Peer-review started: February 28, 2021
First decision: July 30, 2021
Revised: September 6, 2021
Accepted: December 21, 2021
Article in press: December 21, 2021
Published online: January 26, 2022
Processing time: 324 Days and 15.2 Hours
Use of ionizing radiation during cardiac catheterization interventions adversely impacts both the patients and medical staff. In recent years, radiation dose in cardiac catheterization interventions has become a topic of increasing interest in interventional cardiology and there is a strong interest in reducing radiation exposure during the procedures. This review presents the current status of radiation protection in the cardiac catheterization laboratory and summarizes a practical approach for radiation dose management for minimizing radiation exposure. This review also presents recent innovations that have clinical potential for reducing radiation during cardiac interventions.
Core Tip: Radiation safety is of concern to catheterization laboratory personnel. In recent years, radioprotection has become a priority in cardiac catheterization interventions and there is keen interest in reducing radiation exposure during the procedures. This review presents the current status of radiation protection in the cardiac catheterization laboratory and summarizes traditional protection mechanism and innovations.
- Citation: Gutierrez-Barrios A, Cañadas-Pruaño D, Noval-Morillas I, Gheorghe L, Zayas-Rueda R, Calle-Perez G. Radiation protection for the interventional cardiologist: Practical approach and innovations. World J Cardiol 2022; 14(1): 1-12
- URL: https://www.wjgnet.com/1949-8462/full/v14/i1/1.htm
- DOI: https://dx.doi.org/10.4330/wjc.v14.i1.1
Use of ionizing radiation during cardiac catheterization interventions adversely impacts both the patients and medical staff[1]. Radiation exposure can result in long-term health effects, including skin and eye damage, and may cause certain forms of cancer by interacting with and altering cellular DNA[1]. The deleterious effect that ionizing radiation has on human tissue may result in potential stochastic and deterministic sequels[2]. Deterministic effects such as ocular lens defects are characterized by a predictable dose-related increase in severity, which can be evaluated by means of air kerma (kinetic energy released per unit mass, AK)[2]. Stochastic effects follow a linear, no-threshold risk model, in which the risk of damage to the irradiated tissue increases linearly with the amount of exposure. The exposure to low-dose radiation induces a stochastic risk in various malignancies that can be measured by dose area-product (DAP)[2].
Interventional cardiologists, given their chronic radiation exposure in cardiac catheterization laboratories, are exposed to the highest cumulative radiation among health professionals. This issue has been magnified with increased exposure in the long duration of structural or complex coronary intervention like chronic total occlusion (CTO) cases. Cataracts, thyroid cancer, and disproportionate incidence of left-sided brain tumors have been reported in interventional cardiologists[1].
In recent years, radiation dose in cardiac catheterization interventions has become a topic of increasing interest in interventional cardiology and there is a strong interest to reduce radiation exposure during the procedures[1,2].
The level of protection should be the best possible under the prevailing circumstances, maximizing the margin of benefit over harm. Physician responsibilities with regard to radiation safety should be based on the ALARA radiation principle: “as low as reasonably achievable”[1].
The hazards of radiation exposure are not limited to interventional cardiologists. Radiation safety is a multi-disciplinary approach, which should involve all catheterization lab (cathlab) personnel. Traditional lead personal protective equipment (LPPE) and radiation shields are mandatory to enhance the protection of all staff members during interventional procedures. The qualities of protective measures are summa
| Protective measures | Evidence1 | Reduction | Operator protection | Patient protection |
| Technical issues | A[2,3,13,14] | 60% | Yes | Yes |
| Traditional LPPE | B[5-9,51] | 35-95% | Yes | No |
| Surgical caps | D[44] | 3.3% | Yes | No |
| Gloves | D[9] | 20-50% | Yes | No |
| Radiation-blocking cream | D[46] | 85% | Yes | No |
| Shield, curtain and TRPB | B[9,10-12] | 30-70% | Yes | No |
| CathPax® | C[16,20-22] | 70- 80% | Yes | No |
| Zero gravity® | C[19] | 78-93% | Yes | No |
| RadPad® | A[26,29,30] | 30-35% | Yes | No |
| Robotic systems | B[32,34,35,42] | 96% | Yes | No |
Optimizing radiation protocols and implementing a radiation safety program to improve the safety of patients and medical staff should become a priority during interventional procedures. The primary goal is to reduce radiation doses wherever and whenever reasonably achievable, thereby reducing the health risk that is assumed to be proportional to the radiation dose[3,4]. Dose Limits Recommended by International Commission on Radiological Protection are presented in Table 2.
| Type of dose limit | Limit on dose from occupational exposure1 |
| Effective dose | 20 mSv/yr averaged over 5 consecutive years and 50 mSv in a single year |
| Effective dose on pregnancy | The dose to embryo/fetus should not exceed 1mV during remainder of pregnancy |
| Equivalent dose: Lens of the eye | 20 mSv/yr averaged over 5 consecutive years and 50 mSv in a single year |
| Equivalent dose: Skin | 500 mSv/yr |
| Equivalent dose: Extremities (hands and feet) | 500 mSv/yr |
All staff members working in interventional x-ray rooms are required to wear lead-equivalent radiation protection garments. Traditional LPPE consists of the following. (1) Leaded glasses. A higher incidence of cataracts (specifically posterior subcapsular) has been reported in interventional cardiologists[1]. Leaded glasses reduce eye radiation by 35%-90%[5-7]. (2) Thyroid collar. The thyroid gland is a radiosensitive organ and thyroid cancer is a known consequence of radiation exposure. Conse
The main shield mounted on the equipment consists of shields suspended from the ceiling and curtains suspended from the table. Radiation shields must be discreetly placed and actively managed both before and during the procedure to be effective[4].
Ceiling-mounted shields are made of leaded clear plastic that is adjustable during the procedure and if positioned correctly can reduce the radiation dose to the operator's head and neck[4,9]. The shield should be as close as possible to the patient to stop the scatter at the source for the greatest degree of attenuation.
The lower region of the operator receives the most exposure during a procedure. Protecting the pelvic region containing the reproductive organs is essential due to their radiosensitivity. Table suspended lead drapes between the X-ray tube and the operator provide a significant lower radiation dose to operators at pelvic and thorax level[4,9,10]. Recently, a clinical trial showed that the combination of pelvic drapes and under-table shields on top of the standard protective measures of the cathlab reduced the operator radiation exposure at thorax to negligible levels[11].
Transradial radiation protection board (TRPB) is a grooved arm board with a detachable 0.5 mm lead equivalent shield designed to rest between the patient’s arm and side. TRPB reduces radiation operator dose during radial approach procedures in addition to standard protection[12].
Mobile leaded shield consists of a 200 cm x 80 cm lead-equivalent mobile shield with a thickness of 2 mm positioned between the patient and the operator. A transparent 2-mm lead-equivalent window offers a permanent view of the patient. A mobile leaded shield, combined with standard preventive measures, significantly reduces operator exposure to ionizing radiation during interventional procedures.
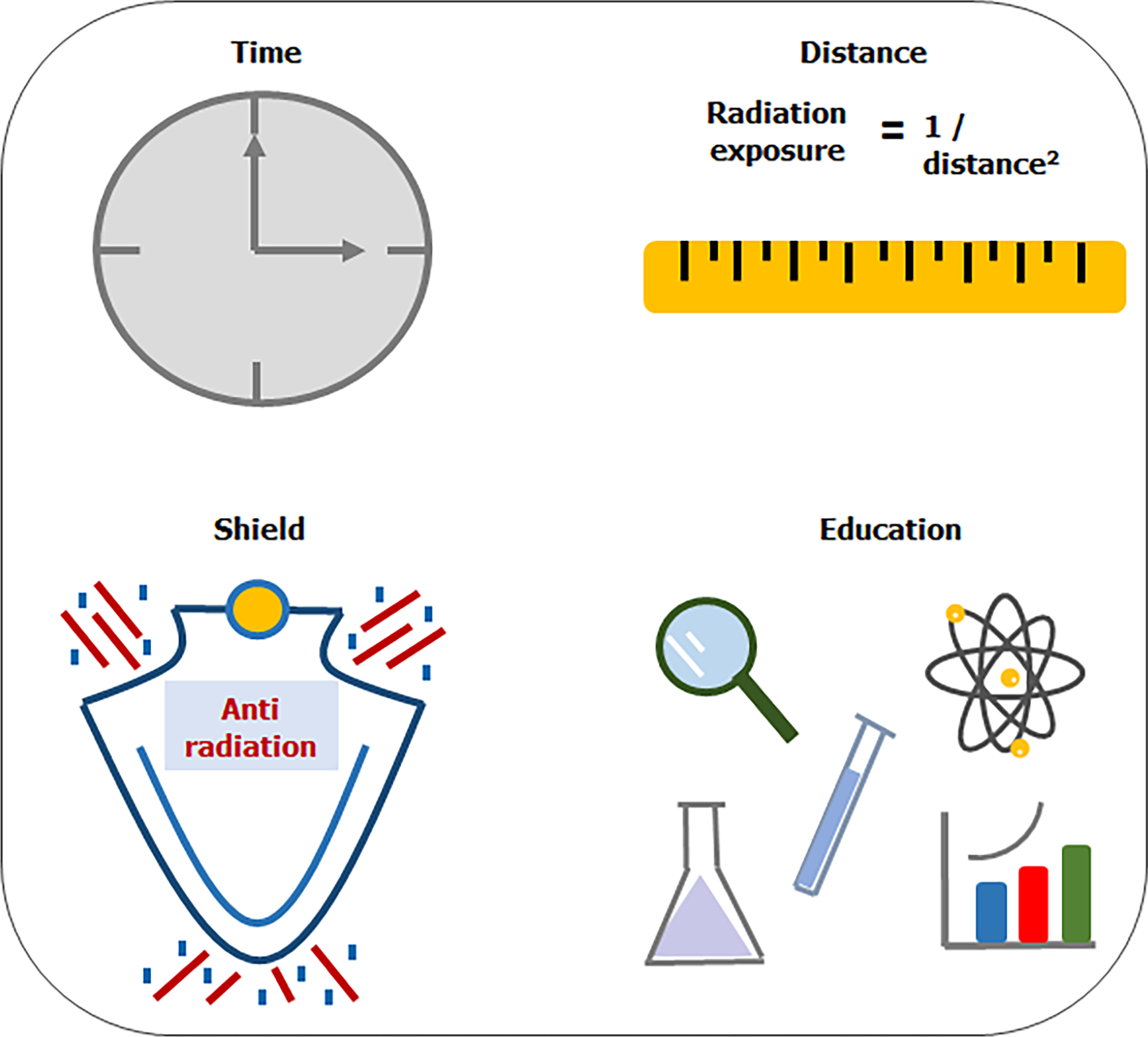
The guiding principle with regard to radiation safety should be based on the ALARA radiation principle. ALARA stands for “as low as reasonably achievable.” This principle means that even if it is a small dose, if receiving that dose has no direct benefit, it should be avoided. Effective strategies to minimize patient and operator exposure during interventions are based on four principles (Figure 1)[2,3,11,13-15].
Utilize radiation only when imaging is necessary to support clinical care and limit fluoroscopy time to only when the operator is looking at the monitor.
Keep the image receptor as low as possible on the patient’s chest. Optimal table positioning can reduce patient radiation dose. The patient is ideally placed as close as possible to the image receptor and further away from the X-ray source.
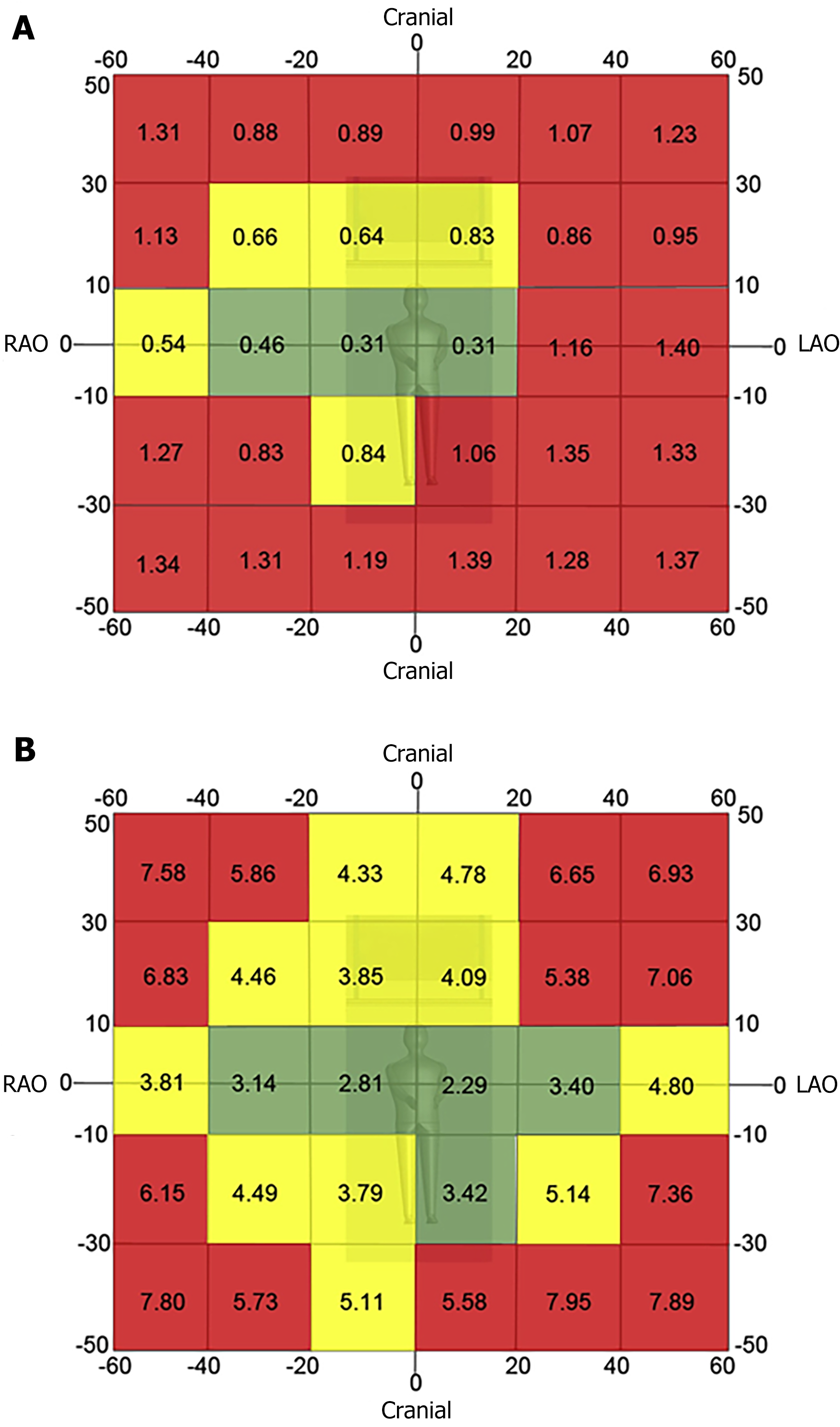
Minimize use of steep angles of the X-ray beam. Extreme angulations are associated with high air kerma values. Paying close attention to the angulation and placing the C-arm in 0° to 20° angulation will result in a more than 3-fold reduction in the amount of radiation scattered during fluoroscopic acquisition. The left anterior oblique view cranial angulation has the highest degree of scatter exposure to the operator (Figure 2)[3].
Minimize use of cine and shorten each cine acquisition as much as possible.
Minimize the use of magnification modes. Most modern systems have software magnification algorithms that allow for magnification without additional radiation. Nevertheless, hardware magnification should still be used when clinically indicated.
Utilize collimation to the fullest extent possible.
Monitor radiation dose in real time to assess the operator’s and patient’s risk/benefit ratio during the procedure. Use of real-time radiation monitoring devices that provide auditory feedback can significantly reduce radiation exposure during cardiac catheterization (Figure 3).
Adjust the fluoroscopy frame rate. Fine-tuning radiation safety protocols could reduce radiation doses without compromising the effectiveness of catheterization procedures in patients. Frame rate is typically set at 15 frames-per-second and decreasing it to 7.5 frames-per-second results in significant radiation dose reduction[2,13].
Increase the distance between the operator and radiation source. Radiation exposure is inversely proportional to the square of the distance from the X ray source. Increasing the distance between the cardiologist and the patient to 1 m can decrease a physician’s occupational radiation dose by about a half.
Educate in radiation protection. Training and education in radiation protection is widely recognized as one of the basic principles of optimization programs for medical exposures. These provide practitioners of interventional cardiology adequate theoretical and practical training in radiation safety to help minimize occupational radiation dose.
Traditional LPPE with lead aprons, thyroid shields and lead glasses are only partially effective. This protection equipment leaves body parts such as arms, hands and heads unprotected[16-18]. In recent years, new concepts in individual or semi-individual radioprotection were being marketed to reduce scatter radiation.
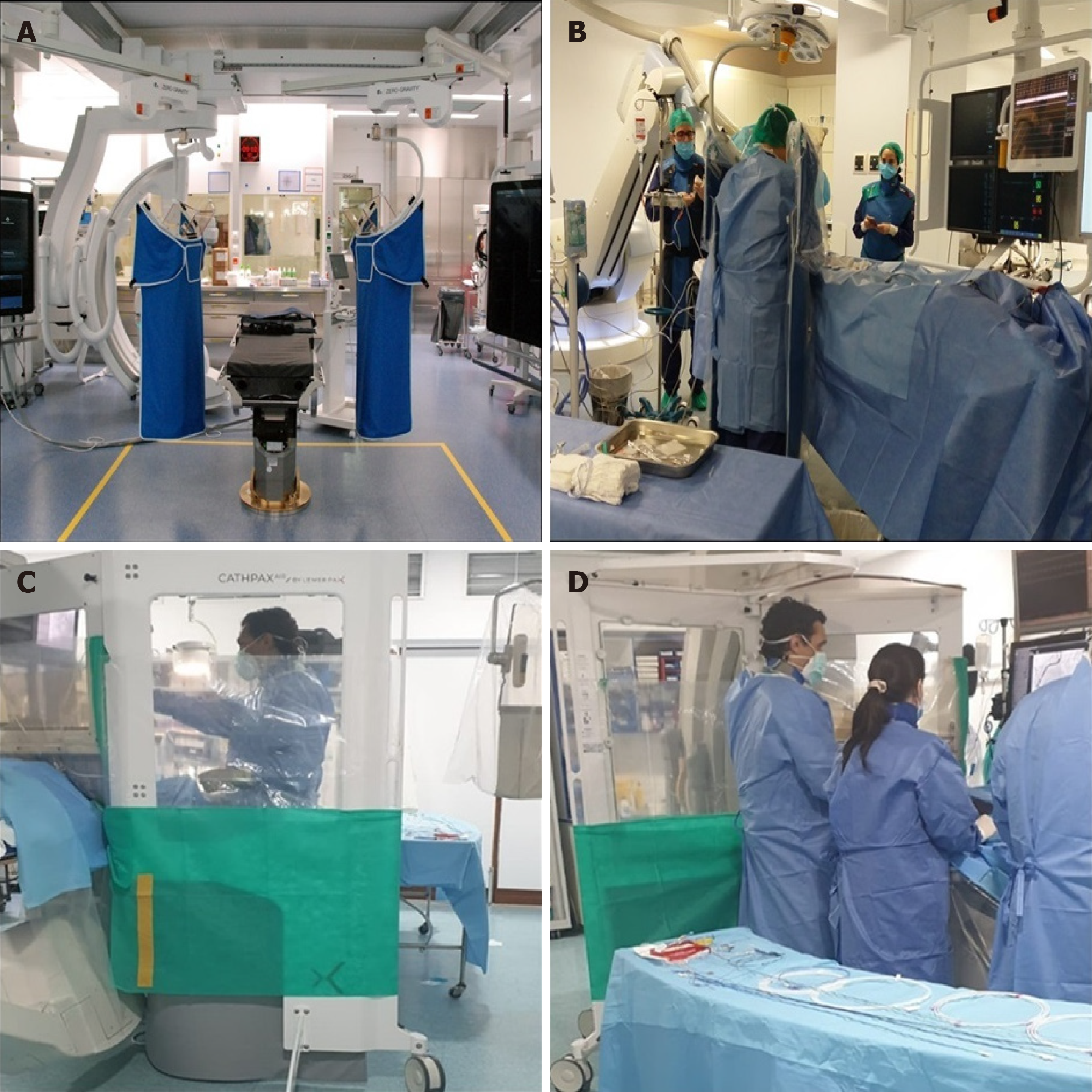
The Zero-Gravity suspended radiation protection system is designed to increase the level of radiation protection while at the same time eliminating the weight burden for the operator. Compared to conventional lead aprons with under-table or ceiling-mounted shields, Zero-Gravity provides superior operator protection during fluoroscopy. This system reduces fatigue and orthopedic injuries resulted from routinely wearing heavy protective apparel as well as allowing clinicians freedom of movement, especially during challenging procedures (Figure 4A and B)[19].
In the last decade, radiation protection cabins (RPCs) have become used in interventional procedures. RPCs significantly reduce radiation exposure in different interventional procedures[16,17,20-23]. The cathpax® cabin (Lemer Pax, Carquefou, France) is a mobile and height-adjustable RPC that comes in three ranges of radiation protection tailored to the specific needs of cardiac interventional operators. The CathPax® AF and the Cathpax® CRM cabins are particularly adapted for electrophysiology procedures and for cardiac devices implantations respectively. The Cathpax® AIR (Figure 4C and D) shielded with 2 mm lead-equivalent is adapted to all interventional cardiology procedures, (coronarography, complex percutaneous coronary interventions (PCIs) as well as structural ones like TAVI or left appendage closure).
One of the main concerns regarding RPCs is its comfortability and workability in a real-world setting, especially in complex scenarios such as CTOs. Recently, the Cathpax® AIR confirmed its feasibility and efficacy in a real-world setting by reducing first-operator relative radiation exposure by 78%. This effect was consistent during different types of procedures including emergent procedures, complex PCIs and structural procedures[24].
Disposable radioprotective drapes can be particularly useful in complex interventional procedures associated with higher radiation exposure, such as CTO interventions[25,26]. They contain metallic elements (bismuth, barium, and tungsten-antimony) and are placed over the patient during fluoroscopically guided interventions. Radioprotective drapes reduce attenuate scatter radiation 12-fold for the eyes, 25-fold for the thyroid, and 29-fold for the hands[27].
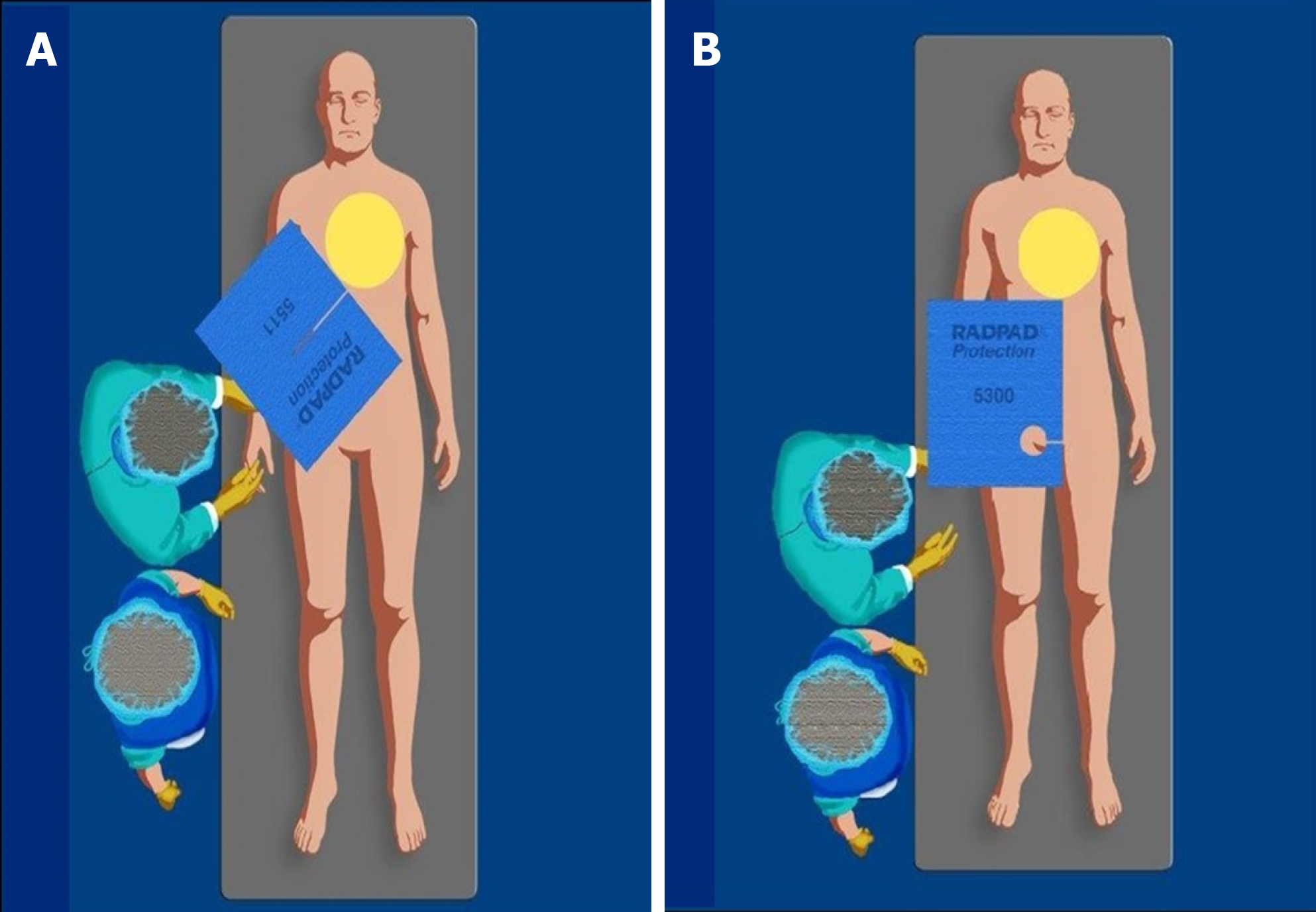
The Radpad® is a disposable drape lead-free shield (Worldwide Innovations and Technologies Inc, Lenexa, KS) (Figure 5) that reduces operator radiation exposure in several studies by 20%-59% including a real-world randomized trial[16-18,26,28-30].
However, the disposable drapes shield should not be placed within the imaging field during radial angiography as such an action may trigger an automatic increase in dose rate, significantly increasing patient dose[25,26].
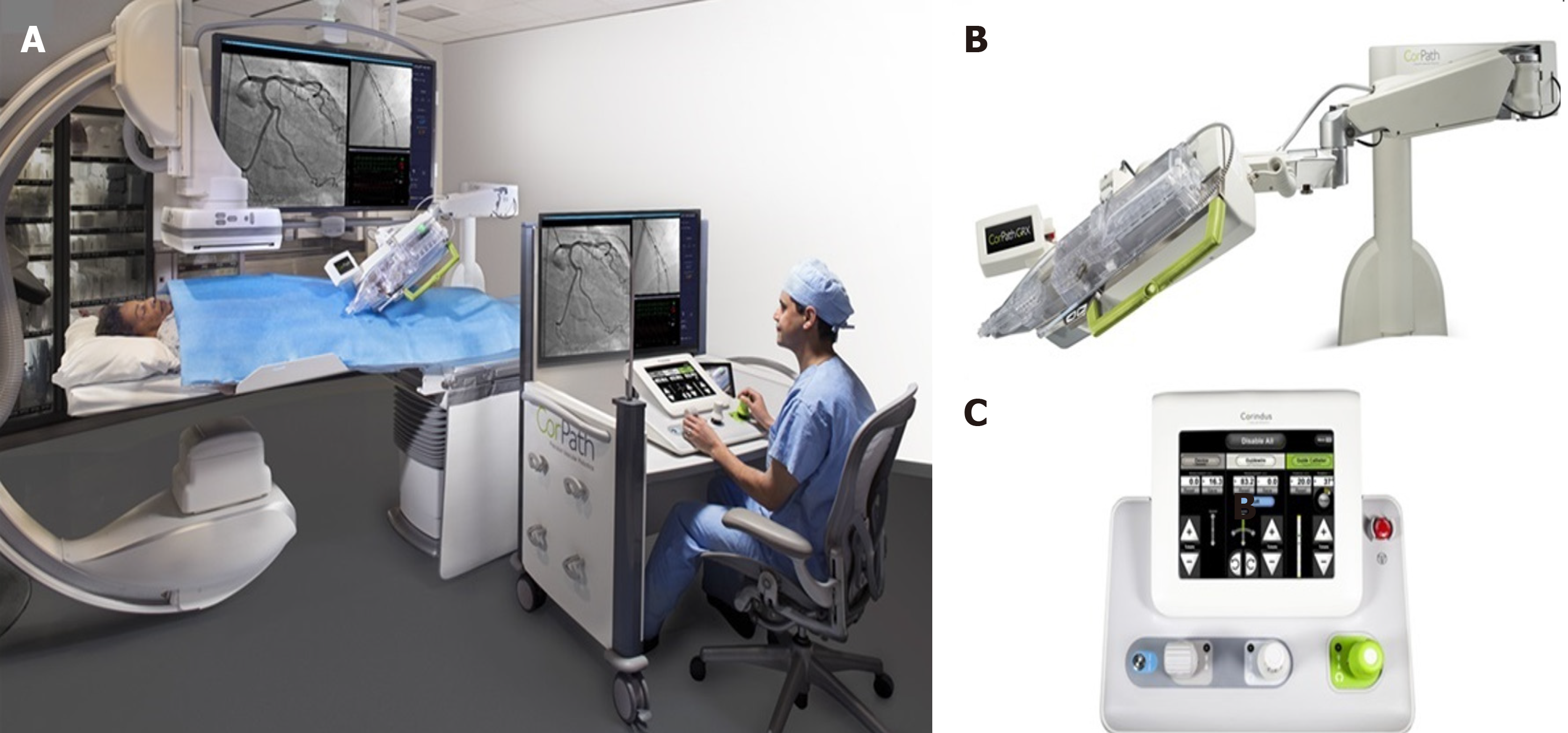
Robotic PCI (R-PCI) is an emerging technology with significant potential for transforming PCI[31]. In 2006, Beyar et al[32] developed and reported the first remote controlled robotic system to address the occupational hazards of interventional cardiology[31,32]. Five years later, Granada and colleagues reported the first in-human experience in a series of eight patients with the CorPath® 200 robotic system (Corindus, Inc., Natick, Massachusetts, United States)[33]. At present, the second generation of CorPath is available. The CorPath GRX system (Figure 6) has new features that further facilitate R-PCI in complex anatomies, including remote manipulation of the guide catheter to help augment support after engagement or incorporation of wiring algorithms such as “rotate on retract and simplified device exchanges.” The new system achieves tremendous reduction in radiation exposure to operators with high rates of clinical and technical success even in complex PCI scenarios such as laser atherectomy or left main stem disease[31,34,35]. The R-One (Robocath) is a new R-PCI platform that recently received regulatory approval for use in Europe. This new system has functional capabilities similar to those of the CorPath 200 system.
