Published online May 26, 2022. doi: 10.4330/wjc.v14.i5.297
Peer-review started: October 17, 2021
First decision: January 25, 2022
Revised: February 6, 2022
Accepted: April 24, 2022
Article in press: April 24, 2022
Published online: May 26, 2022
Processing time: 212 Days and 16.7 Hours
The use of pre-closure suture-based devices represents a widely access-site hemostasis technique in percutaneous transfemoral transcatheter-aortic-valve-replacement (TF-TAVR); yet this technique is associated with the risk of a device failure that may result in clinically relevant residual bleeding. Thus, a bailout intervention is needed. So far, the best management of pre-closure device failure has not been recognized.
To report the first clinical results obtained using a novel bailout hemostasis technique for patients with double suture-based vascular closure device failure in the setting of TF-TAVR.
We developed a “pledget-assisted hemostasis” technique to manage residual access-site bleeding. This consists of the insertion of a surgical, non-absorbable, polytetrafluoroethylene pledget over the sutures of the two ProGlide (Abbott Vascular, CA, United States). The ProGlide’s knot-pushers are used to push down the pledget and the hand-made slipknot to seal the femoral artery leak. This technique was used as a bailout strategy in patients undergoing TF-TAVR with a systematic double pre-closure technique. Post-procedural access-site angiography was systematically performed. In-hospital complications were systematically detected and classified according to Valve Academic Research Consortium-2 criteria.
Out of 136 consecutive patients who underwent TF-TAVR, 15 patients (mean age 80.0 ± 7.2 years, 66.7% female) with access-site bleeding after double pre-closure technique failure were treated by pledget-assisted hemostasis. In the majority of patients, 16F sheath was used (n = 12; 80%). In 2 cases (13%), a peripheral balloon was also inflated in the iliac artery to limit blood loss during pledget preparation. Angiography-confirmed hemostasis (primary efficacy endpoint) was achieved in all patients. After the procedure, 1 patient required blood transfusion (2 units), and no other bleeding or major ischemic complication was noticed.
The “pledget assisted hemostasis” might be considered as a possible bailout technique to treat patients with residual access site bleeding. Further studies are needed to compare this approach with other bail-out techniques.
Core Tip: This is a retrospective pilot study to report the first clinical results obtained using a novel bailout hemostasis technique for patients with double suture-based vascular closure device failure in the setting of trans-femoral transcatheter-aortic-valve-replacement. The “pledget-assisted hemostasis” technique consists of the insertion of a surgical, non-absorbable, polytetrafluoroethylene pledget over the sutures of two ProGlide (Abbott Vascular, CA, United States). The ProGlide’s knot-pushers are used to push down the pledget and the hand-made slipknot to seal the femoral artery leak. This technique was used as a bailout strategy in patients undergoing trans-femoral transcatheter aortic valve replacement with systematic double preclosure technique.
- Citation: Burzotta F, Aurigemma C, Kovacevic M, Romagnoli E, Cangemi S, Bianchini F, Nesta M, Bruno P, Trani C. Pledget-assisted hemostasis to fix residual access-site bleedings after double pre-closure technique. World J Cardiol 2022; 14(5): 297-306
- URL: https://www.wjgnet.com/1949-8462/full/v14/i5/297.htm
- DOI: https://dx.doi.org/10.4330/wjc.v14.i5.297
Despite increased operator experience and device improvement, access site complications still pose a significant concern regarding procedural safety of trans-femoral transcatheter aortic valve replacement (TF-TAVR)[1]. Accordingly, strategies to minimize the occurrence and the clinical sequelae of access-site complications are continuously investigated.
When practicing percutaneous TF-TAVR, in addition to proper access site selection and precise puncture of common femoral artery (CFA)[2], the use of vascular closure devices (VCD) is actually widely adopted. Within different VCD-based technical options, pre-implantation of suture-based closure devices has gained popularity. However, vascular complications are not abolished, and residual access site bleeding is often detected (in up to one-third of patients)[3-5] due to significant blood leakage at the level of arterial entry site. Thus, as a part of TF-TAVR procedures, strategies to bailout manage VCD failures are applied daily according to various local expertise. The best technique to manage residual bleeding after suture-based VCD failure has not yet been recognized. Thus, we herein report the description and the results obtained in the early clinical practice of a novel “pledget-assisted hemostasis” technique.
According to our local practice, TF-TAVR is systematically performed under conscious sedation according to the previously described “less-invasive totally-endovascular” (LITE) technique[6]. Briefly, the LITE technique combines a series of technical solutions aimed to minimize vascular complications and includes radial approach as the “secondary access” (to guide valve positioning, to check femoral-access hemostasis, and to manage eventual access-site complications) and precise CFA puncture using angiographic-guidewire-ultrasound guidance[7]. Femoral hemostasis was systematically attempted using a double pre-closure technique with two suture devices (ProGlide, Abbott Vascular, CA, United States). After prosthesis implantation and TAVR sheath removal, hemostasis with parallel double ProGlide sutures was done[8].
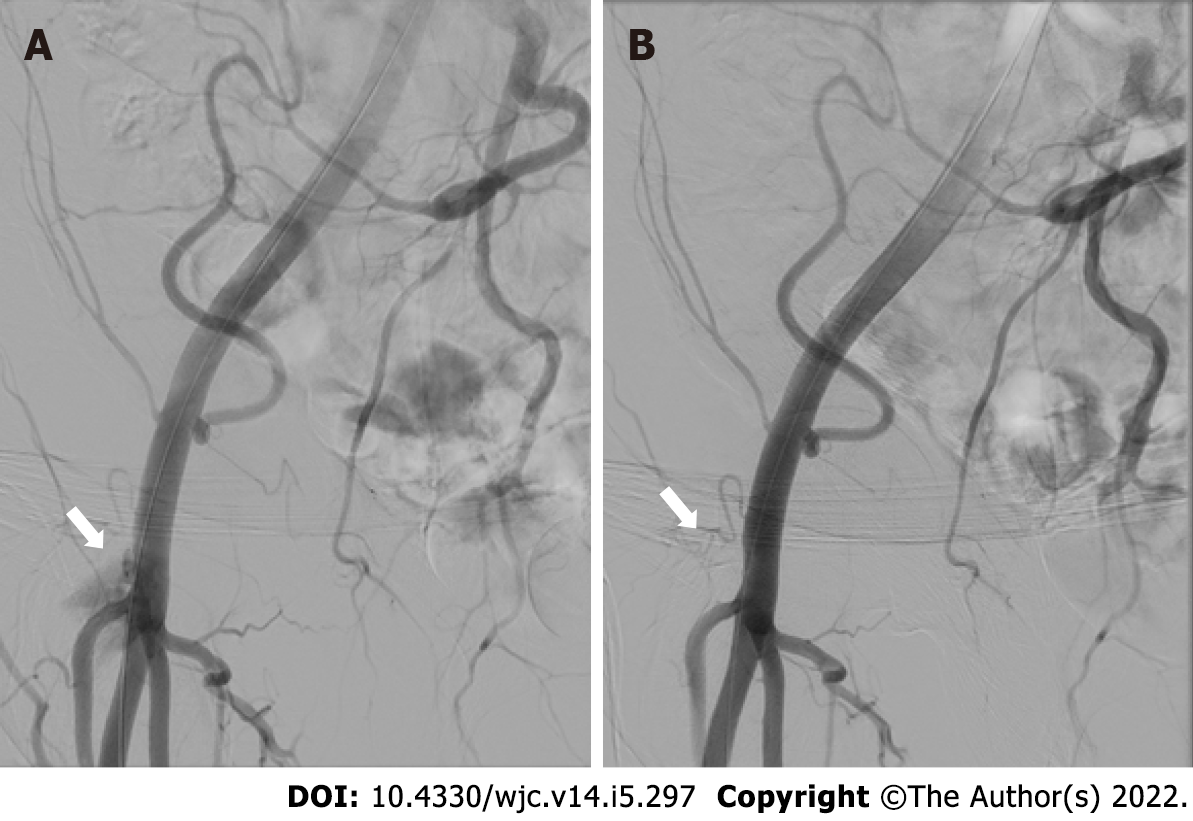
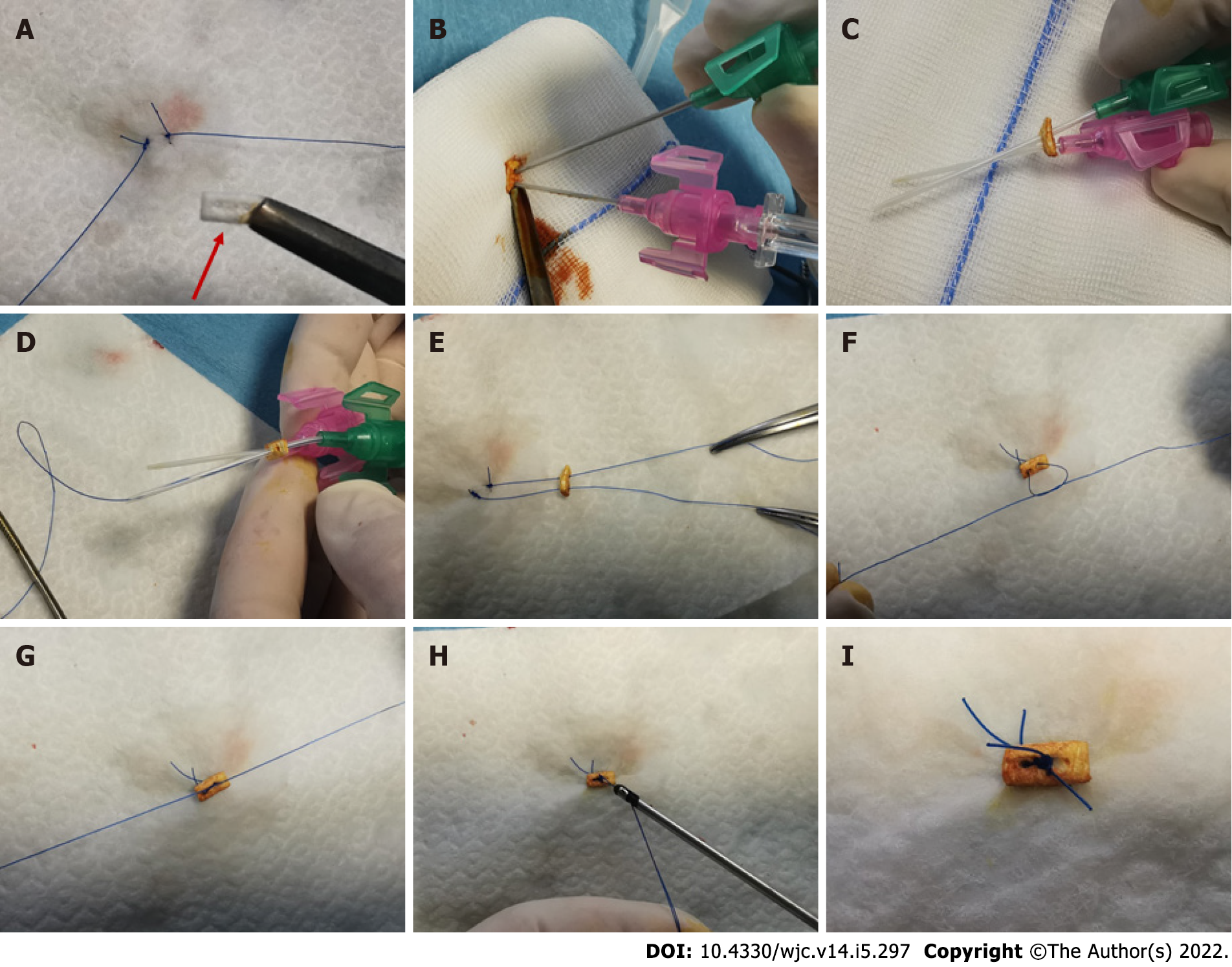
At this stage, before the suture threads of the ProGlide device were cut down, hemostasis was checked by selective iliac-femoral angiography performed by radial access with a multipurpose guiding catheter[6]. Digital subtraction angiography of the iliac-femoral arteries allowed to assess vascular integrity or to diagnose the occurrence of vascular damages or bleeding complications. At this stage, when significant residual bleeding at the site of femoral entry was recognized, a new “pledget-assisted hemostasis” technique was applied (Figure 1A). It consists of the application of a surgical non-absorbable polytetrafluoroethylene 6.5 mm x 4 mm x 1.5 mm pledget over the two ProGlide sutures (one of each device). The steps practiced to mount the pledget over the suture threads are depicted in Figure 2. Then, the pledget was pushed down over the two sutures using the ProGlide knot-pusher, and tied with a hand-made sliding knot to achieve a stable approximation to the surface of the vessel wall.
After pledget application, selective iliac-femoral digital subtraction angiography was repeated to check for hemostasis achievement (Figure 1B).
When massive bleeding was noticed at the first angiographic check such that manual compression was considered insufficient, a peripheral balloon was advanced and inflated in the iliac-femoral artery by radial route to prevent significant blood loss during pledget-assisted hemostasis performance.
According to the standard practice of our center, all patients were referred for TAVR based on formal, multidisciplinary, heart-team discussion. For each patient, the peripheral computed tomography was revised by at least two operators to assess the feasibility of TF approach. Clinical data and procedure details were prospectively entered into a dedicated database that allowed to assess previously the impact of EuroSCORE on coronary interventions[9] and the safety of transradial procedures[10]. At the time of heart-team consultation, patients’ surgical risk was graded according to the Society of Thoracic Surgeons (STS) predicted operative mortality[11]. TAVR risk was graded according to the STS-American College of Cardiology Transcatheter Valve Therapy (STS-ACC TVT)[12] using the online TAVR in-hospital mortality risk calculator (https://tools.acc.org/tavrrisk/#!/content/evaluate/).
The antiplatelet/anticoagulant regimen was individualized according to the patient’s characteristics, and no standardized protocol was available. As a general approach, most of the patients received double antiplatelet therapy, while the patients with the need for anticoagulation were kept on anticoagulant therapy plus 1 mo of single antiplatelet therapy. All procedures were performed under systemic anticoagulation with unfractionated heparin (70 UI/kg, reversed with protamine sulfate at the end of the procedure, before hemostasis).
In-hospital clinical outcomes were prospectively recorded, since the continuous monitoring of in-hospital clinical outcomes for TAVR is part of the Institutional clinical pathway dedicated to patients with heart valve diseases (http://www.policlinicogemelli.it/Policlinico_Gemelli.aspx?p=21C1F922-73FF-4B2F-A2FF-022DE91A6586) according to the European recommendations[13]. Bleeding or vascular complications were defined according to Valve Academic Research Consortium-2 (VARC-2) criteria[14].
Out of consecutive patients who underwent TF-TAVR from October 2019 to September 2020, we selected all patients with residual access site bleeding who underwent pledget-assisted hemostasis attempt after the failure of double pre-closure technique with ProGlide suture. These patients constituted the study population of the present pilot study[15].
The primary efficacy end-point was the achievement of angiographically-confirmed hemostasis in the operative room without the need for further bail-out interventions (surgery or endovascular).
The primary safety end-point was the occurrence of life-threatening bleedings, major bleedings, or major vascular complications as defined according to VARC-2 classification[14].
During the study period, 136 patients underwent TF-TAVR. The TAVR systems used included Medtronic Evolute R (n = 40, 29%), Medtronic Evolut Pro (n = 81, 60%), Edwards Sapien3 (n = 10, 7.3%), and Abbott Portico (n = 5, 3.7 %).
A total of 15 patients (mean age 80.0 ± 7.2 years, 66.7% female) with residual access site bleeding after double pre-closure in TF-TAVR were prospectively included in the pilot study. The main characteristics of the study population are summarized in Table 1. The average STS mortality score was 3.7 ± 2.5, and TAVR score was 2.69 ± 0.7. In the majority of patients, 16F sheath was used (n = 12; 80%), while 14F sheath was used in 2 patients (6.7%) and 18F in 1 patient (6.7%). Direct valve implantation was done only in 1 patient. In 6 (40.0%) patients, valve post-dilatation was done. Balloon inflation in the iliac artery was performed in 2 cases (13.3%) to limit blood loss during pledget preparation and in 2 cases (13.3%) to treat an intimal flap (Table 2).
| Patients | |
| Patient number | 15 |
| Age, yr (mean ± SD) | 80.0 ± 7.2 |
| Female gender | 10 (66.7%) |
| BMI, kg/m2 (mean ± SD) | 27.41 ± 3.6 |
| Risk factors | |
| Diabetes | 2 (13.3%) |
| Hypertension | 13 (86.7%) |
| Dyslipidemia | 6 (40.0%) |
| Smoking | 0 |
| Medical history/comorbidities | |
| Chronic kidney disease (not on dialysis) | 3 (20.0%) |
| Chronic dialysis | 0 |
| Peripheral artery disease | 2 (13.3%) |
| Atrial Fibrillation | 8 (53.3%) |
| Previous stroke | 2 (13.3%) |
| Chronic pulmonary disease | 2 (13.3%) |
| Previous myocardial infarction | 2 (13.3%) |
| Previous PCI | 4 (26.7%) |
| Previous CABG | 1 (6.7%) |
| STS mortality | 3.7 ± 2.5 |
| TAVR score | 2.69 ± 0.7 |
| Anticoagulant and antiplatelet therapy | |
| Anticoagulants | 7 (46.6%) |
| Dual antiplatelet therapy | 6 (40%) |
| Clopidogrel | 11 (73.3%) |
| Acetyl salicylic acid | 8 (53.3%) |
| Adverse events | n (%) | Adverse event description and management |
| Bleeding complications | ||
| Life-threatening bleeding (bleeding in a critical organ or causing hypovolemic shock or severe hypotension requiring vasopressors or surgery or overt source of bleeding with drop in hemoglobin ≥ 5 g/dL or transfusion ≥ 4 units) | 0 | |
| Major bleeding (bleeding either associated with a drop in the hemoglobin level of at least 3.0 g/dL or requiring transfusion of 2-3 units, or causing hospitalization or permanent injury, or requiring surgery but does not meet criteria of life-threatening or disabling bleeding) | 1 (6.7%) | 1 patient requiring post-operative blood transfusion (2 units) without further bleeding source |
| Minor bleeding (any bleeding worthy of clinical mention that does not qualify as life-threatening, disabling, or major) | 0 | |
| Vascular complications | ||
| Major vascular complications | 0 | |
| Minor vascular complications | 2 (13.3%) | |
| Access site or access-related vascular injury (not leading to death, life-threatening or major bleeding, visceral ischemia, or neurological impairment) | 2 (13.3%) | Two femoral artery non-occlusive dissections successfully treated by balloon angioplasty during the index procedure |
| Distal embolization | 0 | |
| Any unplanned vascular intervention (endovascular stenting or unplanned surgical intervention not meeting the criteria for a major vascular complication) | ||
| Need for vascular repair (via surgery, ultrasound-guided compression, transcatheter embolization, or stent-graft) | 0 | |
| Primary safety end-point (life-threatening bleedings or major bleedings or major vascular complications) | 1 (6.7%) |
Angiographically-confirmed hemostasis was achieved in all patients (100%).
After TAVR, 1 patient required blood transfusion (2 units) (Table 2), and no other bleeding or vascular complication were noticed (Table 2). All patients were discharged after 7 ± 5 d.
The complete percutaneous approach in TF-TAVR represents a less invasive technique to treat patients with aortic valve stenosis. Suture-based VCD use according to pre-closure technique is actually widely adopted to achieve arterial haemostasis but is associated with the possibility of residual blood leakage. Thus, as a part of TF-TAVR procedures, strategies to bailout manage VCD failures are daily applied according to various local expertise.
In the present study: (1) We describe a novel technique (based on “pledget” use) to manage double suture-based device failure; and (2) We report the efficacy and safety observed in a pilot clinical observational study.
According to VARC-2 position paper, “access-related” complications are defined as any adverse clinical event possibly associated with any of the access sites used during the procedure[14]. Across the literature, wide variations regarding the occurrence of vascular complications and their impact on clinical outcomes exist[16-18]. Different sizes of the valve delivery systems used over time, evolving closure techniques, and operator experience might play a major role. In such context, the occurrence of VCD failure might determine different clinical consequences ranging from life-threatening bleedings to the absence of any significant blood loss. According to recent studies[16-18], up to 70% of VARC-2 major vascular complications were related to VCD failure. Puncture site optimization and VCD selection might modulate VCD failure occurrence. Regarding entry-site optimization, the “perfect puncture” of CFA within spots free from calcium and above the bifurcation may be pivotal in reducing complications. To select a proper puncture site, either ultrasound guidance[19] or angio-guidewire-ultrasound technique[7] might be considered. Furthermore, different vascular closure devices (VCD) are available to diminish bleeding complications and to make TF-TAVR totally percutaneous. Percutaneous haemostasis of the large-bore devices used during TAVR, requires the “preclosure” technique, which is based on the deployment of the sutures before the introduction of the large sheaths. Then, after the valve implantation at the end of the procedure, sutures are tied by pushing down the knots after introducer removal.
Regarding VCD selection, several devices entered the clinical practice and include suture-based closure devices such as 6F ProGlide, 10 F Prostar XL (both Abbott Vascular Inc, Santa Clara, CA, United States), and plug-based 14 F or 18 F MANTA (Essential Medical Inc., Malvern, PA, United States). Among suture-based closure devices, Prostar XL is associated with a higher rate of major bleeding compared to Proglide, as demonstrated in previous studies[20-24] and meta-analysis[25]. The novel collagen-based MANTA (14 and 18F) appears to be an effective and safe device for large-bore access closure, reporting only 4% of major and 5.6% of minor access site complications in the prospective MARVEL registry[5]. Initial data comparing MANTA with Proglide did not show clear advantages for MANTA device in the terms of access site bleeding complications[26-29]. Thus, the preclosure technique with ProGlide is still popular, and prompt hemostasis failure recognition and effective bailout management strategies might be pivotal to limit the clinical impact of VCD failure. Depending on the characteristics of the access site complications, different methods and materials for bailout endovascular interventions are proposed[2], mainly to avoid the risk of urgent vascular surgery. One possible solution is the crossover balloon occlusion technique (CBOT), which has been associated with a lower risk of VARC-2 major vascular bleeding complications[30]. Of note, CBOT might be effectively performed not only from the contralateral femoral artery, but it can be done ipsilaterally by superficial femoral artery access[31] or remotely by radial access[6].
When a failure of VCD is recognized and wire is still left through the arteriotomy, either a third ProGlide device or an Angio-Seal (Abbott Vascular, Redwood City, CA, United States) can be utilized with great effectiveness and safety[32,33]. Yet, if a wire is no longer available, only prolonged manual compression or endovascular techniques through other arterial accesses can be practiced. Thus, we introduced the novel option of using the Proglide’s sutures to deliver a surgical pledget in order to seal residual leak. According to our experience, this “pledget-assisted hemostasis” was highly effective, allowing early achievement of complete hemostasis. This translated into the smooth clinical post-procedural course in all but 1 patient (who received blood transfusion in the absence of further blood loss source documentation).
The present paper should be regarded just as a pilot study for a novel technique practiced by experienced interventional cardiologists in a limited number of procedures. Important limitations are evident (beyond the sample size) in this study.
First, the long-term safety of this technique has still to be ascertained, since specific complications (like local infections) might theoretically be triggered by the use of additional devices and we limited our follow-up to the in-hospital period.
Second, the study lacked a comparative arm. Thus, it is not possible to speculate regarding the possible benefit as compared with other bailout management strategies.
We have proposed a novel strategy to guarantee post TF-TAVR access site hemostasis using the Proglide sutures to deliver a surgical pledget in order to seal residual leak. The “pledget assisted hemostasis” might be considered as a possible bailout technique to treat patients with residual access site bleeding. Further studies are needed to compare this approach with other bail-out techniques.
The most common technique used for hemostasis in transfemoral transcatheter aortic valve replacement (TF-TAVR) is the use of pre-closure devices. Despite favorable results in terms of successful hemostasis, sometimes it can be followed by device failure and residual bleeding.
Although there are different possibilities to manage residual bleeding after hemostasis device failure, such as bailout additional closure device use, balloon-assisted hemostasis, or surgery, the best management is still unclear.
To describe and report the results of an original technique for managing residual access site bleeding after vascular closure devices failure.
The authors developed a novel technique to resolve residual access-site bleeding named “pledget assisted hemostasis”. If residual bleeding was noticed, “pledget assisted hemostasis” with surgical non-absorbable polytetrafluoroethylene 6.5 mm x 4 mm x 1.5 mm pledget was done on the top of double pre-closure device. Proper hemostasis without residual bleeding was confirmed with control angiography.
A total of 15 consecutive patients (mean age 80.0 ± 7.2 years, 66.7% female) with residual access site bleeding after double pre-closure in TF-TAVR were prospectively included in this pilot study. In the majority of patients 16F sheath was used (n = 12; 80%), 14F sheath was used in 2 patients (6.7%), and 18F in 1 patient (6.7%). Hemostasis with the pledget technique was achieved in all patients (100%) immediately after implantation. Major bleeding defined by Valve Academic Research Consortium-2 definition did not occur. No access site infection was observed in the follow-up period.
“Pledget assisted hemostasis” after pre-closure vascular device failure might be considered as a possible bailout technique to treat patients with residual access site bleeding. Further studies are needed to compare this approach with other bail-out techniques.
“Pledget assisted hemostasis” might be considered as a possible bailout technique for vascular closure device failure.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jani K, India; Mzhavanadze ND, Russia; Nashawi M, United States S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Piccolo R, Pilgrim T, Franzone A, Valgimigli M, Haynes A, Asami M, Lanz J, Räber L, Praz F, Langhammer B, Roost E, Windecker S, Stortecky S. Frequency, Timing, and Impact of Access-Site and Non-Access-Site Bleeding on Mortality Among Patients Undergoing Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2017;10:1436-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 2. | Dato I, Burzotta F, Trani C, Crea F, Ussia GP. Percutaneous management of vascular access in transfemoral transcatheter aortic valve implantation. World J Cardiol. 2014;6:836-846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Barbanti M, Binder RK, Freeman M, Wood DA, Leipsic J, Cheung A, Ye J, Tan J, Toggweiler S, Yang TH, Dvir D, Maryniak K, Lauck S, Webb JG. Impact of low-profile sheaths on vascular complications during transfemoral transcatheter aortic valve replacement. EuroIntervention. 2013;9:929-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Hayashida K, Lefèvre T, Chevalier B, Hovasse T, Romano M, Garot P, Mylotte D, Uribe J, Farge A, Donzeau-Gouge P, Bouvier E, Cormier B, Morice MC. True percutaneous approach for transfemoral aortic valve implantation using the Prostar XL device: impact of learning curve on vascular complications. JACC Cardiovasc Interv. 2012;5:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Kroon HG, Tonino PAL, Savontaus M, Amoroso G, Laine M, Christiansen EH, Toggweiler S, Ten Berg J, Sathananthan J, Daemen J, de Jaegere PP, Brueren GBRG, Malmberg M, Slagboom T, Moriyama N, Terkelsen CJ, Moccetti F, Gheorghe L, Webb J, Wood D, Van Mieghem NM. Dedicated plug based closure for large bore access -The MARVEL prospective registry. Catheter Cardiovasc Interv. 2021;97:1270-1278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Burzotta F, Aurigemma C, Romagnoli E, Shoeib O, Russo G, Zambrano A, Verdirosi D, Leone AM, Bruno P, Trani C. A less-invasive totally-endovascular (LITE) technique for trans-femoral transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2020;96:459-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Burzotta F, Shoeib O, Aurigemma C, Trani C. Angio-Guidewire-Ultrasound (AGU) Guidance for Femoral Access in Procedures Requiring Large Sheaths. J Invasive Cardiol. 2019;31:E37-E39. [PubMed] |
| 8. | Ott I, Shivaraju A, Schäffer NR, Frangieh AH, Michel J, Husser O, Hengstenberg C, Mayr P, Colleran R, Pellegrini C, Cassese S, Fusaro M, Schunkert H, Kastrati A, Kasel AM. Parallel suture technique with ProGlide: a novel method for management of vascular access during transcatheter aortic valve implantation (TAVI). EuroIntervention. 2017;13:928-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg. 1999;16:9-13. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2144] [Cited by in RCA: 2193] [Article Influence: 84.3] [Reference Citation Analysis (0)] |
| 10. | Chugh Y, Bavishi C, Mojadidi MK, Elgendy IY, Faillace RT, Brilakis ES, Tamis-Holland J, Mamas M, Chugh SK. Safety of transradial access compared to transfemoral access with hemostatic devices (vessel plugs and suture devices) after percutaneous coronary interventions: A systematic review and meta-analysis. Catheter Cardiovasc Interv. 2020;96:285-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | O'Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB, Normand SL, DeLong ER, Shewan CM, Dokholyan RS, Peterson ED, Edwards FH, Anderson RP; Society of Thoracic Surgeons Quality Measurement Task Force. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2--isolated valve surgery. Ann Thorac Surg. 2009;88:S23-S42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 854] [Cited by in RCA: 936] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 12. | Edwards FH, Cohen DJ, O'Brien SM, Peterson ED, Mack MJ, Shahian DM, Grover FL, Tuzcu EM, Thourani VH, Carroll J, Brennan JM, Brindis RG, Rumsfeld J, Holmes DR Jr; Steering Committee of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Development and Validation of a Risk Prediction Model for In-Hospital Mortality After Transcatheter Aortic Valve Replacement. JAMA Cardiol. 2016;1:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 194] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 13. | Chambers JB, Prendergast B, Iung B, Rosenhek R, Zamorano JL, Piérard LA, Modine T, Falk V, Kappetein AP, Pibarot P, Sundt T, Baumgartner H, Bax JJ, Lancellotti P. Standards defining a 'Heart Valve Centre': ESC Working Group on Valvular Heart Disease and European Association for Cardiothoracic Surgery Viewpoint. Eur Heart J. 2017;38:2177-2183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodés-Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1345] [Cited by in RCA: 1487] [Article Influence: 114.4] [Reference Citation Analysis (0)] |
| 15. | Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, Robson R, Thabane M, Giangregorio L, Goldsmith CH. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1672] [Cited by in RCA: 1812] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 16. | Généreux P, Webb JG, Svensson LG, Kodali SK, Satler LF, Fearon WF, Davidson CJ, Eisenhauer AC, Makkar RR, Bergman GW, Babaliaros V, Bavaria JE, Velazquez OC, Williams MR, Hueter I, Xu K, Leon MB; PARTNER Trial Investigators. Vascular complications after transcatheter aortic valve replacement: insights from the PARTNER (Placement of AoRTic TraNscathetER Valve) trial. J Am Coll Cardiol. 2012;60:1043-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 412] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 17. | Batchelor W, Patel K, Hurt J, Totten J, Burroughs P, Smith G, Cuervo M, Davis L, Damluji AA, Epps K, Sherwood M, Barnett S, Geloo N, Yazdani S, Sarin E, Ryan L, Noel T. Incidence, Prognosis and Predictors of Major Vascular Complications and Percutaneous Closure Device Failure Following Contemporary Percutaneous Transfemoral Transcatheter Aortic Valve Replacement. Cardiovasc Revasc Med. 2020;21:1065-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Ruge H, Burri M, Erlebach M, Lange R. Access site related vascular complications with third generation transcatheter heart valve systems. Catheter Cardiovasc Interv. 2021;97:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Elbaz-Greener G, Zivkovic N, Arbel Y, Radhakrishnan S, Fremes SE, Wijeysundera HC. Use of Two-Dimensional Ultrasonographically Guided Access to Reduce Access-Related Complications for Transcatheter Aortic Valve Replacement. Can J Cardiol. 2017;33:918-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Barbanti M, Capranzano P, Ohno Y, Gulino S, Sgroi C, Immè S, Tamburino C, Cannata S, Patanè M, Di Stefano D, Todaro D, Di Simone E, Deste W, Gargiulo G, Capodanno D, Grasso C. Comparison of suture-based vascular closure devices in transfemoral transcatheter aortic valve implantation. EuroIntervention. 2015;11:690-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Barbash IM, Barbanti M, Webb J, Molina-Martin De Nicolas J, Abramowitz Y, Latib A, Nguyen C, Deuschl F, Segev A, Sideris K, Buccheri S, Simonato M, Rosa FD, Tamburino C, Jilaihawi H, Miyazaki T, Himbert D, Schofer N, Guetta V, Bleiziffer S, Tchetche D, Immè S, Makkar RR, Vahanian A, Treede H, Lange R, Colombo A, Dvir D. Comparison of vascular closure devices for access site closure after transfemoral aortic valve implantation. Eur Heart J. 2015;36:3370-3379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 22. | Jochheim D, Abdel-Wahab M, Baquet M, Zadrozny M, El-Mawardy M, Lange P, Kupatt C, Theiss H, Greif M, Hausleiter J. Comparison of two suture mediated closure devices for access site closure after transfemoral aortic valve implantation. Devices for access site closure after transfemoral aortic valve implantation. J Am Coll Cardiol. 2015;65:A1692. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Seeger J, Gonska B, Rodewald C, Rottbauer W, Wöhrle J. Impact of suture mediated femoral access site closure with the Prostar XL compared to the ProGlide system on outcome in transfemoral aortic valve implantation. Int J Cardiol. 2016;223:564-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Dimitriadis Z, Scholtz W, Börgermann J, Wiemer M, Piper C, Vlachojannis M, Gummert J, Horstkotte D, Ensminger S, Faber L, Scholtz S. Impact of closure devices on vascular complication and mortality rates in TAVI procedures. Int J Cardiol. 2017;241:133-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Maniotis C, Andreou C, Karalis I, Koutouzi G, Agelaki M, Koutouzis M. A systematic review on the safety of Prostar XL versus ProGlide after TAVR and EVAR. Cardiovasc Revasc Med. 2017;18:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | van Wiechen MP, Tchétché D, Ooms JF, Hokken TW, Kroon H, Ziviello F, Ghattas A, Siddiqui S, Laperche C, Spitzer E, Daemen J, de Jaegere PP, Dumonteil N, Van Mieghem NM. Suture- or Plug-Based Large-Bore Arteriotomy Closure: A Pilot Randomized Controlled Trial. JACC Cardiovasc Interv. 2021;14:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 27. | Biancari F, Romppanen H, Savontaus M, Siljander A, Mäkikallio T, Piira OP, Piuhola J, Vilkki V, Ylitalo A, Vasankari T, Airaksinen JKE, Niemelä M. MANTA versus ProGlide vascular closure devices in transfemoral transcatheter aortic valve implantation. Int J Cardiol. 2018;263:29-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 28. | Moriyama N, Lindström L, Laine M. Propensity-matched comparison of vascular closure devices after transcatheter aortic valve replacement using MANTA versus ProGlide. EuroIntervention. 2019;14:e1558-e1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Hoffmann P, Al-Ani A, von Lueder T, Hoffmann J, Majak P, Hagen O, Loose H, Kløw NE, Opdahl A. Access site complications after transfemoral aortic valve implantation - a comparison of Manta and ProGlide. CVIR Endovasc. 2018;1:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | Zaman S, Gooley R, Cheng V, McCormick L, Meredith IT. Impact of routine crossover balloon occlusion technique on access-related vascular complications following transfemoral transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2016;88:276-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Kaluski E, Khan SU, Sattur S, Sporn D, Rogers G, Reitknecht F. Arteriotomy site complication during transcatheter aortic valve replacement: Ipsilateral wire protection and bailout. Cardiovasc Revasc Med. 2018;19:724-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Kiramijyan S, Magalhaes MA, Ben-Dor I, Koifman E, Escarcega RO, Baker NC, Torguson R, Okubagzi P, Bernardo NL, Satler LF, Pichard AD, Waksman R. The adjunctive use of Angio-Seal in femoral vascular closure following percutaneous transcatheter aortic valve replacement. EuroIntervention. 2016;12:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Costa G, Valvo R, Picci A, Criscione E, Reddavid C, Motta S, Strazzieri O, Deste W, Giuffrida A, Garretto V, Cannizzaro MT, Inserra C, Veroux P, Giaquinta A, Sgroi C, Tamburino C, Barbanti M. An upfront combined strategy for endovascular haemostasis in transfemoral transcatheter aortic valve implantation. EuroIntervention. 2021;17:728-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |