INTRODUCTION
Coronary artery bypass grafting (CABG) remains the standard of management for the multi-vessel and left main coronary disease, however it is associated with an increased risk of postoperative complications. Severe circumferential calcification of the aorta is defined as porcelain aorta (PA) and its frequency has been reported as 2% to 9.3% in patients who are undergoing elective CABG[1-4]. PA is a serious atherosclerotic disease that, due to increased risk of perioperative embolic stroke, precludes manipulation of the aorta during CABG operations. It is expected that cardiac surgeons will encounter more patients with PA, due to the gradual aging of the world population, the increase in life expectancy, and the increase in the number of co-morbid diseases such as diabetes mellitus, chronic obstructive pulmonary disease, cerebrovascular disease, and peripheral artery diseases.
A conventional CABG procedure involves cannulation of the ascending aorta, two-stage cannulation of the right atrium, insertion of antegrade cardioplegia cannula for cardiac arrest, application of a cross-clamp (CC) to the aorta, partial clamping of the aorta to create the proximal anastomosis, and finally removing these cannulas in a certain order. Although various mechanisms have been defined in the development of stroke in patients undergoing CABG surgery, embolic events caused by intraoperative aortic manipulations remain the main cause of stroke[5-7]. Embolic signals that are monitored by intraoperative transcranial Doppler (TCD) ultrasonography have demonstrated that most of the emboli in CABG procedures emerge during cross-clamping and side-clamping[5-8]. Perioperative stroke is still one of the most important complications of CABG surgery and causes an increase in economic costs, due to excessive consumption of resources in addition to high patient morbidity and mortality. Stroke after CABG is seen in approximately 0.48%-2.9%[3,9-11] of the operations, and stroke risk increases with the presence and prevalence of the atherosclerotic disease[4,12,13]. Some preoperative variables such as advanced age, peripheral vascular disease, and diabetes have been stated as risk factors for the development of postoperative stroke[4,10,11].
Clamping the aorta during CABG increases the risk of postoperative stroke regardless of the severity of aortic disease[6,14]. The best approach to prevent cerebral embolic complications is to modify the surgical techniques to minimize or eliminate the manipulation of a severely atherosclerotic or fully calcified aorta. To safely perform CABG operations with low morbidity and mortality rates, various methods including the use of axillary/subclavian artery[15-17], femoral artery (FA)[4,18], or innominate artery (IA)[15,19] for cannulation, utilization of aortic “no-touch” or anaortic off-pump CABG surgery[4,20-23], use of in-situ pedicle arterial grafts[24-26], hypothermic ventricular fibrillation[4,21,26,27] and carrying out the proximal anastomoses in different anatomic locations[28-30], have been suggested. This review examines the relevant studies on patients who have a severe atherosclerotic disease of the ascending aorta or PA, and who need to undergo CABG surgery. The surgical strategies for the prevention of perioperative atheroembolic events and management of severe aortic atherosclerotic disease or PA are summarized in detail.
DEFINITION OF PA
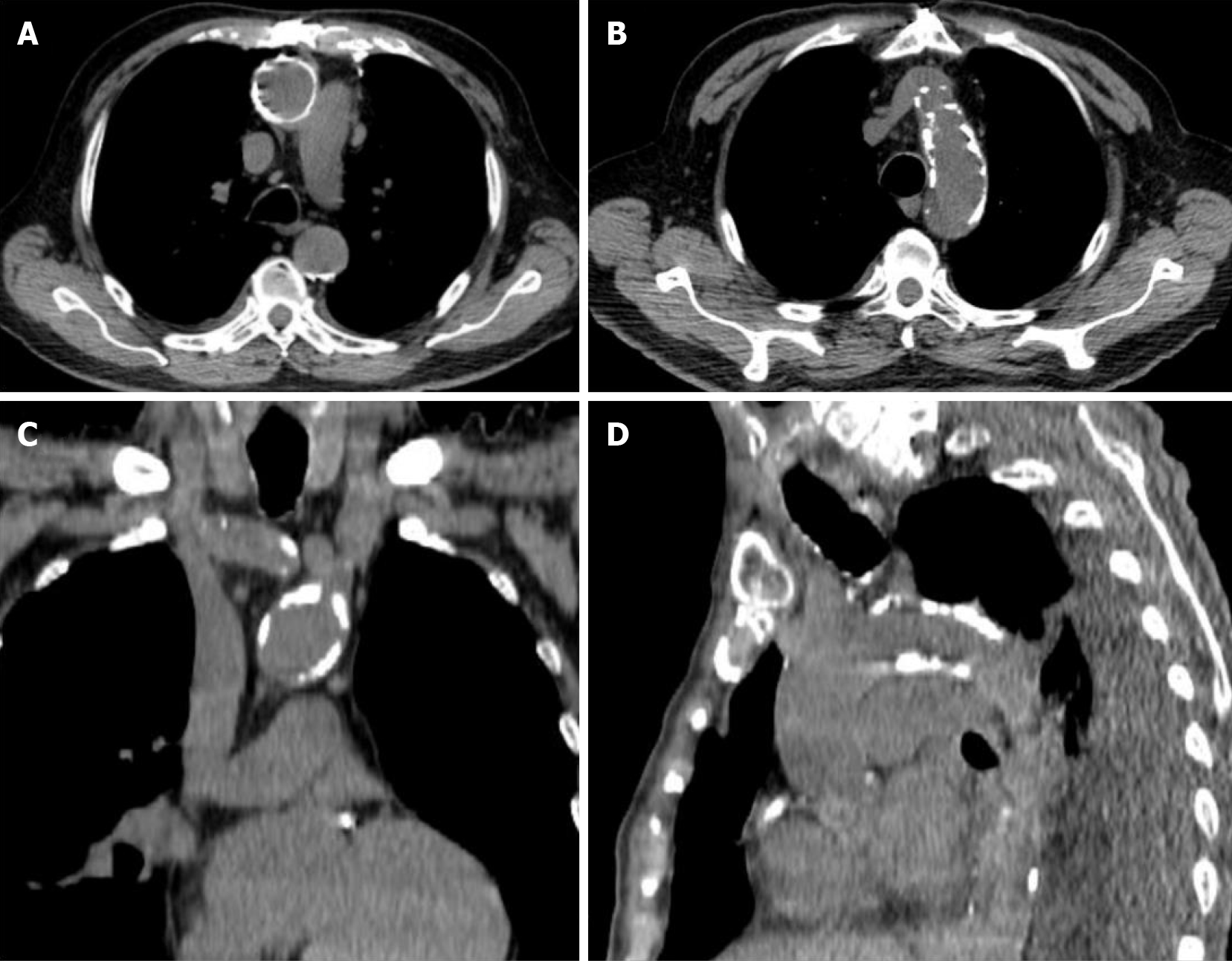
PA is an asymptomatic atherosclerotic disease that is characterized by circumferential calcification of the aorta and which renders manipulation of the ascending aorta impossible (Figure 1). It is detected incidentally in patients evaluated for cardiovascular or pulmonary diseases. The use of different diagnostic methods and differences in the definition makes it difficult to determine the true prevalence of PA[31]. Severe atherosclerosis of the ascending aorta is reported to be present in 2.0% of CABG patients[32]. Studies have shown that atherosclerotic disease most frequently affects the distal part of the ascending aorta, and calcified plaques are significantly more common than complex plaques[12,13]. This may be associated with a large number of patients (75%) treated with lipid-lowering drugs, which can lead to increased calcification of the atherosclerotic plaques.
Figure 1 Porcelain aorta.
Computed tomography reconstructions demonstrate circumferential calcification of the ascending aorta (A) and severe calcification of aortic arch (B-D).
Hangler et al[3] found that atherosclerosis in the ascending aorta was normal or mild in 151 patients (42.9%), moderate in 167 patients (47.5%), and severe in 34 patients (9.6%). Also, by using epiaortic ultrasound (EU) before manipulating the aorta, Daniel et al[2] graded atherosclerotic disease within the ascending aorta using an ordinal variable (grade 1–5), according to the presence and thickness of mobile atheroma. Grade 1: normal (< 2 mm thickness); Grade-2: minimal disease (2–3 mm); Grade-3: moderate disease (3-5 mm); Grade-4: severe disease (> 5 mm); and Grade-5: the presence of a mobile plaque within any part of the ascending aorta. Grade 1-2 aortic disease was detected in 450 (85.7%) patients and grade 3–5 disease in 75 (14.3%) patients. In another study, it was observed that 22% of patients had simple intraluminal atheroma and 2% of them had complex intraluminal atheroma[7].
Amorim et al[1] divided aortic calcifications into two types; Type 1 (circumferential calcification of the ascending aorta) and Type 2 (calcification of the descending aorta including the aortic arch, and no involvement in the ascending aorta). Also, Type I has been divided into two subgroups as Type Ia (the type in which calcified aorta is not likely to be clamped) and Type Ib (calcified aorta with possible clamping).
DIAGNOSIS: PREOPERATIVE AND INTRAOPERATIVE EVALUATION
The presence of an aortic plate thicker than 4 mm is associated with a significant risk of stroke[33,34]. Therefore, a preassessment of atherosclerotic aortic plaques should be performed before procedures such as cannulation, clamping, or proximal anastomosis during which aortic manipulation is unavoidable[13]. Calcification of the arch the ascending aorta may be visible on chest x-ray or fluoroscopy during coronary angiography, however, these are not adequate for evaluating the degree and extent of the disease. Frequently used diagnostic methods are computed tomography (CT), intraoperative epiaortic ultrasonography (EU), and transesophageal echocardiography (TEE).
Chatzikonstantinou et al[35] advocated that conventional CT angiography, with efficacy proven to be superior to TEE, should be the reference method for imaging the ascending aorta. Similarly, Park et al[36] stated that CT angiography significantly affected the perioperative management or follow-up plan of the patients, and they recommended the use of preoperative CT angiography as a routine test unless contraindicated. Park et al[36] obtained a multislice CT scan before CABG in 284 patients and found severely atherosclerotic aorta in 36 patients (12.7%) and severe left subclavian artery stenosis in 18 patients (6.3%) that would alter the conduit selection or grafting strategy. CT accurately diagnoses PA and provides information on the location and extent of aortic calcification. Also, it provides detailed information about the distribution of the disease through 3-dimensional images of the aortic wall[1,31].
There is evidence that EU is superior to manual palpation of the aorta and TEE in the detection of atherosclerotic disease of the ascending aorta and the aortic arch[37-39]. TEE evaluation of the distal ascending aorta, especially the CC and cannulation sites, is limited because the interposition of the left main bronchus between the esophagus and the aorta obstructs the vision[40]. Although many surgeons still rely on manual aortic palpation, this approach has a very low sensitivity and underestimates the severity[41]. EU is a simple and reliable solution to accurately scan the entire ascending aorta for the presence of intraluminal atheroma and has a sensitivity superior to both TEE and palpation[2,42,43].
INTRAOPERATIVE EPIAORTIC ULTRASONOGRAPHY
EU scanning is a fast, non-invasive and sensitive technique that provides information about the ascending aorta. Its use is limited because it does not allow preoperative planning, provides intraoperative information after the surgical incision has been made, extends the duration of surgery, needs special equipment, and requires trained personnel for both uses of the device and interpretation of the acquired data[7,44].
To determine any potential effect on intraoperative surgical decision-making, 6051 consecutive patients, who underwent EU of the aorta during cardiac operations have been analyzed retrospectively by Rosenberger et al[44] The overall effect of the EU on surgical decision-making is 4.1%. This effect was stated as 0.6% for off-pump CABG requirement, 0.5% for the avoidance of aortic CC, 0.2% for change in arterial cannulation area, or avoidance of aortic cannulation. In patients whose surgical plans changed, aortic atheroma was more common in the anterior aspect of the aorta than the posterior. The overall stroke rate is lower in patients in whom intraoperative EU was used, compared to all patients undergoing the surgical procedures[44]. Shapeton et al[7] prospectively evaluated the impact of EU on surgical cannulation strategy and cerebrovascular events, as well as its use in cardiac surgery. EU was performed by the surgeon using a high frequency (> 7 MHz) ultrasound transducer, and two-dimensional images of the ascending aorta were obtained in multiple planes before aortic cannulation and CC application. Aortic cannulation or CC strategy were altered based on EU findings in 7% (19/269) of the cases. No difference was found between patients with and without EU in terms of stroke (1.9% vs 1.2% P = 0.523).
Hangler et al[3] performed EU screening in 352 patients who underwent primary CABG and modified the applied surgical strategy according to the severity of atherosclerosis. In the presence of moderate atherosclerosis (maximum aortic wall thickness between 3 and 5 mm), single aortic CC is preferred first, while in severe atherosclerosis (maximum aortic wall thickness > 5 mm) no-touch techniques (aortic no-touch techniques on the beating heart) were used. The surgical technique was modified in 31% of patients with moderate aortic atherosclerosis and 91% of patients with severe aortic sclerosis. While there was no perioperative mortality for mild disease, mortality rates were 3% for moderate disease and 9% for severe disease (P = 0.005). Stroke rates were 2.0%, 2.4% and 2.9%, respectively (P = 0.935). Similarly, Djaiani et al[45] showed that the use of EU led to changes in intraoperative surgical management in patients undergoing CABG surgery [16 (29%) of 55 patients in the EU group and 7 (12%) of 58 patients in the control group]. However, they showed that the use of EU did not lead to a decrease in the number of cerebral emboli detected by TCD before or during cardiopulmonary bypass (CPB). Bolotin et al[38] also showed that the EU led to a change in the surgical procedure in 28% of 105 patients undergoing CABG procedures.
In a retrospective study involving 2292 patients who underwent isolated off-pump coronary artery bypass (OPCAB), patients were divided into two groups [the non-EU group with OPCAB under intraoperative TEE only (n = 1019) and the EU group with OPCAB under the EU (n = 1273)]. In both groups, OPCAB was performed with or without a partial aortic clamp. There was no statistically significant difference in the incidence of early stroke between the groups with and without EU (non-EU 1.7% (17/1019) vs EU 0.8% (10/1273); P = 0.052). However, in subgroups of patients with partial aortic clamps, the incidence of early stroke was significantly lower in the EU group (2.8% (9/317) vs 0.7% (2/301) P = 0.041)[39].
Konstadt et al[46] reported 100% sensitivity and 60% specificity for TEE in the assessment of the ascending aorta for atherosclerotic disease. A TEE study showed that negative findings for an atherosclerotic plaque suggested that the presence of a significant plaque is not high, whereas when the TEE study showed positive findings the possibility of having a significant disease in the ascending aorta was 34%. Therefore, they stated that the EU should be considered in cases where an atherosclerotic plaque is detected by TEE. On the other hand, it has been reported that routine intraoperative TEE identifies patients with the severe atherosclerotic aortic disease[47], and EU may be required when the insertion of a TEE probe is difficult or contraindicated[48].
CANNULATION STRATEGIES
The standard cannulation site in conventional CABG is the ascending aorta[27,32,49-51]. If the ascending aorta cannot be used safely because of severe atherosclerotic disease or other reasons, alternative cannulation sites may be used. The most preferred sites for the arterial inflow in CPB are the femoral arteries, axillary/subclavian arteries, and IA[51]. When choosing the arterial cannulation area, the risk profiles and benefit/cost ratios of the patients should be evaluated separately.
Femoral artery cannulation
FA cannulation is routinely used for extracorporeal circulation and constitutes the most common alternative to ascending aorta cannulation. The coexistence of severe atherosclerosis in the abdominal aorta or iliofemoral arteries limits its use[27,49,50]. The main disadvantages are the risk of cerebral atheroembolism and retrograde dissection caused by retrograde perfusion[17,27,51]. Grossi et al[47] showed that retrograde perfusion had no significant effect on the incidence of stroke in patients under 50 years of age, but it was an important risk factor for the occurrence of neurologic events in high-risk patients with aortic disease. Intraoperative malperfusion, arterial dissection, vascular injury, or limb ischemia are complications that are associated with cannulation. Femoral cannulation-related local wound complications occurred in only 1% of the patients, while the stroke rate was found to be 2%[18].
Axillary/subclavian artery cannulation
The axillary artery (AA) is a safe and effective path to maintain adequate arterial flow during CPB[17,27,51]. Because they are less affected by the atherosclerotic disease process, the axillary/subclavian arteries are safe access places, even in patients with the severe atherosclerotic disease[51]. However, critical stenosis of the axillary, subclavian, or innominate arteries[52], inadequate AA diameter, AA dissection, and morbid obesity limit its usage[53]. AA cannulation is a safe method that offers shorter mean operative times, shorter average lengths of stay in the intensive care unit and hospital. Minor complications of axillary cannulation include seroma, hematoma, chronic pain, and pectoralis major muscle atrophy[53]. Brachial plexus injury, arterial dissection[16,54], and AA thrombosis requiring thrombectomy[51] are other complications.
The AA can be cannulated either directly or using a side graft[16,55-57]. Sabik et al[16] investigated whether the use of side grafts reduced morbidity in patients with 399 AA cannulations. In more than half of the patients (212 patients, 53%) the AA had been cannulated directly, and the remainder (187 patients / 47%) were cannulated using a side graft. Complications related to cannulation were brachial plexus injury in 7 (1.8%), AA injury in 7 (1.8%), aortic dissection in 3 (0.8%), and arm ischemia in 3 (0.8%) patients. These complications were statistically lower in the side graft group (2.1% vs 7.0%, P = 0.03). Carino et al[55] recommend the open Seldinger-guided approach for axillary cannulation (52/404, 13%) because of its safety, efficacy, speed, and simplicity.
Axillary inflow using a lateral graft reduces stroke and is a preferred method for complicated cardiac operations requiring circulatory arrest[57]. No superiority of AA cannulation over IA cannulation concerning perioperative outcomes in thoracic aortic surgery has been demonstrated[58]. In a study where they compared the early and late postoperative outcomes of patients who underwent axillary (n = 107) and FA(n = 198) cannulation during the repair of acute Type A aortic dissection, Stamou et al[59] found that operative mortality was not affected by the cannulation site (16% for axillary cannulation vs 19% for femoral cannulation, P = 0.64) and that stroke rates were similar between the two techniques. On the contrary, Etz et al[60] reported on their 10-year experience with over 869 patients who were surgically treated for complicated aortic pathology. They compared aortic cannulation (n = 157) with femoral (n = 261), and direct right axillary (n = 451) cannulation. The right axillary cannulation was found to be superior regarding the rates of stroke and mortality. Similarly, Svensson et al[57] found a higher mortality risk in the femoral cannulated group compared to the group that was cannulated using a side graft to the AA.
Innominate artery cannulation
IA cannulation is an easy-to-apply technique that is used as an alternative to AA cannulation and enables selective antegrade cerebral perfusion. Its important advantages are the absence of a need for an extra incision in contrast to femoral and axillary cannulations, and the wide diameter which enables ideal flow without high pump pressures during CPB. Its large diameter is also a safe and advantageous technique for complex aortic repair, allowing faster cooling and rewarming in patients requiring circulatory arrest[15]. Other advantages of IA cannulation include the presence of the cannulation site within the same surgical field, ease of application in obese patients, lack of risk of brachial plexus injury or arm ischemia, prevention of malperfusion and retrograde cerebral atheroembolism associated with femoral cannulation, and the possibility of antegrade cerebral perfusion[15,19]. It can be cannulated either directly[61] as in AA cannulation or using a side graft[19,62].
In a retrospective study evaluating the surgical results of 206 patients with complex aortic pathology, axillary and IA cannulations accounted for 37% (n = 77) and 67% (n = 129) of the cases, respectively. Shorter times for CPB (189 vs 150 min, P < 0.001) and circulatory arrest (22.5 vs 11 min, P < 0.001) were found in the group which underwent IA cannulation. Blood transfusion rates were also found to be lower compared to the AA group[15]. Harky et al[58] reported shorter CPB times (173.12 ± 51.85 min for AA cannulation and 167.45 ± 54.67 min for IA cannulation, P = 0.004) in patients who underwent AA and IA cannulation. However, they did not find a significant difference between the two patient groups in terms of mean deep hypothermic circulatory arrest (DHCA) durations (29.14 ± 23.55 min for right AA cannulation and 38.48 ± 31.32 min for IA cannulation; P = 0.06). It was observed that axillary and IA cannulation were responsible for 3.5% and 4.48% of permanent neurological deficits and 3% and 9.70% of transient neurological deficits, respectively, and there was no significant difference detected between the two groups concerning cannulation-related complications (P > 0.05).
In IA cannulations using side grafts, Preventza et al[19] reported neurological complication rates of 4.4% and mortality rates of 1.5%, while Huang et al[62] reported transient neurological dysfunction in 5 (10.9%) of 46 patients. Di Eusanio et al[54] also reported that their patients who underwent IA cannulation (44 patients) had no morbidity due to cannulation, and neurologic complication and hospital mortality rates in these patients were 6.8%. IA cannulation is a safe and effective alternative to femoral or axillary cannulation for arterial flow in proximal aortic surgery[19]. However, there are no high-quality data to prove that it is superior to AA cannulation in providing cerebral protection[57].
Other cannulation areas
The arch of the aorta[4,63,64], carotid artery[52,65], and brachial artery[66,67] are reported as alternative cannulation sites. Carotid artery cannulation was first performed by Urbanski et al[52] for acute aortic dissection surgery and has become the standard approach for surgical interventions requiring circulatory arrest. It has been noted as a fast, safe, and effective method of arterial cannulation, even in very obese patients.
AORTIC MANIPULATION AND CLAMP RELATED NEUROLOGIC COMPLICATIONS IN PATIENTS UNDERGOING CONVENTIONAL CABG AND OPCAB
It has been demonstrated that most emboli detected during CABG operations occur during the manipulation of the aorta[5,8]. Barbut et al[68] studied the formation of transient microembolisms during different maneuvers in on pump-CABG. They found that, in addition to a much smaller number of microembolisms during clamp application, CC and side-biting clamp removal was responsible for 58% of the total cerebral microembolic load (34% and 24%, respectively). In another study, 39% of the total microembolisms occurred during side-biting clamp application, and 46% during clamp removal[5]. Lev-Ran et al[69] compared 429 patients who underwent the aortic no-touch technique with 271 patients who underwent PC, they found a significantly lower incidence of stroke in the no-touch group and identified PC as an independent predictor of stroke.
In a meta-analysis comparing the results of different CABG techniques; (anaortic off-pump, off-pump with the clampless Heartstring device, off-pump with a PC, traditional on-pump CABG with aortic CC) the anaortic off-pump is an effective treatment method to reduce the risk of postoperative stroke, mortality, renal failure, bleeding complications, atrial fibrillation and to shorten the length of stay in intensive care unit[6]. The degree of EU, postoperative stroke score, operative approach, and aortic clamping were independently associated with an increase in postoperative stroke compared to the no-touch technique. Also, the observed-expected stroke rate increased as the degree of aortic manipulation increased[14].
In a study investigating the effect of different clamping strategies on postoperative stroke incidence during CABG; the double clamp (CC + PC) on-pump strategy showed a 2.5-fold increase in the risk of postoperative stroke compared to the single CC technique. In the OPCAB group, no difference was found between PC and no-clamping. The authors stated that the rate of clamp use generally decreased over the years, nonclamped techniques were used in 93.3% of patients with grade 3–5, and none of these patients developed postoperative stroke. Also, surgery year and surgeon identity were not found to be associated with postoperative stroke risk[2].
Avoiding aortic manipulation may reduce the risk of postoperative stroke, especially in patients at high risk of stroke[6]. In these patients, OPCAB, which is a less invasive method, may be preferred to reduce mortality and morbidity. Motallebzadeh et al[70] randomized a total of 212 patients [on-pump (104 patients) and off-pump (108 patients)] undergoing CABG, and assessed embolic signals from the middle cerebral artery by bilateral TCD ultrasonography. A better neurocognitive score (P = 0.01) and decreased cerebral embolism rates were observed in patients in the off-pump group at discharge, and they found no significant difference in the neurocognitive score at the sixth week and sixth month. Also, age (P = 0.02), length of education (P = 0.03), and on-pump status (P = 0.006) were found to be independent predictors of pre-discharge neurocognitive score. In another study, no significant difference was found between off-pump and on-pump CABG in the 30-day composite result ratio (7.0% and 5.6%, respectively; P = 0.19), the ratio of patients who ended up with less number of completed grafts than previously planned was higher in off-pump patients compared to on-pump CABG (17.8% vs 11.1%, P < 0.001). Follow-up angiograms revealed a lower overall graft patency rate in the off-pump group compared to the on-pump group (82.6% vs 87.8%, P < 0.01)[71]. In contrast, in their study where they shared the results of patients who underwent OPCAB (13 889 patients) and on-pump CABG surgery (35 941 patients), Hannan et al[72] showed that OPCAB patients had a significantly lower 30-day mortality (adjusted OR 0.81, 95% confidence interval [CI] 0.68 to 0.97) and a lower incidence of postoperative stroke (adjusted OR 0.70, 95%CI 0.57 to 0.86). While there was no difference in three-year mortality between the groups (hazard ratio 1.08, 95%CI 0.96 to 1.22), higher rates of subsequent revascularization (hazard ratio 1.55, 95%CI 1.33 to 1.80) were found in OPCAB patients.
In another report; Emmert et al[73] similarly reported that the mortality and stroke rates were significantly lower in patients who underwent OPCAB compared to on-pump CABG. A meta-analysis of seven observational studies comparing unclamped OPCAB with conventional CABG and OPCAB regarding the use of partial clamp avoidance during OPCAB, the conventional CABG (0.38 vs 1.87% RR 0.27; 95%CI 0.14-0.58; P < 0.0001) rate showed a significant reduction in stroke risk compared to OPCAB using partial clamps (0.31 vs 1.35%; RR 0.34; CI 95% 0.18–0.65; P = 0.001)[74]. A similar meta-analysis comparing neurologic complications in off-pump surgery with and without aortic manipulation showed that post-surgical neurologic complications were reduced by half in anaortic OPCAB grafting cases (OR 0.46;% 95 CI, 0.29–0.72; P = 0.008)[22]. Another study compared the patients who underwent CABG without aortic manipulation (1201/1758, 68%) with those who underwent a minimum of one proximal anastomosis using a side-biting aortic clamp or no-clamp proximal anastomotic device (557/1758, 32%). In the anaortic group, the incidence of perioperative neurologic deficits was lower than in the aortic manipulation group (0.25% vs 1.1%, P = 0.037). It was also found that advanced age is associated with peri-operative neurologic injury (OR 1.1, 95%CI 1.01-1.20, P = 0.017)[75].
Douglas and Spaniol[76] emphasized the role of unclamped OPCAB as an important tool in the prevention of postoperative stroke and also reported that assistive techniques (such as anastomotic assist devices) may be an important factor in reducing stroke occurrence for patients with significant atherosclerosis. On the other hand, large randomized controlled trials have reported similar neurologic results after on-pump and off-pump CABG[71,77,78].
PA AND CABG
Various techniques have been described and implemented in patients with atherosclerotic ascending aorta to reduce the risk of atheroembolism that may cause cerebrovascular events. The “no-touch” technique avoids all types of clamps in the aorta. Also when using this technique, no cardioplegia is applied, no grafts are anastomosed to the aorta, and deep hypothermia is not necessary[20,21]. Anaortic or no-touch techniques without aortic manipulation can significantly improve the development of neurologic complications by avoiding aortic maneuvers known to cause embolism[22,23]. Halbersma et al[24] compared the 4-year results with those in the surgical arm of the SYNTAX trial in 400 consecutive non-touch total arterial OPCAB patients without touch, and there was a clear trend towards a reduction in stroke rate in the no-touch group (0.8%) compared to the surgical arm of the SYNTAX trial (2.2%). Also, there was no significant difference in the stroke rates between the non-touch OPCAB group and the PCI arm of the SYNTAX trials. Based on these results, they recommended off-pump in situ grafting as “standard care” to reduce neurologic complications. OPCAB no-touch with total arterial Y-graft and composite graft revascularization is an effective method for preventing thromboembolic events[23-25].
The internal mammary artery (IMA) can be used as an inflow site for saphenous vein (SV) grafts as in the total arterial T, or Y-shaped graft technique[28,32]. Mills et al[32] reported the frequency of severe atherosclerosis of the ascending aorta as 2.0% for patients undergoing CABG, and by using the “no-touch” technique they anastomosed the SV to the IMA in an end-to-side-fashion in 26 patients. They did not encounter any recurrence of angina in the patients. The “no-touch” technique has been used in 18 patients with heavily calcified and atherosclerotic ascending aorta who had myocardial revascularization. With this technique, 37 pedicled arterial grafts (22 IMA and 15 gastroepiploic arteries) were anastomosed proximally to the internal thoracic artery. Fifty-two distal anastomoses were performed using 15 free grafts, and no patients had neurologic complications[20]. However, accompanying subclavian artery stenosis may limit the use of these grafts both in situ and as Y-grafts. In this situation, the preoperative diagnosis becomes even more crucial, and preoperative subclavian artery stenting may be required, as in the cases of Adesanya and Kilic[79].
One of the methods used to achieve optimal coronary revascularization is hypothermic ventricular fibrillation or hypothermic fibrillatory arrest[4,21]. The hypothermic fibrillatory arrest also allows easy proximal anastomosis to a disease-free area of the ascending aorta in patients with a high risk of cerebral embolic complications and significant ascending aortic disease[21,27]. Gaudino et al[4] could not find a difference between hypothermic ventricular fibrillation and beating heart in terms of the overall in-hospital mortality rate. To achieve optimal coronary revascularization and minimize cerebrovascular events, Akpinar et al[80] also used the moderate degree hypothermic fibrillatory arrest technique with pedicled arterial grafts (internal mammary and gastroepiploic artery) in 21 patients with severe calcification of the ascending aorta, and they did not encounter any neurologic complications. However, the hypothermic ventricular fibrillation technique was associated with a greater incidence of neurologic complications (stroke and transient ischemic attack), renal insufficiency, and stay in the intensive care unit and hospital[4].
In conventional and OPCAB, the standard proximal anastomosis site or location is the ascending aorta itself. In advanced stages, the presence of PA makes it difficult to perform proximal anastomoses, and when in situ grafts cannot be used due to limited graft flow resources or T/Y grafting techniques cannot be applied, free grafts that require proximal anastomosis should be used. In this situation, using alternative inflow areas such as IA[27,29,30], subclavian artery, AA[28,30,81] and carotid artery[23,82] may be considered. Extra-anatomic CABG procedure is a safe and reliable method to minimize the prevalence of perioperative stroke and systemic embolization[29,30]. Demirsoy et al[30] preferred IA as the proximal anastomosis site in six of eight patients who underwent extra-anatomic bypass surgery because of the PA, they preferred the AA in one patient and the subclavian artery in another. Postoperative graft patency was evaluated by contrast-enhanced electron beam coronary angiography, and only one saphenous vein graft to the right coronary artery was occluded. Bonatti et al[28] have also demonstrated patency in 11 out of 13 extra-anatomic vein grafts with a 3D multislice CT scan performed in 13 patients during the first postoperative year. Agrifoglio et al[82] have identified a case in which CABG was performed off-pump and the saphenous vein graft was anastomosed proximally to the left common carotid artery. In addition to these methods, the coronary - coronary bypass is an alternative technique that can be used to bypass isolated atherosclerotic coronary lesions in the presence of PA[83,84]. In hemodynamically unstable patients or in whom aortic manipulation cannot be performed due to severely atherosclerotic aorta, performing CABG with the off-pump technique under CPB support and without application of CC can be a good choice to prevent neurologic complications[29,30].
Anastomotic support devices
The use of anastomosis support devices allows proximal aortic anastomoses to be performed without a side-clamp. The use of a sutureless proximal anastomotic device during OPCAB is safe and significantly reduces cerebral microembolism compared with the conventional bypass method[5,8,9]. Guerrieri et al[5] used TCD ultrasound to determine and compare the number and nature of microembolisms in patients. These patients underwent off-pump coronary artery bypass grafting during proximal anastomosis with three different techniques (aortic side-clamps and two separate unclamped devices Enclose II and Heartstring). It was seen that most of the microembolisms occurred during the application/insertion and removal of each device into the ascending aorta. During the anastomosis using the Heartstring device, the median number of total microembolisms was found to be higher than the other two groups, but there was no statistically significant difference (P = 0.239). The solid microembolism rate was significantly higher in the side-clamping group (23%) compared to 6% and 1% in the Enclose and Heartstring groups (P < 0.01). Avoiding aortic side clamping resulted in a significant reduction in the rate of solid microembolism detected by TCD. Similarly, Akpinar et al[80] found that the use of side-biting-clamps causes more microembolic load than Enclose II device [the median number of microemboli: 68 (range 35-290) vs 15 (range 5-48), P < 0.05)]. Hilker et al[9] performed 542 off-pump proximal anastomoses using the Heartstring device in 412 consecutive patients. The overall postoperative stroke incidence was lower than the predicted stroke risk score (0.48% observed vs 1.37% predicted), and a 35% reduction in solid particle embolism has been observed.
In a study in which proximal anastomoses were performed using a sutureless Symmetry aortic connector device (St Jude Medical, St Paul, Minn); OPCAB was compared with conventional coronary artery bypass (cardiopulmonary bypass and hand-stitched proximal anastomoses). Patients were evaluated by intraoperative TCD ultrasonography to determine right- and left-sided cerebral microembolic counts. Patients in the OPCAB group experienced fewer left-sided cerebral embolic events intraoperatively (mean, 24.9 ± 19.2; median, 26.0) compared to the patients who are in the conventional CABG group (mean, 189.9 ± 60.4; median, 180.0; P < 0.0001). A similarly significant difference in embolic counts was also noted in the right cerebral circulation (mean of 21.9 ± 20.7 and median of 18.0 for the OPCAB group vs mean of 181.6 ± 85.3 and median of 173.0 for the S-CABG group, P < 0.0001)[8].
CROSS-CLAMPING, ENDARTERECTOMY OR REPLACEMENT OF PA
Aortic CC is associated with high mortality and morbidity rates in patients with PA. Different techniques have been described to clamp the PA. Hartert et al[63] have reported the results of aortic cross-clamping in 42 patients with PA who underwent valve replacement [aortic valve (n = 33) or mitral valve (n = 9)] by using the open proximal ascending aorta technique. Longitudinal aortotomy was performed under total CPB after distal aortic arch or FA cannulation. The aorta was gently clamped, allowing the mobilized atherosclerotic material to leave the aorta through the open incision. After the main operation, the aorta was opened gradually and the plaques were cleared through the aortotomy which was still open. Surgical revision was required in three patients (7.1%) due to major bleeding and aortic dissection was not observed in any patients. The stroke rate was 7.1% and the 30-day mortality rate was 6.9%. The authors have suggested that cross-clamping with the “open proximal ascending aorta” technique is an effective method, with a low incidence of stroke and low risk of systemic embolization in patients with PA.
Another method used to avoid an embolic event in patients with PA is the “staged aortic clamp” procedure. This procedure includes (1) Short-term cessation of circulation and aortotomy during moderate hypothermia; (2) Balloon occlusion of the ascending aorta during low flow CPB; (3) Endarterectomy using an ultrasonic surgical aspirator to allow aortic CC; and (4) Felt reinforced CC and full-flow CPB steps[85]. The authors stated that they did not encounter any thromboembolic events with this method.
Nishi et al[86] suggested that by carefully selecting the direction of cross-clamping of the aorta with the help of preoperative CT and EU, they could successfully overcome the problem of the severely calcified ascending aorta. They performed a preoperative CT assessment for developing the clamping strategy in a severely calcified aorta. They found that the degree of calcification just below the IA was significantly less compared to the ascending aortic clamp site. After confirming that the extent of calcification in this area is less than 75% of the entire circumference, a soft CC calcification was carefully placed in the ascending aorta. All surgeries were completed under normal CPB with mild hypothermia, and no postoperative neurologic complications were observed in the patients. However, the utility of this technique is questionable, since aortic cross-clamping may cause an increase in the frequency of postoperative cerebrovascular events when calcification covers the entire aortic wall.
In high-risk patients with severe atherosclerosis of the ascending aorta short-period (3.4 ± 1.5 min) and moderately hypothermic (29.0 ± 2.3 ° C) circulatory arrests allow internal inspection or debridement of atheroma plaques, thus allowing safe aortic cross-clamping[87]. Culliford et al[88] have also cooled 13 patients, with excessive calcification in the ascending aorta and transverse arch, to 18 degrees during CPB, and manipulation of the aorta during cooling was not allowed. During DHCA (for 3.5 to 12 min), the aorta was widely opened to remove ulcerated plaques and friable debris and to find a safe place for CC. In 12 patients the presence of PA was known before surgery, whereas one patient was found to have PA after CC. In this latter patient, occipital infarctions developed, and ascending aortic endarterectomy was necessary. Endoaortic balloon occlusion of the atherosclerotic ascending aorta has been proposed as an alternative to conventional CC to prevent vascular damage and distal embolization of atherosclerotic plaques. However, Zingone et al[89] found that endoaortic occlusion was ineffective, and associated with a significantly higher risk of in-hospital death (25%) and a higher risk of stroke (3.8%).
In patients undergoing cardiac surgery, hypothermic circulatory arrest enables safe resection of the severely atherosclerotic ascending aorta and graft replacement with an acceptable postoperative stroke rate and provides long-term protection from subsequent embolic cerebrovascular events[49,90]. Replacement of the atherosclerotic aorta with graft during DHCA is a radical option and has high mortality rates (14%)[64]. In the study by Rokkas et al[90], among 81 patients who were noted to have severe atherosclerosis of the ascending aorta on epiaortic screening and who underwent CABG, 80 received [partial (5) or complete (75)] ascending aorta replacement by using hypothermic circulatory arrest. In the remaining patient, resection of a protruding aortic atheroma was performed. The 30-day mortality rate was reported as 8.6% (for 7 patients) and the incidence of stroke has been reported as 4.9%. Zingone et al[91] replaced the ascending aorta in 36 out of 152 patients who were noted to have severe aortic atherosclerosis which was detected on EU screening and CT. In 13 of them (36.1%), the aortic replacement was extended to the aortic arch. While DHCA was used in 34 patients, proximal aortic disease allowed conventional CC administration in 2 patients. The hospital mortality rate was 5.6%, one patient had a stroke (2.8%) and five patients (13.9%) had a neurocognitive impairment. Four patients (11.1%) experienced excessive bleeding requiring re-exploration.
Aortic endarterectomy may be an option in the management of patients with calcification of the aorta[64,92]. Vogt et al[93] performed complete thromboendarterectomy to the ascending aorta and the aortic arch during hypothermic circulatory arrest on 22 patients (CABG, n = 21 and aortic valve replacement, n = 8), and reported a 4.5% mortality rate and a 9% adverse neurologic event (one early and one late) rate. The follow-up of the patients was performed by magnetic resonance imaging and transthoracic echocardiography, and the endarterectomized aorta did not develop a dilation. The authors above have suggested that for patients with advanced-stage (Grade IV and V) plaques, ascending aorta and transverse arch thromboendarterectomy can be performed with acceptable surgical risk and a low recurrence rate for cerebrovascular events. On the contrary, Takami et al[87] performed reoperations in two patients (2/40, 5%) during the follow-up period, at postoperative 8th month in one and 44th month in the other, due to pseudoaneurysm in the suture line of the aortic patch. The authors suggested that the aortic wall undergoing endarterectomy should be considered as a risk factor for pseudoaneurysm formation and that the aortic suture line should be enforced during surgery.
CONCLUSION
Perioperative stroke is still one of the most crucial complications of CABG surgery with its high patient morbidity and mortality. During CABG, most emboli occur during active aortic clamp applications (cross-clamps, side-biting clamps) or the insertion and removal of unclamped aortic anastomotic devices. Therefore, in procedures that involve cannulation, clamping, or proximal anastomosis where aortic manipulation is inevitable, preassessment of the atherosclerotic aortic plaques is crucial. Imaging methods including preoperative CT or intraoperative EU enable modification of the surgical technique according to the severity of atherosclerosis. The use of anastomosis support devices to prevent lateral clamping and to support the proximal anastomosis may be an important strategy to minimize cerebral damage during proximal anastomoses in, especially high-risk groups. Since a soft or non-calcified aortic segment is needed to use anastomotic support devices, the need for aortic manipulation cannot be eliminated. The best approach to prevent embolic events is the utilization of alternative surgical techniques which aim to minimize or eliminate the manipulation of a severely atherosclerotic or completely calcified aorta.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pan SL S-Editor: Ma YJ L-Editor: A P-Editor: Yuan YY