Published online Sep 26, 2021. doi: 10.4330/wjc.v13.i9.399
Peer-review started: March 25, 2021
First decision: May 13, 2021
Revised: May 30, 2021
Accepted: July 21, 2021
Article in press: July 21, 2021
Published online: September 26, 2021
Processing time: 176 Days and 10.9 Hours
Exercise exerts direct effects on the vasculature via the impact of hemodynamic forces on the endothelium, thereby leading to functional and structural adaptations that lower cardiovascular risk. The patterns of blood flow and endothelial shear stress during exercise lead to atheroprotective hemodynamic stimuli on the endothelium and contribute to adaptations in vascular function and structure. The structural adaptations observed in arterial lumen dimensions after prolonged exercise supplant the need for acute functional vasodilatation in case of an increase in endothelial shear stress due to repeated exercise bouts. In contrast, wall thickness is affected by rather systemic factors, such as transmural pressure modulated during exercise by generalized changes in blood pressure. Several mechanisms have been proposed to explain the exercise-induced benefits in patients with coronary artery disease (CAD). They include decreased progression of coronary plaques in CAD, recruitment of collaterals, enhanced blood rheo
Core Tip: Exercise has beneficial effects on the function and structure of the vasculature, thereby leading to a reduction of the cardiovascular risk. Hemodynamic forces, in particular endothelial shear stress, play a critical role in modulating the endothelial cell phenotype towards atherogenesis or atheroprotection. Exercise improves clinical outcomes in patients with coronary artery disease (CAD). We herein discuss the alterations induced by exercise on vascular function and structure, and the mechanisms involved in the benefits of exercise regarding patients with CAD.
- Citation: Sakellariou XM, Papafaklis MI, Domouzoglou EM, Katsouras CS, Michalis LK, Naka KK. Exercise-mediated adaptations in vascular function and structure: Beneficial effects in coronary artery disease. World J Cardiol 2021; 13(9): 399-415
- URL: https://www.wjgnet.com/1949-8462/full/v13/i9/399.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i9.399
Cardiovascular disease is the main cause of morbidity and mortality in western societies. Although traditional risk factors (hypertension, dyslipidemia, diabetes mellitus, smoking) have a systemic atherogenic effect on the entire vasculature, local hemodynamic factors determine the distribution of atherosclerotic lesions. Endothelial shear stress (ESS), the frictional force acting on the endothelium as the result of blood flow, represents a continuous stimulus eliciting structural and functional effects on the endothelium and plays a critical role in the development of atherosclerosis. Atherosclerotic lesions develop preferentially at areas with disturbed local hemodynamic factors, mainly in regions with low ESS, such as the inner curvature of coronary arteries or in the outer waist of a coronary bifurcation and downstream from a luminal obstruction where ESS is oscillatory. In contrast, arterial regions with physiologic/ increased local flow and ESS are thought to be protected from atherosclerosis[1-3].
Multiple studies suggest that exercise decreases the risk of coronary artery disease (CAD) with the positive impact on both primary and secondary prevention being greater than 30%[4,5]. Exercise-based cardiac rehabilitation has been associated with reduced both all-cause and cardiac mortality as well as hospital admissions[6,7]. Exercise also decreases the risk of cardiovascular events and, in patients with CAD, increases exercise capacity, decreases myocardial ischemia, and delays the onset or inhibits angina pectoris[8,9].
The effects of exercise on traditional risk factors do not fully explain the tremendous impact of exercise on cardiovascular risk: differences in known traditional and novel risk factors explain approximately 60% of exercise-mediated cardiovascular disease risk reduction and only 35% of the heart disease risk reduction[10]. Exercise exerts direct effects on the vasculature through the impact of ESS on the endothelium, which leads to functional and structural adaptations that decrease atherosclerotic risk[9,11]. In addition, exercise-induced changes in flow-mediated dilation (FMD), an index of vascular function, do not correlate well with changes in traditional cardiovascular risk factors[12]. Therefore, it is possible that the cardioprotective effect of exercise is, at least in part, independent of changes in traditional risk factors and is mediated by functional and structural adaptations.
The purpose of this review is to describe how exercise via alterations in hemodynamic factors influences vascular function and structure contributing to cardiovascular risk reduction, and to highlight which mechanisms are involved in the positive effects of exercise on CAD.
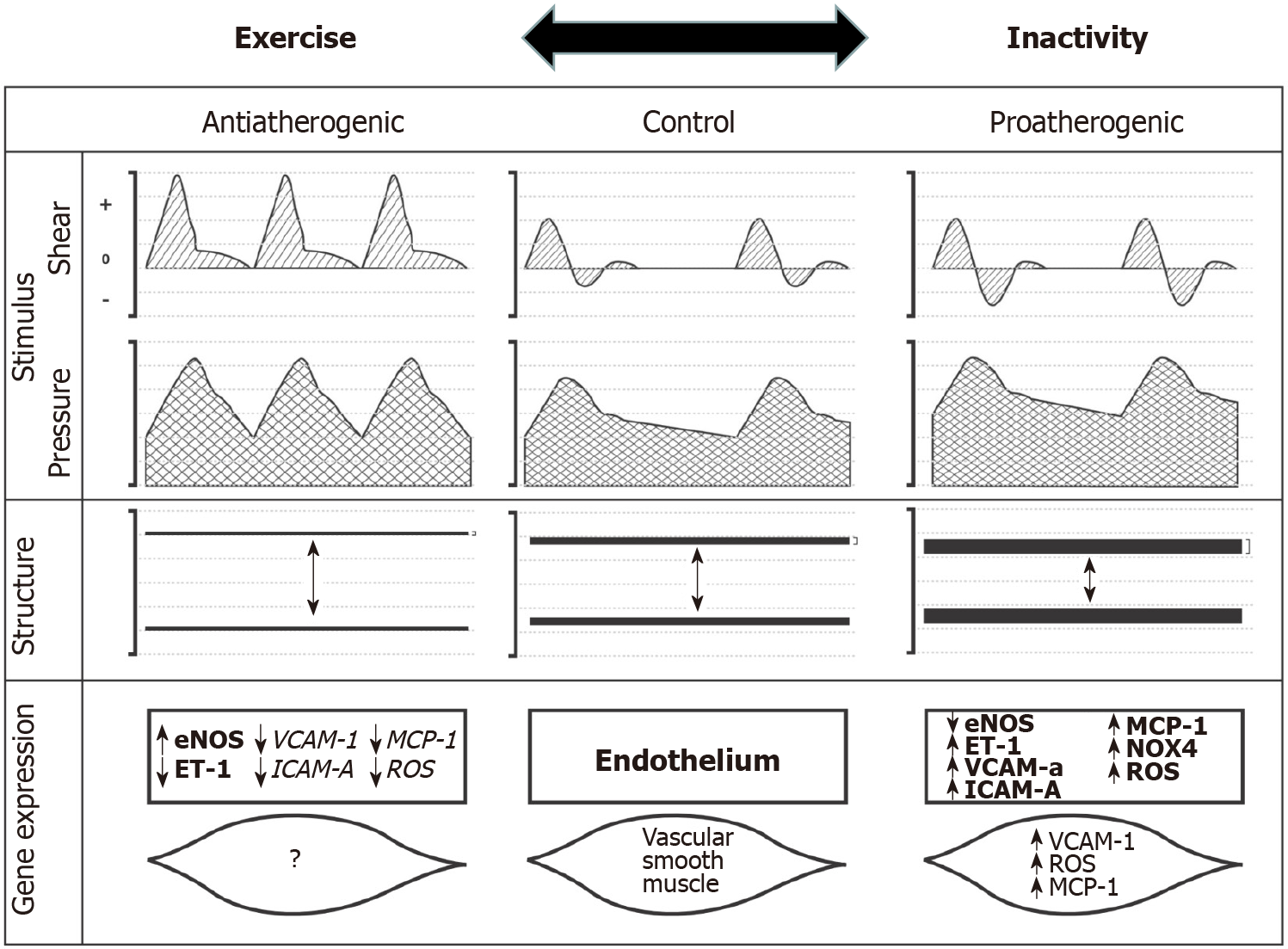
Exercise-induced hemodynamic alterations have been reported to play a major role in cardiovascular disease risk reduction, leading to direct effects on the vasculature that are atheroprotective. These vascular adaptations concern mainly the endothelium and the cross-talk between the endothelium and smooth muscle cells (Figure 1)[11,13,14].
The normal endothelial phenotype is of utmost importance in artery health, and thus, changes in endothelial cell phenotype are related to the development and progression of atherosclerosis. Endothelial dysfunction precedes and is present during the evolution of atherosclerosis, indicating that a proatherogenic endothelial phenotype plays an important role in both the initiation and progression of atherosclerosis[13,15,16]. Furthermore, latest evidence suggests that physical activity exerts beneficial effects by maintaining a normal phenotype of arterial endothelial cells[17-19].
Exercise has beneficial effects in the primary and secondary prevention of CAD, which are closely related to changes in the endothelial cell phenotype[17,20,21]. Exercise augments nitric oxide (NO) bioavailability through a variety of mechanisms; data from cell-culture and animal experiments suggest that NO bioavailability can be affected by many different alterations in the following steps of the NO pathway. Exercise acts as a stimulus for the endothelium to (1) increase the availability of L-arginine (the precursor molecule for NO); (2) promote NO synthase (NOS) activity and expression; and (3) augment the production of extracellular superoxide dismutase, which prevents premature breakdown of NO. These effects likely contribute to amplified exercise capacity and, ultimately, cardiovascular protection. Cardiovascular risk factors, as well as established atherosclerotic disease, are associated with profound impairment of the NO pathway and systems, which may lead to limitations in exercise capacity. Exercise in populations with coronary artery disease can increase NO bioavailability and contribute to secondary prevention[17,22,23]. In addition, the beneficial effects of exercise on preventing atherosclerosis progression and the improvement of endothelial function and phenotype are associated with decreased expression of adhesion molecules as well as inflammatory cell infiltration[24].
The beneficial effects of physical activity on vascular health result from exercise-induced changes in hemodynamic factors. Exercise produces increases in blood flow to the heart and active skeletal muscle, generating shear forces that have been suggested to differentiate gene expression in endothelial and vascular smooth muscle cells[13,25]. Increases in mean ESS positively modify the expression of atheroprotective genes; the beneficial effect of exercise also extends to arteries that do not directly present increased mean ESS during exercise (Figure 1)[14,26]. Accumulating data suggest that elevated ESS is a signal for increased endothelial NOS, decreased endothelin-1 and decreased vascular cell adhesion molecule 1 (VCAM-1) expression. Several intracellular signaling mechanisms have been identified, including G proteins, calcium, and proto-oncogene tyrosine-protein kinase Src (c-Src)[27-30]. Investigation of gene expression patterns of cultured endothelium exposed to different flow waveforms has shown that mean ESS significantly influences the expression of approximately 3000 genes[31]. Data from in vivo models provide a correlation between increases in mean ESS and antiatherogenic effects. Chronic increases in blood flow are associated with upregulation of endothelial NOS mRNA, protein and activity, as well as with decreased endothelin-1 bioavailability[13]. Furthermore, during chronic exercise training, improvement of endothelial and mitochondrial function is found to be mediated by adenosine monophosphate-activated protein kinase alpha-2 (AMPKα2) in studies with AMPKα2 knockout mice[32-34].
Elevated blood flow during exercise is also related to increases in pulse pressure leading to an increase in cyclic strain across the vasculature. However, data obtained from cell culture demonstrated controversial results regarding the effect of cyclic strain. Although some studies reported that cyclic strain produced an antiatherogenic endothelial cell phenotype, other experiments suggested that cyclic strain had the opposite effect leading to a proatherogenic phenotype in cultured endothelial cells[35-38]. A more recent experiment using a whole vessel preparation reported that the decrease of cyclic strain stimulus leads to lower levels of phosphorylation of the endothelial NOS while it increases the production of reactive oxygen species[39].
Exercise has well-documented positive effects on endothelium-dependent vasodilator capacity, and ESS plays a major role in transducing these changes since enhancement in endothelial function is induced by increased endothelium-derived NOS shear-related protein expression[17]. Many studies have been conducted to investigate not only the impact of different patterns of exercise-related ESS on NO-mediated endothelial function, but also the manipulation of the shear stress stimulus during exercise. The modality by which vascular response to exercise training can be influenced in hypertensive individuals has been studied by the SEFRET study, while in spontaneous hypertensive mice the lack of a positive effect of high intensity exercise on endothelial function was found to be related to NO availability imbalance[40,41]. Kim et al[42] enrolled middle-aged marathoners with exercise-induced hypertension and reported increased angiotensin II with a reduction in NO levels. These findings may explain the deterioration of arteries vasodilator capacity and elevated blood pressure during exercise in this group of long-distance runners, as well as the therapeutic effect of angiotensin II inhibitors in patients with exercise-induced hypertension. Experimental data also support the hypothesis that physical exercise combined with the administration of renin-angiotensin-aldosterone system blockers could have beneficial effects in order to prevent hypertensive cardiac alterations (e.g., left ventricular hypertrophy)[43].
Exercise effect on the vasculature has also been investigated in respect to other conditions, considering a load of high fat meal in healthy subjects, or in a different study considering glucose ingestion in adults with pre-diabetes. Both studies concluded via FMD measurements that exercise produces beneficial effects by attenuating the susceptibility to oxidative damage[44,45]. In patients with type 2 diabetes, acute (2 h after exercise) improvement of endothelial function was observed by FMD measurements after a bout of seven 1-minute cycling intervals using leg resistance exercises, while without leg resistance the beneficial effects were observed at 1 h post exercise[46]. No long-term study on patients with diabetes is available yet.
The acute impact of different exercise modalities, and as a consequence different patterns of blood velocity and flow on the upper limb vasculature function, has also been investigated. FMD, a largely NO-mediated vasodilator response, has been simultaneously studied in both brachial arteries of healthy young men before and after 30-min interventions consisting of bilateral forearm heating, recumbent leg cycling, and bilateral handgrip exercise. During each intervention, a cuff was inflated on 1 arm to unilaterally manipulate the shear rate stimulus. These studies revealed a significant difference in the pattern of ESS between the 3 interventions. Post-intervention FMD was significantly increased compared with pre-intervention in response to each intervention[47,48]. Beneficial effects have also been reported in women[49]. Taken these results into account, we speculate that increases in anterograde blood flow and ESS lead to enhanced endothelial function.
Observations that predominantly lower limb exercise induces upper limb vascular adaptation have indicated that a generalized or systemic impact of exercise on endothelial function occurs in vascular beds other than those where the exercise stimulus is focused[50-52]. Therefore, exercise induces both localized improvements in vascular function in the active regions through repetitive increases in ESS and systemic vascular adaptations in case of large muscle group exercise.
Based on the above studies one can speculate that different patterns of blood flow and ESS during exercise lead to different hemodynamic and ESS stimuli to which the endothelium is exposed and as a consequence to different vascular adaptations, including the production and bioavailability of NO (Figure 1)[14].
Flow-mediated dilation in response to reactive hyperemia is, surprisingly, manifested only after blood flow drops back to baseline levels. It remains a puzzle whether this discrepancy (i.e. dissociation of flow and diameter) could be explained by a reduction in transmural pressure produced by high flow. Dedicated studies have shown that blood pressure and transmural pressure fall after cuff release at the time of peak hyperemic flow. Flow interruption 20 s after cuff release (during high flow but no dilation) leads to an immediate increase in artery diameter. These observations suggest that flow-dependent dilation may be offset by a flow-induced fall in local arterial pressure, and thus, in transmural pressure. This observation indicates that the combination of systemic pressure and local ESS may determine the ultimate vasoactive arterial response following acute exercise[53]. The critical role of these parameters is further confirmed by showing that during a single handgrip exercise, the response (measured by FMD 15 minutes after exercise) was blunted by the addition of an inflated pneumatic cuff to the exercising arm, clearly by abruptly influencing the local ESS[54]. Finally, improvement of the endothelial function was the conclusion of a recent systematic review meta-analysis, where the overall effect of exercise training on the endothelial function in heart failure patients was assessed by FMD in a total of 16 studies[55].
Adaptations that occur in human vasculature following acute exercise, as mentioned above, are useful to understand the impact of repeated episodic exposure to exercise. Previous studies conducted in healthy, asymptomatic subjects have reported that there was no adaptation in endothelial function following exercise and, interestingly, this observation was independent of whether exercise involved localized or large muscle systemic exercise[4]. Therefore, it may be difficult to enhance normal endothelial function in healthy subjects. However, there is evidence suggesting that a moderate-to-higher load (intensity, frequency, duration) of exercise may be necessary for improving endothelial function in healthy asymptomatic humans[56]. Campbell et al[57] examined the impact of long-term aerobic exercise during advancing age. They highlighted the importance of remaining active throughout a lifetime, since enhancement of endothelial vascular function is apparent only in athletic older persons, and not in otherwise healthy sedentary individuals following only a period of exercise training. Despite this beneficial effect, it is not clear which exercise load is most appropriate since exercise of high intensity may also cause oxidative stress[58,59]. Qiu et al[60] revealed that chronic aerobic and combined aerobic and resistance exercise training programs improved endothelial function even in patients with type 2 diabetes, whose endothelium is characterized by impaired nitric oxide bioavailability following exercise training. Limited data are available regarding the endothelial function of coronary microcirculation, which suggest that coronary microvascular vasodilator response is enhanced following high-intensity exercise in humans[23]. Finally, we should consider that exercise may induce a transient increase in vascular function in healthy humans, which also influences conduit coronary arteries. FMD may be initially enhanced, but subsequently declined to pre-training levels, as exercise induces changes in arterial structure[52,61,62].
Remodeling of luminal dimension: Exercise induces adaptations in the cross-sectional size of arteries, and athletes are characterized by augmented peak vasodilator capacity, which is associated with luminal expansion of resistance arteries[63,64]. Peak vasodilator capacity is significantly greater in the dominant limb of athletes compared to both their non-dominant limb and non-athletic control subjects[65,66]. These data suggest that resistance artery luminal adaptation is apparent in athletes and can occur as a result of localized and intrinsic vascular stimuli. However, there are some studies in which lower limb exercise induced enhanced peak vasodilator capacity of the upper limb, thereby suggesting a generalized effect of exercise on arterial lumen adaptations[67].
As far as structural adaptations of resistance vessels in the normal heart are concerned, exercise increases arteriolar densities and diameters as suggested by experimental studies in animals[68]. An increase of total cross-sectional area of arterioles in the diameter range of 20-120 μm has been reported, with a higher increase in arterioles of 20-40 μm than in arterioles of 40-120 μm[69]. The impact of aerobic training on the growth of the capillaries of the coronary circulation is also well established. Capillary proliferation is a fundamental response to exercise but is accompanied by concurrent transformation of capillaries into arterioles[70]. Although some experimental studies suggested that exercise causes an increase in myocardial capillary density in prepubescent rats, it seems to have no effect on capillary density in postpubescent ones[71,72]. Exercise-induced angiogenesis may temporarily exceed the increase in left ventricular mass, but with prolonged exercise, angiogenesis matches the left ventricular hypertrophic response[69].
Various methodological approaches suggest that exercise induces growth of epicardial arteries which is in proportion to exercise-induced cardiac hypertrophy (left ventricular mass)[73-75]. Angiographic studies suggest that exercise significantly induces enhanced coronary artery dilating capacity in more active subjects, whereas there is no difference at rest between runners and control subjects[76,77]. For this reason, it is important to elicit dilator responses in order to uncover differences between athletes and control subjects; of note, basal arterial tone in athletes may be enhanced because of alpha-adrenergic and NOS inhibition-induced vasoconstriction, as well as increased resting plasma norepinephrine concentration[78]. Finally, transthoracic echocardiographic assessment has shown similar results since coronary flow reserve, an index of conduit artery vasodilator capacity, is greater in humans undergoing regular exercise[79].
Remodeling of arterial wall: Arterial wall thickness may have implications for cardiovascular risk since common carotid and femoral artery intima-media thickness have been found to be independent predictors of future clinical cardiovascular adverse events[80,81]. Furthermore, arterial wall remodeling may differentiate vascular functional responses since a larger wall-to-lumen ratio is correlated with greater responses to vasoactive stimuli[82].
Studies on the exercise-mediated responses of brachial, carotid, and superficial femoral arteries have shown that arterial wall thickness may be influenced by systemic factors, such as arterial pressure in contrast to shear-mediated impacts on arterial lumen size, which are more localized[64]. A decreased wall thickness is observed in all arteries (carotid, brachial and superficial femoral) of able-bodied athletes compared with control subjects. In contrast to the effects on lumen diameter, a decreased wall thickness was found in both limbs and was not related to exercise type[64,83]. Another study examined bilateral brachial artery wall thickness across an 8-wk period of bilateral handgrip training. ESS was attenuated by cuff inflation around one forearm, but brachial artery pressure responses during exercise were not affected. Handgrip exercise had no effect on baseline brachial artery diameter, blood flow, or shear rate, but significantly decreased brachial artery wall thickness after 6 and 8 wk (similar in cuffed and non-cuffed arm) and wall-to-lumen ratio after 8 wk (also similar in cuffed and non-cuffed arm)[84].
These findings suggest that in contrast to exercise-induced adaptations of arterial luminal dimensions, which are modified by more local mechanisms and mainly by ESS, wall thickness is affected by rather systemic factors, such as transmural pressure, which is modulated during exercise as a result of generalized changes in blood pressure. The interest in wall remodeling has been due to its use as an index of pre-clinical atherosclerosis and estimation of cardiovascular risk. Regarding the time period required for these adaptations, studies have reported that the effects of exercise on atherosclerosis of the carotid artery may require intensive or prolonged exercise, whereas aerobic exercise decreases femoral, popliteal, and brachial artery intima-media thickness in a relatively shorter period[85]. Therefore, a decrease in arterial wall thickness in athletes and healthy young subjects should not necessarily be considered synonymous with a decrease in cardiovascular risk, but as a physiological impact on wall remodeling with yet unknown long-term health implications (Figure 1)[14,86].
Exercise is well known to have a major role in both primary and secondary prevention of CAD, while regular physical activity of 150 min/wk reduces the risk of numerous chronic diseases and decreases cardiovascular mortality[4,9,10,22,87]. In patients with symptomatic CAD, exercise augments physical performance and raises the angina threshold. Endurance exercise has also been shown to attenuate the extent of ischemic ST-segment depression during exercise and decrease perfusion defects on scintigraphy, indicating a possible enhancement in myocardial perfusion[88,89]. The great benefits of exercise are also demonstrated by the fact that physical exercise in selected patients with CAD leads to higher event-free survival and exercise capacity at lower costs compared with percutaneous coronary angioplasty, reflecting the impact of exercise on the whole arterial tree and not only a single location[90].
Several mechanisms have been proposed to explain the exercise-induced benefits in patients with CAD. These include decreased progression (or regression) of coronary plaques/lesions in CAD, recruitment of collaterals, enhanced blood rheological properties, and improvement of endothelial function and coronary blood flow, as well as enhancement of vascular smooth muscle cell function[22,23,91].
Early investigations indicated that the extent of myocardial perfusion was associated with the magnitude of coronary stenosis depicted by angiography. There is evidence that exercise induces enlargement of conduit coronary arteries in normal subjects, and reduces the development or even causes regression of atherosclerotic lesions in coronary arteries in animal CAD models[74,76,77]. Many studies have been conducted to document the regression or decreased progression of atherosclerotic lesions[92,93].
Experimental studies provided controversial results regarding the effect of exercise on reducing the development of coronary artery atherosclerosis or decreasing lesion progression. However, more recent experiments suggest that although exercise has cardio-protective effects and reduces the risk for cardiovascular disease, it may not inhibit progression or reverse coronary artery disease according to angiographic measures of lesion area[91,94,95]. In addition, Kim et al[96] studied veteran marathon runners and reported increased prevalence of coronary artery plaques among those with exercise-induced hypertension, thereby suggesting that exercise-induced hypertension could be a novel risk factor for coronary artery plaque formation.
Several randomized trials in humans evaluated angiographically the impact of exercise and investigated whether exercise has a direct effect on the extent of CAD[97-99]. However, findings derived from these studies need to be carefully interpreted since exercise is only one component of lifestyle and medical interventions.
In the Stanford Coronary Risk Intervention Project, the main angiographic outcome was the rate of change in the minimal diameter of diseased segment. Multifactor risk reduction slowed down the progression of CAD; the rate of narrowing of coronary lesion was 47% less than for subjects in the usual-care group[98]. Studies on the impact of exercise and low-fat diet on coronary morphology and myocardial perfusion found that despite the progression of CAD, patients participating in physical exercise and low-fat diet were characterized by lower stress-induced myocardial ischemia and improvement of myocardial perfusion[100]. In the long-term, when patients were reevaluated 6 years later, in the intervention group, the progression of coronary stenoses had a significantly slower rate than in the control group, and that effect was mediated by chronic physical exercise[97].
The Lifestyle Heart Trial demonstrated that lifestyle changes may induce regression of coronary artery atherosclerosis after only 1 year according to the average percentage diameter stenosis, whereas in the control group there was progression of coronary lesions[101]. After 5 years, these lifestyle changes continued to have impacts on CAD in the experimental group, since further regression of coronary lesions was documented, whereas progression of coronary atherosclerosis continued in the control group[99]. In addition, it appears that physical inactivity is considered as a significant atherosclerotic risk factor and accelerates atherosclerosis development[102]. Additionally, there have also been reports on the potential of different levels of regular–leisure time–exercise to enhance cardio-respiratory fitness and retard progression of (or reverse) CAD. Patients in the exercise intervention group have exhibited an increase in oxygen uptake and in peak exercise while a decrease in the respective parameters was observed in the control group. Decreased progression or regression of CAD lesions was observed, only when CAD patients could sustain a high level of leisure time physical activity for 1 year[103]. Nytrøen et al[104] investigated the effect of high-intensity interval training on cardiac allograft vasculopathy in heart transplant recipients. They demonstrated that 1 year of exercise training resulted in significantly lower atheroma volume (assessed by intravascular ultrasound) compared with the control group. A recent review provides recommendations to physicians regarding high-intensity interval training (i.e. short bouts of high-intensity submaximal exercise interspersed with rest periods) which has become very popular among patients following cardiac rehabilitation programs[105]. In addition, low-volume high-intensity interval training (typically involving less than 15 minutes of high-intensity exercise per session) is a time and energy efficient way of exercise and leads to similar or even greater cardiorespiratory fitness and cardiac function enhancement when compared to traditional ways of aerobic exercise[106].
Exercise may have a greater impact on CAD progression or regression following treatment with percutaneous coronary intervention (PCI) and stent implantation. Diameter restenosis after PCI was significantly higher in untrained compared with trained patients. In addition, myocardial perfusion of patients with angiographic restenosis was enhanced only in the exercise group[107]. Studies in a porcine PCI model suggest that exercise significantly decreases the extent of neointimal hyperplasia lesion and restenosis[108]. A recent meta-analysis demonstrated that rehabilitation exercise programs (including cycle ergometer, jogging, climbing, swimming and treadmill) reduced the incidence of coronary artery restenosis following PCI in patients with CAD[109]. A possible explanation for this beneficial impact of exercise is that coronary hemodynamics are altered by interventional procedures and exercise bouts generate more beneficial “mechanical” signals in the walls of these arteries[91].
Experimental studies have shown that moderate physical exercise reduces the bulk of coronary lesions and spontaneous atherosclerotic plaque rupture leading to prolonged survival[110]. Exercise alters the extracellular matrix composition of the neointima in animal PCI models and decreases neointimal proliferation, which may have a significant impact on preventing restenosis following coronary angioplasty[108]. Differences in the intima/media ratio were observed after exercise, as well as higher collagen and elastin contents of atherosclerotic plaques were detected in exercise groups. Lower macrophage concentration in the atherosclerotic plaques has also been found in the exercise group. Furthermore, a significant decrease in MMP-9/TIMP-1 (matrix metalloproteinases to tissue inhibitor of matrix metalloproteinases) ratio, which has an important role in the atherosclerotic plaque vulnerability, has been reported after exercise[111]. The latest studies support the idea that physical exercise may convert a vulnerable thin-cap atheroma to a more stable lesion, less prone to rupture, which can reduce cardiac mortality[91].
Studies in animals suggest that exercise stimulates coronary collateral development[112-114]. Endothelial progenitor cells are considered to initiate neovascularization in response to ischemia after myocardial infarction; a significant increase of endothelial progenitor cell proliferation and function has been observed in mice both with and without myocardial infarction in the exercise group[111,115]. However, there are some contradictory data that report no development of coronary collaterals in dogs with normal coronary arteries[116,117]. The effect of physical exercise on collateral vessel growth in humans is also disputable. Patients with ischemic heart disease and left ventricular systolic dysfunction who have been randomized to exercise and control groups demonstrated that there is enhanced perfusion and contractile response to dobutamine, which were correlated with an increase in coronary collateralization in the exercise group[118]. Zbinden et al[119] demonstrated that a 3-month endurance exercise training program enhanced coronary collateral supply to normal vessels, as well as to previously stenotic arteries with percutaneous intervention in patients referred for diagnostic coronary angiography due to chest pain or positive treadmill exercise test. However, there are many angiographic studies conducted at rest in patients with CAD that did not confirm this hypothesis[120,121]. A randomized trial including patients with CAD did not show any significant effect of exercise on collateral formation after 1 year, although progression of CAD was significantly slowed in the intervention group[122].
Abnormalities of blood rheological properties are an independent risk factor for cardiovascular disease, and may contribute to athero-thrombogenesis. However, there are limited literature data about the effect of exercise on blood rheological properties. Recent studies suggest that blood becomes more dilute because of expansion of blood volume as a result of exercise training. It has been reported that this hypervolemia and blood dilutional effect may contribute to enhanced cardiac stroke volume during exercise[123]. Blood rheology may be enhanced after regular physical exercise since different experimental approaches, including regular exercise, demonstrate a decrease of blood viscosity[124]. However, blood rheology may also remain unaffected in patients with CAD and heart failure[125]. Further studies are necessary to determine the possible association between exercise and blood rheological profiles, especially since the improvement of blood viscosity remains an interesting therapeutic option for symptoms relief in patients with CAD; enhanced fluidity may facilitate oxygen delivery to the exercising muscles because of a reduced resistance to blood flow within the microcirculation.
None of the above-mentioned mechanisms fully explain the beneficial effect of exercise on cardiovascular mortality and myocardial perfusion, as well as the major role that exercise has gained in cardiac rehabilitation. During the last two decades, endothelial dysfunction has been correlated with major risk factors for CAD, and identified even before coronary stenoses are visible. Endothelial dysfunction is considered a significant predictor of coronary adverse events and has a great role in myocardial ischemia. Coronary endothelial function depends on NO bioavailability, the balance of which is disrupted in CAD. The impairment of NO production, in addition with an increase in oxidative stress, induces the loss of endothelial cells by apoptosis and the deterioration of endothelial function, leading to paradoxical vasoconstriction and myocardial ischemia[18,22,126]. Exercise attenuates paradoxical vasoconstriction in patients with CAD and leads to improvement of endothelial function as it restores the disrupted NO balance.
Patients with CAD are characterized by functional alteration of circulating progenitor cells, which maintain the integrity of the vasculature. Exercise restores the regenerative capacity of circulating progenitor cells in cardiovascular disease and prevents further impairment of vessels vasomotion. A recent meta-analysis demon
Exercise in patients with CAD may enhance coronary endothelial function. To investigate this hypothesis, patients with coronary endothelial dysfunction (according to abnormal acetylcholine-induced vasoconstriction) were randomized to an exercise or a control group. Coronary vasoconstriction in response to acetylcholine was significantly attenuated and adenosine-induced flow-dependent vasodilation was improved after physical exercise, which indicates that exercise had beneficial impacts on the endothelium of epicardial conduit vessels[23]. In addition, exercise induces increases in coronary blood flow reserve, as assessed by adenosine infusion, indicating augmentation in vasodilator capacity of resistance coronary vessels. A recent meta-analysis studied the long-term effects of aerobic exercise in patients with coronary artery disease, suggesting a significant enhancement of vascular vasomotor function and coronary flow velocity reserve[129]. Patients with CAD following a 6-month aerobic exercise training program had higher peak response to acetylcholine when they performed high-frequency exercise compared with low frequency cardiac rehabilitation programs[130]. In addition, a 2-wk twice daily aquatic endurance plus calisthenics exercise training program in patients with a recent myocardial infarction or revascularization intervention improved both aerobic exercise capacity and vascular endothelial function[131]. Kollet et al[132] conducted a randomized pilot study and enrolled post-myocardial infarction patients undergoing PCI who performed a 30-min moderate-intensity aerobic training program. This group of patients demonstrated enhanced endothelial function as determined by improved FMD of the brachial artery after each exercise period. On the contrary, prolonged sitting leads to significant deterioration of vascular function in the lower limbs. However, this deleterious effect on FMD may be reversed by “sitting interruption” strategies including simple resistance and aerobic activities[133]. All these observations suggest that exercise reduces stress-induced myocardial ischemia and improves endothelium-dependent coronary vasodilation in patients with CAD[23]. Patients with newly diagnosed CAD and improved FMD after 6 mo of optimized therapy for reducing cardiovascular risk factors had a lower rate of adverse cardiac events (10% vs 26%, P < 0.01) during 3 years of follow-up, while persistent impairment of endothelial vasomotor function was an independent predictor of adverse outcomes[134].
Exercise does not quickly restore endothelial function to normal levels; restoration of normal endothelial function may require more extended exercise[23]. Improvement of vascular endothelial function of conduit and resistance coronary vessels may occur shortly after the beginning of exercise in patients with CAD; however, augmentation of the capillary bed needs a period of few weeks and collateral formation and regression of coronary lesions requires a much more extended exercise period[22].
The vasodilator response of epicardial arteries to nitroglycerine-induced endothelium-independent coronary vasodilation is not significantly affected following exercise. However, there is evidence that epicardial coronary arteries of highly trained middle-aged endurance runners demonstrate greater dilating capacity to nitroglycerin compared with inactive individuals. Thus, it is possible that high intensity endurance training over a long period may be necessary to enhance endothelium-independent dilation capacity of coronary vessels in patients with CAD[76].
In the majority of cases, the positive impact of exercise is limited to the function of the endothelium, whereas smooth muscle function stays unaltered. However, some studies report that enhancement of smooth muscle function is possible to occur but in more severe disease. Thus, one can speculate that there is a stepwise process of dysfunction and amelioration which begins with the endothelium and migrates to the remaining layers of the vessel wall[135].
Cardiovascular disease is closely related to local hemodynamic factors. Exercise has direct effects on the vasculature via the impact of ESS on the endothelium, leading to decreased atherosclerotic risk. Exercise contributes to maintaining a normal phenotype of arterial endothelial cells which is the result of changes in hemodynamic factors, ultimately leading to beneficial effects. Different patterns of blood flow and ESS exert variable stimuli on the endothelium and result in improved NO bioavailability and adaptations in vascular function and structure. Regarding arterial structure, wall thickness may also be influenced by systemic factors. In patients with CAD, exercise has multiple beneficial effects beyond endothelial function since it contributes to the conversion of vulnerable atherosclerotic plaques to a more stable phenotype, may enhance the recruitment of collaterals, and improves the blood rheological properties. These effects translate to reduced cardiac mortality indicating the value of exercise in cardiac rehabilitation programs. Further research is required to investigate and clarify the molecular mechanisms underlying the structural and vascular adaptations. Finally, it is a great challenge to study the different vascular adaptations which are induced by each type of exercise as well as which training program is most effective for each population group.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: European Society of Cardiology, No. 207984.
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen SM S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Vergallo R, Papafaklis MI, Yonetsu T, Bourantas CV, Andreou I, Wang Z, Fujimoto JG, McNulty I, Lee H, Biasucci LM, Crea F, Feldman CL, Michalis LK, Stone PH, Jang IK. Endothelial shear stress and coronary plaque characteristics in humans: combined frequency-domain optical coherence tomography and computational fluid dynamics study. Circ Cardiovasc Imaging. 2014;7:905-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 2. | Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, Krams R, de Crom R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744-2753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 726] [Cited by in RCA: 734] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 3. | Stone PH, Saito S, Takahashi S, Makita Y, Nakamura S, Kawasaki T, Takahashi A, Katsuki T, Namiki A, Hirohata A, Matsumura T, Yamazaki S, Yokoi H, Tanaka S, Otsuji S, Yoshimachi F, Honye J, Harwood D, Reitman M, Coskun AU, Papafaklis MI, Feldman CL; PREDICTION Investigators. Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: the PREDICTION Study. Circulation. 2012;126:172-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 490] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 4. | Thijssen DH, Maiorana AJ, O'Driscoll G, Cable NT, Hopman MT, Green DJ. Impact of inactivity and exercise on the vasculature in humans. Eur J Appl Physiol. 2010;108:845-875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 206] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 5. | Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. JAMA. 2002;288:1994-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 603] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 6. | Taylor RS, Brown A, Ebrahim S, Jolliffe J, Noorani H, Rees K, Skidmore B, Stone JA, Thompson DR, Oldridge N. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116:682-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1475] [Cited by in RCA: 1391] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 7. | Heran BS, Chen JM, Ebrahim S, Moxham T, Oldridge N, Rees K, Thompson DR, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;CD001800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 388] [Cited by in RCA: 472] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 8. | Thompson PD. Exercise prescription and proscription for patients with coronary artery disease. Circulation. 2005;112:2354-2363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Green DJ, O'Driscoll G, Joyner MJ, Cable NT. Exercise and cardiovascular risk reduction: time to update the rationale for exercise? J Appl Physiol (1985). 2008;105:766-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 207] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 10. | Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116:2110-2118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 750] [Cited by in RCA: 675] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 11. | Green DJ. Exercise training as vascular medicine: direct impacts on the vasculature in humans. Exerc Sport Sci Rev. 2009;37:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | Green DJ, Eijsvogels T, Bouts YM, Maiorana AJ, Naylor LH, Scholten RR, Spaanderman ME, Pugh CJ, Sprung VS, Schreuder T, Jones H, Cable T, Hopman MT, Thijssen DH. Exercise training and artery function in humans: nonresponse and its relationship to cardiovascular risk factors. J Appl Physiol (1985). 2014;117:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol (1985). 2008;104:588-600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 250] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 14. | Newcomer SC, Thijssen DH, Green DJ. Effects of exercise on endothelium and endothelium/smooth muscle cross talk: role of exercise-induced hemodynamics. J Appl Physiol (1985). 2011;111:311-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Vita JA, Keaney JF Jr. Endothelial function: a barometer for cardiovascular risk? Circulation. 2002;106:640-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 548] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 16. | Vita JA, Loscalzo J. Shouldering the risk factor burden: infection, atherosclerosis, and the vascular endothelium. Circulation. 2002;106:164-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Hambrecht R, Adams V, Erbs S, Linke A, Kränkel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107:3152-3158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 634] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 18. | Linke A, Erbs S, Hambrecht R. Exercise and the coronary circulation-alterations and adaptations in coronary artery disease. Prog Cardiovasc Dis. 2006;48:270-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Maiorana A, O'Driscoll G, Taylor R, Green D. Exercise and the nitric oxide vasodilator system. Sports Med. 2003;33:1013-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 221] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 20. | Laughlin MH. Endothelium-mediated control of coronary vascular tone after chronic exercise training. Med Sci Sports Exerc. 1995;27:1135-1144. [PubMed] |
| 21. | Muller JM, Myers PR, Laughlin MH. Vasodilator responses of coronary resistance arteries of exercise-trained pigs. Circulation. 1994;89:2308-2314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 112] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Gielen S, Schuler G, Hambrecht R. Exercise training in coronary artery disease and coronary vasomotion. Circulation. 2001;103:E1-E6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 131] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, Schoene N, Schuler G. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;342:454-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 828] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 24. | Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292:H1209-H1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 595] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 25. | Orr AW, Hastings NE, Blackman BR, Wamhoff BR. Complex regulation and function of the inflammatory smooth muscle cell phenotype in atherosclerosis. J Vasc Res. 2010;47:168-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 213] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 26. | Green D, Cheetham C, Reed C, Dembo L, O'Driscoll G. Assessment of brachial artery blood flow across the cardiac cycle: retrograde flows during cycle ergometry. J Appl Physiol (1985). 2002;93:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Davis ME, Cai H, McCann L, Fukai T, Harrison DG. Role of c-Src in regulation of endothelial nitric oxide synthase expression during exercise training. Am J Physiol Heart Circ Physiol. 2003;284:H1449-H1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Malek AM, Jiang L, Lee I, Sessa WC, Izumo S, Alper SL. Induction of nitric oxide synthase mRNA by shear stress requires intracellular calcium and G-protein signals and is modulated by PI 3 kinase. Biochem Biophys Res Commun. 1999;254:231-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Tuttle JL, Nachreiner RD, Bhuller AS, Condict KW, Connors BA, Herring BP, Dalsing MC, Unthank JL. Shear level influences resistance artery remodeling: wall dimensions, cell density, and eNOS expression. Am J Physiol Heart Circ Physiol. 2001;281:H1380-H1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 173] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 30. | Uematsu M, Ohara Y, Navas JP, Nishida K, Murphy TJ, Alexander RW, Nerem RM, Harrison DG. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am J Physiol. 1995;269:C1371-C1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 390] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 31. | Himburg HA, Dowd SE, Friedman MH. Frequency-dependent response of the vascular endothelium to pulsatile shear stress. Am J Physiol Heart Circ Physiol. 2007;293:H645-H653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Miller VM, Burnett JC Jr. Modulation of NO and endothelin by chronic increases in blood flow in canine femoral arteries. Am J Physiol. 1992;263:H103-H108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Nadaud S, Philippe M, Arnal JF, Michel JB, Soubrier F. Sustained increase in aortic endothelial nitric oxide synthase expression in vivo in a model of chronic high blood flow. Circ Res. 1996;79:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 160] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 34. | Chen X, An X, Chen D, Ye M, Shen W, Han W, Zhang Y, Gao P. Chronic Exercise Training Improved Aortic Endothelial and Mitochondrial Function via an AMPKα2-Dependent Manner. Front Physiol. 2016;7:631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Awolesi MA, Sessa WC, Sumpio BE. Cyclic strain upregulates nitric oxide synthase in cultured bovine aortic endothelial cells. J Clin Invest. 1995;96:1449-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 243] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 36. | Awolesi MA, Widmann MD, Sessa WC, Sumpio BE. Cyclic strain increases endothelial nitric oxide synthase activity. Surgery. 1994;116:439-44; discussion 444. [PubMed] |
| 37. | Ziegler T, Bouzourène K, Harrison VJ, Brunner HR, Hayoz D. Influence of oscillatory and unidirectional flow environments on the expression of endothelin and nitric oxide synthase in cultured endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 225] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 38. | Ziegler T, Silacci P, Harrison VJ, Hayoz D. Nitric oxide synthase expression in endothelial cells exposed to mechanical forces. Hypertension. 1998;32:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 163] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Thacher T, Gambillara V, da Silva RF, Silacci P, Stergiopulos N. Reduced cyclic stretch, endothelial dysfunction, and oxidative stress: an ex vivo model. Cardiovasc Pathol. 2010;19:e91-e98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Pedralli ML, Waclawovsky G, Camacho A, Markoski MM, Castro I, Lehnen AM. Study of endothelial function response to exercise training in hypertensive individuals (SEFRET): study protocol for a randomized controlled trial. Trials. 2016;17:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Battault S, Singh F, Gayrard S, Zoll J, Reboul C, Meyer G. Endothelial function does not improve with high-intensity continuous exercise training in SHR: implications of eNOS uncoupling. Hypertens Res. 2016;39:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Kim CH, Park Y, Chun MY, Kim YJ. Exercise-induced hypertension is associated with angiotensin II activity and total nitric oxide. Medicine (Baltimore). 2020;99:e20943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Tomaz de Castro QJ, Araujo CM, Watai PY, de Castro E Silva SS, de Lima WG, Becker LK, Locatelli J, Guimarães HN, Grabe-Guimarães A. Effects of physical exercise combined with captopril or losartan on left ventricular hypertrophy of hypertensive rats. Clin Exp Hypertens. 2021;43:536-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Lopes Krüger R, Costa Teixeira B, Boufleur Farinha J, Cauduro Oliveira Macedo R, Pinto Boeno F, Rech A, Lopez P, Silveira Pinto R, Reischak-Oliveira A. Effect of exercise intensity on postprandial lipemia, markers of oxidative stress, and endothelial function after a high-fat meal. Appl Physiol Nutr Metab. 2016;41:1278-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Malin SK, Rynders CA, Weltman JY, Jackson Roberts L 2nd, Barrett EJ, Weltman A. Endothelial function following glucose ingestion in adults with prediabetes: Role of exercise intensity. Obesity (Silver Spring). 2016;24:1515-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Francois ME, Durrer C, Pistawka KJ, Halperin FA, Little JP. Resistance-based interval exercise acutely improves endothelial function in type 2 diabetes. Am J Physiol Heart Circ Physiol. 2016;311:H1258-H1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 47. | Tinken TM, Thijssen DH, Hopkins N, Black MA, Dawson EA, Minson CT, Newcomer SC, Laughlin MH, Cable NT, Green DJ. Impact of shear rate modulation on vascular function in humans. Hypertension. 2009;54:278-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 245] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 48. | Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension. 2009;53:986-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 240] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 49. | Carrick-Ranson G, Sloane NM, Howden EJ, Bhella PS, Sarma S, Shibata S, Fujimoto N, Hastings JL, Levine BD. The effect of lifelong endurance exercise on cardiovascular structure and exercise function in women. J Physiol. 2020;598:2589-2605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 50. | Green DJ, Maiorana AJ, Cable NT. Point: exercise training does induce vascular adaptations beyond the active muscle beds. J Appl Physiol (1985). 2008;105:1002-4; discussion 1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | Green D, Cheetham C, Mavaddat L, Watts K, Best M, Taylor R, O'Driscoll G. Effect of lower limb exercise on forearm vascular function: contribution of nitric oxide. Am J Physiol Heart Circ Physiol. 2002;283:H899-H907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 52. | Birk GK, Dawson EA, Atkinson C, Haynes A, Cable NT, Thijssen DH, Green DJ. Brachial artery adaptation to lower limb exercise training: role of shear stress. J Appl Physiol (1985). 2012;112:1653-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 53. | Jiang B, Seddon M, Fok H, Donald A, Chowienczyk P. Flow-mediated dilation of the radial artery is offset by flow-induced reduction in transmural pressure. Hypertension. 2011;57:1145-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Paiva FM, Vianna LC, Fernandes IA, Nóbrega AC, Lima RM. Effects of disturbed blood flow during exercise on endothelial function: a time course analysis. Braz J Med Biol Res. 2016;49:e5100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 55. | Pearson MJ, Smart NA. Effect of exercise training on endothelial function in heart failure patients: A systematic review meta-analysis. Int J Cardiol. 2017;231:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 56. | Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561:1-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 673] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 57. | Campbell A, Grace F, Ritchie L, Beaumont A, Sculthorpe N. Long-Term Aerobic Exercise Improves Vascular Function Into Old Age: A Systematic Review, Meta-Analysis and Meta Regression of Observational and Interventional Studies. Front Physiol. 2019;10:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 58. | Goto C, Higashi Y, Kimura M, Noma K, Hara K, Nakagawa K, Kawamura M, Chayama K, Yoshizumi M, Nara I. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: role of endothelium-dependent nitric oxide and oxidative stress. Circulation. 2003;108:530-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 395] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 59. | Bergholm R, Mäkimattila S, Valkonen M, Liu ML, Lahdenperä S, Taskinen MR, Sovijärvi A, Malmberg P, Yki-Järvinen H. Intense physical training decreases circulating antioxidants and endothelium-dependent vasodilatation in vivo. Atherosclerosis. 1999;145:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 123] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 60. | Qiu S, Cai X, Yin H, Sun Z, Zügel M, Steinacker JM, Schumann U. Exercise training and endothelial function in patients with type 2 diabetes: a meta-analysis. Cardiovasc Diabetol. 2018;17:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 61. | Tinken TM, Thijssen DH, Black MA, Cable NT, Green DJ. Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol. 2008;586:5003-5012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 206] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 62. | Wang J, Wolin MS, Hintze TH. Chronic exercise enhances endothelium-mediated dilation of epicardial coronary artery in conscious dogs. Circ Res. 1993;73:829-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 193] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 63. | Naylor LH, O'Driscoll G, Fitzsimons M, Arnolda LF, Green DJ. Effects of training resumption on conduit arterial diameter in elite rowers. Med Sci Sports Exerc. 2006;38:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | Rowley NJ, Dawson EA, Birk GK, Cable NT, George K, Whyte G, Thijssen DH, Green DJ. Exercise and arterial adaptation in humans: uncoupling localized and systemic effects. J Appl Physiol (1985). 2011;110:1190-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 65. | Sinoway LI, Musch TI, Minotti JR, Zelis R. Enhanced maximal metabolic vasodilatation in the dominant forearms of tennis players. J Appl Physiol (1985). 1986;61:673-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 114] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 66. | Sinoway LI, Shenberger J, Wilson J, McLaughlin D, Musch T, Zelis R. A 30-day forearm work protocol increases maximal forearm blood flow. J Appl Physiol (1985). 1987;62:1063-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 90] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 67. | Maiorana A, O'Driscoll G, Dembo L, Cheetham C, Goodman C, Taylor R, Green D. Effect of aerobic and resistance exercise training on vascular function in heart failure. Am J Physiol Heart Circ Physiol. 2000;279:H1999-H2005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 150] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 68. | Breisch EA, White FC, Nimmo LE, McKirnan MD, Bloor CM. Exercise-induced cardiac hypertrophy: a correlation of blood flow and microvasculature. J Appl Physiol (1985). 1986;60:1259-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 69. | White FC, Bloor CM, McKirnan MD, Carroll SM. Exercise training in swine promotes growth of arteriolar bed and capillary angiogenesis in heart. J Appl Physiol (1985). 1998;85:1160-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 113] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 70. | Brown MD. Exercise and coronary vascular remodelling in the healthy heart. Exp Physiol. 2003;88:645-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 71. | Jacobs TB, Bell RD, McClements JD. Exercise, age and the development of the myocardial vasculature. Growth. 1984;48:148-157. [PubMed] |
| 72. | Tomanek RJ. Effects of age and exercise on the extent of the myocardial capillary bed. Anat Rec. 1970;167:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 94] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 73. | Pelliccia A, Spataro A, Granata M, Biffi A, Caselli G, Alabiso A. Coronary arteries in physiological hypertrophy: echocardiographic evidence of increased proximal size in elite athletes. Int J Sports Med. 1990;11:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 74. | Windecker S, Allemann Y, Billinger M, Pohl T, Hutter D, Orsucci T, Blaga L, Meier B, Seiler C. Effect of endurance training on coronary artery size and function in healthy men: an invasive followup study. Am J Physiol Heart Circ Physiol. 2002;282:H2216-H2223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 75. | Zandrino F, Molinari G, Smeraldi A, Odaglia G, Masperone MA, Sardanelli F. Magnetic resonance imaging of athlete's heart: myocardial mass, left ventricular function, and cross-sectional area of the coronary arteries. Eur Radiol. 2000;10:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | Haskell WL, Sims C, Myll J, Bortz WM, St Goar FG, Alderman EL. Coronary artery size and dilating capacity in ultradistance runners. Circulation. 1993;87:1076-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 142] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 77. | Nguyen PK, Terashima M, Fair JM, Varady A, Taylor-Piliae RE, Iribarren C, Go AS, Haskell WL, Hlatky MA, Fortmann SP, McConnell MV. Physical activity in older subjects is associated with increased coronary vasodilation: the ADVANCE study. JACC Cardiovasc Imaging. 2011;4:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 78. | Sugawara J, Komine H, Hayashi K, Yoshizawa M, Otsuki T, Shimojo N, Miyauchi T, Yokoi T, Maeda S, Tanaka H. Systemic alpha-adrenergic and nitric oxide inhibition on basal limb blood flow: effects of endurance training in middle-aged and older adults. Am J Physiol Heart Circ Physiol. 2007;293:H1466-H1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 79. | Hildick-Smith DJ, Johnson PJ, Wisbey CR, Winter EM, Shapiro LM. Coronary flow reserve is supranormal in endurance athletes: an adenosine transthoracic echocardiographic study. Heart. 2000;84:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 80. | Lekakis JP, Papamichael CM, Cimponeriu AT, Stamatelopoulos KS, Papaioannou TG, Kanakakis J, Alevizaki MK, Papapanagiotou A, Kalofoutis AT, Stamatelopoulos SF. Atherosclerotic changes of extracoronary arteries are associated with the extent of coronary atherosclerosis. Am J Cardiol. 2000;85:949-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 131] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 81. | Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2110] [Cited by in RCA: 2211] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 82. | Thijssen DH, Willems L, van den Munckhof I, Scholten R, Hopman MT, Dawson EA, Atkinson G, Cable NT, Green DJ. Impact of wall thickness on conduit artery function in humans: is there a "Folkow" effect? Atherosclerosis. 2011;217:415-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 83. | Rowley NJ, Dawson EA, Hopman MT, George KP, Whyte GP, Thijssen DH, Green DJ. Conduit diameter and wall remodeling in elite athletes and spinal cord injury. Med Sci Sports Exerc. 2012;44:844-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 84. | Thijssen DH, Dawson EA, van den Munckhof IC, Tinken TM, den Drijver E, Hopkins N, Cable NT, Green DJ. Exercise-mediated changes in conduit artery wall thickness in humans: role of shear stress. Am J Physiol Heart Circ Physiol. 2011;301:H241-H246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 85. | Dinenno FA, Tanaka H, Monahan KD, Clevenger CM, Eskurza I, DeSouza CA, Seals DR. Regular endurance exercise induces expansive arterial remodelling in the trained limbs of healthy men. J Physiol. 2001;534:287-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 179] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 86. | Green DJ, Spence A, Rowley N, Thijssen DH, Naylor LH. Vascular adaptation in athletes: is there an 'athlete's artery'? Exp Physiol. 2012;97:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 87. | Blair SN, Morris JN. Healthy hearts--and the universal benefits of being physically active: physical activity and health. Ann Epidemiol. 2009;19:253-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 249] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 88. | Ehsani AA, Heath GW, Hagberg JM, Sobel BE, Holloszy JO. Effects of 12 mo of intense exercise training on ischemic ST-segment depression in patients with coronary artery disease. Circulation. 1981;64:1116-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 129] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 89. | Schuler G, Hambrecht R, Schlierf G, Grunze M, Methfessel S, Hauer K, Kübler W. Myocardial perfusion and regression of coronary artery disease in patients on a regimen of intensive physical exercise and low fat diet. J Am Coll Cardiol. 1992;19:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 90. | Hambrecht R, Walther C, Möbius-Winkler S, Gielen S, Linke A, Conradi K, Erbs S, Kluge R, Kendziorra K, Sabri O, Sick P, Schuler G. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation. 2004;109:1371-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 458] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 91. | Laughlin MH, Bowles DK, Duncker DJ. The coronary circulation in exercise training. Am J Physiol Heart Circ Physiol. 2012;302:H10-H23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 92. | Okabe TA, Kishimoto C, Murayama T, Yokode M, Kita T. Effects of exercise on the development of atherosclerosis in apolipoprotein E-deficient mice. Exp Clin Cardiol. 2006;11:276-279. [PubMed] |
| 93. | Link RP, Pedersoli WM, Safanie AH. Effect of exercise on development of atherosclerosis in swine. Atherosclerosis. 1972;15:107-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 94. | Turk JR, Laughlin MH. Physical activity and atherosclerosis: which animal model? Can J Appl Physiol. 2004;29:657-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 95. | Williams JK, Kaplan JR, Suparto IH, Fox JL, Manuck SB. Effects of exercise on cardiovascular outcomes in monkeys with risk factors for coronary heart disease. Arterioscler Thromb Vasc Biol. 2003;23:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 96. | Kim CH, Park Y, Chun MY, Kim YJ. Exercise-induced hypertension can increase the prevalence of coronary artery plaque among middle-aged male marathon runners. Medicine (Baltimore). 2020;99:e19911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 97. | Niebauer J, Hambrecht R, Velich T, Hauer K, Marburger C, Kälberer B, Weiss C, von Hodenberg E, Schlierf G, Schuler G, Zimmermann R, Kübler W. Attenuated progression of coronary artery disease after 6 years of multifactorial risk intervention: role of physical exercise. Circulation. 1997;96:2534-2541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 206] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 98. | Haskell WL, Alderman EL, Fair JM, Maron DJ, Mackey SF, Superko HR, Williams PT, Johnstone IM, Champagne MA, Krauss RM. Effects of intensive multiple risk factor reduction on coronary atherosclerosis and clinical cardiac events in men and women with coronary artery disease. The Stanford Coronary Risk Intervention Project (SCRIP). Circulation. 1994;89:975-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 555] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 99. | Ornish D, Scherwitz LW, Billings JH, Brown SE, Gould KL, Merritt TA, Sparler S, Armstrong WT, Ports TA, Kirkeeide RL, Hogeboom C, Brand RJ. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280:2001-2007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1039] [Cited by in RCA: 921] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 100. | Schuler G, Hambrecht R, Schlierf G, Niebauer J, Hauer K, Neumann J, Hoberg E, Drinkmann A, Bacher F, Grunze M. Regular physical exercise and low-fat diet. Effects on progression of coronary artery disease. Circulation. 1992;86:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 590] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 101. | Ornish D, Brown SE, Scherwitz LW, Billings JH, Armstrong WT, Ports TA, McLanahan SM, Kirkeeide RL, Brand RJ, Gould KL. Can lifestyle changes reverse coronary heart disease? Lancet. 1990;336:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1450] [Cited by in RCA: 1149] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 102. | Mury P, Chirico EN, Mura M, Millon A, Canet-Soulas E, Pialoux V. Oxidative Stress and Inflammation, Key Targets of Atherosclerotic Plaque Progression and Vulnerability: Potential Impact of Physical Activity. Sports Med. 2018;48:2725-2741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 103. | Hambrecht R, Niebauer J, Marburger C, Grunze M, Kälberer B, Hauer K, Schlierf G, Kübler W, Schuler G. Various intensities of leisure time physical activity in patients with coronary artery disease: effects on cardiorespiratory fitness and progression of coronary atherosclerotic lesions. J Am Coll Cardiol. 1993;22:468-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 230] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 104. | Nytrøen K, Rustad LA, Erikstad I, Aukrust P, Ueland T, Lekva T, Gude E, Wilhelmsen N, Hervold A, Aakhus S, Gullestad L, Arora S. Effect of high-intensity interval training on progression of cardiac allograft vasculopathy. J Heart Lung Transplant. 2013;32:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 105. | Taylor JL, Holland DJ, Keating SE, Bonikowske AR, Coombes JS. Adherence to High-Intensity Interval Training in Cardiac Rehabilitation: A REVIEW AND RECOMMENDATIONS. J Cardiopulm Rehabil Prev. 2021;41:61-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 106. | Sabag A, Little JP, Johnson NA. Low-volume high-intensity interval training for cardiometabolic health. J Physiol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 107. | Belardinelli R, Paolini I, Cianci G, Piva R, Georgiou D, Purcaro A. Exercise training intervention after coronary angioplasty: the ETICA trial. J Am Coll Cardiol. 2001;37:1891-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 191] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 108. | Fleenor BS, Bowles DK. Exercise training decreases the size and alters the composition of the neointima in a porcine model of percutaneous transluminal coronary angioplasty (PTCA). J Appl Physiol (1985). 2009;107:937-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 109. | Fu C, Wang H, Wei Q, He C, Zhang C. Effects of rehabilitation exercise on coronary artery after percutaneous coronary intervention in patients with coronary heart disease: a systematic review and meta-analysis. Disabil Rehabil. 2019;41:2881-2887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 110. | Napoli C, Williams-Ignarro S, de Nigris F, Lerman LO, D'Armiento FP, Crimi E, Byrns RE, Casamassimi A, Lanza A, Gombos F, Sica V, Ignarro LJ. Physical training and metabolic supplementation reduce spontaneous atherosclerotic plaque rupture and prolong survival in hypercholesterolemic mice. Proc Natl Acad Sci U S A. 2006;103:10479-10484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 111. | Kadoglou NP, Kostomitsopoulos N, Kapelouzou A, Moustardas P, Katsimpoulas M, Giagini A, Dede E, Boudoulas H, Konstantinides S, Karayannacos PE, Liapis CD. Effects of exercise training on the severity and composition of atherosclerotic plaque in apoE-deficient mice. J Vasc Res. 2011;48:347-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 112. | Cohen MV, Yipintsoi T, Scheuer J. Coronary collateral stimulation by exercise in dogs with stenotic coronary arteries. J Appl Physiol Respir Environ Exerc Physiol. 1982;52:664-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 39] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 113. | Knight DR, Stone HL. Alteration of ischemic cardiac function in normal heart by daily exercise. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 114. | Scheel KW, Ingram LA, Wilson JL. Effects of exercise on the coronary and collateral vasculature of beagles with and without coronary occlusion. Circ Res. 1981;48:523-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 43] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 115. | Guo Y, Peng R, Liu Q, Xu D. Exercise training-induced different improvement profile of endothelial progenitor cells function in mice with or without myocardial infarction. Int J Cardiol. 2016;221:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 116. | Cohen MV. Training in dogs with normal coronary arteries: lack of effect on collateral development. Cardiovasc Res. 1990;24:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 117. | Neill WA, Oxendine JM. Exercise can promote coronary collateral development without improving perfusion of ischemic myocardium. Circulation. 1979;60:1513-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 118. | Belardinelli R, Georgiou D, Ginzton L, Cianci G, Purcaro A. Effects of moderate exercise training on thallium uptake and contractile response to low-dose dobutamine of dysfunctional myocardium in patients with ischemic cardiomyopathy. Circulation. 1998;97:553-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 97] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 119. | Zbinden R, Zbinden S, Meier P, Hutter D, Billinger M, Wahl A, Schmid JP, Windecker S, Meier B, Seiler C. Coronary collateral flow in response to endurance exercise training. Eur J Cardiovasc Prev Rehabil. 2007;14:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 120. | Ferguson RJ, Petitclerc R, Choquette G, Chaniotis L, Gauthier P, Huot R, Allard C, Jankowski L, Campeau L. Effect of physical training on treadmill exercise capacity, collateral circulation and progression of coronary disease. Am J Cardiol. 1974;34:764-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 121. | Franklin BA. Exercise training and coronary collateral circulation. Med Sci Sports Exerc. 1991;23:648-653. [PubMed] |
| 122. | Niebauer J, Hambrecht R, Marburger C, Hauer K, Velich T, von Hodenberg E, Schlierf G, Kübler W, Schuler G. Impact of intensive physical exercise and low-fat diet on collateral vessel formation in stable angina pectoris and angiographically confirmed coronary artery disease. Am J Cardiol. 1995;76:771-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 123. | El-Sayed MS, Ali N, El-Sayed Ali Z. Haemorheology in exercise and training. Sports Med. 2005;35:649-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 124. | Ernst E. Influence of regular physical activity on blood rheology. Eur Heart J. 1987;8 Suppl G:59-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 125. | Reinhart WH, Dziekan G, Goebbels U, Myers J, Dubach P. Influence of exercise training on blood viscosity in patients with coronary artery disease and impaired left ventricular function. Am Heart J. 1998;135:379-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 126. | Gielen S, Erbs S, Schuler G, Hambrecht R. Exercise training and endothelial dysfunction in coronary artery disease and chronic heart failure. From molecular biology to clinical benefits. Minerva Cardioangiol. 2002;50:95-106. [PubMed] |
| 127. | Cavalcante SL, Lopes S, Bohn L, Cavero-Redondo I, Álvarez-Bueno C, Viamonte S, Santos M, Oliveira J, Ribeiro F. Effects of exercise on endothelial progenitor cells in patients with cardiovascular disease: A systematic review and meta-analysis of randomized controlled trials. Rev Port Cardiol. 2019;38:817-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 128. | Linke A, Erbs S, Hambrecht R. Effects of exercise training upon endothelial function in patients with cardiovascular disease. Front Biosci. 2008;13:424-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 129. | Ahmadi A, Dabidi Roshan V, Jalali A. Coronary vasomotion and exercise-induced adaptations in coronary artery disease patients: A systematic review and meta-analysis. J Res Med Sci. 2020;25:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 130. | Borges JP, Nascimento AR, Lopes GO, Medeiros-Lima DJM, Coelho MP, Nascimento PMC, Kopiler DA, Matsuura C, Mediano MFF, Tibirica E. The impact of exercise frequency upon microvascular endothelium function and oxidative stress among patients with coronary artery disease. Clin Physiol Funct Imaging. 2018;38:840-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 131. | Vasić D, Novaković M, Božič Mijovski M, Barbič Žagar B, Jug B. Short-Term Water- and Land-Based Exercise Training Comparably Improve Exercise Capacity and Vascular Function in Patients After a Recent Coronary Event: A Pilot Randomized Controlled Trial. Front Physiol. 2019;10:903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 132. | Kollet DP, Marenco AB, Bellé NL, Barbosa E, Boll L, Eibel B, Waclawovsky G, Lehnen AM. Aerobic exercise, but not isometric handgrip exercise, improves endothelial function and arterial stiffness in patients with myocardial infarction undergoing coronary intervention: a randomized pilot study. BMC Cardiovasc Disord. 2021;21:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 133. | Paterson C, Fryer S, Zieff G, Stone K, Credeur DP, Barone Gibbs B, Padilla J, Parker JK, Stoner L. The Effects of Acute Exposure to Prolonged Sitting, With and Without Interruption, on Vascular Function Among Adults: A Meta-analysis. Sports Med. 2020;50:1929-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 134. | Kitta Y, Obata JE, Nakamura T, Hirano M, Kodama Y, Fujioka D, Saito Y, Kawabata K, Sano K, Kobayashi T, Yano T, Nakamura K, Kugiyama K. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J Am Coll Cardiol. 2009;53:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 249] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 135. | Green DJ, Spence A, Halliwill JR, Cable NT, Thijssen DH. Exercise and vascular adaptation in asymptomatic humans. Exp Physiol. 2011;96:57-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |