Published online Oct 26, 2022. doi: 10.4330/wjc.v14.i10.546
Peer-review started: July 2, 2022
First decision: August 1, 2022
Revised: August 27, 2022
Accepted: September 15, 2022
Article in press: September 15, 2022
Published online: October 26, 2022
Processing time: 109 Days and 4.4 Hours
Haemophilus parainfluenzae (HPI) belongs to the HACEK (Haemophilus spp., Aggregatibacter spp., Cardiobacterium spp., Eikenella spp., and Kingella spp.) group of organisms. The HACEK group of organisms are a part of the oropharyngeal flora and can cause invasive opportunistic infection such infective endocarditis (IE) in hosts with compromised immunological barriers.
To perform a 20-year systematic review of the literature characterizing the clinical presentation, epidemiology and prognosis of HPI IE.
We performed a systematic review of Medline, Pubmed, Scopus and Embase from 2000 to 2022 to identify all cases of HPI IE.
Thirty-nine adult cases were identified. HPI IE was found to affect males slightly more than females and is common in patients with predisposing risk factors such as underlying valvular abnormalities. It mostly affected the mitral valve and had an indolent course; significantly sized vegetations (> 1 cm) developed in most cases. Central nervous system septic embolization was common. It had a favo
Clinicians should be attentive to the indolent course of HPI IE and the presence of predisposing risk factors in order to allow for timely management.
Core tip: This review and illustrative case show a temporal change in the epidemiology of Haemophilus parainfluenzae (HPI) infective endocarditis in 2000–2022. Compared with a review of 26 HPI endocarditis cases from 1984–1995 by Darras-Joly et al, this review reported younger mean age, similar rate of infection in both genders, shorter time to diagnosis, higher association with intravenous drug use (IVDU), higher rate of embolic events in general, and tricuspid and pulmonic valve involvement. The rate of mitral valve involvement has remained steady over the past three decades, while there has been a decrease in the rate of aortic valve involvement. There has been a decrease in valvular vegetation rates and incidence of congestive heart failure as complications, while the mortality rate remained similar. These findings indicate improvement in diagnosis and treatment of HPI over the past three decades; however, they also suggest an increase in its virulence and an association with the rising rate of IVDU highlighted by the involvement of the right-sided heart valves.
- Citation: Olagunju A, Martinez J, Kenny D, Gideon P, Mookadam F, Unzek S. Virulent endocarditis due to Haemophilus parainfluenzae: A systematic review of the literature. World J Cardiol 2022; 14(10): 546-556
- URL: https://www.wjgnet.com/1949-8462/full/v14/i10/546.htm
- DOI: https://dx.doi.org/10.4330/wjc.v14.i10.546
Infective endocarditis (IE) remains a significant cause of morbidity and mortality. The incidence has increased from 5–7 cases per 100000 of the population in 2000 to 15 cases per 100000 person-years in 2011[1-3]. Common risk factors include an immunocompromised state, intravenous drug use (IVDU), underlying valvular disorders, prosthetic valves, and implanted cardiac devices[1-3]. The microbiology of IE is important and affects clinical presentation and prognosis[1-3]. Skin flora, including Staphylococcus, Enterococcus and Streptococcus spp., are the most common causative organisms in IE, accounting for 80%–90% of cases, with a mortality rate as high as 30%[1-3].
Although less common, the oropharyngeal flora is also an important cause of IE, particularly the HACEK (Haemophilus spp., Aggregatibacter spp., Cardiobacterium spp., Eikenella spp., and Kingella spp.) group[1,4-6]. This group has been identified in 1.5%–2% of all IE cases, with a mortality rate of 2%[4-6]. They are fastidious Gram-negative bacilli known for their slow growth in routine blood culture media, which may cause a delay in diagnosis[4,5]. Reported risk factors for the development of HACEK group IE include recent dental procedures and abnormal heart valves[4,5]. The most common organism implicated is Aggregatibacter spp[4,6]; however, IE due to Haemophilus parainfluenzae (HPI) is gaining increasing attention in the literature. Here, we present an illustrative case of endocarditis in a healthy young man with no predisposing risk factors, and a systematic review of HPI IE cases reported in the literature within the last 20 years to characterize its clinical presentation, epidemiology and prognosis.
A 25-year-old man with no significant past medical history presented to the emergency department with a 2-mo history of worsening frontal headache and 1 wk of fever and watery diarrhea. Physical examination was significant for a fever of 39.1 °C, heart rate of 109 beats/min, blood pressure of 118/63 mmHg, and holosystolic murmur auscultated at the cardiac apex. Laboratory results were remarkable for white blood count (WBC) count of 12.9 K/L (normal: 4–11), hemoglobin 8.5 g/dL (normal: 13.5–17.0), mean corpuscular volume 77 fL (normal: 78–100), relative distribution width of 16.2% (normal: 11–15), procalcitonin 2.33 ng/mL (normal: 0.49), C-reactive protein 218 mg/L (normal: 4.9), and erythrocyte sedimentation rate 56 mm/h (normal: 0–15). Intravenous (IV) vancomycin, ceftriaxone and acyclovir were initiated due to concern for meningitis. Chest radiograph and head computed tomography were negative for acute abnormalities. A lumbar puncture was performed, with cerebrospinal spinal fluid (CSF) analysis positive for WBC count of 231/mm3 (normal: 0–5), with 67% neutrophils and 21% lymphocytes, glucose 51 mg/dL (normal: 40–70), and protein f 49.9 mg/dL (normal: 15–40). CSF herpes simplex virus polymerase chain reaction was negative, and acyclovir was discontinued.
Blood cultures on hospital day 4 showed Gram-negative rods, which speciated to HPI on hospital day 6. CSF cultures remained negative, and antibiotics were de-escalated to IV ceftriaxone for HPI bacteremia. Additionally, esophagogastroduodenoscopy, colonoscopy and subsequent biopsies were normal. Iron studies were significant for serum iron of 9 g/dL (normal: 40–190), transferrin 119 mg/dL (normal: 200–390), transferrin saturation 6% (normal: 15–50), total iron binding capacity 167.8 g/dL (normal: 250–435), and ferritin 1083 ng/mL (normal: 25–506).
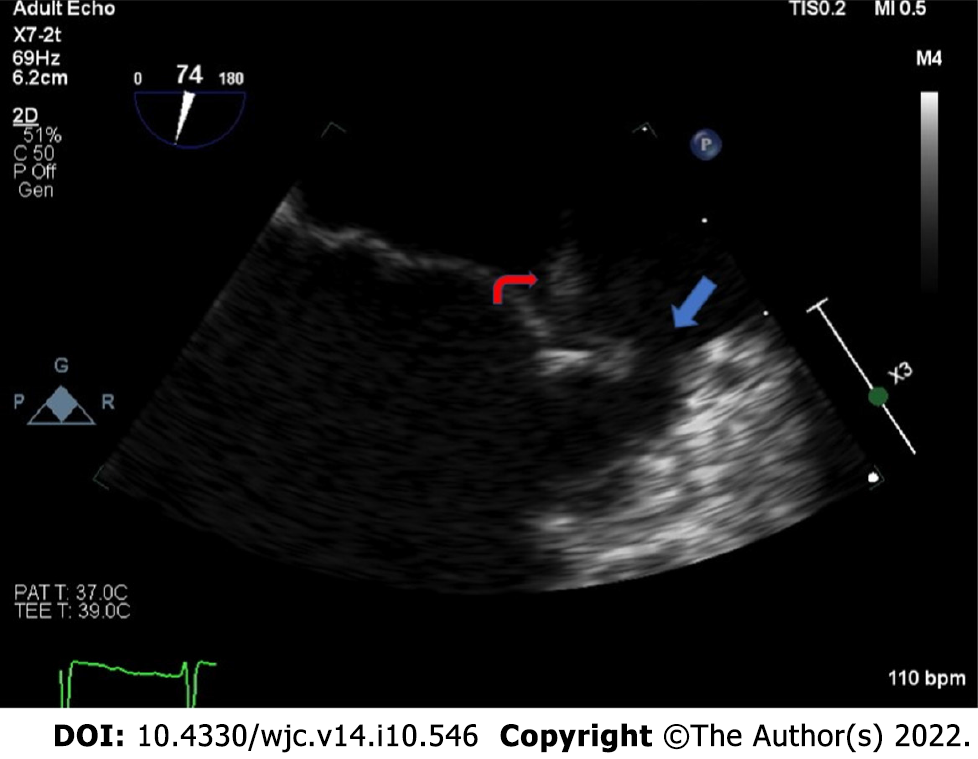
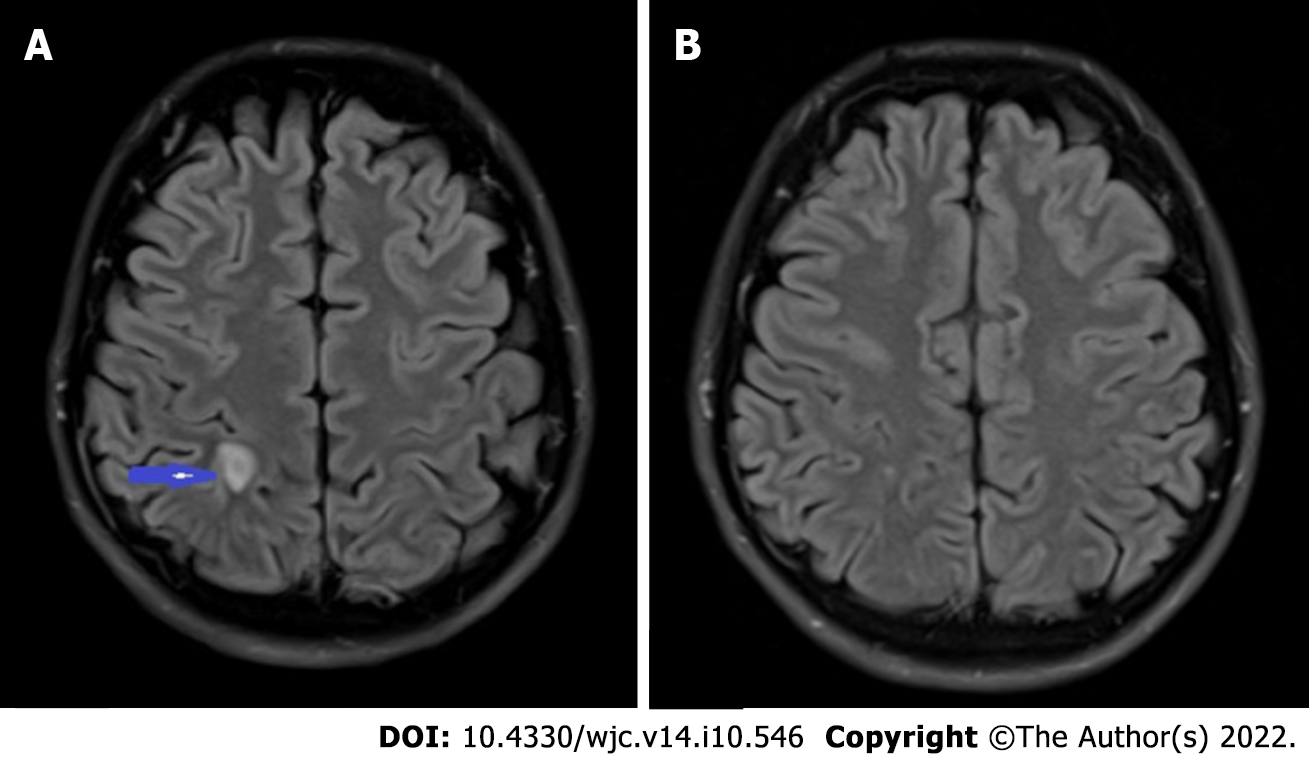
Transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) revealed two mobile echodensities on the atrial side of the mitral valve, consistent with vegetations on the A2 and P2 segments of the mitral leaflets (Figure 1), with an anterior leaflet perforation and a severe mitral regurgitation (Videos 1 and 2). Magnetic resonance imaging (MRI) of the brain revealed a 1.0 0.5 cm ring enhancing lesion in the right parietal lobe with surrounding vasogenic edema, suggestive of an abscess secondary to septic emboli (Figure 2A). Repeat MRI brain at 4 wk revealed near resolution of the right parietal lobe lesion (Figure 2B). After completing 8 wk of ceftriaxone, he underwent mitral valve repair with edge-to-edge repair of A1 and P1 segments. Postoperative TEE revealed adequate A1 and P1 fusion. The postoperative course was complicated by left-sided proximal muscle weakness and paresthesia, which resolved within 48 h. He completed cardiac rehabilitation successfully and had no further complications.
Two authors (AO and DK) independently searched Medline, Pubmed, Scopus, Embase and Reference Citation Analysis from January 1, 2000 to March 30, 2022 using the following keywords: Haemophilus parainfluenzae and infective endocarditis. An independent search was conducted by a qualified librarian using similar terms. Only articles published in English were included.
Inclusion criteria included IE due to HPI, patients aged > 18 years, positive blood or pathology specimens for HPI, and clinical and echocardiographic evidence of IE. Articles not meeting these criteria were excluded. The study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA)[7] (PRISMA 2009 Checklist).
Extracted data included patient demographics (age and gender), symptoms at initial presentation, comorbidities (prior valvular disorder, structural heart defects, recent dental, and gastrointestinal or genitourinary procedures), affected valves, severity of valvular damage, patient management, and complications.
We conducted a qualitative systematic analysis using descriptive statistics. A meta-analysis could not be performed due to the differences among individual cases and the small sample sizes (i.e. 1 patient) included in the case reports.
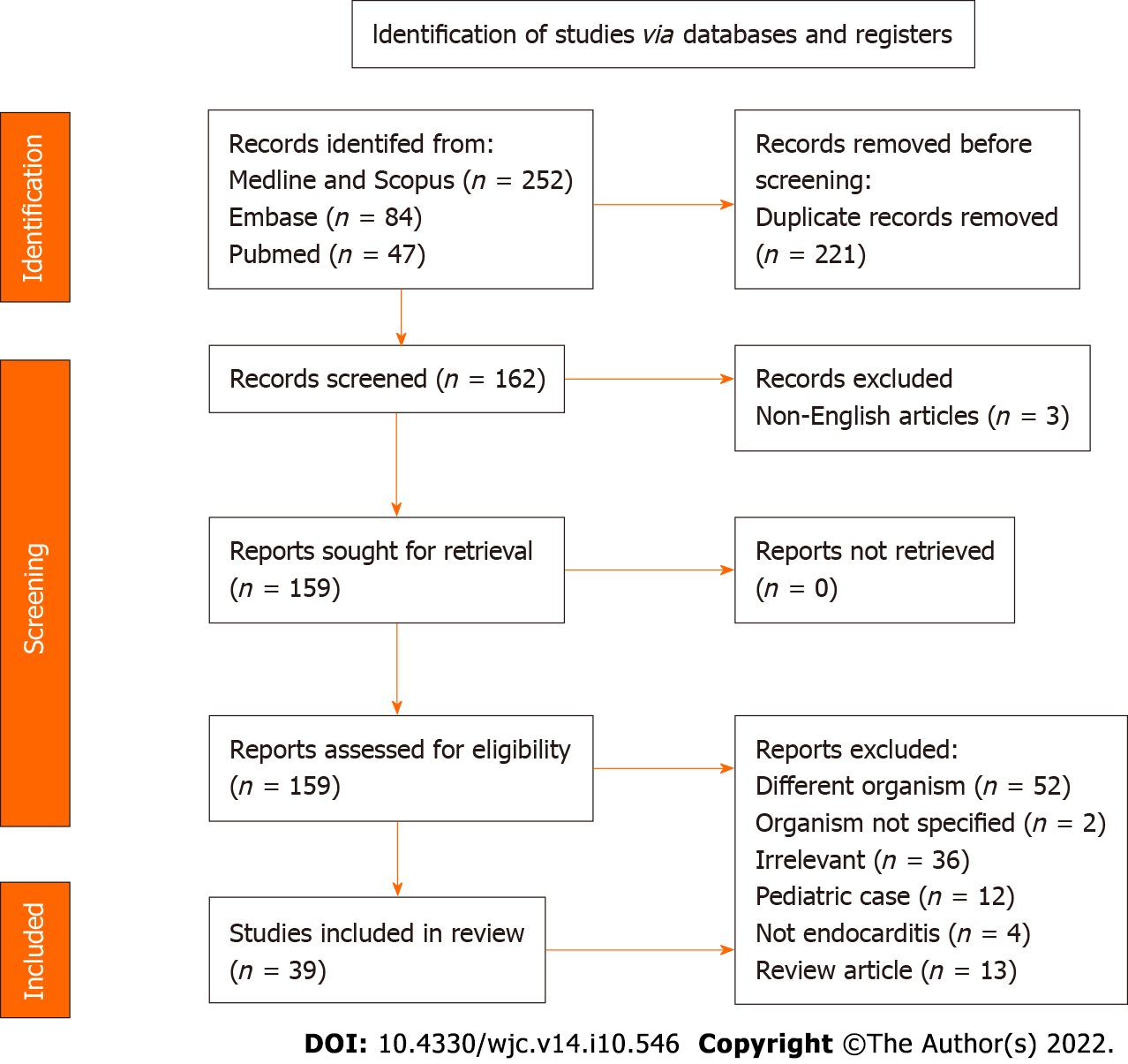
Our initial search generated 383 articles. After excluding 221 duplicates, the remaining 162 articles were screened for inclusion (Figure 3). Of these, 39 articles[8-46] were systematically reviewed. The remaining articles were excluded because they were irrelevant to the topic (36 articles), discussing IE with organisms other than HPI (52 articles), review articles on HACEK organisms and IE (13 articles), pediatric case reports on HPI IE (12 articles), or not published in English (3 articles).
Age and gender: A total of 39 patients were identified. The mean age was 39 years, with a range of 18–69 years. There was a slight predominance towards men (52.5%).
Predisposing risk factors for IE: Approximately 10% of the patients reported a history of IE. About 17.5% had a history of valve replacement (4 with bioprosthetic valves, 2 with mechanical valves, and 1 with unspecified valve type). Twenty percent had mitral valve disorders (3 with mitral valve prolapse, 2 with rheumatic heart disease, and 1 with mitral regurgitation). Eighteen percent had aortic valve disorders (3 with bicuspid aortic valve and 3 with aortic stenosis). Current IVDU was reported in 17.5%. Approximately 10% had poor dentition. Thirteen percent had a history of pacemaker and implanted cardiac defibrillator placement. Two patients had a recent gastrointestinal or genitourinary tract procedure. Three patients were immunocompromised, 2 of which were pregnant (Table 1). Seven of the 39 patients had no predisposing risk factors.
| Characteristics | Present review, n (%) | Previous review, n (%) | |
| Mean duration from sx to dx | 18.9 (3-49) d | 28.2 (5-80) d | |
| Demographics | Age, yr (range) | 39 (18-69) | 43.9 (21-79) |
| Male-to-female ratio | Approximately 1:1 | Approximately 2:1 | |
| Predisposing risk factors | History of IE | 4 (10) | 1 (3.8) |
| Valve disorder and/or replacement | 10 (25) | 18 (69.2) | |
| IVDU | 8 (20) | 1 (2.4) | |
| Poor dentition | 4 (10) | NR | |
| Cardiac devices | 5 (13) | 4 (15.4) | |
| History of recent GI/GU procedure | 2 (5) | NR | |
| Immunocompromised | 3 (8) | 3 (7.1) | |
| Symptoms and signs | Fever | 36 (90) | 40 (95.2) |
| Fatigue | 24 (60) | NR | |
| Vegetations | 24 (60) | 19 (73.1) | |
| Abscesses | 7 (17.5) | 2 (7.7) | |
| Embolic events | 29 (73) | 15 (35.7) | |
| CNS | 20 (50) | 9 (21.4) | |
| Lungs | 4 (10) | 2 (4.8) | |
| Spleen | 3 (7.5) | 2 (4.8) | |
| Kidney | 2 (5) | 1 (2.4) | |
| Valves affected | Mitral | 29 (73) | 19 (73) |
| Aorta | 8 (20) | 8 (30.7) | |
| Tricuspid | 7 (17.5) | 1 (3.8) | |
| Pulmonic | 1 (2.5) | 0 (0) | |
| Multiple valves | 6 (15) | 3 (11.5) | |
| Outcomes | Recovery with antibiotics and/or surgery | 35 (88) | NR |
| CHF | 3 (8) | 13 (30.9) | |
| Death | 2 (5) | 2 (4.8) |
Presenting symptoms and signs: The most common presenting symptom was fever, reported in most patients (36 patients), followed by fatigue (25 patients). Seven patients had shortness of breath, and four reported weight loss. Twenty-eight of 39 patients also presented with one or more manifestations of septic emboli, including embolic stroke (20 patients), septic pulmonary embolism (4 patients), renal emboli (2 patients), and splenic infarct (3 patients). Cutaneous manifestations were noted including Janeway lesions (3 patients), splinter hemorrhages (4 patients), petechiae or purpura (2 patients), or Osler nodes (1 patient) (Table 1).
Valve involvement: Valvular regurgitation was by far the most common abnormality; reported in 28 patients. Of these, 14 had severe regurgitation, eight had moderate regurgitation, and two had mild regurgitation. Mitral regurgitation and stenosis were reported in one case, and mitral valve prolapse was reported in one case. The mitral valve was the most common valve to be affected; noted in 28 patients. Eight patients had aortic valve involvement. The tricuspid valve was affected in seven patients, and only one patient had pulmonary valve involvement (Table 1).
Echocardiography: TTE was the main diagnostic modality, utilized in 36 (90%) patients, followed by TEE for confirmation in 33 patients. Valvular vegetations were reported in 23 patients, with an estimated mean size of 1.9 cm. Cardiac abscesses were reported in 17.5%, but abscess size was reported in only one of the cases as 1.6 1.8 cm. The abscess locations included the aortic root, mitral–aortic intervalvular fibrosa, near a prosthetic aortic valve, left ventricular endocardium, and myocardium. Three patients developed a fistulous connection between the atrium and ventricle. Valvular perforation was reported in 2 cases.
Treatment: The majority (28 patients) were treated both medically and surgically. Nine patients underwent valve repair, while six underwent replacement. Two patients underwent pacemaker removal. Eleven patients had unspecified surgical intervention. Sixty-two percent of patients were treated with ceftriaxone. Ten percent received other antibiotics including levofloxacin, ciprofloxacin, gentamicin, cefotaxime and rifampin. The antibiotic therapy utilized in the remaining 28% of patients was not specified.
Outcome: Two patients reportedly developed congestive heart failure (CHF) and two patients died. The remaining 35 patients recovered adequately.
HPI is a part of the oropharyngeal and genitourinary tract flora and has been implicated as a cause of opportunistic infections such as meningitis, IE, and septic arthritis[47]. It is a fastidious Gram-negative coccobacillus and belongs to the genus Haemophilus which consists of the H. influenzae, H. parainfluenzae and H. ducreyi groups[47]. They require beta-nicotinamide adenine dinucleotide (NAD) and/or heme to supplement in vitro growth[47]. An important differentiating feature of HPI is its ability to synthesize heme and hence does not require heme supplementation to grow[47].
The virulence of HPI is not well characterized[47,48]. In general, the H. parainfluenzae group has some degree of resistance to beta-lactam antibiotics, particularly penicillins[47,48]. Isolates have been iden
In our review, the majority of patients had at least one predisposing risk factor for IE, such as a history of IE, an underlying valve disorder, a prosthetic or mechanical valve or a cardiac device, poor dentition, recent dental procedure within 2 wk, IVDU, or an immunocompromised state (including use of steroids or pregnancy). This is important for clinicians to recognize, as eradication or control of the predisposing factor may help prevent recurrent HPI infection.
The average duration between symptom onset and diagnosis was 18.9 d, and surgical intervention (due to the presence of large vegetations ~2 cm) was required in most of the patients (69%). These features highlight the indolent course of HPI IE and signify the need for prompt diagnosis, which may reduce the need for surgical intervention. The resolution of IE with cephalosporin, aminoglycoside and fluoroquinolone antibiotics suggests that the majority of HPI bacteria in the past 20 years are not multidrug resistant.
The risk of embolic events in IE is common with Staphylococcus aureus, Candida spp., and HACEK organisms[51]. The reported incidence ranges between 28% and 66% for S. aureus, with central nervous system (CNS) embolism being the most common[53,54]. In this review, ~70% of embolic complications were in the CNS. This is notable as previously, Kingella spp. appeared to have the highest rate of CNS embolism of all HACEK organisms, with a rate of 20%–30%[55]. Embolic events have been associated with worse prognosis in IE, with the risk proportional to vegetation size > 10 mm[53,54]. The indolent or subacute course of HPI IE may explain why the mortality remains lower compared to IE involving other organisms, despite significant vegetation size. The in-hospital mortality rates of S. aureus and Streptococcus spp. IE are 20%–30% and 11%, respectively[53,56]. The mortality rate of HPI IE in this review was 5%. Of the HACEK organisms that cause IE in adults, Actinobacillus actinomycetemcomitans (a member of the Aggregatibacter spp.) and Cardiobacterium spp. have the highest reported mortality rates of 18% and 10%, respectively[57,58]. While both are associated more with aortic valve endocarditis[57,58], HPI more commonly affects the mitral valve.
Our findings and the illustrative case show a temporal change in the epidemiology of HPI within 2000–2022. Compared to a review of 26 HPI endocarditis cases from 1984 to 1995 by Darras-Joly et al[58], this review reported a younger mean age, similar rate of infection in both genders, shorter time to diagnosis, higher association with IVDU, higher rate of embolic events, and tricuspid and pulmonic valve involvement. The rate of mitral valve involvement has remained steady over the past three decades, while there has been a decrease in the rate of aortic valve involvement. Valvular vegetation rates and CHF incidence have decreased, while the mortality rate remained similar (Table 1). These findings might indicate the improvement in the diagnosis and treatment of HPI over the past three decades. However, the increased involvement of right-sided valves suggests an increase in its virulence and an association with the rising rate of IVDU. Notably, the review by Darras-Joly et al[58] was not systematic because it was limited to cases in France. To the best of our knowledge, this is the first systematic review HPI IE to be published in the English language literature.
Our patient’s presentation of subacute IE highlights the typical features of HPI IE. It is indolent, has a predilection for the mitral valve, and is commonly associated with septic emboli involving the CNS. However, multiple features were present suggesting HPI may be more virulent in the current era, including the patient’s absence of risk factors, HPI induced leaflet perforation (which was not noted in our review), and valvular destruction requiring surgery.
A noteworthy limitation of this review is that it did not account for unreported cases of HPI IE; therefore, we cannot ascertain an exact incidence and prevalence.
This systematic review of reported adult HPI IE cases spanning the last two decades highlights the subacute course of HPI IE, its preference for the mitral valve, and favorable prognosis compared to IE caused by the other HACEK organisms, Staphylococcus, and Streptococcus. Clinicians should be attentive to its indolent course and the presence of predisposing risk factors in order to allow for timely management.
Existing data indicate that the incidence of infective endocarditis (IE) continues to rise steadily. Although components of the skin flora including Staphylococcus spp., Streptococcus spp., and Enterococcus spp. are the most implicated organisms particularly in virulent IE, the oropharyngeal flora including the HACEK group of are a significant cause of IE.
An interesting presentation of Haemophilus parainfluenza (HPI) IE in a 25-year-old man with no significant past medical history and no predisposing risk factor for IE was the basis for this systematic review. It aimed to determine if there have been temporal changes in the presentation and prognosis of IE caused by HPI over the past two decades.
To characterize the risk factors, signs and symptoms, echocardiographic findings and the prognosis of IE caused HPI.
A search of Medline, Pubmed, Scopus and Embase was conducted to identify the cases of HPI IE published in 2000–2022. A systematic review of these cases was performed to analyze the trends in the presentation and prognosis of HPI IE.
This systematic review of 39 HPI IE cases in the English literature highlights the slight male predominance of the disease, the nonspecific presentation with constitutional symptoms, the predilection for the mitral valve, a high rate of central nervous system embolic events and a lower mortality rate compared to IE caused by microbes of the skin flora.
HPI IE is an indolent disease that requires a high index of suspicion to diagnose and is associated with a favorable prognosis with timely intervention.
We have illustrated a case and conducted a two-decade systematic review of the HPI IE cases published in the English language literature. In doing so, we have highlighted its indolent course, presentation and prognosis. We have also compared our findings with those of a review of HPI IE cases between 1984 and 1995; in doing so, we have enumerated some temporal changes in this disease entity. These include a younger mean age of presentation, identical rate of infection between males and females, improvement in diagnosis, a higher rate of embolic events and an increasing association with intravenous drug use.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jayaweera J, Sri Lanka; Tousoulis D, Greece S-Editor: Liu JH L-Editor: Kerr C P-Editor: Liu JH
| 1. | Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O'Gara P, Taubert KA; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015;132:1435-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1569] [Cited by in RCA: 2098] [Article Influence: 209.8] [Reference Citation Analysis (1)] |
| 2. | Ambrosioni J, Hernandez-Meneses M, Téllez A, Pericàs J, Falces C, Tolosana JM, Vidal B, Almela M, Quintana E, Llopis J, Moreno A, Miro JM; Hospital Clinic Infective Endocarditis Investigators. The Changing Epidemiology of Infective Endocarditis in the Twenty-First Century. Curr Infect Dis Rep. 2017;19:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 3. | Rajani R, Klein JL. Infective endocarditis: A contemporary update. Clin Med (Lond). 2020;20:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 152] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 4. | Ambrosioni J, Martinez-Garcia C, Llopis J, Garcia-de-la-Maria C, Hernández-Meneses M, Tellez A, Falces C, Almela M, Vidal B, Sandoval E, Fuster D, Quintana E, Tolosana JM, Marco F, Moreno A, Miró JM; Hospital Clinic Infective Endocarditis Investigators. HACEK infective endocarditis: Epidemiology, clinical features, and outcome: A case-control study. Int J Infect Dis. 2018;76:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Chambers ST, Murdoch D, Morris A, Holland D, Pappas P, Almela M, Fernández-Hidalgo N, Almirante B, Bouza E, Forno D, del Rio A, Hannan MM, Harkness J, Kanafani ZA, Lalani T, Lang S, Raymond N, Read K, Vinogradova T, Woods CW, Wray D, Corey GR, Chu VH; International Collaboration on Endocarditis Prospective Cohort Study Investigators. HACEK infective endocarditis: characteristics and outcomes from a large, multi-national cohort. PLoS One. 2013;8:e63181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Bläckberg A, Morenius C, Olaison L, Berge A, Rasmussen M. Infective endocarditis caused by HACEK group bacteria-a registry-based comparative study. Eur J Clin Microbiol Infect Dis. 2021;40:1919-1924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13349] [Article Influence: 834.3] [Reference Citation Analysis (0)] |
| 8. | Nwaohiri N, Urban C, Gluck J, Ahluwalia M, Wehbeh W. Tricuspid valve endocarditis caused by Haemophilus parainfluenzae: a case report and review of the literature. Diagn Microbiol Infect Dis. 2009;64:216-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Ho HH, Cheung CW, Yeung CK. Septic peripheral embolization from Haemophilus parainfluenzae endocarditis. Eur Heart J. 2006;27:1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Bath AS, Zoltowska DM, Agrawal Y, Gupta V. Rare cause of subarachnoid haemorrhage. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Koshkelashvili N, Shah M, Codolosa JN, Climaco A. Polymicrobial infective endocarditis caused by Neisseria sicca and Haemophilus parainfluenzae. IDCases. 2016;4:3-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Todoran TM, Sobieszczyk PS, Levy MS, Perry TE, Shook DC, Kinlay S, Davidson MJ, Eisenhauer AC. Percutaneous extraction of right atrial mass using the Angiovac aspiration system. J Vasc Interv Radiol. 2011;22:1345-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Pai RK, Pergam SA, Kedia A, Cadman CS, Osborn LA. Pacemaker lead infection secondary to Haemophilus parainfluenzae. Pacing Clin Electrophysiol. 2004;27:1008-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Menegueti MG, Machado-Viana J, Gaspar GG, Nicolini EA, Basile-Filho A, Auxiliadora-Martins M. Ischemic Stroke and Septic Shock After Subacute Endocarditis Caused by Haemophilus parainfluenzae: Case Report. J Clin Med Res. 2017;9:71-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Bae JY, Murugiah K, McLeod GX, Anwer M, Howes CJ. Haemophilus Parainfluenzae mural endocarditis with large atrial septal defect and peripheral embolization. J Cardiol Cases. 2022;25:149-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Gagliardi R, Sensi C, Flaminio G, De Canale E, Vettor R, De Carlo E. Haemophilus parainfluenzae endocarditis in a low-risk woman: a case report. Clin Case Rep. 2021;9:e05066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Gabaldón-Pérez A, De la Espriella R, Merenciano-Gonzalez H, Santas E, Chorro FJ. Native mitral valve endocarditis complicated with abscess and fistulas: Diagnosis by three-dimensional transesophageal echocardiography. Rev Port Cardiol (Engl Ed). 2021;40:803-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Finch LC, Gerdzhikov S, Buttery R. Haemophilus parainfluenzae endocarditis presenting with symptoms of COVID-19. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Cannon JW, Hayanga JWA, Drvar TB, Ellison M, Cook C, Salman M, Roberts H, Badhwar V, Hayanga HK. A 34-Year-Old Male Intravenous Drug User with a Third Episode of Tricuspid Valve Endocarditis Treated with Repeat Valve Surgery. Am J Case Rep. 2021;22:e927385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Bridwell RE, Cibrario A, Long B, Cho AM. Multisystem organ failure secondary to Haemophilus parainfluenzae infective endocarditis on an ICD lead: A case report. Am J Emerg Med. 2019;37:1602.e1-1602.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | De Castro A, Abu-Hishmeh M, El Husseini I, Paul L. Haemophilus parainfluenzae endocarditis with multiple cerebral emboli in a pregnant woman with coronavirus. IDCases. 2019;18:e00593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Costescu Strachinaru DI, Chaumont M, Gobin D, Sattar L, Strachinaru M, Karakike E, Roman A, Konopnicki D. Hemophagocytic lymphohistiocytosis associated to Haemophilus parainfluenzae endocarditis- a case report. Acta Clin Belg. 2018;73:220-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | McCann N, Barakat MF, Schafer F. An aggressive form of Haemophilus parainfluenzae infective endocarditis presenting with limb weakness. BMJ Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Litmathe J, Fussen R, Heinzel A, Müller M, Sucker C, Tewarie L, Dafotakis M. An unusual agent for an unusual localization of infective endocarditis. Perfusion. 2017;32:691-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Duzenli AE, Dwyer J, Carey J. Haemophilus parainfluenzae Endocarditis Associated With Maxillary Sinusitis and Complicated by Cerebral Emboli in a Young Man. J Investig Med High Impact Case Rep. 2017;5:2324709617704003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Faure E, Cannesson O, Schurtz G, Coisne A, Vincentelli A, Faure K, Guery B. Haemophilus parainfluenzae endocarditis in young adults. Med Mal Infect. 2017;47:58-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Maruyama R, Yamada A, Sugiyama T, Ujihira K, Nishioka N, Iba Y, Hatta E, Kurimoto Y, Asaoka K, Nakanishi K, Sakai K. Mitral valve repair for endocarditis can be performed 3 days after repair of a bleeding mycotic brain aneurysm. J Thorac Cardiovasc Surg. 2016;151:e59-e61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Yong MS, Saxena P, Killu AM, Coffey S, Burkhart HM, Wan SH, Malouf JF. The Preoperative Evaluation of Infective Endocarditis via 3-Dimensional Transesophageal Echocardiography. Tex Heart Inst J. 2015;42:372-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Cunha BA, Brahmbhatt K, Raza M. Haemophilus parainfluenzae aortic prosthetic valve endocarditis (PVE) successfully treated with oral levofloxacin. Heart Lung. 2015;44:317-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Mehrzad R, Sublette M, Barza M. Polymicrobial endocarditis in intravenous heroin and fentanyl abuse. J Clin Diagn Res. 2013;7:2981-2985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Liang JJ, Swiecicki PL, Killu AM, Sohail MR. Haemophilus parainfluenzae prosthetic valve endocarditis complicated by septic emboli to brain. BMJ Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Saby L, Le Dolley Y, Laas O, Tessonnier L, Cammilleri S, Casalta JP, Raoult D, Habib G, Thuny F. Early diagnosis of abscess in aortic bioprosthetic valve by 18F-fluorodeoxyglucose positron emission tomography-computed tomography. Circulation. 2012;126:e217-e220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Wang'ondu RW, Murray TS. Relapse of polymicrobial endocarditis in an intravenous drug user. Yale J Biol Med. 2011;84:321-324. [PubMed] |
| 34. | Miquel-Goulenok T, Le Tohic A, Laurichesse JJ, Iung B, Leport C, Longuet P. Haemophilus parainfluenzae infective endocarditis associated with pelvic abscess: an uncommon complication of endometriosis. Clin Exp Obstet Gynecol. 2010;37:324-325. [PubMed] |
| 35. | Christou L, Economou G, Zikou AK, Saplaoura K, Argyropoulou MI, Tsianos EV. Acute Haemophilus parainfluenzae endocarditis: a case report. J Med Case Rep. 2009;3:7494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Wijesinghe N, Mills G, Lin Z, Waymouth S, Sebastian C, McAlister H. A case of Haemophilus Parainfluenzae endocarditis during pregnancy. Scott Med J. 2008;53:65. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Choi D, Thermidor M, Cunha BA. Haemophilus parainfluenzae mitral prosthetic valve endocarditis in an intravenous drug abuser. Heart Lung. 2005;34:152-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Friedel JM, Stehlik J, Desai M, Granato JE. Infective endocarditis after oral body piercing. Cardiol Rev. 2003;11:252-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Kelesidis T, Kelesidis I, Lewinski MA, Humphries R. Establishing diagnosis of Haemophilus parainfluenzae as etiology of culture-negative endocarditis using DNA sequence analysis on tissue specimen. Am J Med. 2011;124:e9-e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Redmond AM, Meiklejohn C, Kidd TJ, Horvath R, Coulter C. Endocarditis after use of tongue scraper. Emerg Infect Dis. 2007;13:1440-1441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Barreto Cortes M, Teixeira V, Fernandes SR, Rego F. Haemophilus parainfluenzae endocarditis with systemic embolisation following maxillary sinusitis. BMJ Case Rep. 2016;2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Alp E, Yildiz O, Aygen B, Sumerkan B. Haemophilus parainfluenzae endocarditis. Turk J Med Sci. 2003;33:397-400. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Giurgea LT, Lahey T. Haemophilus parainfluenzae Mural Endocarditis: Case Report and Review of the Literature. Case Rep Infect Dis. 2016;2016:3639517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 44. | Fazili T, James O, Wani L. A sixty-five-year-old female with pneumonia, endocarditis and meningitis. J Okla State Med Assoc. 2009;102:269-270. [PubMed] |
| 45. | Nguyen S, Fayad G, Modine T, Leroy O. Mitral acute bacterial endocarditis caused by HACEK microorganisms. J Heart Valve Dis. 2009;18:353-354. [PubMed] |
| 46. | Kiss G, Braunberger E. Emergency Valve Replacement Under Minimal Cardiopulmonary Bypass for a Patient With Infective Endocarditis and Large Brain Hematoma: A Case Report. A A Pract. 2018;10:144-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 47. | González-Díaz A, Tubau F, Pinto M, Sierra Y, Cubero M, Càmara J, Ayats J, Bajanca-Lavado P, Ardanuy C, Marti S. Identification of polysaccharide capsules among extensively drug-resistant genitourinary Haemophilus parainfluenzae isolates. Sci Rep. 2019;9:4481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Sierra Y, González-Díaz A, Tubau F, Imaz A, Cubero M, Càmara J, Ayats J, Martí S, Ardanuy C. Emergence of multidrug resistance among Haemophilus parainfluenzae from respiratory and urogenital samples in Barcelona, Spain. Eur J Clin Microbiol Infect Dis. 2020;39:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | García-Cobos S, Arroyo M, Campos J, Pérez-Vázquez M, Aracil B, Cercenado E, Orden B, Lara N, Oteo J. Novel mechanisms of resistance to β-lactam antibiotics in Haemophilus parainfluenzae: β-lactamase-negative ampicillin resistance and inhibitor-resistant TEM β-lactamases. J Antimicrob Chemother. 2013;68:1054-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Soriano F, Granizo JJ, Coronel P, Gimeno M, Ródenas E, Gracia M, García C, Fernández-Roblas R, Esteban J, Gadea I. Antimicrobial susceptibility of Haemophilus influenzae, Haemophilus parainfluenzae and Moraxella catarrhalis isolated from adult patients with respiratory tract infections in four southern European countries. The ARISE project. Int J Antimicrob Agents. 2004;23:296-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | Mayo JB, McCarthy LR. Antimicrobial susceptibility of Haemophilus parainfluenzae. Antimicrob Agents Chemother. 1977;11:844-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Oill PA, Chow AW, Guze LB. Adult bacteremic Haemophilus parainfluenzae infections. Seven reports of cases and a review of the literature. Arch Intern Med. 1979;139:985-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 53. | Thuny F, Di Salvo G, Belliard O, Avierinos JF, Pergola V, Rosenberg V, Casalta JP, Gouvernet J, Derumeaux G, Iarussi D, Ambrosi P, Calabró R, Riberi A, Collart F, Metras D, Lepidi H, Raoult D, Harle JR, Weiller PJ, Cohen A, Habib G. Risk of embolism and death in infective endocarditis: prognostic value of echocardiography: a prospective multicenter study. Circulation. 2005;112:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 435] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 54. | Joye R, Ceroni D, Beghetti M, Aggoun Y, Sologashvili T. Fulminant Infective Endocarditis Due to Kingella Kingae and Several Complications in a 6-Year-Old Girl: A Case Report. Front Pediatr. 2021;9:707760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Chamat-Hedemand S, Dahl A, Oestergaard L, Arpi M, Fosboel E, Boel J, Kaur K, Lauridsen T, Gislason G, Torp-Pedersen C, Bruun N. Independent risk factors of mortality in streptococcal infective endocarditis. Eur Heart J. 2021;42:(Supplement_1).. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 56. | Paturel L, Casalta JP, Habib G, Nezri M, Raoult D. Actinobacillus actinomycetemcomitans endocarditis. Clin Microbiol Infect. 2004;10:98-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 57. | Steinberg J, Burd E. Other Gram-Negative and Gram-Variable Bacilli. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. J Steinberg E Burd. 2015;2667-2683.e4. [DOI] [Full Text] |
| 58. | Darras-Joly C, Lortholary O, Mainardi JL, Etienne J, Guillevin L, Acar J. Haemophilus endocarditis: report of 42 cases in adults and review. Haemophilus Endocarditis Study Group. Clin Infect Dis. 1997;24:1087-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 2.8] [Reference Citation Analysis (0)] |