A key potential advancement that R-PCI could bring is in the field of tele-R-PCI. This will allow for the treatment of patients who are in geographically distant locations[36]. Performing long distance tele-R-PCI in type A coronary lesion is currently feasible with predictably successful outcomes if reliable network connectivity and local cardiac catheterization facilities are available[37].
In addition to mitigating occupational hazards for interventional cardiologists, R-PCI offers the potential advantages of more precise measurements of lesion length and more stable deployment of angioplasty balloons and stents[38].
Finally, the ongoing worldwide coronavirus disease 2019 (COVID-19) pandemic has imposed severe restrictions on such an interventional environment. In this setting, R-PCI can provide an additional layer of protection to the healthcare personnel par
The current robotic systems are in the early stages of development compared to standard manual PCI. Despite being a promising technology, there are still some important issues to address before its use spreads beyond a few limited centers. Most importantly, there is a need for clinical evidence from large-scale randomized clinical trials showing improved radiation safety for the operators and non-inferior angiographic and clinical results[40]. Additionally, the initial CorPath 200 system had several limitations; the subsequent version, the CorPath GRX, has overcome some of these limitations but there are still multiple technical limitations to current R-PCI technology compared to manual PCI. These include the need for operators to obtain arterial access and manually engage guide catheters prior to utilization of R-PCI systems; current R-PCI systems are limited to 0.014″ wires and rapid exchange devices; thus, rotational atherectomy or two stent deployment cannot currently be performed[41]. The absence of tactile feedback is another important issue in complex PCIs, where the interaction among the wire, lesion, and operator is key to subsequent technical success[40]. Finally, at least one team member needs to remain at the bedside for equipment exchanges and the procedure duration is significantly increased compared to traditional interventions[32,35,42].
The effectiveness of other radiation safety innovations, such as radiation-blocking hats, gloves, or radiation-blocking cream, still remain uncertain[17,20,43].
A recent study showed that radiation scattering comes predominantly from under the head of the operator and surgical radio-absorbing caps do not cover this area, so the brain protection demonstrated by a surgical lead cap is minimal. It has been shown to decrease radiation dose to the brain by only 3.3%[4,44].
The hands of the fluoroscope operator should only be placed in the field of view when required by the procedure. The best way to protect the hands is to keep them away from the direct radiation beam whether protective gloves are used or not[9]. Notably, the use of protective gloves when the hands are placed in the field may trigger an automatic increase in dose rate, significantly increasing patient dose. Nevertheless, the use of radio protective gloves to reduce the exposure of the hands to scattered radiation when the hands remain outside the field is not contraindicated[45].
Radiation-blocking cream (BloXR®) is a hand lotion designed to offer radiation protection from X-rays[46]. The cream is applied prior to donning gloves, or over a glove with another glove on top. However, the commercially available cream comes with an United States Food and Drug Administration black box warning. The radiation-blocking cream could pose a radiation exposure risk to healthcare professionals due to the lack of radiation attenuation, which can occur due to inadequate or inconsistent cream formulation.
Women are particularly underrepresented in cardiology procedural subspecialties, and account for < 10% of the physician workforce in interventional cardiology[47]. However, currently, the proportion of women who choose intervention cardiology as a career is increasing. The Women in the Innovations group of Cardiologists aim to provide guidance by describing the risk of radiation exposure to pregnant physicians and cardiac catheterization personnel[48].
Radiation exposure during pregnancy poses a risk to the fetus leading to two types of adverse effects: deterministic and stochastic effects. Deterministic effects consist of intrauterine growth retardation, pregnancy loss, mental retardation, small head size, reduced intelligence quotient and congenital malformations. Stochastic effects consist of risk of childhood cancer and hereditary diseases in the descendants[49]. The risk of each effect depends on the gestational age at the time of exposure with the first trimester being the period of greatest risk. Doses below 50 mGy have not been associated with an increase in fetal anomalies or pregnancy losses[50]. The fetal radiation exposure for most women who work in the cardiac catheterization laboratory is extremely low, and is much lower than the recommended limit. Protective garments specifically for pregnant women must provide at least 0.5 mm lead-equivalent protection throughout the entire pregnancy with a double thickness protective garment, specific maternity lead apron or maternity bib (for an additional lead protection layer). Additionally, an extra dosimeter at waist level under the lead apron to monitor fetal radiation exposure monthly is also recommended[48].
Although perceptions of radiation exposure risk remain widespread, with standard radiation safety measures practiced routinely, there is no statistically significant or convincing evidence of an increased risk of pregnancy-related complications for female cardiologists exposed to radiation. This suggests that female interventionists can integrate pregnancy safely into their careers. All operators should follow common sense measures under the ALARA principle[47].
Optimal use of ionizing radiation in cardiovascular interventions is the responsibility of the healthcare professionals working in the cathlab. Efforts to reduce radiation exposure and participation in radiation safety educational programs should be encouraged by all the professionals involved in interventional procedures exposed to radiation. Professionals endeavor should be focused on the correct use of LPPE, optimal positioning and distancing to the table, the image intensifier and the operator. It is vitally important to optimize the X-ray settings, use fluoroscopy judiciously, and ensure appropriate shielding. Proper use of personnel dosimeters ensures correct radiation monitoring limiting exposure.
Based on the ALARA radiation principle, a priority should be to minimize radiation exposure in every clinical circumstance reducing radiation hazards.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang SY, Zeng C S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Elmaraezy A, Ebraheem Morra M, Tarek Mohammed A, Al-Habaa A, Elgebaly A, Abdelmotaleb Ghazy A, Khalil AM, Tien Huy N, Hirayama K. Risk of cataract among interventional cardiologists and catheterization lab staff: A systematic review and meta-analysis. Catheter Cardiovasc Interv. 2017;90:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 2. | Wassef AW, Hiebert B, Ravandi A, Ducas J, Minhas K, Vo M, Kass M, Parmar G, Hussain F. Radiation dose reduction in the cardiac catheterization laboratory utilizing a novel protocol. JACC Cardiovasc Interv. 2014;7:550-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Agarwal S, Parashar A, Bajaj NS, Khan I, Ahmad I, Heupler FA Jr, Bunte M, Modi DK, Tuzcu EM, Kapadia SR. Relationship of beam angulation and radiation exposure in the cardiac catheterization laboratory. JACC Cardiovasc Interv. 2014;7:558-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Fetterly KA, Magnuson DJ, Tannahill GM, Hindal MD, Mathew V. Effective use of radiation shields to minimize operator dose during invasive cardiology procedures. JACC Cardiovasc Interv. 2011;4:1133-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Marshall NW, Faulkner K, Clarke P. An investigation into the effect of protective devices on the dose to radiosensitive organs in the head and neck. Br J Radiol. 1992;65:799-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Burns S, Thornton R, Dauer LT, Quinn B, Miodownik D, Hak DJ. Leaded eyeglasses substantially reduce radiation exposure of the surgeon's eyes during acquisition of typical fluoroscopic views of the hip and pelvis. J Bone Joint Surg Am. 2013;95:1307-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Thornton RH, Dauer LT, Altamirano JP, Alvarado KJ, St Germain J, Solomon SB. Comparing strategies for operator eye protection in the interventional radiology suite. J Vasc Interv Radiol. 2010;21:1703-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Ron E, Lubin JH, Shore RE, Mabuchi K, Modan B, Pottern LM, Schneider AB, Tucker MA, Boice JD Jr. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res. 1995;141:259-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Badawy MK, Deb P, Chan R, Farouque O. A Review of Radiation Protection Solutions for the Staff in the Cardiac Catheterisation Laboratory. Heart Lung Circ. 2016;25:961-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Sciahbasi A, Sarandrea A, Rigattieri S, Patrizi R, Cera M, Di Russo C, Zezza L, Fedele S, Ferraiuolo G. Extended Protective Shield Under Table to Reduce Operator Radiation Dose in Percutaneous Coronary Procedures. Circ Cardiovasc Interv. 2019;12:e007586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Anadol R, Brandt M, Merz N, Knorr M, Ahoopai M, Geyer M, Krompiec D, Wenzel P, Münzel T, Gori T. Effectiveness of additional X-ray protection devices in reducing scattered radiation in radial intervention: the ESPRESSO randomised trial. EuroIntervention. 2020;16:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Behan M, Haworth P, Colley P, Brittain M, Hince A, Clarke M, Ghuran A, Saha M, Hildick-Smith D. Decreasing operators' radiation exposure during coronary procedures: the transradial radiation protection board. Catheter Cardiovasc Interv. 2010;76:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Gutiérrez-Barrios A, Camacho-Galán H, Medina-Camacho F, Cañadas-Pruaño D, Jimenez-Moreno A, Calle-Perez G, Vázquez-García R. Effective Reduction of Radiation Exposure during Cardiac Catheterization. Tex Heart Inst J. 2019;46:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Christopoulos G, Papayannis AC, Alomar M, Kotsia A, Michael TT, Rangan BV, Roesle M, Shorrock D, Makke L, Layne R, Grabarkewitz R, Haagen D, Maragkoudakis S, Mohammad A, Sarode K, Cipher DJ, Chambers CE, Banerjee S, Brilakis ES. Effect of a real-time radiation monitoring device on operator radiation exposure during cardiac catheterization: the radiation reduction during cardiac catheterization using real-time monitoring study. Circ Cardiovasc Interv. 2014;7:744-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Vano E, Gonzalez L. Accreditation in radiation protection for cardiologists and interventionalists. Radiat Prot Dosimetry. 2005;117:69-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Schernthaner C, Danmayr F, Strohmer B. Significant reduction of radiation exposure using a protection cabin for electrophysiological procedures. Medical Imaging and Radiology 2013; 1.. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Brilakis ES. Innovations in Radiation Safety During Cardiovascular Catheterization. Circulation. 2018;137:1317-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Marcusohn E, Postnikov M, Musallam A, Yalonetsky S, Mishra S, Kerner A, Poliakov A, Roguin A. Usefulness of Pelvic Radiation Protection Shields During Transfemoral Procedures-Operator and Patient Considerations. Am J Cardiol. 2018;122:1098-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Marichal DA, Anwar T, Kirsch D, Clements J, Carlson L, Savage C, Rees CR. Comparison of a suspended radiation protection system vs standard lead apron for radiation exposure of a simulated interventionalist. J Vasc Interv Radiol. 2011;22:437-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Guersen J, Karmouche K, Moyon JB, Osmond E, Poulin M, Gabrillargues J, Jean B, Chabert E, Dutheil F, Cassagnes L, Boyer L. Use of a prototype radioprotection cabin in vascular neuroradiology: Dosimetry and ergonomics. J Neuroradiol. 2015;42:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Dragusin O, Weerasooriya R, Jaïs P, Hocini M, Ector J, Takahashi Y, Haïssaguerre M, Bosmans H, Heidbüchel H. Evaluation of a radiation protection cabin for invasive electrophysiological procedures. Eur Heart J. 2007;28:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Ploux S, Jesel L, Eschalier R, Amraoui S, Ritter P, Haïssaguerre M, Bordachar P. Performance of a radiation protection cabin during extraction of cardiac devices. Can J Cardiol. 2014;30:1602-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Ploux S, Ritter P, Haïssaguerre M, Clementy J, Bordachar P. Performance of a radiation protection cabin during implantation of pacemakers or cardioverter defibrillators. J Cardiovasc Electrophysiol. 2010;21:428-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Gutierrez-Barrios A, Angulo-Pain E, Noval-Morillas I, Cañadas-Pruaño D, de la Lastra IA, Gheorghe L, Zayas-Rueda R, Calle-Perez G, Vázquez-García R. The radioprotective effect of the Cathpax® AIR cabin during interventional cardiology procedures. Catheter Cardiovasc Interv. 2021;98:E523-E530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Shorrock D, Christopoulos G, Wosik J, Kotsia A, Rangan B, Abdullah S, Cipher D, Banerjee S, Brilakis ES. Impact of a Disposable Sterile Radiation Shield on Operator Radiation Exposure During Percutaneous Coronary Intervention of Chronic Total Occlusions. J Invasive Cardiol. 2015;27:313-316. [PubMed] |
| 26. | Murphy JC, Darragh K, Walsh SJ, Hanratty CG. Efficacy of the RADPAD protective drape during real world complex percutaneous coronary intervention procedures. Am J Cardiol. 2011;108:1408-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | King JN, Champlin AM, Kelsey CA, Tripp DA. Using a sterile disposable protective surgical drape for reduction of radiation exposure to interventionalists. AJR Am J Roentgenol. 2002;178:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Ertel A, Nadelson J, Shroff AR, Sweis R, Ferrera D, Vidovich MI. Radiation Dose Reduction during Radial Cardiac Catheterization: Evaluation of a Dedicated Radial Angiography Absorption Shielding Drape. ISRN Cardiol. 2012;2012:769167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Kherad B, Jerichow T, Blaschke F, Noutsias M, Pieske B, Tschöpe C, Krackhardt F. Efficacy of RADPAD protective drape during coronary angiography. Herz. 2018;43:310-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Vlastra W, Delewi R, Sjauw KD, Beijk MA, Claessen BE, Streekstra GJ, Bekker RJ, van Hattum JC, Wykrzykowska JJ, Vis MM, Koch KT, de Winter RJ, Piek JJ, Henriques JPS. Efficacy of the RADPAD Protection Drape in Reducing Operators' Radiation Exposure in the Catheterization Laboratory: A Sham-Controlled Randomized Trial. Circ Cardiovasc Interv. 2017;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Chakravartti J, Rao SV. Robotic Assisted Percutaneous Coronary Intervention: Hype or Hope? J Am Heart Assoc. 2019;8:e012743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Beyar R, Gruberg L, Deleanu D, Roguin A, Almagor Y, Cohen S, Kumar G, Wenderow T. Remote-control percutaneous coronary interventions: concept, validation, and first-in-humans pilot clinical trial. J Am Coll Cardiol. 2006;47:296-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Anuwatworn A, Ali Kahn M, Stys T, Petrasko M, Stys A. Robotic-Assisted Percutaneous Coronary Intervention Through Transradial Approach: Experience in 4 Patients with Complex Lesions. Tex Heart Inst J. 2020;47:144-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Smitson CC, Ang L, Pourdjabbar A, Reeves R, Patel M, Mahmud E. Safety and Feasibility of a Novel, Second-Generation Robotic-Assisted System for Percutaneous Coronary Intervention: First-in-Human Report. J Invasive Cardiol. 2018;30:152-156. [PubMed] |
| 35. | Kagiyama K, Mitsutake Y, Ueno T, Sakai S, Nakamura T, Yamaji K, Ishimatsu T, Sasaki M, Chibana H, Itaya N, Sasaki KI, Fukumoto Y. Successful introduction of robotic-assisted percutaneous coronary intervention system into Japanese clinical practice: a first-year survey at single center. Heart Vessels. 2021;36:955-964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | De Silva K, Myat A, Strange J, Weisz G. Iterative Improvement and Marginal Gains in Coronary Revascularisation: Is Robot-assisted Percutaneous Coronary Intervention the New Hope? Interv Cardiol. 2020;15:e18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Madder RD, VanOosterhout SM, Jacoby ME, Collins JS, Borgman AS, Mulder AN, Elmore MA, Campbell JL, McNamara RF, Wohns DH. Percutaneous coronary intervention using a combination of robotics and telecommunications by an operator in a separate physical location from the patient: an early exploration into the feasibility of telestenting (the REMOTE-PCI study). EuroIntervention. 2017;12:1569-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Bezerra HG, Mehanna E, W Vetrovec G, A Costa M, Weisz G. Longitudinal Geographic Miss (LGM) in Robotic Assisted Versus Manual Percutaneous Coronary Interventions. J Interv Cardiol. 2015;28:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Tabaza L, Virk HUH, Janzer S, George JC. Robotic-assisted percutaneous coronary intervention in a COVID-19 patient. Catheter Cardiovasc Interv. 2021;97:E343-E345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Wegermann ZK, Swaminathan RV, Rao SV. Cath Lab Robotics: Paradigm Change in Interventional Cardiology? Curr Cardiol Rep. 2019;21:119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Harrison J, Ang L, Naghi J, Behnamfar O, Pourdjabbar A, Patel MP, Reeves RR, Mahmud E. Robotically-assisted percutaneous coronary intervention: Reasons for partial manual assistance or manual conversion. Cardiovasc Revasc Med. 2018;19:526-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Weisz G, Metzger DC, Caputo RP, Delgado JA, Marshall JJ, Vetrovec GW, Reisman M, Waksman R, Granada JF, Novack V, Moses JW, Carrozza JP. Safety and feasibility of robotic percutaneous coronary intervention: PRECISE (Percutaneous Robotically-Enhanced Coronary Intervention) Study. J Am Coll Cardiol. 2013;61:1596-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 208] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 43. | Kuon E, Birkel J, Schmitt M, Dahm JB. Radiation exposure benefit of a lead cap in invasive cardiology. Heart. 2003;89:1205-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 44. | Karadag B, Ikitimur B, Durmaz E, Avci BK, Cakmak HA, Cosansu K, Ongen Z. Effectiveness of a lead cap in radiation protection of the head in the cardiac catheterisation laboratory. EuroIntervention. 2013;9:754-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Pasciak AS, Jones AK. Time to take the gloves off: the use of radiation reduction gloves can greatly increase patient dose. J Appl Clin Med Phys. 2014;15:5002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 46. | Subramanian S, Waller BR, Winders N, Bird LE, Agrawal V, Zurakowski D, Kuhls-Gilcrist A, Khandkar A, Sathanandam SK. Clinical evaluation of a radio-protective cream for the hands of the pediatric interventional cardiologist. Catheter Cardiovasc Interv. 2017;89:709-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Sarma AA, Nkonde-Price C, Gulati M, Duvernoy CS, Lewis SJ, Wood MJ; American College of Cardiology Women in Cardiology Leadership Council. Cardiovascular Medicine and Society: The Pregnant Cardiologist. J Am Coll Cardiol. 2017;69:92-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 48. | Best PJ, Skelding KA, Mehran R, Chieffo A, Kunadian V, Madan M, Mikhail GW, Mauri F, Takahashi S, Honye J, Hernández-Antolín R, Weiner BH; Women in Innovations (WIN) group of the Society of Cardiac Angiography and Intervention. SCAI consensus document on occupational radiation exposure to the pregnant cardiologist and technical personnel. Heart Lung Circ. 2011;20:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 49. | McCollough CH, Schueler BA, Atwell TD, Braun NN, Regner DM, Brown DL, LeRoy AJ. Radiation exposure and pregnancy: when should we be concerned? Radiographics. 2007;27:909-17; discussion 917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 337] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 50. | ACOG Committee on Obstetric Practice. ACOG Committee Opinion. Number 299, September 2004 (replaces No. 158, September 1995). Guidelines for diagnostic imaging during pregnancy. Obstet Gynecol. 2004;104:647-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 192] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 51. | Schultz FW, Zoetelief J. Dosemeter readings and effective dose to the cardiologist with protective clothing in a simulated interventional procedure. Radiat Prot Dosimetry. 2008;129:311-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |