Published online Apr 26, 2022. doi: 10.4330/wjc.v14.i4.266
Peer-review started: November 11, 2021
First decision: December 27, 2021
Revised: December 29, 2021
Accepted: March 16, 2022
Article in press: March 16, 2022
Published online: April 26, 2022
Processing time: 158 Days and 14.4 Hours
Diabetes mellitus (DM) is a health condition characterized by glucose dysregulation and affects millions of people worldwide. The presentation of heart failure in diabetic cardiomyopathy extends over a wide phenotypic spectrum, comm
Core Tip: Dipeptidyl peptidase-4 inhibitors are a safe and effective glucose lowering treatment option in patients with type 2 diabetes mellitus. However, their effect on cardiovascular outcomes in diabetic patients has been neutral. The potential correlation between atrial flutter and dipeptidyl peptidase-4 inhibitors administration is an interesting finding, but since currently there is no sheer underlying pathophysiologic mechanism to justify it, more evidence is required.
- Citation: Bourazana A, Giamouzis G, Skoularigis J, Triposkiadis F, Xanthopoulos A. Glucose lowering does not necessarily reduce cardiovascular risk in type 2 diabetes. World J Cardiol 2022; 14(4): 266-270
- URL: https://www.wjgnet.com/1949-8462/full/v14/i4/266.htm
- DOI: https://dx.doi.org/10.4330/wjc.v14.i4.266
Diabetes mellitus (DM) is a health condition characterized by glucose dysregulation and affects 237.9 million males and 222 million females worldwide[1]. It is an established risk factor of cardiovascular disease, atrial and ventricular arrhythmias, as well as sudden cardiac death[2,3]. Dipeptidyl peptidase-4 (DPP-4) inhibitors are a safe and effective glucose lowering treatment option in patients with type 2 DM (T2DM). Nevertheless, pooled recent data on the effect of DPP-4 inhibitors on cardiovascular outcomes and major cardiac arrhythmias are lacking.
We read with great interest the paper by Patoulias et al[4], who attempted to close the abovementioned knowledge gap by performing a meta-analysis of six randomized controlled trials (52520 patients) concerning the impact of dipeptidyl peptidase-4 (DPP-4) inhibitors on “hard” cardiovascular outcomes and major cardiac arrhythmias. The authors concluded that DPP-4 inhibitors, compared to placebo, had no effect on fatal or non-fatal myocardial infarction, fatal and non-fatal stroke, hospitalization for heart failure, hospitalization for unstable angina, hospitalization for coronary revascularization, and cardiovascular death and did not seem to confer any significant risk for major cardiac arrhythmias, with the exception of atrial flutter, which was associated with an increased risk equal to 52% (relative risk = 1.52, 95% confidence interval: 1.03-2.24, I2 = 0%).
We agree with the authors’ insight that the presence of DM per se increases the risk of adverse cardiovascular outcomes and arrhythmias and results in cellular destabilization of myocardial tissue altogether. For example, it has been demonstrated that diabetic patients present an increased propensity for developing heart failure[5]. Diabetic cardiomyopathy, defined as ventricular dysfunction in the absence of hypertension or coronary artery disease, has been attributed to the deregulated immune response in type-1 DM (T1DM) and to the background of obesity in the majority of T2DM patients. Τhe amplified immune response of T1DM patients to myocardial injury, leads to the expansion of proinflammatory CD4+ T cells specific to myosin and the development of autoantibodies to MYH6 and other cardiac antigens. On the other hand, obesity that predominates over T2DM patients reduces the palliative actions of circulating natriuretic peptides on ventricular stress, pressure overload, and sympathetic activation. In the absence of natriuretic peptides’ favorable actions, left ventricle hypertrophy, fibrosis, and insulin desensitization in skeletal muscles are more frequently observed in obese patients[5]. The presentation of heart failure in diabetic cardiomyopathy extends over a wide phenotypic spectrum, commencing from asymptomatic, subclinical structural abnormalities, developing progressively to severely symptomatic biventricular dysfunction with advanced mortality risk. Similarly, the spectrum of systolic dysfunction in diabetic-induced heart failure is diverse, originating in the heterogeneous risk factors that diabetes comes along, such as hypertension, hyperlipidemia, cardiovascular disease, and chronic kidney disease[6].
Except for the morphological implications on ventricular myocardium that account for the wide spectrum of left ventricular dysfunction, DM leads to cardiac electrical remodeling reacting on various targets. Among the diabetes-induced electrical disturbances, reduced conduction velocity, prolonged repolarization, and increased QT dispersion have been recognized, all predisposing to ventricular arrhythmias[7,8]. T1DM and T2DM lead to action potential duration prolongation, which becomes prominent on electrocardiography with a QRS prolongation in some diabetic patients[9]. QT duration prolongation subsequently predisposes to early after depolarizations development and an enhanced risk of torsade de pointes[10]. The proposed mechanisms are diabetes-exerted alterations in the function of several proteins involved in ion handling. More specifically, DM modifies ion channels responsible for depolarization as well as repolarization and resting phase[11]. Therefore, DM affects essentially all phases of action potential and correlates strongly to ventricular arrhythmias emergence.
The culprit pathophysiological mechanisms for the occurrence of atrial arrhythmias in DM substrate are not yet in depth elucidated. In atrial myocardium, DM favors the phenotypic switch of fibroblasts to myofibroblasts[12]. Mighty it is that diabetes induced atrial neuropathy as well as diabetes generated advanced myofibroblast differentiation promote atrial remodeling and lead to atrial cardiomyopathy overall[13]. Nonetheless, on epidemiological basis, DM is a strong independent risk factor for atrial fibrillation (AF) and atrial flutter occurrence.
Several studies have demonstrated that antidiabetic drugs may have differing effects on the risk of new-onset AF[14]. Metformin has been associated with anti-atrial arrhythmic benefits[15]. A case control study revealed no association between sulfonylurea and incident AF, whereas the use of insulin was associated with increased risk of new-onset AF[16]. A recent meta-analysis showed that DPP-4 inhibitor treatment resulted in a non-significant decrease in the risk for AF, whereas both glucagon-like peptide-1 receptor agonists (GLP1-RA) and sodium-glucose cotransporter 2 inhibitors (SGLT2-i) were associated with a significant decrease in the risk for AF, equal to 14% and 19%, respectively[17]. Liraglutide (a GLP1-RA) demonstrated favorable effects on electrophysiological changes regarding AF inducibility and conduction velocity decrease[18].
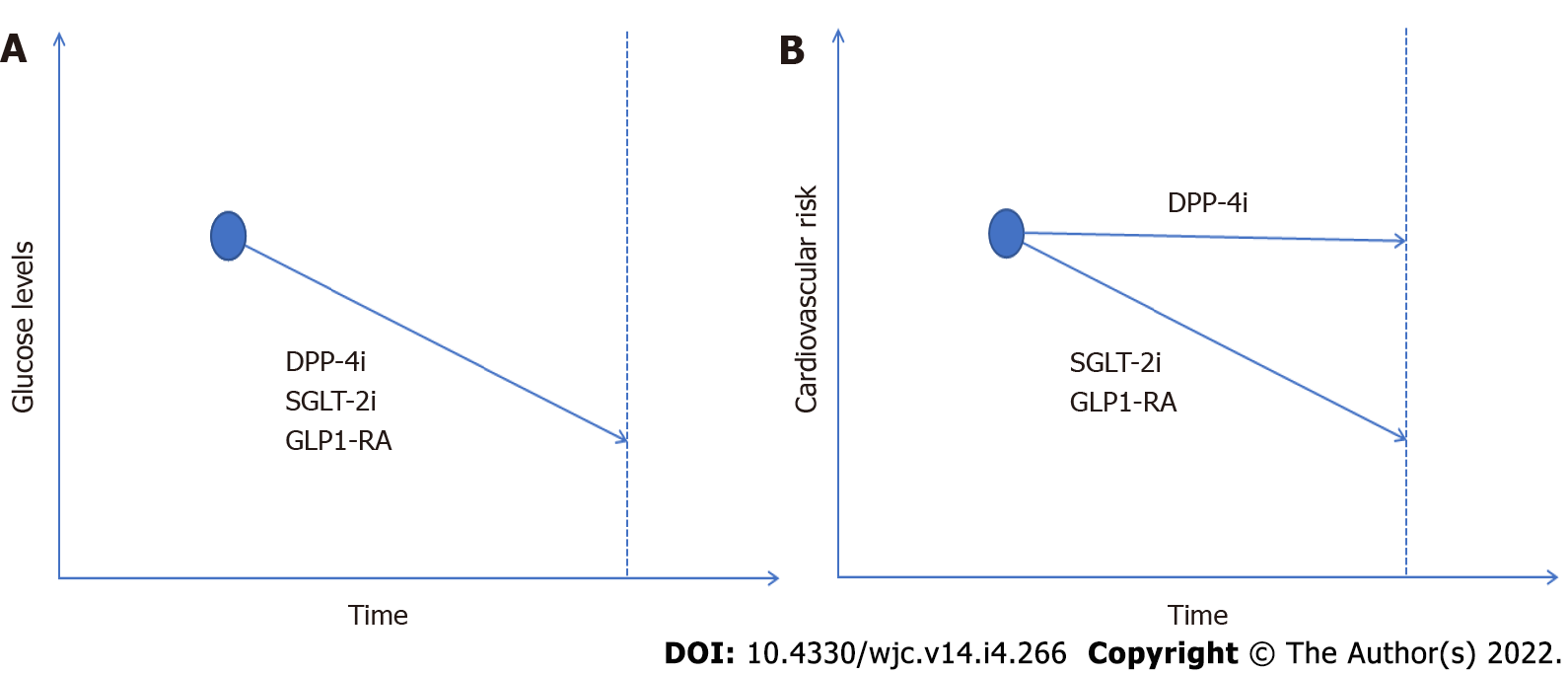
DPP-4 inhibitors reduce glucagon and blood glucose levels by raising levels of the endogenous hormones glucagon-like-peptide 1 and glucose-dependent insulinotropic peptide, which are called incretins. Subsequently incretins inhibit glucagon release and increase insulin secretion. Despite DPP-4 inhibitors’ indisputable efficacy regarding glycemic control, their effect on cardiovascular outcomes in diabetic patients, as denoted in the aforementioned and previous studies, has been neutral[19,20]. Furthermore, the risk of hypoglycemia is admitted to be increased compared to SGLT2-i. SGLT2-i reduce hyperglycemia through inhibition of glucose reabsorption in the renal proximal tubules. Acting on glucose/Na co-transporter they promote natriuresis and display not solely hypoglycemic effects but also reduce major adverse cardiovascular events (cardiovascular and total mortality, fatal or nonfatal myocardial infarction, or stroke) and hospitalization for heart failure and improve outcome in chronic kidney disease in diabetic and non-diabetic patients[21]. GLP1-RA are oral hypoglycemic drugs that mimic the effects of the incretin hormone glucagon-like-peptide 1. GLP-RA stimulate insulin release, inhibit glucagon secretion, and slow gastric emptying. Liraglutide, albiglutide, and dualiglutide have all shown significant decreases in adverse cardiovascular events[22]. In line with this evidence, the European Society of Cardiology guidelines recommend the administration of SGLT-2i or GLP1-RA as a first option in the presence of high or remarkably high cardiovascular risk or of cardiovascular disease[23].
The potential correlation between atrial flutter and DPP-4 inhibitors administration is an interesting finding, but since there is no sheer underlying pathophysiologic mechanism to justify it, more evidence is required to establish this thesis as a widely accepted knowledge admissible in clinical practice. The authors speculated that the abovementioned correlation may stem from the inherent higher risk of atrial flutter that patients with DM carry[24]. However, it is also well known that DM per se is a risk factor of AF[24], which in the current meta-analysis was not associated with DPP-4 inhibitor use.
In conclusion, the authors should be congratulated on their attempt to provide state of the art data on the association between DPP-4 inhibitors and cardiovascular outcomes as well as major cardiac arrhythmias. The reported increased risk of atrial flutter in patients receiving DPP-4 inhibitors needs further investigation (Figure 1).
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ma JH, China; Zhang J, China S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, Samad Z, Satou GM, Schroeder EB, Shah SH, Shay CM, Stokes A, VanWagner LB, Wang NY, Tsao CW; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021;143:e254-e743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 3598] [Article Influence: 899.5] [Reference Citation Analysis (0)] |
| 2. | Bell DSH, Goncalves E. Atrial fibrillation and type 2 diabetes: Prevalence, etiology, pathophysiology and effect of anti-diabetic therapies. Diabetes Obes Metab. 2019;21:210-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 3. | Lynge TH, Svane J, Pedersen-Bjergaard U, Gislason G, Torp-Pedersen C, Banner J, Risgaard B, Winkel BG, Tfelt-Hansen J. Sudden cardiac death among persons with diabetes aged 1-49 years: a 10-year nationwide study of 14 294 deaths in Denmark. Eur Heart J. 2020;41:2699-2706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 4. | Patoulias DI, Boulmpou A, Teperikidis E, Katsimardou A, Siskos F, Doumas M, Papadopoulos CE, Vassilikos V. Cardiovascular efficacy and safety of dipeptidyl peptidase-4 inhibitors: A meta-analysis of cardiovascular outcome trials. World J Cardiol. 2021;13:585-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 5. | Triposkiadis F, Xanthopoulos A, Bargiota A, Kitai T, Katsiki N, Farmakis D, Skoularigis J, Starling RC, Iliodromitis E. Diabetes Mellitus and Heart Failure. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 6. | Triposkiadis F, Butler J, Abboud FM, Armstrong PW, Adamopoulos S, Atherton JJ, Backs J, Bauersachs J, Burkhoff D, Bonow RO, Chopra VK, de Boer RA, de Windt L, Hamdani N, Hasenfuss G, Heymans S, Hulot JS, Konstam M, Lee RT, Linke WA, Lunde IG, Lyon AR, Maack C, Mann DL, Mebazaa A, Mentz RJ, Nihoyannopoulos P, Papp Z, Parissis J, Pedrazzini T, Rosano G, Rouleau J, Seferovic PM, Shah AM, Starling RC, Tocchetti CG, Trochu JN, Thum T, Zannad F, Brutsaert DL, Segers VF, De Keulenaer GW. The continuous heart failure spectrum: moving beyond an ejection fraction classification. Eur Heart J. 2019;40:2155-2163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 196] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 7. | Ninkovic VM, Ninkovic SM, Miloradovic V, Stanojevic D, Babic M, Giga V, Dobric M, Trenell MI, Lalic N, Seferovic PM, Jakovljevic DG. Prevalence and risk factors for prolonged QT interval and QT dispersion in patients with type 2 diabetes. Acta Diabetol. 2016;53:737-744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Gallego M, Zayas-Arrabal J, Alquiza A, Apellaniz B, Casis O. Electrical Features of the Diabetic Myocardium. Arrhythmic and Cardiovascular Safety Considerations in Diabetes. Front Pharmacol. 2021;12:687256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Singleton MJ, German C, Hari KJ, Saylor G, Herrington DM, Soliman EZ, Freedman BI, Bowden DW, Bhave PD, Yeboah J. QRS duration is associated with all-cause mortality in type 2 diabetes: The diabetes heart study. J Electrocardiol. 2020;58:150-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Maruyama M, Lin SF, Xie Y, Chua SK, Joung B, Han S, Shinohara T, Shen MJ, Qu Z, Weiss JN, Chen PS. Genesis of phase 3 early afterdepolarizations and triggered activity in acquired long-QT syndrome. Circ Arrhythm Electrophysiol. 2011;4:103-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Yu P, Hu L, Xie J, Chen S, Huang L, Xu Z, Liu X, Zhou Q, Yuan P, Yan X, Jin J, Shen Y, Zhu W, Fu L, Chen Q, Yu J, Hu J, Cao Q, Wan R, Hong K. O-GlcNAcylation of cardiac Nav1.5 contributes to the development of arrhythmias in diabetic hearts. Int J Cardiol. 2018;260:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Fowlkes V, Clark J, Fix C, Law BA, Morales MO, Qiao X, Ako-Asare K, Goldsmith JG, Carver W, Murray DB, Goldsmith EC. Type II diabetes promotes a myofibroblast phenotype in cardiac fibroblasts. Life Sci. 2013;92:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Wang A, Green JB, Halperin JL, Piccini JP Sr. Atrial Fibrillation and Diabetes Mellitus: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;74:1107-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 247] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 14. | Lee TW, Lee TI, Lin YK, Chen YC, Kao YH, Chen YJ. Effect of antidiabetic drugs on the risk of atrial fibrillation: mechanistic insights from clinical evidence and translational studies. Cell Mol Life Sci. 2021;78:923-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Nantsupawat T, Wongcharoen W, Chattipakorn SC, Chattipakorn N. Effects of metformin on atrial and ventricular arrhythmias: evidence from cell to patient. Cardiovasc Diabetol. 2020;19:198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Liou YS, Yang FY, Chen HY, Jong GP. Antihyperglycemic drugs use and new-onset atrial fibrillation: A population-based nested case control study. PLoS One. 2018;13:e0197245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Patoulias D, Toumpourleka M, Papadopoulos C, Doumas M. Meta-analysis Evaluating the Risk of Atrial Fibrillation With Newer Antidiabetics Across the Cardiovascular and Renal Outcome Trials. Am J Cardiol. 2021;139:139-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Nakamura H, Niwano S, Niwano H, Fukaya H, Murakami M, Kishihara J, Satoh A, Yoshizawa T, Ishizue N, Igarashi T, Fujiishi T, Ako J. Liraglutide suppresses atrial electrophysiological changes. Heart Vessels. 2019;34:1389-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Sinha B, Ghosal S. Meta-analyses of the effects of DPP-4 inhibitors, SGLT2 inhibitors and GLP1 receptor analogues on cardiovascular death, myocardial infarction, stroke and hospitalization for heart failure. Diabetes Res Clin Pract. 2019;150:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 20. | Liu D, Jin B, Chen W, Yun P. Dipeptidyl peptidase 4 (DPP-4) inhibitors and cardiovascular outcomes in patients with type 2 diabetes mellitus (T2DM): a systematic review and meta-analysis. BMC Pharmacol Toxicol. 2019;20:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Xiao L, Nie X, Cheng Y, Wang N. Sodium-Glucose Cotransporter-2 Inhibitors in Vascular Biology: Cellular and Molecular Mechanisms. Cardiovasc Drugs Ther. 2021;35:1253-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Sheahan KH, Wahlberg EA, Gilbert MP. An overview of GLP-1 agonists and recent cardiovascular outcomes trials. Postgrad Med J. 2020;96:156-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 23. | Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Jüni P, Lettino M, Marx N, Mellbin LG, Östgren CJ, Rocca B, Roffi M, Sattar N, Seferović PM, Sousa-Uva M, Valensi P, Wheeler DC; ESC Scientific Document Group. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1670] [Cited by in RCA: 2633] [Article Influence: 658.3] [Reference Citation Analysis (0)] |
| 24. | Movahed MR, Hashemzadeh M, Jamal MM. Diabetes mellitus is a strong, independent risk for atrial fibrillation and flutter in addition to other cardiovascular disease. Int J Cardiol. 2005;105:315-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 273] [Article Influence: 13.7] [Reference Citation Analysis (1)] |