Published online Oct 26, 2022. doi: 10.4330/wjc.v14.i10.537
Peer-review started: May 29, 2022
First decision: June 8, 2022
Revised: June 18, 2022
Accepted: September 6, 2022
Article in press: September 6, 2022
Published online: October 26, 2022
Processing time: 144 Days and 0.2 Hours
Cryoballoon ablation (CBA) is recommended for patients with paroxysmal atrial fibrillation (AF) refractory to antiarrhythmic drugs. However, only 80% of patients benefit from initial CBA. There is growing evidence that pretreatment with angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) decreases the recurrence of AF postablation, particularly in nonparoxysmal AF undergoing radiofrequency ablation. The role of ACEIs and ARBs in patients with paroxysmal AF in CBA remains unknown. We decided to investigate the role of ACEIs and ARBs in preventing the recurrence of atrial arrhythmia (AA) following CBA for paroxysmal AF.
To investigate the role of ACEIs and ARBs in preventing recurrence of AA following CBA for paroxysmal AF.
We followed 103 patients (age 60.6 ± 9.1 years, 29% women) with paroxysmal AF undergoing CBA 1-year post procedure. Recurrence was assessed by documented AA on electrocardiogram or any form of long-term cardiac rhythm monitoring. A multivariable Cox proportional hazard model was used to assess if ACEI or ARB treatment predicted the risk of AA recurrence.
After a 1-year follow-up, 19 (18.4%) participants developed recurrence of AA. Use of ACEI or ARB therapy was noted in the study population. Patients on ACEI/ARB had a greater prevalence of hypertension and coronary artery disease. On a multivariate model adjusted for baseline demo
In our study population, preablation treatment with an ACEI or ARB had no influence on the recurrence of AA following CBA for paroxysmal AF.
Core tip: We investigated the role of angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) in preventing recurrence of atrial arrhythmias following cryoballoon ablation (CBA) for paroxysmal atrial fibrillation (AF). Outcomes of 103 patients were evaluated in a retrospective chart review. Preablation treatment with an ACEI or ARB had no influence on recurrence of AA following CBA for paroxysmal AF. To our knowledge, this study is the first of its kind to examine the effect of ACEI/ARB use in this exclusive subset of patients.
- Citation: Al-Seykal I, Bose A, Chevli PA, Hashmath Z, Sharma N, Mishra AK, Laidlaw D. Role of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in cryoballoon ablation outcomes for paroxysmal atrial fibrillation. World J Cardiol 2022; 14(10): 537-545
- URL: https://www.wjgnet.com/1949-8462/full/v14/i10/537.htm
- DOI: https://dx.doi.org/10.4330/wjc.v14.i10.537
Atrial fibrillation (AF) is a common arrhythmia that represents an evolving, global epidemic[1]. It is estimated that the number of Americans afflicted by AF will increase from the current 2.3 million to more than 10 million by 2050[2]. Due to a substantial increase in incidence and prevalence of AF over the past few decades, it presents a significant economic burden on the health care system[3]. In 2014, in the United States alone, an estimated 599790 emergency department visits, 453060 hospitalizations, and 21712 deaths were associated with AF as a primary medical diagnosis. Furthermore, the mean cost per hospitalization for patients with a primary diagnosis of AF was $88194[4].
Even though cryoballoon ablation (CBA) is beneficial in patients with paroxysmal AF refractory to antiarrhythmic drugs, only 80% of patients benefit from initial CBA[5-8]. Myocardial fibrosis is a known risk factor for the development of AF and angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are known agents that prevent remodeling. There is growing evidence that pretreatment with ACEIs and ARBs decreases the recurrence of AF postablation, particularly in nonparoxysmal AF undergoing radiofrequency ablation (RFA) [9]. The role of ACEIs and ARBs in patients with paroxysmal AF in CBA remains unknown. In this study, we aimed to investigate the role of ACEIs and ARBs in preventing recurrence of atrial arrhythmia (AA) following CBA for paroxysmal AF.
We performed a single-center, retrospective, cross-sectional study in a community-based tertiary care center in Worcester, MA, USA. Paroxysmal AF was defined as nonsustained episodes of AF converting to sinus rhythm in < 7 d. Patients were required to be on an ACEI or ARB for a minimum duration of 4 wk. Institutional review board approval was obtained before initiation of the study.
To be included in this study, participants had to be aged ≥ 18 years and have had CBA as a first or repeat procedure for paroxysmal AF between January 2015 and April 2018. We excluded all patients with a diagnosis of permanent and persistent AF. Following inclusion data on baseline demographics, concomitant comorbidities, clinical, laboratory, echocardiographic, pharmacological and ablation procedural details and outcome details were obtained by trained physicians from chart review.
All CBA were conducted under general anesthesia. Two sheaths (7 and 9 Fr) were placed in the left femoral vein following ultrasound-guided vascular access. With the fluoroscopic guidance, an intracardiac echocardiography (ICE) catheter was advanced into the right atrium and a decapolar mapping catheter was positioned within the coronary sinus. A trans-septal sheath was placed through the right femoral vein approach. Heparin was administered intravenously for an activated clotting time > 300 s to prevent intraprocedural thromboembolic events. Trans-septal access to the left atrium was obtained under fluoroscopic and ICE guidance. The trans-septal sheath was exchanged for a 12 Fr Flexcath sheath (Medtronic, Minneapolis, MN, USA) which was then utilized to advance a 28-mm Arctic Front Advance Cryoballoon ablation catheter (Medtronic) with the Achieve spiral mapping catheter into the left atrium. An electroanatomic map of the left atrium and all pulmonary veins was performed utilizing 3-dimentional mapping software (Abbott Cardiovascular, Santa Clara, CA, USA), guided by a three-dimensional computed tomography recreation of the left atrium. Each of the pulmonary veins were then isolated using CBA. During the right-sided pulmonary vein ablation, the decapolar catheter was withdrawn to the superior vena cava to pace the diaphragm and allow for monitoring of phrenic nerve injury. For pulmonary veins with incomplete isolation following CBA, local RFA was performed as needed to provide complete isolation. All pulmonary veins have demonstrated a bidirectional conduction block and a postablation voltage map was created using the mapping software.
The patients with paroxysmal AF undergoing CBA were followed for 1 year postprocedure for any development of AA. The recurrence of AA was assessed through a medical records review by documented self-reported patient symptoms, supplemented by an electrocardiogram or any form of documented long-term rhythm monitoring such as a cardiac event or a Holter monitor. A 3-mo blanking period was used postablation to allow for recurrence of AF after the initial procedure, with the exception for symptomatic patients with early recurrence that required electrical cardioversion or repeat ablation.
We reported continuous variables as mean with standard deviation and categorical variables as frequency and percentage. A multivariable Cox proportional hazard model was used to assess if ACEI or ARB treatment predicted the risk of AA recurrence. For the multivariate analysis, we utilized two models. In the first model, analysis was adjusted for nonmodifiable variables including age and gender. In the second model, analysis was adjusted for both nonmodifiable (Model 1) and modifiable variables including diabetes mellitus, hypertension, hyperlipidemia, coronary artery disease (CAD), heart failure (HF), chronic kidney disease stage ≥ 3, and at least moderate degree of any valvular heart disease. The χ2 test was used to analyze the significance of the categorical variables and Student’s t-test was used to analyze the significance of continuous variables. To look for the association between ACEI/ARB use and recurrence of AA, we used the multivariable Cox- proportional analysis and calculated hazard ratio (HR) and 95% confidence interval (CI). We used the Kaplan–Meier method to obtain survival curves and a log-rank test for their comparison. SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for all these statistical analyses. P < 0.05 was considered to be statistically significant.
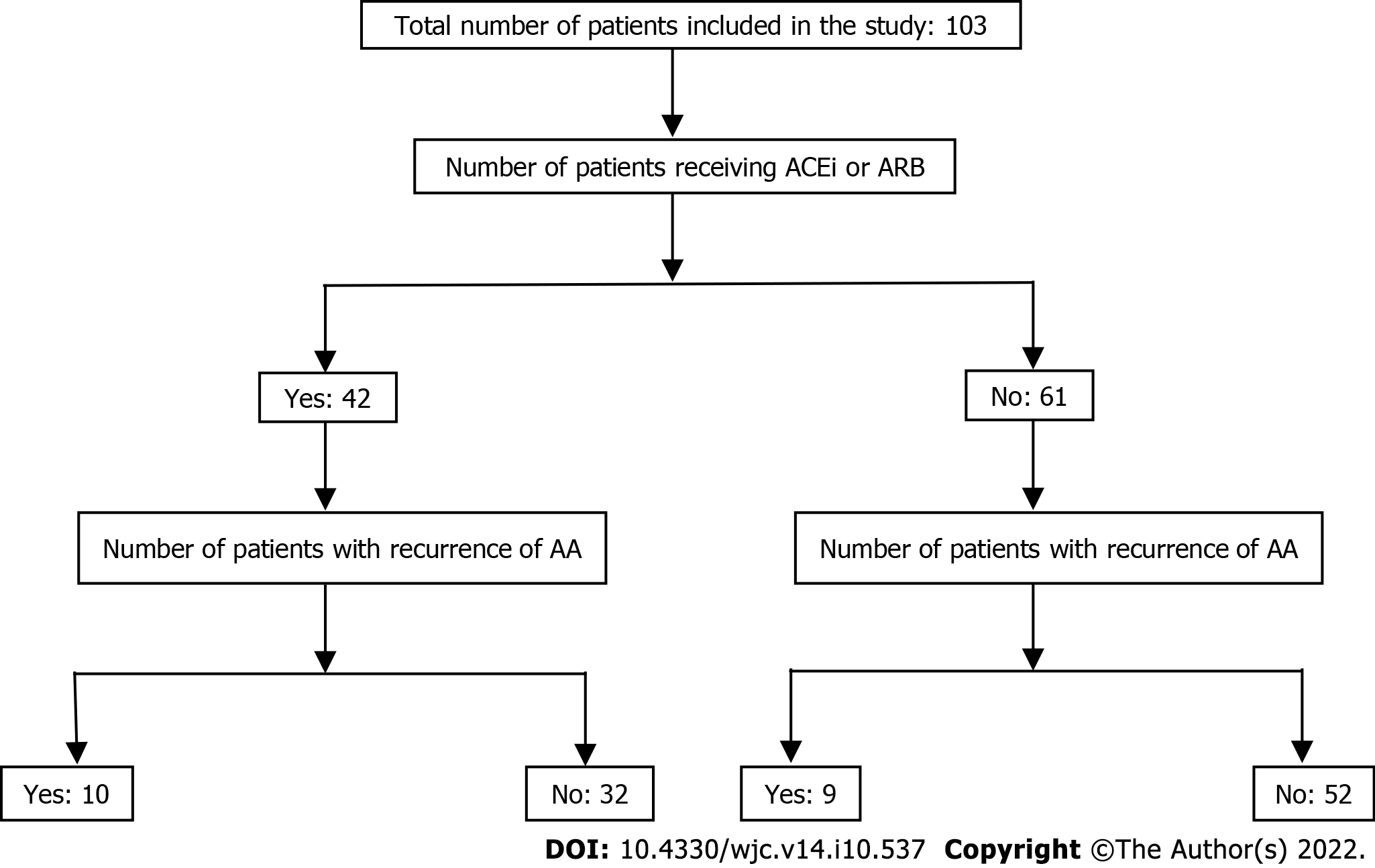
Between January 2015 and April 2018, 103 patients undergoing CBA as a first or repeat procedure for paroxysmal AF were included in this study. They were divided into two groups based on the use of ACEIs/ARBs at the time of CBA. Their baseline characteristics are presented in Table 1. Out of the 103 patients, 42 were receiving ACEIs/ARBs at the time of CBA. The mean age was similar in both groups (61.7 ± 8.6 years in the ACEI/ARB group vs 59.9 ± 9.5 years in the other group). Patients in the ACEI/ARB group were more likely to be male (78.6% vs 65.6%) and have hypertension (86% vs 54.1%) and CAD (26.2% vs 9.8%). Out of the 42 patients, 21 (58%) were taking ACEIs and 15 (42%) were taking ARBs. In the ACEI group, all patients were taking lisinopril. In the ARB group, 87% of patients were taking losartan. The mean dose for lisinopril was 16 mg orally daily and the median dose was 10 mg orally daily. For patients taking losartan, the mean and median doses were 57 and 50 mg orally daily, respectively.
| Characteristics | No ACEI / ARB | ACEI / ARB | P value |
| mean ± SD or n (%) | n = 61 | n = 42 | |
| Age (yr) | 59.9 ± 9.5 | 61.7 ± 8.6 | 0.32 |
| Male | 40 (65.6%) | 33 (78.6%) | 0.15 |
| Baseline heart rate | 70 ± 15.7 | 63 ± 17.8 | 0.06 |
| Body mass index (kg/m2) | 30.9 ± 76.6 | 32.3 ± 6.9 | 0.33 |
| Coronary artery disease | 6 (9.8%) | 11 (26.2%) | 0.028 |
| Congestive heart failure | 6 (9.8%) | 9 (21.4%) | 0.10 |
| Diabetes mellitus | 9 (14.8%) | 12 (28.6%) | 0.09 |
| Hypertension | 33 (54.1%) | 36 (85.7%) | < 0.001 |
| Valvular heart disease | 25 (41.0%) | 14 (33.3%) | 0.43 |
| Hyperlipidemia | 40 (65.6%) | 31 (73.8%) | 0.37 |
| CHA2DS2VASC score | 1.5 ± 1.4 | 2.0 ± 1.3 | 0.08 |
| Recurrence of AA | 9 (14.8%) | 10 (24.4%) | 0.22 |
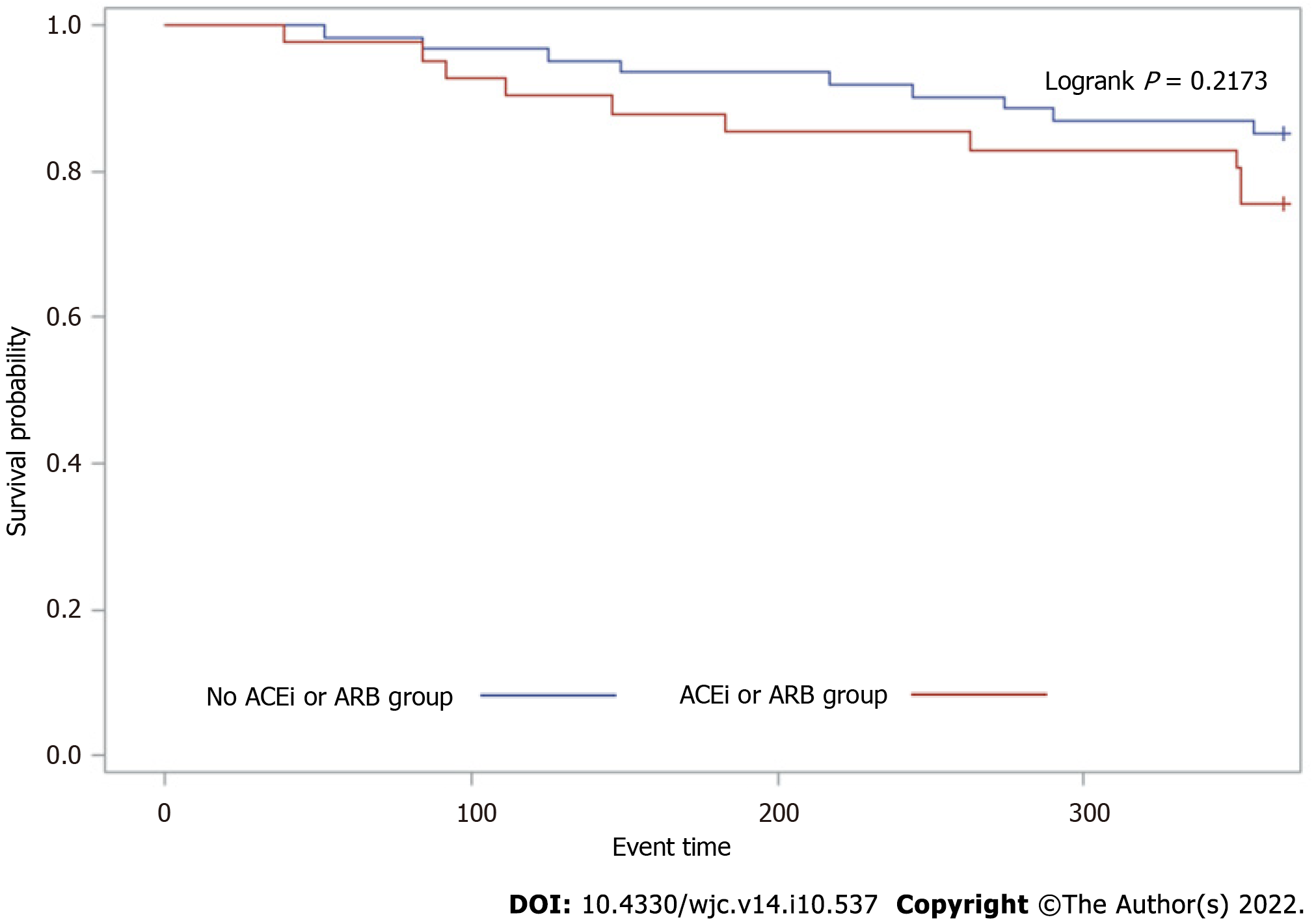
At 1-year follow-up, 19 (8.4%) patients developed recurrence of AA (Figure 1). The role of ACEIs and ARBs in preventing the recurrence of AA following CBA for paroxysmal AF was assessed using a multivariable Cox proportional hazard model (Table 2). In the initial model adjusted for age and sex, ACEI/ARB use did not have a significant impact on AA recurrence after CBA for paroxysmal AF (HR 1.78, 95%CI 0.72–4.42, P = 0.66). In a second model adjusted for CAD, congestive HF, diabetes mellitus, hypertension, and valvular heart disease, ACEI/ARB use still did not have a significant impact on AA recurrence after CBA for paroxysmal AF (HR 1.37, 95%CI 0.51–3.7, P = 0.72). On Kaplan–Meier analysis, ACEI/ARB use did not predict the time to first recurrence of AA (Figure 2, P = 0.2173) in these patients.
In paroxysmal AF patients undergoing CBA, the use of preprocedural ACEI or ARB had no effect on the recurrence of AA at 1 year follow-up. To our knowledge, this study is the first of its kind to examine the effect of ACEI/ARB use in this subset of patients. It is important to note that the ACEI/ARB group of patients that underwent CBA for paroxysmal AF had significantly higher prevalence of hypertension and CAD, which are known to contribute to myocardial fibrosis. Additionally, both hypertension and CAD contributed to increased left ventricular compliance and left atrial filling pressure. Subsequent left atrial enlargement further promotes AA. These comorbidities, however, were adjusted for in multivariate analysis in Model 2 shown above.
The pulmonary veins are the most common substrate for AF initiation[10,11]. Isolated firing of the atrial myocardium can lead to AF initiation initially as an ectopic focus subsequently progressing to a single-circuit re-entry, and eventually to a multiple-circuit re-entry[12,13]. The process correlates with the duration of AF as it progresses from paroxysmal to persistent, and eventually permanent AF5. Myocardial remodeling is one of the key factors in the pathophysiology of AF and is defined as a group of molecular, cellular and interstitial changes that clinically manifest as changes in size, shape and function of the heart resulting from cardiac injury[14]. Additionally, myocardial remodeling can be classified into electrical and structural remodeling. which in turn, can be physiological (adaptive) or pathological[15]. There are several ways in which remodeling could lead to arrhythmia development. The first mechanism involves ion channel changes such as inactivation of sodium ion channels, changes in calcium and potassium ion channels, and alteration in the sodium/calcium exchanger function[5,16-18]. Another mechanism includes changes in the junctional intercellular communication, particularly in protein connexin that is responsible for contact between adjacent cells and electrical coupling[19]. Finally, there is structural cardiac remodeling that involves cell death and fibroblast proliferation that promotes extracellular matrix production, and, eventually, fibrosis[20,21]. Fibrotic lesions impede electrical propagation and eventually promote mechanisms for re-entrant arrhythmias[15].
In recent times, there has been a renewed interest in investigating the role of the renin– angiotensin–aldosterone system in AF. There are several ways hypothesized in which ACEIs and ARBs can potentially affect clinical outcomes. These drugs not only have direct effects on the functional remodeling or electrical properties but also indirect effects by controlling hypertension and HF symptoms which are known risk factors for AF[22]. Atrial myocardium is sensitive to increased hemodynamic stressors due to volume and pressure overload from hypertension and HF. These stressors can in turn promote electrical alterations such as decreased resting potential and delayed afterdepolarizations contributing to increased myocardial excitability[23]. Additionally, increased hemodynamic load and myocardial mechanical stretch can activate genetic pathways that rapidly augment the secretion of angiotensin II[24]. Angiotensin II can successively contribute to arrhythmogenicity by promoting an increase net inward calcium current in affected cardiomyocytes and by inducing myocardial hypertrophy and fibrosis[25]. Increased fibrosis of the atrial myocardium is known to be associated with AF[5,20]. G protein-coupled receptor agonists like angiotensin II induce cellular differentiation processes and activation of fibroblasts, and the development of interstitial fibrosis through activation of extracellular signal-regulating kinases (ERKs). These findings were confirmed in a small study by Goette et al[26] where patients with AF undergoing cardiac surgery were found to have a significant increase in atrial fibrosis along with an increased expression of ERK1 and ERK2 in atrial interstitial cells.
The use of ACEIs and ARBs has also been shown to be associated with a decreased AF burden in certain population groups. Anné et al[27] investigated 196 patients undergoing RFA for atrial flutter and evaluated outcomes associated with the use of an ACEI or ARB. Predictably, more than half of these patients eventually developed AF, but it was found that the use of an ACEI/ARB was associated with reduced incidence of AF after atrial flutter ablation (P = 0.04)[27]. Furthermore, the use of ARBs was evaluated in conjunction with antiarrhythmic medications and showed better outcomes in comparison to antiarrhythmics alone. In a prospective randomized trial by Madrid et al[28], 159 patients were randomized to either amiodarone or amiodarone plus irbesartan to evaluate the role of ACEI in maintaining sinus rhythm in persistent AF. Patients in the irbesartan plus amiodarone combination group had a significantly higher rate of sinus rhythm maintenance at 360 d in comparison to the group on amiodarone alone.
In patients with HF and AF, preablation use of an ACEI resulted in an improved ablation success rate in nonparoxysmal AF with low ejection fraction. In a single center, Mohanty et al[9] investigated 703 consecutive patients with preserved left ventricular ejection fraction (LVEF > 45%) and 345 patients with reduced EF (< 45%) undergoing RFA for AF. At 24 ± 7 mo of follow-up, in patients with nonparoxysmal AF and reduced EF, the ACEI pretreatment group had lower recurrence of AF post-RFA compared to the non-ACEI group (76% vs 64%, P = 0.015). Among paroxysmal AF patients regardless of LVEF, ACEI use was not observed to be associated with improved RFA outcomes (80% vs 77%, P = 0.82). These findings are similar to our study, which demonstrated that in patients with paroxysmal AF the use of an ACEI or ARB did not affect outcomes and event-free survival following CBA. Currently, the guidelines do not recommend the use of ARB or ACEI for the sole purpose of preventing the recurrence of AF, due to a lack of substantial literature supporting it. However, there is growing evidence that in patients with nonparoxysmal AF undergoing RFA, the use of ACEIs or ARBs decreases the recurrence of AF.
Our study had its inherent limitations. Firstly, it was a single center retrospective study with a small sample size limiting the generalizability of outcomes. Secondly, the study population was underpowered for the detection of benefits in patients with paroxysmal AF. Thirdly, we examined an exclusive population of patients with paroxysmal AF and not persistent AF who are known to have a higher degree of electrical and structural remodeling with the potential for superior benefits with ACEIs/ARBs. Since this was a small retrospective study, we could not perform a power analysis. Lastly, our patient population within the ACEI/ARB group had a higher rate of hypertension and CAD. Although these factors were accounted for in the multivariate analysis, we did not collect the data on whether CAD was optimally treated or required a revascularization procedure. Despite these limitations, our study is the first of its kind to examine the role of ACEI/ARB use in an exclusive population of paroxysmal AF patients undergoing CBA.
In paroxysmal AF patients undergoing CBA, the use of ACEIs or ARBs was not associated with decreased recurrence of AA. Larger, multicenter, controlled studies, particularly in patients with persistent AF and those at risk for significant myocardial fibrosis such as cardiomyopathy, HF or valvular disease are necessary to fully evaluate the effect of ACEIs, ARBs, or angiotensin receptor neprilysin inhibitors such as sacubitril/valsartan in patients undergoing CBA for AF.
Cryo-balloon ablation (CBA) is recommended for patients with paroxysmal atrial fibrillation (AF) refractory to antiarrhythmic drugs. However, only 80% of patients benefit from initial CBA.
Myocardial fibrosis is a known risk factor for the development of AF and angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are known agents that prevent remodeling. There is growing evidence that pretreatment with ACEIs and ARBs decreases the recurrence of AF postablation, particularly in nonparoxysmal AF undergoing radiofrequency ablation.
To investigate the role of ACEIs and ARBs in preventing the recurrence of atrial arrhythmia (AA) following CBA for paroxysmal AF.
We performed a single-center, retrospective, cross-sectional study. All patients aged 18 years or older, with a diagnosis of paroxysmal AF, undergoing CBA as a first or repeat procedure between January 2015 and April 2018 were included. We followed these patients with paroxysmal AF undergoing CBA for 1 year post-procedure. Recurrence was assessed by documented AA on electrocardiogram or any form of long-term cardiac rhythm monitoring.
After 1-year follow-up, out of 103 patients, 19 (18.4%) developed recurrence of AA. Of these, 42 patients were receiving ACEIs/ARBs at the time of CBA. 21 (58%) patients were taking ACEIs and 15 (42%) ARBs. Patients on ACEIs/ARBs had a greater prevalence of hypertension and coronary artery disease. On a multivariate model adjusted for baseline demographics and risk factors for AF, ACEI or ARB therapy did not prevent the recurrence of AA following CBA (P = 0.72). Similarly, on Kaplan–Meier analysis pretreatment with ACEIs/ARBs did not predict the time to first recurrence of AA (P = 0.2173).
In paroxysmal AF patients undergoing CBA, the use of ACEIs or ARBs was not associated with decreased recurrence of AA.
Future studies, particularly in patients with persistent AF and those at risk for significant myocardial fibrosis such as cardiomyopathy, heart failure or valvular disease are necessary to fully evaluate the effect of ACEIs, ARBs, or angiotensin receptor neprilysin inhibitors such as sacubitril/valsartan in patients undergoing CBA for AF.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barve P, United States; Karim HMR, India S-Editor: Wang LL L-Editor: Kerr C P-Editor: Wang LL
| 1. | Ball J, Carrington MJ, McMurray JJ, Stewart S. Atrial fibrillation: profile and burden of an evolving epidemic in the 21st century. Int J Cardiol. 2013;167:1807-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 467] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 2. | Chen LY, Shen WK. Epidemiology of atrial fibrillation: a current perspective. Heart Rhythm. 2007;4:S1-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Jackson SL, Tong X, Yin X, George MG, Ritchey MD. Emergency Department, Hospital Inpatient, and Mortality Burden of Atrial Fibrillation in the United States, 2006 to 2014. Am J Cardiol. 2017;120:1966-1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Sheikh A, Patel NJ, Nalluri N, Agnihotri K, Spagnola J, Patel A, Asti D, Kanotra R, Khan H, Savani C, Arora S, Patel N, Thakkar B, Pau D, Badheka AO, Deshmukh A, Kowalski M, Viles-Gonzalez J, Paydak H. Trends in hospitalization for atrial fibrillation: epidemiology, cost, and implications for the future. Prog Cardiovasc Dis. 2015;58:105-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124:2264-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 626] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 6. | Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, Chen PS, Chen SA, Chung MK, Cosedis Nielsen J, Curtis AB, Davies DW, Day JD, d'Avila A, Natasja de Groot NMS, Di Biase L, Duytschaever M, Edgerton JR, Ellenbogen KA, Ellinor PT, Ernst S, Fenelon G, Gerstenfeld EP, Haines DE, Haissaguerre M, Helm RH, Hylek E, Jackman WM, Jalife J, Kalman JM, Kautzner J, Kottkamp H, Kuck KH, Kumagai K, Lee R, Lewalter T, Lindsay BD, Macle L, Mansour M, Marchlinski FE, Michaud GF, Nakagawa H, Natale A, Nattel S, Okumura K, Packer D, Pokushalov E, Reynolds MR, Sanders P, Scanavacca M, Schilling R, Tondo C, Tsao HM, Verma A, Wilber DJ, Yamane T; Document Reviewers. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018;20:e1-e160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1458] [Cited by in RCA: 1538] [Article Influence: 192.3] [Reference Citation Analysis (0)] |
| 7. | Andrade JG, Champagne J, Dubuc M, Deyell MW, Verma A, Macle L, Leong-Sit P, Novak P, Badra-Verdu M, Sapp J, Mangat I, Khoo C, Steinberg C, Bennett MT, Tang ASL, Khairy P; CIRCA-DOSE Study Investigators. Cryoballoon or Radiofrequency Ablation for Atrial Fibrillation Assessed by Continuous Monitoring: A Randomized Clinical Trial. Circulation. 2019;140:1779-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 416] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 8. | Morillo CA, Verma A, Connolly SJ, Kuck KH, Nair GM, Champagne J, Sterns LD, Beresh H, Healey JS, Natale A; RAAFT-2 Investigators. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA. 2014;311:692-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 525] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 9. | Mohanty S, Mohanty P, Trivedi C, Gianni C, Bai R, Burkhardt JD, Gallinghouse JG, Horton R, Sanchez JE, Hranitzky PM, Al-Ahmad A, Bailey S, Di Biase L, Natale A. Association of pretreatment with angiotensin-converting enzyme inhibitors with improvement in ablation outcome in atrial fibrillation patients with low left ventricular ejection fraction. Heart Rhythm. 2015;12:1963-1971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Mahida S, Sacher F, Derval N, Berte B, Yamashita S, Hooks D, Denis A, Amraoui S, Hocini M, Haissaguerre M, Jais P. Science Linking Pulmonary Veins and Atrial Fibrillation. Arrhythm Electrophysiol Rev. 2015;4:40-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Bose A, Chevli PA, Berberian G, Januszkiewicz J, Ahmad G, Hashmath Z, Mishra AK, Laidlaw D. Presence of a left common pulmonary vein and pulmonary vein anatomical characteristics as predictors of outcome following cryoballoon ablation for paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2021;62:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Allessie MA, Boyden PA, Camm AJ, Kléber AG, Lab MJ, Legato MJ, Rosen MR, Schwartz PJ, Spooner PM, Van Wagoner DR, Waldo AL. Pathophysiology and prevention of atrial fibrillation. Circulation. 2001;103:769-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 446] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 13. | Wakili R, Voigt N, Kääb S, Dobrev D, Nattel S. Recent advances in the molecular pathophysiology of atrial fibrillation. J Clin Invest. 2011;121:2955-2968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 437] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 14. | Azevedo PS, Polegato BF, Minicucci MF, Paiva SA, Zornoff LA. Cardiac Remodeling: Concepts, Clinical Impact, Pathophysiological Mechanisms and Pharmacologic Treatment. Arq Bras Cardiol. 2016;106:62-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 203] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 15. | Cutler MJ, Jeyaraj D, Rosenbaum DS. Cardiac electrical remodeling in health and disease. Trends Pharmacol Sci. 2011;32:174-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Obreztchikova MN, Patberg KW, Plotnikov AN, Ozgen N, Shlapakova IN, Rybin AV, Sosunov EA, Danilo P Jr, Anyukhovsky EP, Robinson RB, Rosen MR. I(Kr) contributes to the altered ventricular repolarization that determines long-term cardiac memory. Cardiovasc Res. 2006;71:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Plotnikov AN, Yu H, Geller JC, Gainullin RZ, Chandra P, Patberg KW, Friezema S, Danilo P Jr, Cohen IS, Feinmark SJ, Rosen MR. Role of L-type calcium channels in pacing-induced short-term and long-term cardiac memory in canine heart. Circulation. 2003;107:2844-2849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Thomsen MB, Wang C, Ozgen N, Wang HG, Rosen MR, Pitt GS. Accessory subunit KChIP2 modulates the cardiac L-type calcium current. Circ Res. 2009;104:1382-1389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Patel PM, Plotnikov A, Kanagaratnam P, Shvilkin A, Sheehan CT, Xiong W, Danilo P Jr, Rosen MR, Peters NS. Altering ventricular activation remodels gap junction distribution in canine heart. J Cardiovasc Electrophysiol. 2001;12:570-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 938] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 21. | Yue L, Xie J, Nattel S. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc Res. 2011;89:744-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 300] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 22. | Ehrlich JR, Hohnloser SH, Nattel S. Role of angiotensin system and effects of its inhibition in atrial fibrillation: clinical and experimental evidence. Eur Heart J. 2006;27:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 221] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 23. | Franz MR. Mechano-electrical feedback. Cardiovasc Res. 2000;45:263-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol. 1997;59:551-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 604] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 25. | Kass RS, Blair ML. Effects of angiotensin II on membrane current in cardiac Purkinje fibers. J Mol Cell Cardiol. 1981;13:797-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Goette A, Staack T, Röcken C, Arndt M, Geller JC, Huth C, Ansorge S, Klein HU, Lendeckel U. Increased expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation. J Am Coll Cardiol. 2000;35:1669-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 404] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 27. | Anné W, Willems R, Van der Merwe N, Van de Werf F, Ector H, Heidbüchel H. Atrial fibrillation after radiofrequency ablation of atrial flutter: preventive effect of angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, and diuretics. Heart. 2004;90:1025-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Madrid AH, Bueno MG, Rebollo JM, Marín I, Peña G, Bernal E, Rodriguez A, Cano L, Cano JM, Cabeza P, Moro C. Use of irbesartan to maintain sinus rhythm in patients with long-lasting persistent atrial fibrillation: a prospective and randomized study. Circulation. 2002;106:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 474] [Article Influence: 20.6] [Reference Citation Analysis (0)] |