Published online Jan 26, 2022. doi: 10.4330/wjc.v14.i1.54
Peer-review started: April 2, 2021
First decision: June 25, 2021
Revised: July 14, 2021
Accepted: December 21, 2021
Article in press: December 21, 2021
Published online: January 26, 2022
Processing time: 291 Days and 20 Hours
Cornelia de Lange syndrome (CdLS) is a congenital multisystemic genetic disorder. The expected lifespan of children with this disorder has been prolonged in parallel with the advances in medicine in recent years. However, they still more frequently undergo cardiac surgery. There are some challenges for clinicians when faced with CdLS patients. We present the perioperative management of a child with CdLS undergoing open-heart surgery.
Severe pulmonic and subpulmonic valvular stenosis, enlargement of the right side of the heart, mild tricuspid regurgitation, atrial septal defect, and patent ductus arteriosus were diagnosed in a 14-month-old boy with manifested cyanosis, developmental delay, and malnutrition. Attempted balloon valvuloplasty was unsuccessful due to a severe stenotic pulmonary valve, therefore it was decided to perform an open surgical repair. Following a successful and uncomplicated intraoperative course, the patient was extubated on postoperative day 5, and adrenalin and dopamine infusions were gradually decreased and stopped on postoperative days 6 and 10, respectively. Moderate laryngomalacia and subo
This is the first report of the perioperative anesthetic and clinical management of a CdLS patient undergoing open-heart surgery.
Core tip: Cornelia de Lange Syndrome (CdLS) is a congenital multisystemic genetic disorder with multiple congenital abnormalities. The expected lifespan of children with CdLS has been prolonged in parallel with the advances in medicine in recent years. Patients with CdLS undergo cardiac surgery more frequently. In any patients with multiple medical challenges, anesthesiologists, cardiovascular surgeons and pediatricians may face unexpectedly unusual perioperative courses with additional difficulties when undergoing congenital open-heart surgery. The case presented here demonstrates an example of a challenging perioperative management period of a child with multisystemic congenital disease undergoing multiple high-risk surgeries.
- Citation: Arun O, Oc B, Metin EN, Sert A, Yilmaz R, Oc M. Anesthetic management of a child with Cornelia de Lange Syndrome undergoing open heart surgery: A case report. World J Cardiol 2022; 14(1): 54-63
- URL: https://www.wjgnet.com/1949-8462/full/v14/i1/54.htm
- DOI: https://dx.doi.org/10.4330/wjc.v14.i1.54
Cornelia de Lange Syndrome (CdLS), also known as Brachmann–de Lange Syndrome, is a genetic developmental disorder with a prevalence of 1.24 per 100 000 births[1]. It is characterized by congenital craniofacial, gastrointestinal, cardiac, musculoskeletal, genitourinary, behavioral and neurodevelopmental anomalies[2]. Although CdLS patients are expected to have growth retardation, intellectual disabilities and a shorter lifespan due to these multiple severe malformations, there have been no previous population-based studies on survival, and some of the patients (particularly with the milder forms) have been reported to reach adulthood[3,4]. Most patients need diagnostic and/or interventional procedures and surgical operations under general anesthesia (GA) to survive. Previous research has described anesthetic implementations in non-cardiac surgery. We present the perioperative management of a child with CdLS undergoing open-heart surgery.
A 14-month-old male patient presented with severe cardiac manifestations including pulmonic and subpulmonic valvular stenosis with 91 mmHg gradient, enlargement of the right side of the heart, mild tricuspid regurgitation, atrial septal defect (ASD), and patent ductus arteriosus (PDA).
After genetic evaluation, CdLS diagnosis was approved, and the SMC3 gene was reported to be implicated. When the patient was aged 14 mo, the decision to undertake pulmonary valvuloplasty was taken.
The patient was born at 37 wk gestation after a standard spontaneous vaginal delivery, weighing 2009 g due to intrauterine growth retardation because of ABO maternal–fetal incompatibility. The baby was admitted to the neonatal intensive care unit for 15 d (intubated in the first 2 d) due to respiratory distress and a cleft lip and palate. In the first month after delivery, severe pulmonic stenosis (PS) was diagnosed, and the patient was admitted to a cardiology follow-up program. Lip adhesion surgery was conducted for the cleft lip at age 5 mo.
The patient was born in Syria, and no information was available regarding his family history.
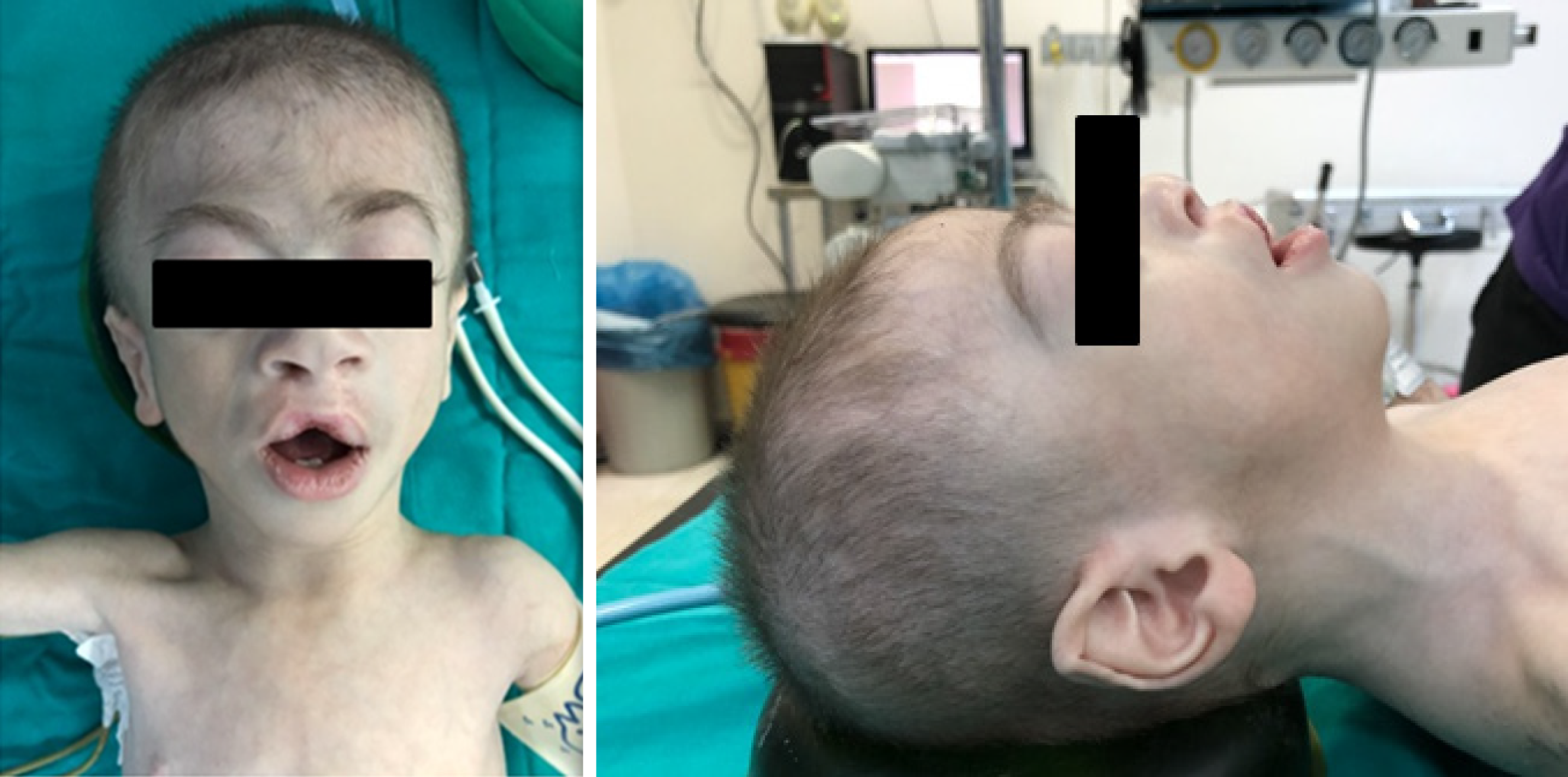
The patient was a 14-month-old boy weighing 4700 g, 60 cm in height (< 3rd percentile) and 39 cm head circumference (< 3rd percentile). Mild exertional dyspnea, cyanosis, developmental delay, and malnutrition were manifest. Synophrys, brachycephaly, long and thick eyelashes, depressed nasal bridge, repaired cleft lip, micrognathia, short neck, and thickened helices in both ears were distinguishing craniofacial features (Figure 1). There was no apparent renal, musculoskeletal, gastrointestinal and neurological involvement. There was a systolic thrill at the left upper sternal border. The auscultation of the patient revealed a grade 2–3/6 midsystolic (ejection systolic) murmur, systolic ejection click, and a widely split, fixed S2 at the upper left sternal border. Also, there was widened S2 and delayed pulmonic component of S2 due to the increased duration of systole and late closing of the pulmonary valve.
In the preoperative laboratory examination all parameters were normal except: white blood cell count 13.1 103/μL, hemoglobin 11.0 g/dL, hematocrit 32.7%, platelet count 504 K/μL, aspartate aminotransferase 71 U/L, and urea 58 mg/dL.
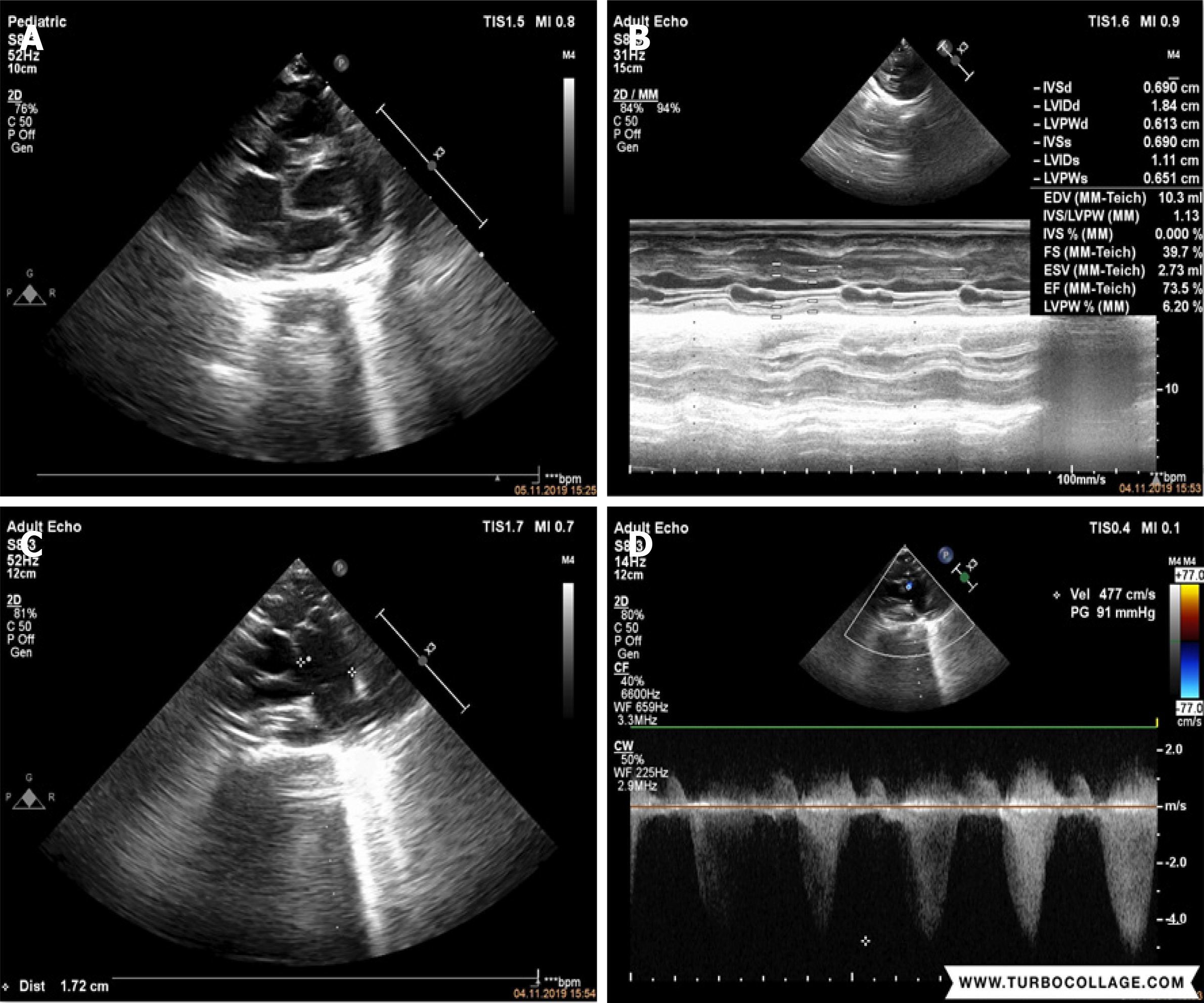
Echocardiographic evaluation revealed severe cardiac manifestations including severe PS and sub-PS with 91 mmHg gradient, enlargement on the right side of the heart, mild tricuspid regurgitation, atrial septal ASD, and PDA (Figure 2).
The ASA Class III patient was scheduled for percutaneous pulmonary valvuloplasty under general anesthesia.
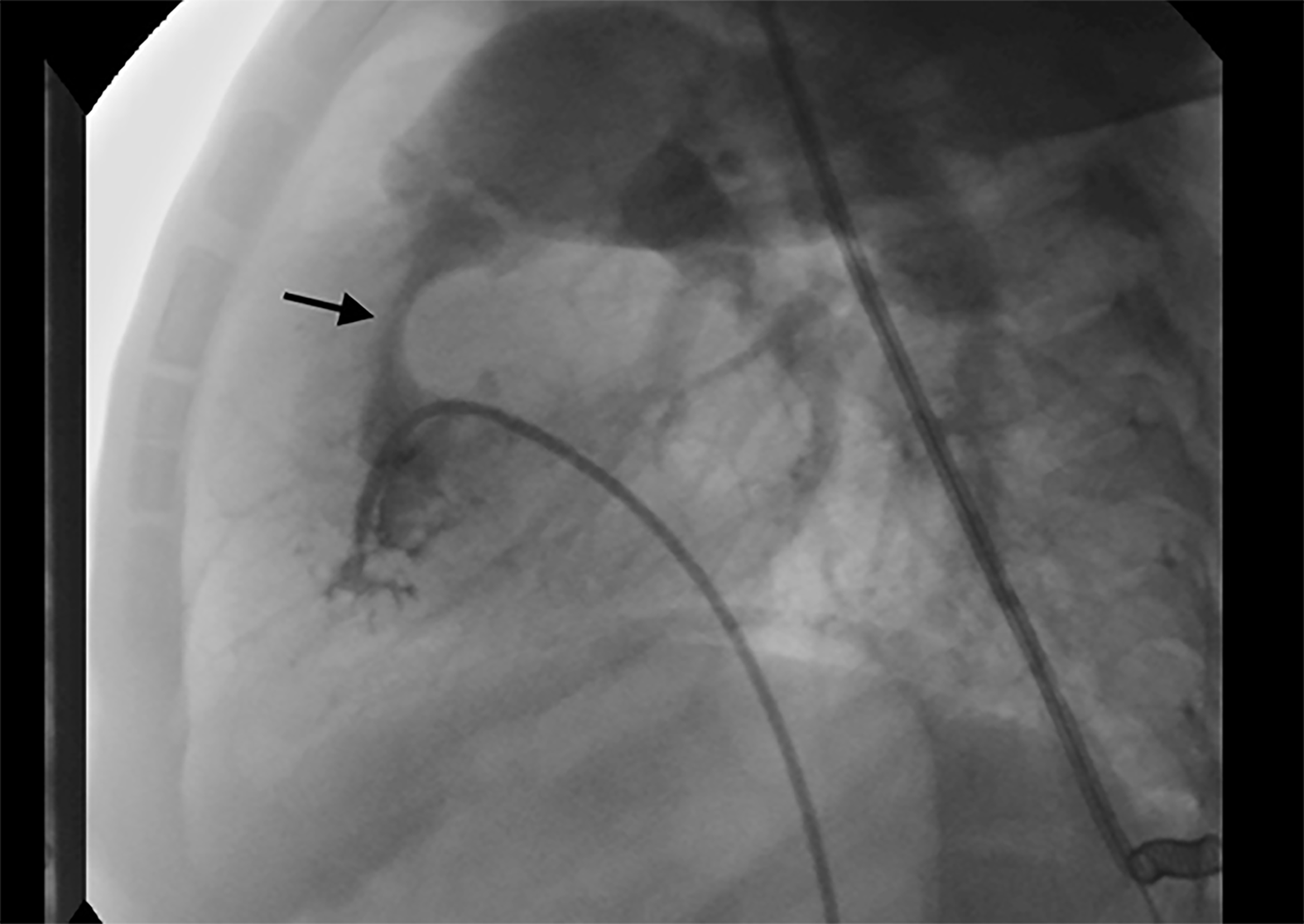
The patient was transferred to the catheter laboratory with an O2 supply without premedication. After standard monitoring with ECG, oxygen saturation (SpO2) and noninvasive blood pressure, anesthesia was induced with 2 mg/kg intravenous (iv) 1% propofol followed by 1.5 mg/kg iv fentanyl and maintained with 2% sevoflurane in 50:50% O2 in air. I-Gel No. 2 was used in airway management without any problems. Due to the severe stenotic pulmonary valve (Figure 3), the attempted balloon valvuloplasty was unsuccessful. Therefore, the decision to perform an open surgical repair was taken.
Nine days after valvuloplasty, the patient was transferred to the cardiac theater with supplemental nasal O2 without premedication. After standard monitoring with ECG, SpO2 and NIBP, anesthesia induction was begun with 8% sevoflurane in 100% O2 followed by 3 mg/kg iv fentanyl, 0.75 mg/kg iv midazolam, 1 mg/kg iv rocuronium, and 1.2 mg/kg iv dexamethasone after insertion of a 20 G cannula. Intubation was easily achieved with a 4.5-mm uncuffed endotracheal tube (grade 1 Cormack–Lehane). Anesthesia was maintained with 2% sevoflurane in 50:50% O2 in air and 0.5–1.0 mg/kg iv rocuronium, 0.5 mg/kg iv midazolam, and 2–5 mg/kg iv fentanyl intermittently as needed. Canulations were completed with the placement of a 20 G 5-cm arterial cannula with floswitch (BD Arterial Cannula; Becton Dickinson Infusion Therapy Systems Inc, Sandy, UT, USA) placed in the left femoral vein; a 4 F 8-cm double lumen central venous catheter (Royal Fornia Medical Equipment Co. Ltd., Guangdong, China) placed in the right femoral vein; and a 20 G 5-cm single lumen catheter (FMTO; Royal Fornia Medical Equipment Co. Ltd.) placed in the left femoral artery.
After standard anticoagulation with heparin (3 mg/kg) and ensuring activated clotting time of > 400 s, cardiopulmonary bypass (CPB) was established by aortic and bicaval cannulations. Under hypothermic (28 °C) CPB and cold crystalloid cardioplegic arrest, transatrial and transpulmonary incisions were performed. A 15 mm 25 mm ASD, subpulmonic infundibular stenosis and pulmonic valvular stenosis were present. Intracardiac repair with excision of the infundibular membrane and pulmonary valvular commissurotomy were achieved. At the pulmonic level, the outlet admitted a size 12 Hegar’s dilator (normal size 11). The ASD closure was done through the right atrium using an autologous pericardial patch. After rewarming to 35 °C and ensuring normal serum electrolytes, the patient was removed from CPB. The child had a stable sinus rhythm after discontinuing heart–lung machine support. For heparin neutralization, 3 mg/kg protamine was applied. CPB was terminated with 52 min aorta cross-clamp time and 81 min of CPB. During anesthesia, iv infusions of 5 g/kg/min dopamine, 0.1 g/kg/min adrenaline, and 0.5 g/kg/min milrinone (after 50 g/kg loading for 1 h) were begun. Intravenous methylprednisolone (5 g/kg/min) and 10 mg/kg iv tranexamic acid were given in the off-pump period. Arterial blood gas analysis was conducted intermittently during surgery (Table 1). The total amount of saline and gelatin fluid (Gelofusine; B. Braun Medical AG, Crissier, Switzerland) and erythrocyte suspension given were 60 mL, 20 mL and 70 mL, respectively. Fresh frozen plasma was not used. The total urinary output was recorded as 350 mL. The hemodynamically stable patient was transported intubated to the pediatric cardiac intensive care unit (CICU).
| Parameters | Beginning of surgery | Beginning of bypass | End of surgery | End of bypass |
| pH | 7.43 | 7.46 | 7.52 | 7.38 |
| PCO2 (mmHg) | 30.8 | 29.1 | 27.6 | 38.1 |
| PO2 (mmHg) | 200 | 172 | 186 | 68.3 |
| Hb (g.dL-1) | 9.7 | 11.4 | 12.2 | 13.3 |
| Htc (%) | 30 | 35.1 | 37.4 | 40.9 |
| SaO2 (%) | 99.6 | 98.9 | 99.3 | 92.8 |
| K+ (mmol/L) | 3.4 | 3.3 | 3.1 | 2.7 |
| Na+ (mmol/L) | 149 | 143 | 146 | 150 |
| Ca2+ (mmol/L) | 1.25 | 1.16 | 1.14 | 0.87 |
| Glucose (mg/dL) | 77 | 191 | 196 | 177 |
| Lactate (mmol/L) | 1.0 | 1.9 | 1.8 | 1.1 |
| Base (mmol/L) | -3.1 | -2.6 | 0.3 | -2.2 |
| HCO3- (mmol/L) | 22.2 | 22.9 | 25.6 | 22.6 |
The patient was extubated on postoperative day 5; adrenalin and dopamine infusions were gradually decreased and stopped on postoperative days 6 and 10, respectively. On postoperative day 14, the patient was transferred to the pediatric ICU (PICU). On the first day in the PICU, continuous positive airway pressure (CPAP) support (due to respiratory distress) and total parenteral nutrition (TPN) were started. Endoscopic evaluation by the ear, nose and throat (ENT) consultant revealed moderate laryngomalacia and suboptimal vocal cord movements. Intermittent CPAP therapy was terminated upon recovery of respiratory functions after 4 d. Enteral nutritional (EN) was begun. With the improvement of the general medical condition, a tracheotomy (based on the ENT consultant’s recommendation) and a percutaneous endoscopic gastrostomy (PEG) to provide a convenient way of feeding were performed under GA in the same session on postoperative day 32.
The patient was discharged on postoperative day 85 after the parental educational discharge program. The general medical condition of the patient was good at the time of discharge. The patient was breathing spontaneously via tracheotomy cannula without requiring additional oxygen therapy with SpO2 95–96%. The hemodynamics were within the normal range without inotropic support. The nutritional support was achieved with enteral nutritional products. The parental educational discharge program included content for feeding and general care of the patient at home. The patient’s medical care was planned to be implemented within the home health service program of the Ministry of Health.
The present case is the first report of perioperative management of a CdLS patient undergoing open-heart surgery. CdLS is a multiple congenital anomaly and mental retardation syndrome accompanied by multiple disorders in different clinical forms, including classical and mild forms. Although the estimated prevalence of CdLS has been reported as 0.5–1.0 per 100 000 Live births, when the mild forms are also taken into account, the prevalence has been reported to be as high as 1 per 10 000 live births. Due to problems in the diagnosis of the syndrome, particularly for the mild forms because of the lack of objective diagnostic criteria for this subgroup, the exact prevalence is still unknown. Barisic et al[1] have given the overall prevalence including mild and classical forms of CdLS as 1.6–2.2 per 100 000 births.
Prior to the definitive molecular studies, CdLS was thought to be caused by defective expression of a multifunctional protein involved in chromosomal function, gene regulation, and DNA repair[5]. More recently, CdLS has been genetically found to be a cohesinopathy disorder caused by autosomal heterozygous or X-linked mutations in the cohesion core subunits of the genes of SMCA1, SMC3, RAD21, or in the cohesion-associated factors NIPBL and HDAC8[6]. The phenotype of this syndrome is a spectrum that is formed by classical forms as well as nonclassical variants that are caused by pathogenic alternatives in genes involved in cohesion functioning[7].
The characteristic phenotype of patients with CdLS includes thick eyebrows that meet in the midline, a short nose with a depressed or wide nasal ridge, anteverted nares with upturned nasal tip, a long and smooth philtrum, a thin upper lip and downturned corners of the mouth[6]. These phenotypic features may overlap with the appearance of patients with other chromatin disorders such as Wiedemann–Steiner syndrome, Rubinstein–Taybi syndrome, and Coffin–Siris syndrome[8]. Also, patients with this syndrome may present different phenotypic features that are completely dissimilar to each other. This phenotypic diversity poses a major challenge in diagnosing patients with this syndrome in clinical practice.
CdLS is characterized by multisystem involvement. Common craniofacial features of classic form are synophrys, micro-brachycephaly, long and thick eyelashes, a high and arched palate with clefts, micrognathia, short neck, and hairy ears with thickened helices[9]. The most prominent and existing comorbidity that can be seen in almost every patient, particularly in the neonatal period, is gastroesophageal reflux and related complications. Congenital cardiac abnormalities such as ventricular septal defects, ASD, PS, tetralogy of Fallot, hypoplastic left heart syndrome, and bicuspid aortic valve can be diagnosed in approximately 25% of patients[10]. Syndactyly, clinodactyly, bradydactyly, oligodactyly, clubbed feet, poikilothermia, pectus exca
Patients with CdLS with all genetic variants may have global developmental delays, intellectual disabilities, and prenatal and postnatal growth retardation. When evaluated from this point of view, prenatal diagnosis becomes even more important. The major indications for prenatal diagnosis are a history of having an earlier child with CdLS, a recent pregnancy in a family with a known genetic problem in a CdLS gene, and suggestive features of CdLS on fetal ultrasonography[7]. Since our patient and his family were Syrian immigrants and only his delivery was performed in a state hospital in Turkey, there was no prenatal care according to the information we received from the parents. The diagnosis of CdLS was made after the genetic examination performed in our university hospital when our patient was aged 5 mo. The absence of prenatal care and the presence of other pathologies that may cause growth retardation can cause delays in diagnosis, as in our patient.
There are some challenges for clinicians when facing CdLS patients, and our patient underwent four different surgical operations under general anesthesia in 85 d. Airway management is of particular interest. Different authors have referred to difficult airway probabilities, and various reports have mentioned some device suggestions instead of a conventional laryngoscope such as a laryngeal mask airway, fiberoptic endoscopes, and a Pentax Airway Scope GlideScope video laryngoscope[13-15]. Uncomplicated airway management, particularly in the intraoperative period has also been reported[16,17]. In our case, micro-brachycephaly, short neck, macroglossia, micrognathia, and a cleft lip and palate were some craniofacial features that could increase the probability of difficulties in airway management. Although no airway difficulties were encountered during the intraoperative period, our patient suffered postoperative respiratory problems, failed to reach adequate extubating criterion during the weaning period, and could not be extubated until postoperative day 5. Despite intermittent CPAP treatments, the patient underwent a tracheotomy in line with ENT consultant’s recommendations after diagnosing moderate tracheomalacia and suboptimal vocal cord movement.
Gastroesophageal reflux and related complications such as esophagitis, aspiration, chemical pneumonitis, and irritability can be seen in almost every CdLS patient, particularly in the neonatal period[18]. Gastroesophageal reflux and intestinal malformations may lead to regurgitation and aspiration of gastric contents, particularly in the anesthesia induction period. Although rapid sequence induction and intubation should be considered as a precaution to avoid regurgitation and aspiration of gastric contents, we did not have a chance to apply these until a secure intravenous line had been achieved.
Another possible severe risk is the presence of perioperative nutritional problems and underfeeding in the postoperative course. Malnutrition is an accepted problem in children with congenital heart disease because of unmatched energy requirements with poor feeding, and inadequate caloric intake[19]. Major surgery (mainly upper gastrointestinal surgery and cardiac surgery) and existing gastrointestinal abnor
PS occurs in 0.6–0.8 per 1000 live births, and its prevalence is 8%–12% of all congenital heart defects[21]. It can be an isolated lesion or associated with other congenital heart defects such as ASD, ventricular septal defect, PDA, and tetralogy of Fallot[22]. There are three different morphological types of valvular PS. In the classic or dome-shaped pulmonary valve, there is a narrowed central orifice with a preserved mobile valve mechanism. In the dysplastic pulmonary valve that represents approximately 20% of all cases, there are poorly mobile and marked myxomatoses-thickened leaflets without commissural fusion. In the third type, the pulmonary valve is unicuspid or bicuspid and mostly seen in the context of tetralogy of Fallot[23]. Obstruction of the right ventricular outflow tract leads to a rise in right ventricular afterload, which also induces ventricular muscle hypertrophy producing thicker chamber walls, decreased compliance, increased ventricular stiffness, and higher right atrial filling pressures[24]. As the obstruction increases, cardiac output and the patient’s physical activity is increasingly limited due to impaired compliance and worsening diastolic dysfunction. When obstruction becomes critically severe, right ventricular systolic and diastolic dysfunction and ischemia can occur. At this point, chest pain, dyspnea, arrhythmia, syncope, and even sudden cardiac death can be seen. For patients with severe PS (peak-to-peak transcatheter gradient > 50 mmHg), who have not undergone surgical correction, poor long-term outcomes have been reported. In contrast, excellent survival rates have been achieved in patients with > 80 mmHg gradient after surgical valvotomy[25]. In our patient, the cardiac pathology was severe due to the severe PS with 91 mmHg gradient, which was combined with PDA and ASD, and the main purpose of the surgical operation was to prolong the lifespan of the patient.
The severity and characteristics of stenosis determine the clinical consequences and optimal treatment modality. An obstruction in the right ventricular outflow tract with a gradient of > 64 mmHg (peak velocity > 4 m/s) on Doppler imaging indicates repair[22]. Although balloon valvuloplasty is the first-choice treatment option, in some circumstances, such as hypoplastic and severely dysplastic valves, infundibular stenosis, and associations of other congenital lesions, surgical repair may be required. Due to the various risks associated with the surgical intervention such as procedural complications, prolonged hospitalization and recovery times, and a higher cost than nonsurgical interventions, surgical intervention is reserved only for more complex diagnoses or in cases in which intervention is not possible[26]. In our case, the guidewire could not be passed through the severe stenotic pulmonary valve, and the decision to undertake a surgical repair was made due to procedural failure.
Severe PS and pulmonary hypertension increase right ventricular work while decreasing left ventricular output, and are significant independent risk factors for mortality and morbidity in both cardiac and noncardiac surgery[27]. The essential targets of anesthetic management are the maintenance of adequate right ventricular preload and contractility and left ventricular afterload with decreasing systemic and pulmonary vascular resistance. In the intraoperative period, right ventricular filling pressure is significant in optimizing myocardial contractility and maintaining hemodynamic stability[28]. Intravascular volume status is also crucial as acute right heart failure and cardiac arrhythmias can easily be precipitated by excessive intravenous fluid. The total amount of intravenous fluids and erythrocyte suspension is limited during surgery. Although all volatile anesthetics may worsen right ventricular dysfunction by reducing preload, afterload, and contractility, desflurane and nitrous oxide, unlike others, are reported to increase pulmonary vascular resistance and should be avoided[29].
CdLS is a complex disease in which many organs and systems are affected. The existence of milder forms of CdLS has been demonstrated by advanced molecular and genetic diagnostic methods. In addition, with the medical developments in recent years, the expected lifespan of children with CdLS has been prolonged, with these patients undergoing cardiac surgery more frequently. In CdLS patients who present various anesthetic challenges, anesthesiologists, cardiovascular surgeons, and pediatricians may face unexpectedly unusual perioperative courses with additional difficulties in congenital open-heart surgeries.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Dong HJ, Fromes Y, Spartalis M, Wong KL S-Editor: Ma YJ L-Editor: Kerr C P-Editor: Ma YJ
| 1. | Barisic I, Tokic V, Loane M, Bianchi F, Calzolari E, Garne E, Wellesley D, Dolk H; EUROCAT Working Group. Descriptive epidemiology of Cornelia de Lange syndrome in Europe. Am J Med Genet A. 2008;146A:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Hirai T, Nitahara K, Higa K, Iwakiri S, Shono S, Katori K. [Anesthetic management of an infant with Cornelia de Lange syndrome]. Masui. 2006;55:454-456. [PubMed] |
| 3. | Kline AD, Grados M, Sponseller P, Levy HP, Blagowidow N, Schoedel C, Rampolla J, Clemens DK, Krantz I, Kimball A, Pichard C, Tuchman D. Natural history of aging in Cornelia de Lange syndrome. Am J Med Genet C Semin Med Genet. 2007;145C:248-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Torres MD, Calvo E, Fernández Esplá F, Gilsanz F. Anesthetic management of an adult patient with Cornelia de Lange Syndrome. Minerva Anestesiol. 2010;76:229-231. [PubMed] |
| 5. | Strachan T. Cornelia de Lange Syndrome and the link between chromosomal function, DNA repair and developmental gene regulation. Curr Opin Genet Dev. 2005;15:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Avagliano L, Parenti I, Grazioli P, Di Fede E, Parodi C, Mariani M, Kaiser FJ, Selicorni A, Gervasini C, Massa V. Chromatinopathies: A focus on Cornelia de Lange syndrome. Clin Genet. 2020;97:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Kline AD, Moss JF, Selicorni A, Bisgaard AM, Deardorff MA, Gillett PM, Ishman SL, Kerr LM, Levin AV, Mulder PA, Ramos FJ, Wierzba J, Ajmone PF, Axtell D, Blagowidow N, Cereda A, Costantino A, Cormier-Daire V, FitzPatrick D, Grados M, Groves L, Guthrie W, Huisman S, Kaiser FJ, Koekkoek G, Levis M, Mariani M, McCleery JP, Menke LA, Metrena A, O'Connor J, Oliver C, Pie J, Piening S, Potter CJ, Quaglio AL, Redeker E, Richman D, Rigamonti C, Shi A, Tümer Z, Van Balkom IDC, Hennekam RC. Diagnosis and management of Cornelia de Lange syndrome: first international consensus statement. Nat Rev Genet. 2018;19:649-666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 244] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 8. | Parenti I, Teresa-Rodrigo ME, Pozojevic J, Ruiz Gil S, Bader I, Braunholz D, Bramswig NC, Gervasini C, Larizza L, Pfeiffer L, Ozkinay F, Ramos F, Reiz B, Rittinger O, Strom TM, Watrin E, Wendt K, Wieczorek D, Wollnik B, Baquero-Montoya C, Pié J, Deardorff MA, Gillessen-Kaesbach G, Kaiser FJ. Mutations in chromatin regulators functionally link Cornelia de Lange syndrome and clinically overlapping phenotypes. Hum Genet. 2017;136:307-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Deardorff MA, Noon SE, Krantz ID. Cornelia de Lange Syndrome. 2005 Sep 16. In: GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993. [PubMed] |
| 10. | Tsukahara M, Okamoto N, Ohashi H, Kuwajima K, Kondo I, Sugie H, Nagai T, Naritomi K, Hasegawa T, Fukushima Y, Masuno M, Kuroki Y. Brachmann-de Lange syndrome and congenital heart disease. Am J Med Genet. 1998;75:441-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Jackson L, Kline AD, Barr MA, Koch S. de Lange syndrome: a clinical review of 310 individuals. Am J Med Genet. 1993;47:940-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 219] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Washington V, Kaye AD. Anesthetic management in a patient with Cornelia de Lange syndrome. Middle East J Anaesthesiol. 2010;20:773-778. [PubMed] |
| 13. | Fernández-García R, Pérez Mencía T, Gutiérrez-Jodra A, López García A. Anesthetic management with laryngeal mask in a child with Brachmann-de Lange syndrome. Paediatr Anaesth. 2006;16:698-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Sugiyama T, Okutani R. Difficult tracheal intubation in a child with Cornelia de Lange syndrome using a paediatric Intlock installed in a Pentax Airway Scope. Anaesthesia. 2012;67:1411-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | August DA, Sorhabi S. Is a difficult airway predictable in Cornelia de Lange syndrome? Paediatr Anaesth. 2009;19:707-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Corsini LM, De Stefano G, Porras MC, Galindo S, Palencia J. Anaesthetic implications of Cornelia de Lange syndrome. Paediatr Anaesth. 1998;8:159-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Moretto A, Scaravilli V, Ciceri V, Bosatra M, Giannatelli F, Ateniese B, Mariani M, Cereda A, Sosio S, Zanella A, Pesenti A, Selicorni A. Sedation and general anesthesia for patients with Cornelia De Lange syndrome: A case series. Am J Med Genet C Semin Med Genet. 2016;172:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Bull MJ, Fitzgerald JF, Heifetz SA, Brei TJ. Gastrointestinal abnormalities: a significant cause of feeding difficulties and failure to thrive in Brachmann-de Lange syndrome. Am J Med Genet. 1993;47:1029-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Vaidyanathan B, Nair SB, Sundaram KR, Babu UK, Shivaprakasha K, Rao SG, Kumar RK. Malnutrition in children with congenital heart disease (CHD) determinants and short term impact of corrective intervention. Indian Pediatr. 2008;45:541-546. [PubMed] |
| 20. | Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S, Laviano A, Ljungqvist O, Lobo DN, Martindale R, Waitzberg DL, Bischoff SC, Singer P. ESPEN guideline: Clinical nutrition in surgery. Clin Nutr. 2017;36:623-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 859] [Cited by in RCA: 1060] [Article Influence: 132.5] [Reference Citation Analysis (0)] |
| 21. | Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3680] [Cited by in RCA: 3718] [Article Influence: 161.7] [Reference Citation Analysis (1)] |
| 22. | Cuypers JA, Witsenburg M, van der Linde D, Roos-Hesselink JW. Pulmonary stenosis: update on diagnosis and therapeutic options. Heart. 2013;99:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Margey R, Inglessis-Azuaje I. Percutaneous Therapies in the Treatment of Valvular Pulmonary Stenosis. Interv Cardiol Clin. 2012;1:101-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, Del Nido P, Fasules JW, Graham TP Jr, Hijazi ZM, Hunt SA, King ME, Landzberg MJ, Miner PD, Radford MJ, Walsh EP, Webb GD. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e143-e263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 993] [Cited by in RCA: 1016] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 25. | Earing MG, Connolly HM, Dearani JA, Ammash NM, Grogan M, Warnes CA. Long-term follow-up of patients after surgical treatment for isolated pulmonary valve stenosis. Mayo Clin Proc. 2005;80:871-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Patel AB, Ratnayaka K, Bergersen L. A review: Percutaneous pulmonary artery stenosis therapy: state-of-the-art and look to the future. Cardiol Young. 2019;29:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Forrest P. Anaesthesia and right ventricular failure. Anaesth Intensive Care. 2009;37:370-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Sanikop CS, Umarani VS, Ashwini G. Anaesthetic management of a patient with isolated pulmonary stenosis posted for caesarean section. Indian J Anaesth. 2012;56:66-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Ciofolo MJ, Reiz S. Circulatory effects of volatile anesthetic agents. Minerva Anestesiol. 1999;65:232-238. [PubMed] |