New Approaches in Myocarditis and Inflammatory Cardiomyopathy. J Am Coll Cardiol. 2016;68:2348-2364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 249] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 29. | Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 424] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 30. | Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19:75-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 180] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 31. | Siripanthong B, Nazarian S, Muser D, Deo R, Santangeli P, Khanji MY, Cooper LT Jr, Chahal CAA. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17:1463-1471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 527] [Cited by in RCA: 503] [Article Influence: 100.6] [Reference Citation Analysis (0)] |
| 32. | Liu PP, Blet A, Smyth D, Li H. The Science Underlying COVID-19: Implications for the Cardiovascular System. Circulation. 2020;142:68-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 587] [Article Influence: 117.4] [Reference Citation Analysis (0)] |
| 33. | Fischinger S, Boudreau CM, Butler AL, Streeck H, Alter G. Sex differences in vaccine-induced humoral immunity. Semin Immunopathol. 2019;41:239-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 278] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 34. | Bozkurt B, Kamat I, Hotez PJ. Myocarditis With COVID-19 mRNA Vaccines. Circulation. 2021;144:471-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 556] [Cited by in RCA: 621] [Article Influence: 155.3] [Reference Citation Analysis (0)] |
| 35. | Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021;6:1078-1094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 931] [Cited by in RCA: 1867] [Article Influence: 466.8] [Reference Citation Analysis (0)] |
| 36. | Milane L, Amiji M. Clinical approval of nanotechnology-based SARS-CoV-2 mRNA vaccines: impact on translational nanomedicine. Drug Deliv Transl Res. 2021;11:1309-1315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |