Published online Dec 26, 2022. doi: 10.4330/wjc.v14.i12.657
Peer-review started: September 8, 2022
First decision: October 13, 2022
Revised: November 3, 2022
Accepted: November 22, 2022
Article in press: November 22, 2022
Published online: December 26, 2022
Processing time: 101 Days and 11.5 Hours
Wild-type transthyretin amyloidosis (ATTRwt) is the most common form of transthyretin amyloid cardiomyopathy, occurring mostly over age of 60 years (mean age of 80 years). Mean survival without treatment is 3.6 years, making early detection imperative. We report an unusual case of a 58-year-old patient with ATTRwt cardiomyopathy requiring heart transplantation.
A 58-year-old male presented with progressive fatigue, shortness of breath, weight gain, leg swelling, orthopnoea, and paroxysmal nocturnal dyspnoea for several months. Approximately ten months before this clinical presentation, the patient had first received a diagnosis of heart failure with reduced ejection fraction (EF) of 15% to 20%. The patient was started on appropriate guideline-directed medical therapy with only mild improvement in his EF. Upon further investigation, echocardiogram, technetium pyrophosphate scan (Tc PYP), and cardiac magnetic resonance imaging (cMRI) suggested a diagnosis of amyloidosis, and ATTRwt was subsequently confirmed with native heart tissue biopsy, congo red staining, liquid chromatography-tandem mass spectrometry, and genetic testing. The patient was successfully treated with heart transplantation and is doing well post-transplant.
Wild-type ATTR amyloidosis should be kept on differentials in all patients (even less than 60 years old) with non-ischemic cardiomyopathy, especially in the setting of increased ventricular wall thickness and other classic echocardiogram, cMRI, and Tc PYP findings. Early diagnosis and management can be consequential in improving patient outcomes.
Core Tip: Wild-type transthyretin amyloidosis (ATTRwt) continues to be an underdiagnosed condition. Although rare in patients under 60 years of age, physicians should include the condition in their differential as early diagnosis and early management can impact patient outcomes. Physicians should be aware of the findings on non-invasive testing that supports ATTRwt. Classically on echocardiogram, cardiac amyloidosis can present as thickened ventricular walls, small lehigh valley chamber, biatrial enlargement, apical sparing on longitudinal strain and signs of elevated filling pressures and restrictive diastolic physiology (increased E/A ratio, E/e’ and reduced mitral annular tissue velocities). Cardiac magnetic resonance imaging classically shows late gadolinium enhancement. A technetium pyrophosphate study shows an increased heart-to-contralateral ratio and increased Perugini visual grade.
- Citation: Boda I, Farhoud H, Dalia T, Goyal A, Shah Z, Vidic A. Early and aggressive presentation of wild-type transthyretin amyloid cardiomyopathy: A case report. World J Cardiol 2022; 14(12): 657-664
- URL: https://www.wjgnet.com/1949-8462/full/v14/i12/657.htm
- DOI: https://dx.doi.org/10.4330/wjc.v14.i12.657
Amyloid deposition is commonly found in aging populations. Transthyretin amyloid cardiomyopathy (ATTR-CM) is an infiltrative, progressive, and potentially fatal cardiomyopathy that is caused by extracellular deposition of misfolded transthyretin-derived insoluble amyloid fibrils in the myocardium[1]. Wild-type transthyretin amyloidosis (ATTRwt) is the most common type of ATTR-CM[2]. ATTRwt has increasingly been identified in patients greater than the age of 60-65 with an average age of diagnosis of 80 years old[2,3]. A retrospective review of 360 patients diagnosed with ATTRwt showed that 93% of the patients with an antemortem diagnosis were Caucasian males greater than 70 years old[4]. Another retrospective review showed only a handful of patients that were diagnosed with ATTRwt in their forties[2]. A recent case of ATTRwt was identified in a 34-year-old male in India[5]. Our case report highlights an unusual case of ATTRwt cardiomyopathy presenting in a relatively young, 58-year-old patient with end stage heart failure (HF) symptoms.
Progressive fatigue, shortness of breath, weight gain, leg swelling, orthopnoea, and paroxysmal nocturnal dyspnoea.
A 58-year-old Caucasian man presented to the heart failure clinic with progressive fatigue, shortness of breath, weight gain, leg swelling, orthopnoea, and paroxysmal nocturnal dyspnoea for several months. On review of symptoms, the patient had no palpitations, carpal tunnel symptoms, neuropathy, or back pain.
Approximately ten months before this clinic presentation, the patient had first received a diagnosis of HF with reduced ejection fraction (EF) of 15% to 20%. At that time, he underwent left heart catheterization which showed no obstructive coronary artery disease. The patient was started on appropriate guideline-directed medical therapy (GDMT) with only mild improvement in his EF. The patient was followed in the heart failure clinic and had recurrences of acute on chronic heart failure episodes. Unfortunately, due to borderline hypotension, he was unable to reach maximum doses of GDMT which ultimately had to be stopped.
His past medical history was significant for non-ischemic cardiomyopathy (EF 15%-20%), left ventricular hypertrophy, implantable cardioverter defibrillator in situ, atrial fibrillation, moderate to severe mitral regurgitation, prior left atrial thrombus, and prior tobacco use disorder. Other than atrial fibrillation, he had no significant history of other arrhythmias. He worked as a construction worker. His family history was notable only for his father who underwent a coronary artery bypass graft at age 59 years.
Physical exam was notable for the patient appearing well-nourished; however, jugular venous distension, a holosystolic murmur consistent with known mitral regurgitation, abdominal distention, and bilateral lower extremity edema were present. Vital signs depicted borderline systolic blood pressure of 95/70 mmHg with a mean of 78 mmHg, normal heart rate, normal respiratory rate and saturation on room air, and normal temperature.
The patient’s presentation to the clinic with progressively worsening heart failure symptoms prompted admission to the hospital.
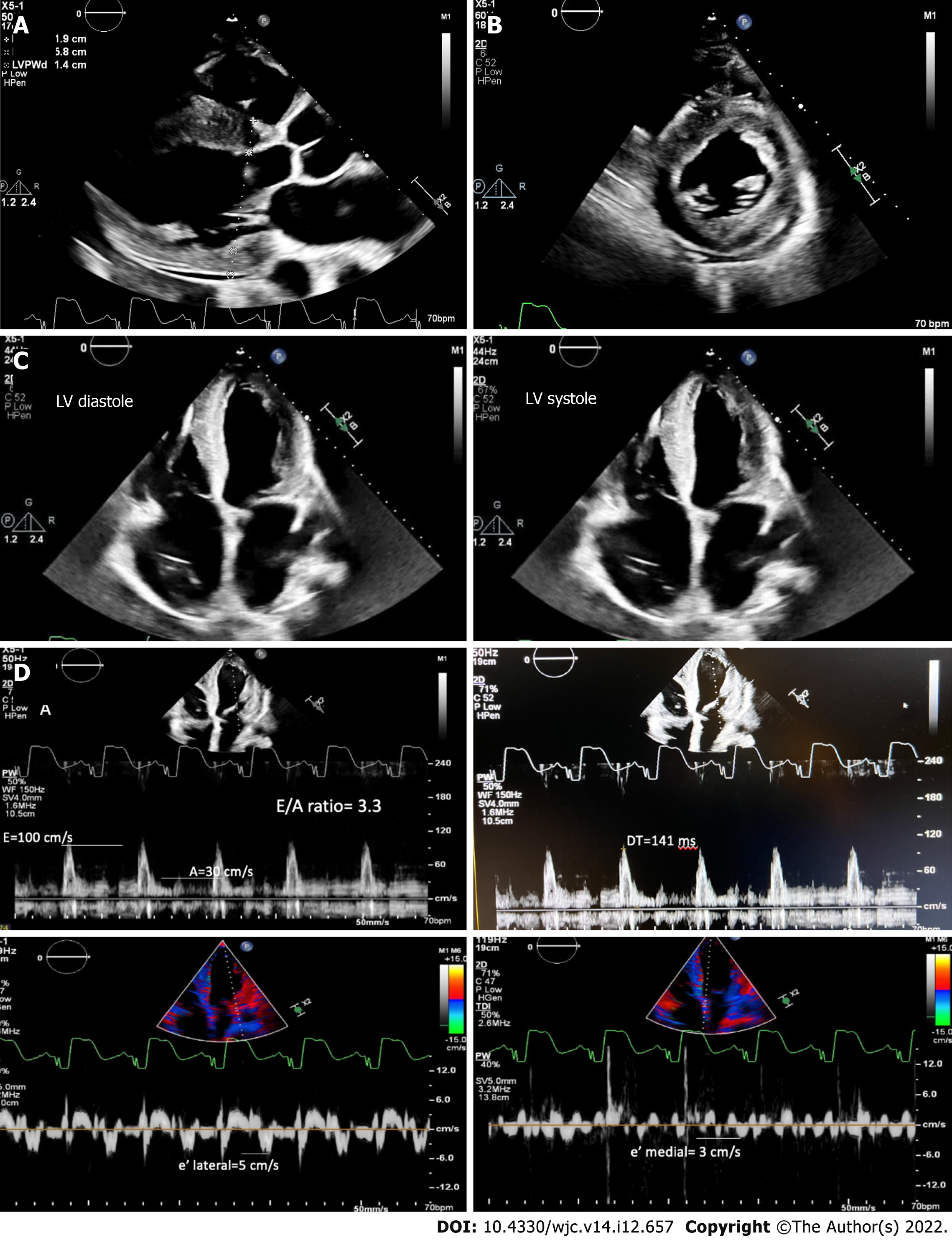
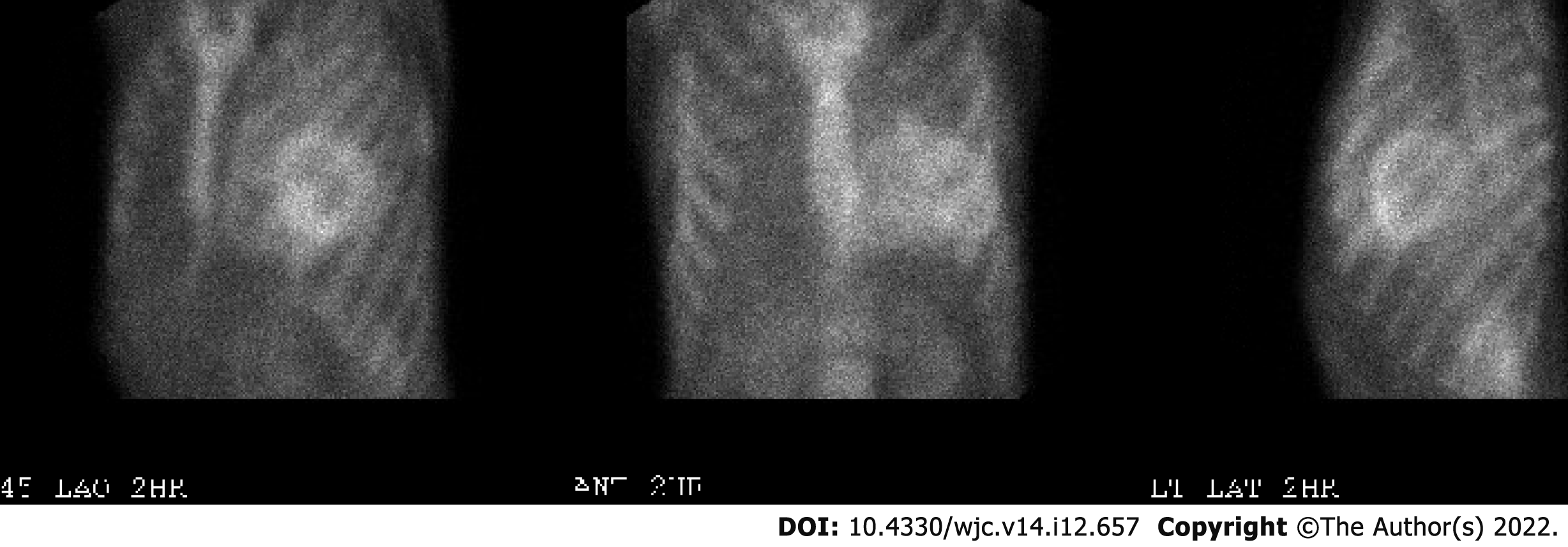
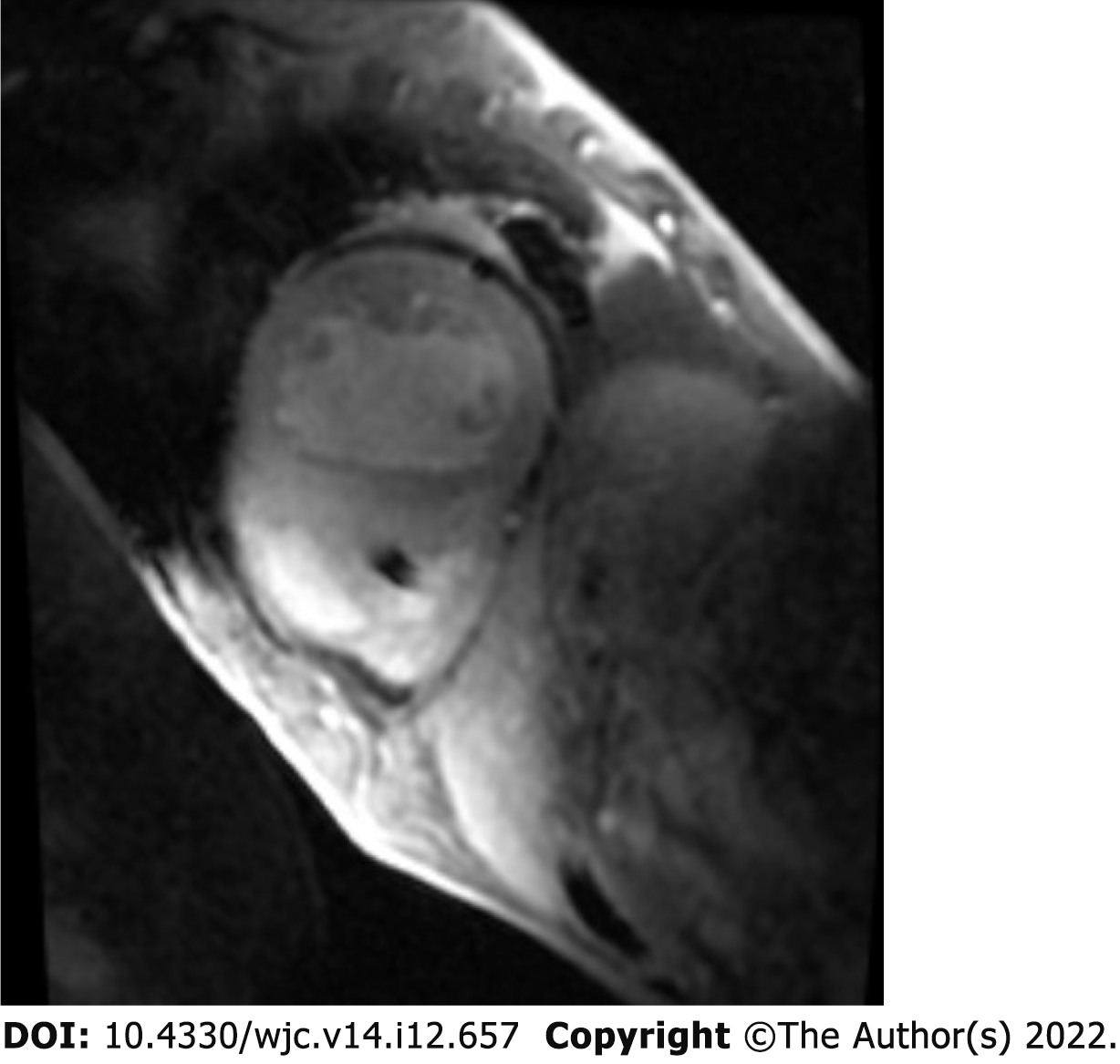
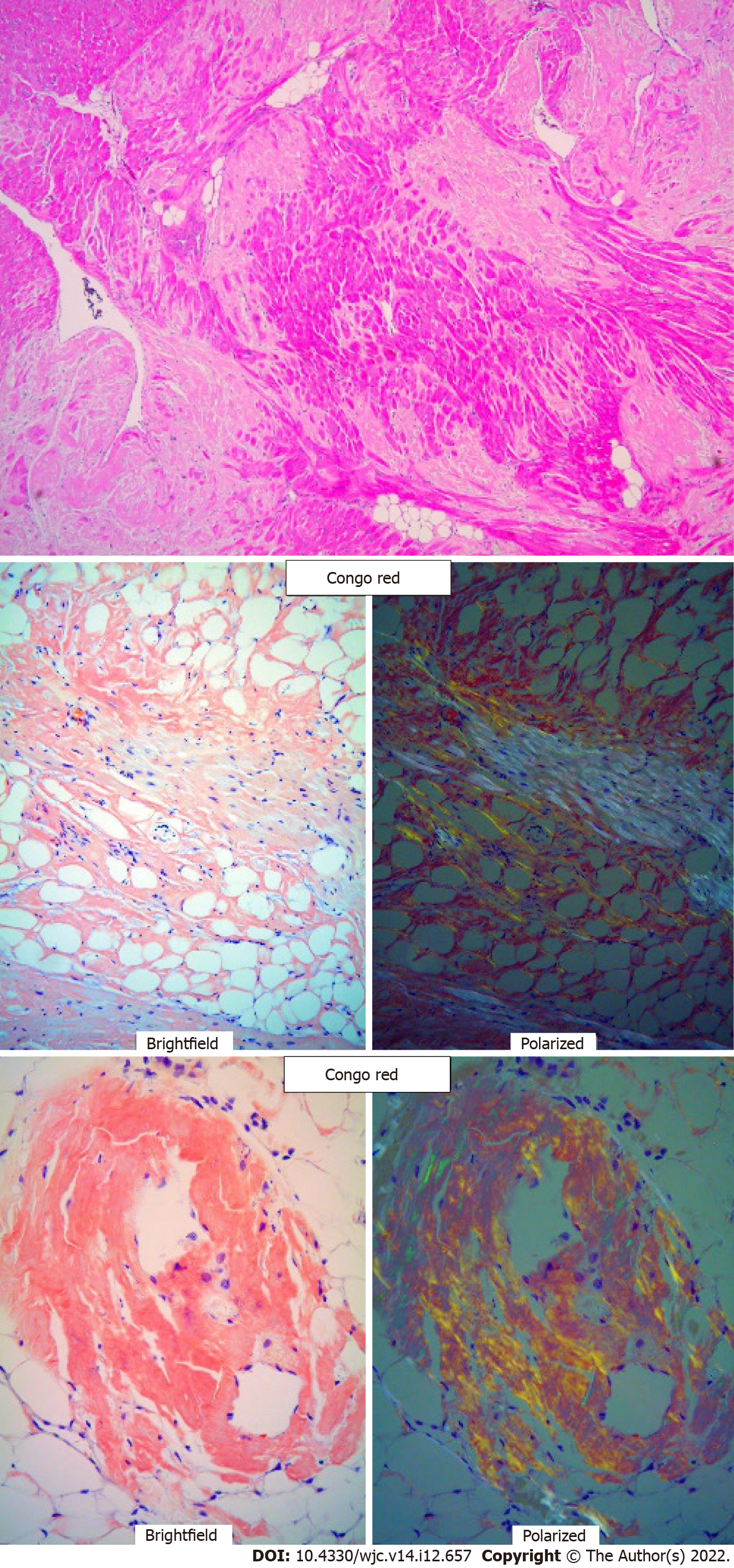
Electrocardiogram showed AV-paced complexes and low voltage in both limb and precordial leads. He underwent right heart catheterization which was consistent with cardiogenic shock with central venous pressure 18 mmHg, polymeraseacidicprotein 43/25 with mean of 32 mmHg, pulmonary capillary wedge pressure 23 mmHg, fick cardiac output of 4.6 L/min, and cardiac index of 2.1 L/min/m2. Transthoracic echocardiogram demonstrated a reduced EF of 10%, concentric hypertrophy with interventricular septal thickness of 1.9 cm and posterior wall thickness of 1.4 cm, moderately reduced right ventricle (RV) function with a dilated RV, moderate mitral regurgitation, and trivial pericardial effusion (Figure 1A-C). Diastology additionally showed an E/A ratio of 3.3, severely reduced mitral annular tissue velocities (e' medial of 3 cm/s and e' lateral of 5 cm/s), E/e' of 25, and deceleration time of 141 ms (Figure 1D). The increased myocardial wall thickness on echocardiogram as well as parameters consistent with restrictive pathology led to a detailed amyloidosis workup. A technetium pyrophosphate scan (Tc PYP) showed a heart/contralateral lung ratio of 1.77 and visual grade 3 as per the Perugini scale, highly suggestive of cardiac amyloidosis (Figure 2). Cardiac magnetic resonance imaging (cMRI) showed global myocardial delayed hyperenhancement and failure to null most likely due to cardiac amyloidosis (Figure 3). Amyloid light chain amyloidosis work-up including kappa and lambda light chains levels, serum protein electrophoresis, and urine protein electrophoresis was unremarkable. The native heart tissue pathology showed diffuse amyloid deposits between the myocardial fibrils, confirmed on Congo red staining (Figure 4). Liquid chromatography-tandem mass spectrometry was also performed on peptides extracted from the Congo red-positive areas of tissue and was consistent with ATTR-CM. The spectrometry did not detect an amino acid sequence abnormality in the transthyretin protein, suggesting ATTRwt as the diagnosis. Genetic testing was performed and was unremarkable, showing two variants of unknown significance, c.3262-3C>G in the LAMA4 gene and p.K963E in the RBM20 gene. Screening of the TTR gene was unremarkable ruling out hereditary TTR cardiomyopathy.
In conclusion, the history, exam, and workup, including genetic testing, confirmed ATTRwt as the final diagnosis.
The patient was initially supported with inotropes; however, given the concern for end-stage cardiac amyloidosis and risk for progressive cardiogenic shock in the setting of biventricular failure, orthotopic heart transplant evaluation was initiated. He was deemed a suitable candidate for heart transplantation by our multidisciplinary transplant committee. The patient was maintained on milrinone and epinephrine for inotropic support until he underwent transplantation.
Unfortunately, a few days following the transplant, the patient had pericardial bleeding with tamponade followed by successful evacuation of blood. He had no other postoperative complications and was discharged home on postoperative day eight. The patient has followed closely in the heart failure clinic and is doing very well post-transplant.
ATTRwt has increasingly been identified in patients greater than the age of 60-65 with an average age of diagnosis of 80 years old. Although ATTRwt is rare in younger populations, physicians should consider ATTRwt in their differential. Our patient not only developed ATTRwt below the age of 60, but also within 1 year of the patient’s HF diagnosis that rapidly progressed to end-stage cardiomyopathy. After review of the literature, it remains uncertain why some individuals may be predisposed to developing ATTRwt at an earlier age. However, early diagnosis can lead to improved management and outcomes as ATTRwt continues to be an underdiagnosed condition.
Classically on echocardiogram, cardiac amyloidosis can present as thickened ventricular walls, small lehigh valley chamber, valve thickening, atrial enlargement, apical sparing on strain and signs of elevated filling pressures, restrictive diastolic physiology (increased E/A ratio, E/e’ and reduced mitral annular tissue velocities)[6]. In patients with ATTR-CM amyloidosis, late gadolinium enhancement (LGE) is almost always present on cMRI. Studies have shown with LGE cMRI, an inability to suppress or “null” the myocardial signal or the presence of diffuse subendocardial or transmural enhancement patterns, which suggests amyloidosis with a sensitivity and specificity that approach 85%-90%[7,8]. cMRI also shows elevated native T1 values and increased extracellular volume[7]. Bone scans using Tc PYP scan show increased 99mTc-PYP uptake in the heart of patients with amyloid infiltration leading to increased heart-to-contralateral ratio and visual grading[3]. The non-biopsy diagnosis of ATTR-CM with TcPYP scan (first described by Gillmore et al[9]) is now widely accepted and has replaced the historical endocardial biopsy provided AL amyloidosis is ruled out.
In ATTR-CM, several new pharmaceutical therapies that target the disease at various levels have emerged, including TTR stabilizers (example: Tafamidis), and antisense oligonucleotides, RNA interference (example: Patisiran, Vutrisiran and Inotersen). Tafamidis is the only drug approved for ATTR cardiomyopathy so far, although trials with patisiran, vutrisiran, inotersen and eplontersen are underway. All of these are most effective when administered prior to significant cardiac dysfunction[3,10]. There are rare and isolated case reports of amyloid cardiac deposition recurrence post-transplant[11]. Since there are very few cases of heart transplantation for ATTR-CM reported in the literature given that most patients have a median age of 80, it is unknown how to best prevent the deposition of amyloid again in the transplanted heart. Our patient remains on Tafamidis post-transplant with the hope of stabilizing TTR protein tetramer and preventing amyloid fibrils from depositing in the myocardium.
Hence, it is imperative to diagnose ATTR-CM at earlier stages with available non-invasive testing and FDA approved treatments. Heart transplantation can be considered in select patients with Stage D HF[12]. The current allocation system provides priority as Status 4 to these stage D ATTR-CM patients due to a lack of durable mechanical support options[10].
Although ATTRwt is rare in younger populations, physicians should consider ATTRwt in their differential, especially in non-ischemic cardiomyopathy patients with thickened interventricular septum, posterior wall thickness, and arrhythmias especially atrial fibrillation. Early diagnosis of ATTRwt combined with newer therapies can be consequential in increasing patients’ quality of life and survival[3].
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Teragawa H, Japan; Wixner J, Sweden S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Yamamoto H, Yokochi T. Transthyretin cardiac amyloidosis: an update on diagnosis and treatment. ESC Heart Fail. 2019;6:1128-1139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 2. | Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin Amyloid Cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:2872-2891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 692] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 3. | Griffin JM, Rosenthal JL, Grodin JL, Maurer MS, Grogan M, Cheng RK. ATTR Amyloidosis: Current and Emerging Management Strategies: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2021;3:488-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 4. | Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, Klarich KW, Miller WL, Maleszewski JJ, Dispenzieri A. Natural History of Wild-Type Transthyretin Cardiac Amyloidosis and Risk Stratification Using a Novel Staging System. J Am Coll Cardiol. 2016;68:1014-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 530] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 5. | Ghosh S, Khanra D, Krishna V, Thakur AK. Wild type transthyretin cardiac amyloidosis in a young individual: A case report. Medicine (Baltimore). 2021;100:e25462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126:1286-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 512] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 7. | Martinez-Naharro A, Treibel TA, Abdel-Gadir A, Bulluck H, Zumbo G, Knight DS, Kotecha T, Francis R, Hutt DF, Rezk T, Rosmini S, Quarta CC, Whelan CJ, Kellman P, Gillmore JD, Moon JC, Hawkins PN, Fontana M. Magnetic Resonance in Transthyretin Cardiac Amyloidosis. J Am Coll Cardiol. 2017;70:466-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 305] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 8. | Zhao L, Tian Z, Fang Q. Diagnostic accuracy of cardiovascular magnetic resonance for patients with suspected cardiac amyloidosis: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2016;16:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 9. | Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, Wechalekar AD, Berk JL, Quarta CC, Grogan M, Lachmann HJ, Bokhari S, Castano A, Dorbala S, Johnson GB, Glaudemans AW, Rezk T, Fontana M, Palladini G, Milani P, Guidalotti PL, Flatman K, Lane T, Vonberg FW, Whelan CJ, Moon JC, Ruberg FL, Miller EJ, Hutt DF, Hazenberg BP, Rapezzi C, Hawkins PN. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation. 2016;133:2404-2412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 927] [Cited by in RCA: 1404] [Article Influence: 156.0] [Reference Citation Analysis (0)] |
| 10. | Kittleson MM, Maurer MS, Ambardekar AV, Bullock-Palmer RP, Chang PP, Eisen HJ, Nair AP, Nativi-Nicolau J, Ruberg FL; American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology. Cardiac Amyloidosis: Evolving Diagnosis and Management: A Scientific Statement From the American Heart Association. Circulation. 2020;142:e7-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 417] [Article Influence: 83.4] [Reference Citation Analysis (0)] |
| 11. | Fermin DR, Cohle SD, Twydell PT, Dickinson MG. Early Recurrence of Myocardial Transthyretin Amyloid Deposition Three Years Post Heart Transplantation for Hereditary V40I Amyloidosis. J Card Fail. 2019;25:S170. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Witteles RM. Cardiac Transplantation and Mechanical Circulatory Support in Amyloidosis: JACC: CardioOncology Primer. Cardio Oncology. 2021;3:516-521. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |