Published online Apr 26, 2022. doi: 10.4330/wjc.v14.i4.250
Peer-review started: December 5, 2021
First decision: January 25, 2022
Revised: February 9, 2022
Accepted: March 27, 2022
Article in press: March 27, 2022
Published online: April 26, 2022
Processing time: 134 Days and 16.1 Hours
Vasoplegia is a common complication of cardiac surgery but its causal relationship with preoperative use of renin angiotensin system (RAS) blockers [angiotensin converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARB)] is still debated.
To update and summarize data on the effect of preoperative use of RAS blockers on incident vasoplegia.
All published studies from MEDLINE, EMBASE, and Web of Science providing relevant data through January 13, 2021 were identified. A random-effects meta-analysis method was used to pool estimates, and post-cardiac surgery shock was differentiated from vasoplegia.
Ten studies reporting on a pooled population of 15672 patients (none looking at ARBs exclusively) were included in the meta-analysis. All were case-control studies. Use of ACEIs was associated with an increased risk of vasoplegia [pooled adjusted odds ratio (Aor) of 2.06, 95%CI: 1.45-2.93] and increased inotropic/vasopressor support requirement (pooled aOR 1.19, 95%CI: 1.10-1.29). Post-cardiac surgery shock was increased in the presence of left ventricular dysfunction (pooled aOR 2.32, 95%CI: 1.60-3.36; I2 49%) but not increased by the use of beta blockers (pooled aOR 0.78, 95%CI: 0.36-1.69; I2 77%). Two randomized control trials (RCTs), not eligible for the meta-analysis, did not show an association between continuation of RAS blockers and vasoplegia.
Preoperative continuation of ACEIs is associated with an increased need for inotropic support postoperatively and with an increased risk of vasoplegia in observational studies but not in RCTs. The absence of a consensus definition of vasoplegia should lead to the use of perioperative cardiovascular monitoring when designing RCTs to better understand this discrepancy.
Core Tip: Vasoplegia is a common complication of cardiac surgery but its causal relationship with preoperative use of renin angiotensin system blockers, mainly angiotensin converting enzyme inhibitors (ACEIs), is still debated. The meta-analysis of observational studies suggests that preoperative continuation of ACEIs is associated with an increased risk of vasoplegia and of the use of inotropic support postoperatively. However, these associations were not observed in two included randomized controlled trials with limited power. These findings support the potential benefit of holding ACEIs prior to cardiac surgery to reduce the risk of vasoplegia and associated adverse outcomes. However, well-powered randomized controlled trials using a consensus definition of vasoplegia are still needed to properly assess management strategies of RAS blockers in the perioperative setting.
- Citation: Noubiap JJ, Nouthe B, Sia YT, Spaziano M. Effect of preoperative renin-angiotensin system blockade on vasoplegia after cardiac surgery: A systematic review with meta-analysis. World J Cardiol 2022; 14(4): 250-259
- URL: https://www.wjgnet.com/1949-8462/full/v14/i4/250.htm
- DOI: https://dx.doi.org/10.4330/wjc.v14.i4.250
Shock is a frequent complication of major cardiac surgery, occurring in approximately a quarter of procedures, especially those with cardiopulmonary bypass (CPB)[1-3]. Vasoplegia is a form of distributive shock characterized by persistent hypotension, reduced systemic vascular resistance (SVR) with normal or elevated cardiac output[1]. It is due to reduced vascular smooth cell contraction resulting from several mechanisms including the alteration of the endothelial glycocalyx, impaired receptor signaling, changes in endothelial cell metabolism, and depletion and decreased response to endogenous vasopressors[1,4]. This impairment of vascular reactivity is worsened by a systemic inflammatory response to surgical trauma, ischemia-reperfusion syndrome, and exposure of blood to the foreign surfaces during extracorporeal circulation[1,4]. The use of some medications prior to surgery is also thought to contribute to inappropriate vasodilatation in vasoplegia[1,4].
The association of continuation of renin angiotensin system (RAS) blockers and vasoplegia following cardiac surgery is still a matter of debate[2,5-7]. Preoperative administration of ACEIs has been associated with poor outcomes including acute kidney injury and increased mortality[8]. This systematic review and meta-analysis aimed to comprehensively summarize data on the effect of preoperative use of RAS blockers on vasoplegia in patients undergoing cardiac surgery.
This review is reported in accordance with the Meta-analyses Of Observational Studies in Epidemiology guidelines[9]. It was registered with PROSPERO (CRD42017072923).
PubMed/MEDLINE, Excerpta Medica Database (EMBASE), and Web of Science were searched to identify all cohort studies, case-control studies or randomized controlled trials (RCTs) reporting primary data on the association between ACEIs or ARBs and the incidence of vasoplegia after cardiac surgery, published by January 13, 2021 (date of the last search), without language restriction. The search strategy used a combination of terms or their synonyms related to vasoplegia, a detailed list of cardiac surgical procedures, and names of ACEIs or ARBs (Supplementary Table 1). The reference lists of relevant research and review articles were also screened to identify potential additional data sources.
We included: (1) Cohort studies, case-control studies or RCTs; (2) With 30 participants or more; and (3) Reporting on risk of vasoplegia associated with ACEIs or ARBs, or studies with enough data to compute these estimates. We excluded studies: (1) Which included patients undergoing non-cardiac surgery; (2) Lacking explicit description of the perioperative follow-up of patients; and (3) Not reporting primary data (reviews, commentaries, editorials). For duplicate publication of the same group/cohort of patients, we included the single most comprehensive report with the largest sample size. Two investigators (JJN and BN) independently selected records from bibliographic searches based on titles and abstracts screening. Full texts of articles deemed potentially eligible were retrieved and screened independently by the same investigators for final inclusion. Disagreements were resolved via discussion and consensus.
Data were extracted using a standard data abstraction form by one investigator (JJN) and cross-checked by a second investigator (BN). We collected data on study characteristics, type of cardiac surgery, definition of vasoplegia, sample size, mean or median age, sex proportion, proportion of patients with co-morbidities such as hypertension, diabetes, heart failure, or previous cardiac surgery, proportion of patients taking relevant medications (ACEIs, ARBs, beta blockers, calcium channel blockers, nitrates, number of participants with vasoplegia, and risk estimate [odds ratio (OR) or relative risk (RR)] with the 95% confidence interval (95%CI) for the association between vasoplegia and ACEIs or ARBs. We also extracted adjusted risk estimates (from multivariable regression analysis) for other risk factors for vasoplegia as complementary information. We assessed the risk of bias using the tools for case-control studies and randomized controlled trial developed by the CLARITY group at McMaster University[10,11]. We separated studies with or without perioperative cardiac index monitoring in an attempt to further differentiate post-cardiac surgery shock from vasoplegia.
Analyses were conducted with R statistical software (version 3.6.2, The R Foundation for statistical computing, Vienna, Austria). The generic inverse variance method was used to pool adjusted risk estimates (OR or RR) and their standard errors with the random-effects meta-analysis model using the metagen function. Heterogeneity was assessed by the χ² test on Cochrane's Q statistic, which was quantified by I² values, assuming I² values of 25%, 50% and 75% respectively representing low, moderate and high heterogeneity[12]. We assessed the presence of publication bias related to small study effect by funnel plot inspection and by linear regression test of funnel plot asymmetry (Egger’s test)[13]. All statistical tests were two-tailed and statistical significance defined as P value ≤ 0.05.
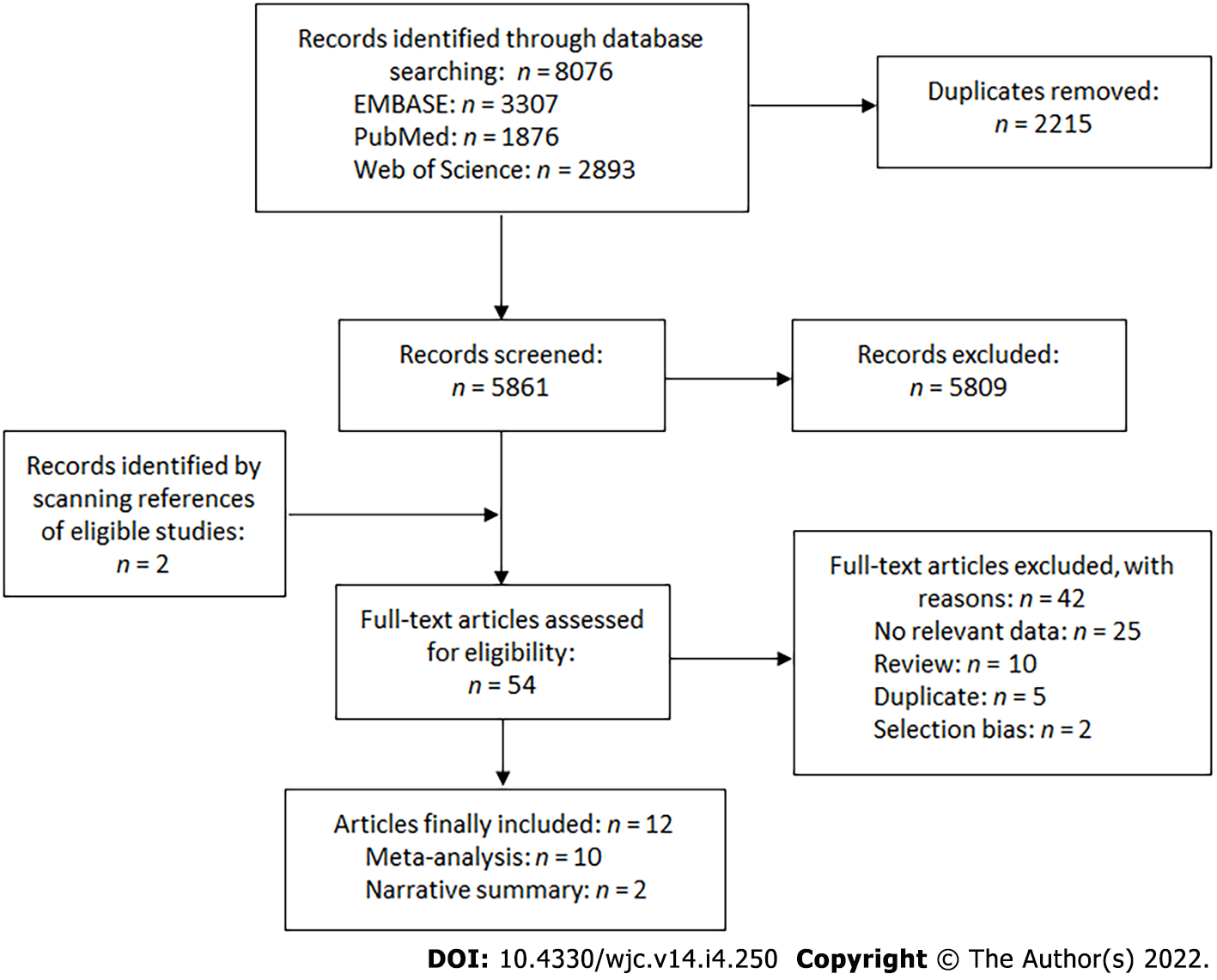
Bibliographic searches retrieved 8076 records and 12 articles were finally included[2,5-7,14-21]. Ten studies were included in the meta-analysis[2,5-7,14-19] and 2 were summarized narratively[20,21]. The study selection is summarized in Figure 1. The observational studies included in the meta-analysis reported data from a pooled sample of 15672 patients undergoing a cardiac surgery. Half of them focused on patients undergoing coronary artery bypass (CABG)[6,7,15,18,19], while the other studies included patients undergoing various procedures including CABG or valvular surgery[2,5,14,16,17]. All observational studies were case-control studies, with data collection done prospectively in most of them[5-7,14-17,19]. The two studies summarized narratively were RCTs[20,21]. The characteristics of the included studies are presented in the appendix (Supplementary Table 2). Most of the studies had a low risk of bias in the majority of assessment items (Supplementary Tables 3 and 4).
The definition of vasoplegia varied across studies (Table 1). Nine studies were considered having acceptable definitions of vasoplegia[2,5-7,14-17,20]. In the remaining three studies, the need for inotropic support was the outcome variable and the definition of vasoplegia seemed to encompass other causes of shock[18,19,21]. We therefore separated studies reporting on vasoplegia and those reporting on post-cardiac surgery shock. The proportions of patients who developed post-cardiac surgery shock varied from 4.5% to 34.0% (Table 1). The proportion of patients who used ACEIs preoperatively varied from 9.1% to 64.0% in observational studies. Data on the use of other medications such as ARBs, beta blockers, calcium channel blockers, and nitrates are reported in the appendix (Supplementary Table 2), along with the distribution of co-morbidities across study populations. One of the 2 RCTs reported on an aggregated use of ACEIs and ARBs[20].
| Ref. | Design | Procedure | Definition of vasoplegia | Total sample | Vasoplegia (%) | ACEI (%) |
| Tuman et al[14] | Case-control | Coronary artery and/or valve surgery requiring CPB | Post-CPB ≥ 2 vasoconstrictors with adequate cardiac output | 4301 | 4.5 | 12.1 |
| Bruce et al[16] | Case-control | Cardiac surgery requiring CPB | MAP ≤ 50 mmHg, indexed SVR ≤ 1400 dynes s/cm5/m2, cardiac index ≥ 2.2 L/min/m2, requiring norepinephrine infusion | 188 | 34.0 | 42.0 |
| Carrel et al[17] | Case-control | CABG or AVR | SVR < 600 dynes s/cm5 with adequate cardiac output | 800 | 7.5 | 43.1 |
| Mekontso-Dessap et al[7] | Case-control | CABG | MAP < 70 mmHg, indexed SVR ≤ 1400 dynes s/cm5/m2, normal cardiac output, requiring vasoconstrictor | 108 | 33.3 | 31.5 |
| Sun et al[15] | Case-control | CABG | MAP ≤ 70 mmHg, indexed SVR ≤ 1400 dynes s/cm5/m2, cardiac index ≥ 2.5 L/min/m2, and central venous pressure ≥ 10 mmHg | 696 | 4.7 | 38.5 |
| Levin et al[2] | Case-control | Cardiac surgery | Epinephrine/norepinephrine (≥ 150 ng/kg/min), dopamine (≥ 10 μg/kg/min) or vasopressin (≥ 4 U/h) | 2823 | 20.4 | 19.7 |
| Shahzamani et al[6] | Case-control | CABG | MAP < 65 mmHg, normal cardiac output, requiring vasoconstrictor | 300 | 17.0 | 64.0 |
| Radaelli et al[19] | Case-control | Cardiac surgery | 3 of these 4: MAP < 65 mmHg, indexed SVR ≤ 1600 dynes s/cm5/m2, cardiac index ≥ 2.5/min/m2, and requirement of norepinephrine (> 0.03 μg/kg/min) or vasopressin | 3139 | 32.5 | 52.1 |
| Suga et al[5] | Case-control | CABG | Inotropic support post-CABG | 562 | 11.7 | 9.1 |
| Miceli et al[18] | Case-control | CABG | Inotropic support post-CABG | 2655 | 43.5 | 51.0 |
| Pigot et al[21] | RCT | CABG | Inotropic support post-CABG | 40 | 15.0 | 100 |
| van Diepen et al[20] | RCT | CABG or valve surgery | MAP < 60 mmHg requiring vasopressor administration for at least 4 h and a central venous pressure ≥ 8 mmHg | 121 | 29.8 | 76.9 |
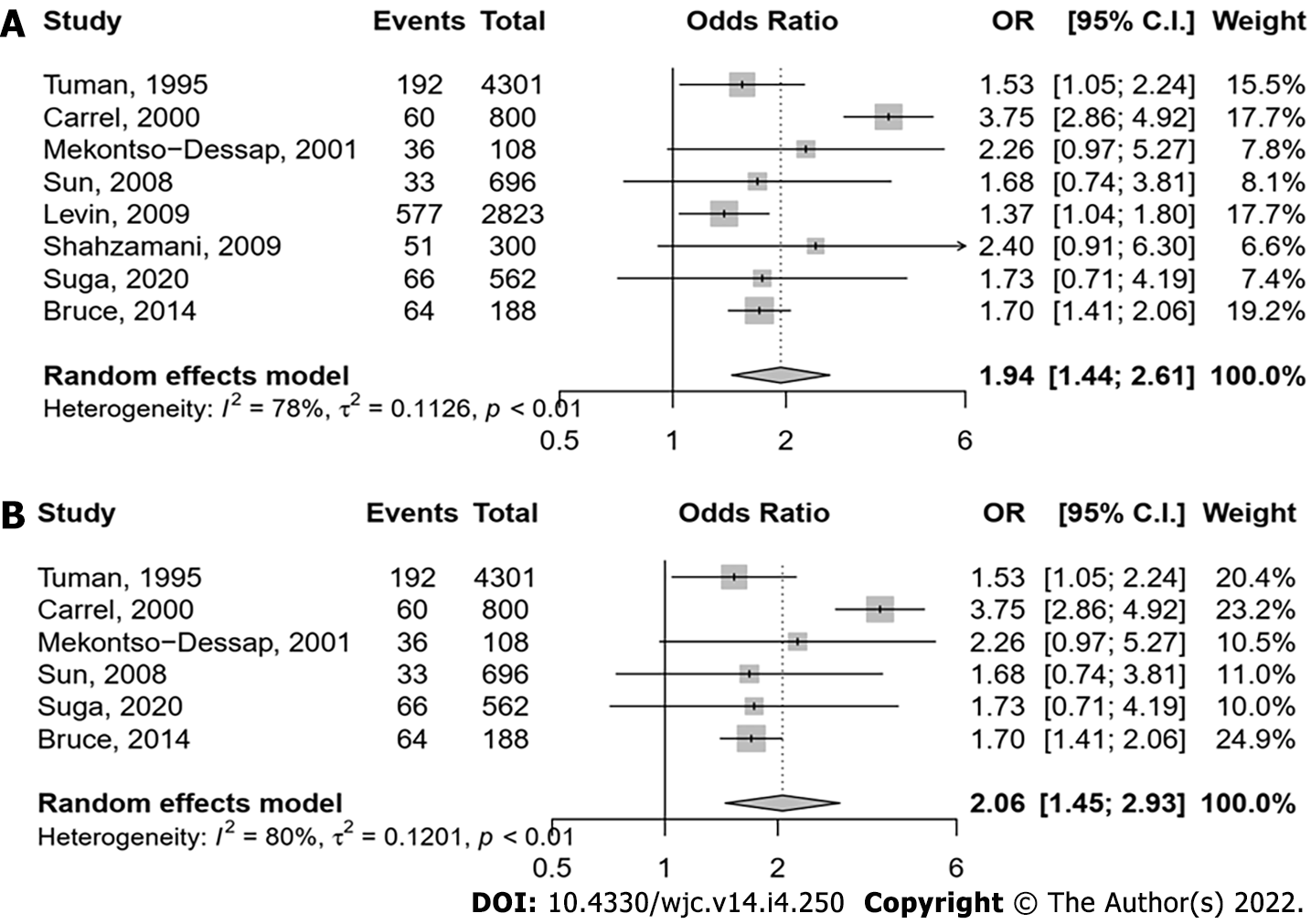
From the 10 observational studies selected, 1076 out of 9778 patients had post-cardiac surgery shock (Figure 2A). In the 6 studies with perioperative cardiac monitoring, 755 patients (12.0%) developed vasoplegia and the risk was increased by preoperative continuation of ACEIs [pooled adjusted odds ratio (aOR) 2.06, 95%CI: 1.45-2.93] (Figure 2B). Considering the high heterogeneity (I2= 80%), we performed influencer analysis using a leave-one-out approach. Omission of the study by Carrel et al[17] resulted in a pooled aOR that changed to 1.61 (95%CI: 1.41-1.85) (Supplementary Figure 1). There was no evidence of small study effect on funnel plot inspection (Supplementary Figure 2), and according to the Egger’s test (P = 0.906).
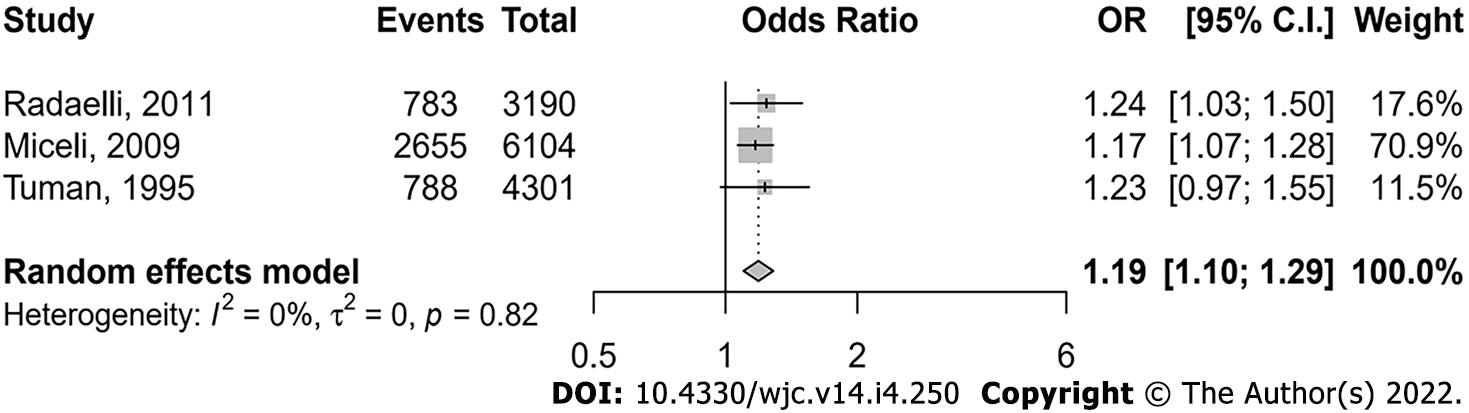
Three studies reported data on the association of omitting ACEIs with the need of inotropic support (use of at least one inotropic drug). A total of 4226 (31.1%) patients required inotropic support from a pooled population of 13595 patients undergoing cardiac surgery. Preoperative continuation of ACEI was associated with an increased risk of inotropic support requirement (pooled aOR 1.19, 95%CI: 1.10-1.29) (Figure 3). There was no heterogeneity (I2= 0), and no evidence of publication bias both on funnel plot inspection (Supplementary Figure 3) and based on the Egger’s test (P = 0.2).
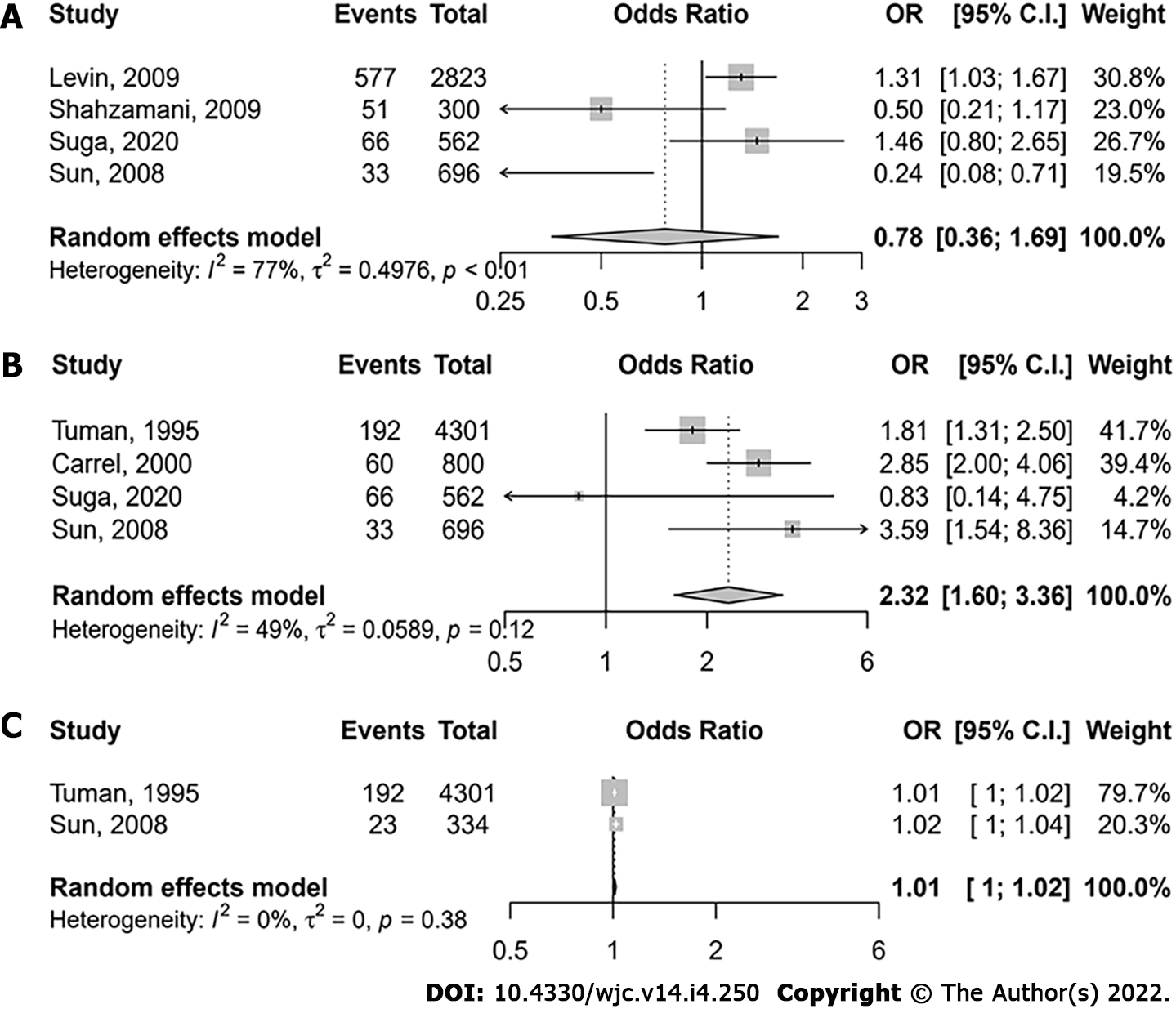
There was no association between beta-blockers and post-cardiac surgery shock (pooled aOR 0.78, 95%CI: 0.36-1.69; I277%) (Figure 4A). The presence of left ventricular dysfunction (ejection fraction < 40%) increased the risk of post-cardiac surgery shock (pooled aOR 2.32, 95%CI: 1.60-3.36; I249%) (Figure 4B). The risk of post-cardiac surgery shock increased with CPB time (pooled aOR 1.012 per 1 min increase, 95%CI: 1.003-1.021, P = 0.008; I20%) (Figure 4C). There was no significant association between age and post-cardiac surgery shock (pooled aOR 1.02 per 1 year increase, 95%CI: 1.00-1.04, P = 0.052; I20%) (Supplementary Figure 4). There was no evidence of publication bias on funnel plot inspection (Supplementary Figures 5 and 6), and according to the Egger’s test for studies reporting on the association between beta blockers and vasoplegia (P = 0.906), and between left ventricular dysfunction and vasoplegia (P = 0.193).
Two RCTs reported on the risk of refractory hypotension with preoperative use of ACEIs[20,21]. The study by van Diepen et al[20] included perioperative cardiac monitoring in their definition of vasoplegia and had a larger sample size (61 patients taking RAS blockers)[21]. Incidence of post-cardiac surgery shock was 5%-15% in the study by Pigott et al[21] and in the RCT by van Diepen et al[20], vasoplegia was found in 29.8% of patients. Preoperative continuation of ACEIs (RAS blockers) was not associated with an increased risk of vasoplegia.
This study aimed to summarize data on the effect of preoperative use of ACEIs on incident vasoplegia in patients undergoing cardiac surgery using all relevant studies. Higher odds of vasoplegia (even when defined using perioperative cardiac monitoring) and more frequent use of inotropic support postoperatively were observed in patients who did not discontinue ACEIs. Other factors associated with the risk of post-cardiac surgery hypotension included left ventricular dysfunction and longer duration of CPB, whereas beta blockers use preoperatively was not. Interestingly, the 2 RCTs which evaluated the risk of vasoplegia and continuation of ACEIs (RAS blockers) before cardiac surgery did not show any association.
Hypotension is very common in cardiac surgery, especially with CPB. In response to reduced systemic blood pressure, the kidneys release renin that cleaves angiotensinogen to yield angiotensin I, further converted into angiotensin II by the angiotensin–converting enzyme which causes systemic vasoconstriction. It also increases the secretion of arginine vasopressin and aldosterone, and potentiates the release of norepinephrine by direct action on postganglionic sympathetic fibers. The increased risk of vasoplegia attributable to preoperative ACEI use reported in our study is also consistent with the evidence supporting a hemodynamic benefit of treatment with angiotensin II in patients undergoing cardiac surgery. For instance, a post-hoc analysis of the Angiotensin II for the Treatment of High-Output Shock (ATHOS-3) multinational, randomized, double-blind trial showed that patients with vasoplegia after cardiac surgery with CPB rapidly responded to angiotensin II, reducing significantly vasopressor use[22].
Whether RAS blockers and specifically ACEIs should be held or not before cardiac surgery has been a matter of debate for more than two decades. The benefits of ACEIs and ARBs in patients with cardiovascular disease are well-established[23]. These positive effects are particularly prominent in the long-term management of patients with ischemic heart disease, especially those undergoing CABG, as evidenced in the QUinapril On Vascular Ace and Determinants of Ischemia (QUO VADIS) RCT[24]. A meta-analysis of 29 studies, mostly retrospective, showed that preoperative use of RAS blockers was associated with increased odds of postoperative acute kidney injury and mortality in patients undergoing cardiovascular surgery[25]. Despite the theoretical favorable mechanisms for RAS blockers in reducing post-operative atrial fibrillation, an increased incidence of post-operative atrial fibrillation has been shown in patients on preoperative RAS blockers, with an adverse effect on survival[26]. These data have motivated the recommendation to omit ACEIs or ARBs before cardiac surgery as a rational strategy to reduce the risk of postoperative vasoplegia and other adverse outcomes[27]. The two RCTs included in this review did not confirm an increased risk of vasoplegia, increased inotropic use or acute kidney injury when ACEIs (RAS blockers) were not discontinued before cardiac surgery[20,21]. However, these trials were not well-powered to be conclusive. They highlight the importance of conducting large multicenter randomized trials to examine the impact of preoperative RAS blockers discontinuation and of its timing on postoperative hemodynamic and clinical outcomes. A previous review already mentioned the weakness of the association between ACEIs and post-cardiac surgery vasoplegia, but a causal relationship has been widely accepted in some cardiovascular anesthesiology communities[28].
This study has some limitations. The definition of vasoplegia was not exactly the same across studies, thereby introducing a potential bias. This is probably due to the fact that there is still no consensus definition of vasoplegia. To address this issue, we pooled together data from studies that used similar definitions and as a result, the level of heterogeneity was low in most analyses. Next, it is possible that the effect of RAS blockers on vasoplegia and inotrope use were confounded by LVEF. Patients receiving RAS blockers are more likely to have low LVEF as these drugs are guideline-recommended in this population. As patients with low LVEF are more likely to have a more complicated post-operative course, it is possible that it is the low LVEF that drove the results, rather than the use of RAS blockers. Unfortunately, stratified analyses by LVEF were not available in the included studies. Moreover, there was no standardized perioperative medication management across studies. It is unclear whether patients reportedly not on ACEI ever took one, or whether some of them were chronically on ACEI but stopped few days before surgery. Another limitation is the relatively low number of eligible studies, which makes some of our estimates less robust. Furthermore, the association between vasoplegia and other factors such as age, CPB time, left ventricular dysfunction or preoperative beta blockers were not preplanned. These variables were not specifically included in the search strategy. Therefore, it is possible that some studies reporting on their attributable risk of vasoplegia were missed. Our findings related to these variables should therefore be interpreted with caution. Despite these limitations, our study is the first to systematically present the discrepancy between observational studies and RCT’s on the effect of preoperative RAS blockage and the risk of vasoplegia.
Our meta-analysis shows that preoperative continuation of ACEIs is associated with an increased risk of vasoplegia and of the use of inotropic support postoperatively. These findings support the potential benefit of holding ACEIs prior to cardiac surgery to reduce the risk of vasoplegia and associated adverse outcomes. A consensus definition of vasoplegia may help future RCTs properly assess management strategies of RAS blockers in the perioperative setting.
Vasoplegia is a common complication of cardiac surgery. The use of some medications prior to surgery is thought to contribute to inappropriate vasodilatation in vasoplegia. The causal relationship between preoperative use of renin angiotensin system (RAS) blockers [angiotensin converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARB)] between vasoplegia is unclear.
If perioperative use of RAS blockers is associated with vasoplegia, withholding these medications in patients undergoing cardiac surgery might help preventing vasoplegia after cardiac surgery.
To update and summarize data on the effect of preoperative use of RAS blockers on incident vasoplegia.
The authors performed a systematic review of the literature, and summarized available data using a random-effects meta-analysis.
Ten studies reported on a pooled population of 15672 patients were included in the meta-analysis. Use of ACEIs was associated with an increased risk of vasoplegia and increased inotropic/vasopressor support requirement. Left ventricular dysfunuction increased the risk of post-cardiac surgery shock. There was no association between continuation of RAS blockers and vasoplegia in the two included randomized control trials (RCTs) .
Preoperative continuation of ACEIs is associated with an increased risk of the use of inotropic support postoperatively and vasoplegia in observational studies but not in RCTs.
Further studies are needed to clarify the relationship between perioperative use of RAS blockers and vasoplegia after cardiac surgery. Such studies should use a consensus definition of vasoplegia and conduct appropriate perioperative cardiovascular monitoring.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: World Heart Federation; Heart Rhythm Society.
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cure E, Turkey; Glumac S, Croatia S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Lambden S, Creagh-Brown BC, Hunt J, Summers C, Forni LG. Definitions and pathophysiology of vasoplegic shock. Crit Care. 2018;22:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 2. | Levin MA, Lin HM, Castillo JG, Adams DH, Reich DL, Fischer GW. Early on-cardiopulmonary bypass hypotension and other factors associated with vasoplegic syndrome. Circulation. 2009;120:1664-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 3. | Weis F, Kilger E, Beiras-Fernandez A, Nassau K, Reuter D, Goetz A, Lamm P, Reindl L, Briegel J. Association between vasopressor dependence and early outcome in patients after cardiac surgery. Anaesthesia. 2006;61:938-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Barnes TJ, Hockstein MA, Jabaley CS. Vasoplegia after cardiopulmonary bypass: A narrative review of pathophysiology and emerging targeted therapies. SAGE Open Med. 2020;8:2050312120935466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Suga M, Kawakami D, Ueta H, Shimozono T, Ito J, Seo R, Nakamori Y, Korenaga A, Morimoto T, Mima H. Longer term hemodialysis-dependent chronic renal failure increases the risk of post-cardiac surgery vasoplegic syndrome. J Anesth. 2020;34:243-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Shahzamani M, Yousefi Z, Frootaghe AN, Jafarimehr E, Froughi M, Tofighi F, Azadani AN, Pourhoseingholi MA, Azadani PN. The effect of angiotensin-converting enzyme inhibitor on hemodynamic instability in patients undergoing cardiopulmonary bypass: results of a dose-comparison study. J Cardiovasc Pharmacol Ther. 2009;14:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Mekontso-Dessap A, Houël R, Soustelle C, Kirsch M, Thébert D, Loisance DY. Risk factors for post-cardiopulmonary bypass vasoplegia in patients with preserved left ventricular function. Ann Thorac Surg. 2001;71:1428-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 110] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Disque A, Neelankavil J. Con: ACE Inhibitors Should Be Stopped Prior to Cardiovascular Surgery. J Cardiothorac Vasc Anesth. 2016;30:820-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14425] [Cited by in RCA: 16804] [Article Influence: 672.2] [Reference Citation Analysis (0)] |
| 10. | CLARITY Group. Tool to Assess Risk of Bias in Case Control Studies. Available from: https://www.evidencepartners.com/resources/methodological-resources/tool-to-assess-risk-of-bias-in-case-control-studies-distillersr. |
| 11. | CLARITY Group. Tool to Assess Risk of Bias in Randomized Controlled Trials. Available from: https://www.evidencepartners.com/resources/methodological-resources/tool-to-assess-risk-of-bias-in-randomized-controlled-trials-distillersr. |
| 12. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 25818] [Article Influence: 1122.5] [Reference Citation Analysis (0)] |
| 13. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 40577] [Article Influence: 1449.2] [Reference Citation Analysis (2)] |
| 14. | Tuman KJ, McCarthy RJ, O'Connor CJ, Holm WE, Ivankovich AD. Angiotensin-converting enzyme inhibitors increase vasoconstrictor requirements after cardiopulmonary bypass. Anesth Analg. 1995;80:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Sun X, Zhang L, Hill PC, Lowery R, Lee AT, Molyneaux RE, Corso PJ, Boyce SW. Is incidence of postoperative vasoplegic syndrome different between off-pump and on-pump coronary artery bypass grafting surgery? Eur J Cardiothorac Surg. 2008;34:820-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Durán Bruce M, Gomar Sancho C, Holguera JC, Muliterno Español E. [Factors involved in the development of vasoplegia after cardiac surgery with extracorporeal circulation. A prospective observational study]. Rev Esp Anestesiol Reanim. 2014;61:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Carrel T, Englberger L, Mohacsi P, Neidhart P, Schmidli J. Low systemic vascular resistance after cardiopulmonary bypass: incidence, etiology, and clinical importance. J Card Surg. 2000;15:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 111] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Miceli A, Capoun R, Fino C, Narayan P, Bryan AJ, Angelini GD, Caputo M. Effects of angiotensin-converting enzyme inhibitor therapy on clinical outcome in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2009;54:1778-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Radaelli G, Bodanese LC, Guaragna JC, Borges AP, Goldani MA, Petracco JB, Piccoli Jda C, Albuquerque LC. The use of inhibitors of angiotensin-converting enzyme and its relation to events in the postoperative period of CABG. Rev Bras Cir Cardiovasc. 2011;26:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | van Diepen S, Norris CM, Zheng Y, Nagendran J, Graham MM, Gaete Ortega D, Townsend DR, Ezekowitz JA, Bagshaw SM. Comparison of Angiotensin-Converting Enzyme Inhibitor and Angiotensin Receptor Blocker Management Strategies Before Cardiac Surgery: A Pilot Randomized Controlled Registry Trial. J Am Heart Assoc. 2018;7:e009917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Pigott DW, Nagle C, Allman K, Westaby S, Evans RD. Effect of omitting regular ACE inhibitor medication before cardiac surgery on haemodynamic variables and vasoactive drug requirements. Br J Anaesth. 1999;83:715-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 91] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Klijian A, Khanna AK, Reddy VS, Friedman B, Ortoleva J, Evans AS, Panwar R, Kroll S, Greenfeld CR, Chatterjee S. Treatment With Angiotensin II Is Associated With Rapid Blood Pressure Response and Vasopressor Sparing in Patients With Vasoplegia After Cardiac Surgery: A Post-Hoc Analysis of Angiotensin II for the Treatment of High-Output Shock (ATHOS-3) Study. J Cardiothorac Vasc Anesth. 2021;35:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 23. | López-Sendón J, Swedberg K, McMurray J, Tamargo J, Maggioni AP, Dargie H, Tendera M, Waagstein F, Kjekshus J, Lechat P, Torp-Pedersen C; Task Force on ACE-inhibitors of the European Society of Cardiology. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease. The Task Force on ACE-inhibitors of the European Society of Cardiology. Eur Heart J. 2004;25:1454-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 183] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 24. | Oosterga M, Voors AA, Pinto YM, Buikema H, Grandjean JG, Kingma JH, Crijns HJ, van Gilst WH. Effects of quinapril on clinical outcome after coronary artery bypass grafting (The QUO VADIS Study). QUinapril on Vascular Ace and Determinants of Ischemia. Am J Cardiol. 2001;87:542-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 99] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Yacoub R, Patel N, Lohr JW, Rajagopalan S, Nader N, Arora P. Acute kidney injury and death associated with renin angiotensin system blockade in cardiothoracic surgery: a meta-analysis of observational studies. Am J Kidney Dis. 2013;62:1077-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | Bandeali SJ, Kayani WT, Lee VV, Pan W, Elayda MA, Nambi V, Jneid HM, Alam M, Wilson JM, Birnbaum Y, Ballantyne CM, Virani SS. Outcomes of preoperative angiotensin-converting enzyme inhibitor therapy in patients undergoing isolated coronary artery bypass grafting. Am J Cardiol. 2012;110:919-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Sousa-Uva M, Head SJ, Milojevic M, Collet JP, Landoni G, Castella M, Dunning J, Gudbjartsson T, Linker NJ, Sandoval E, Thielmann M, Jeppsson A, Landmesser U. 2017 EACTS Guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53:5-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 267] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 28. | Raja SG, Fida N. Should angiotensin converting enzyme inhibitors/angiotensin II receptor antagonists be omitted before cardiac surgery to avoid postoperative vasodilation? Interact Cardiovasc Thorac Surg. 2008;7:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |