Published online Jun 26, 2021. doi: 10.4330/wjc.v13.i6.163
Peer-review started: February 5, 2021
First decision: February 28, 2021
Revised: March 12, 2021
Accepted: May 22, 2021
Article in press: May 22, 2021
Published online: June 26, 2021
Processing time: 136 Days and 12 Hours
Sarcopenia or cachexia is often complicated in heart failure. Nutritional support, particularly branched-chain amino acid (BCAA) supplementation, is a candidate treatment for improving sarcopenia or cachexia in elderly patients. However, the efficacy of BCAA supplementation in patients with heart failure has not been established, and the issue is comparatively more complex. Indeed, there are conflicting reports on the efficacy of BCAA supplementation. The evidence for including BCAA supplementation in treating patients with heart failure was reviewed, and the complexity of the issue was discussed.
Core Tip: The pros and cons of branched-chain amino acid (BCAA) supplementation can vary depending on the patient and their specific conditions. Particularly, BCAA supplementation for patients with cardiac dysfunction, who could easily be presumed to have metabolic dysfunction, should be carefully considered.
- Citation: Narita K, Amiya E. Is branched-chain amino acid nutritional supplementation beneficial or detrimental in heart failure? World J Cardiol 2021; 13(6): 163-169
- URL: https://www.wjgnet.com/1949-8462/full/v13/i6/163.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i6.163
Sarcopenia or cachexia is often complicated in heart failure, which aggravates the clinical course of the disease. Sarcopenia and cachexia were reported to be present in approximately 20% of patients with heart failure; however, there were differences in their percentages among different studies[1]. Also, both of them sometimes coexist in approximately 10% of patients with heart failure[2]. Low physical performance and reduced cardiopulmonary capacity influence sarcopenia and cachexia[3]. These comorbidities are independent predictors of the clinical course of patients with heart failure[4]. Therefore, the therapeutic strategy for sarcopenia or cachexia is a critical issue in managing heart failure. However, there is no standard management strategy at this time.
Nutritional support might be one candidate treatment for the improvement of sarcopenia or cachexia. Amino acid supplementation was effective for sarcopenia in elderly patients. Rondanelli et al[5] demonstrated nutritional supplementation with whey protein, essential amino acids, and vitamin D for twelve weeks, significantly increasing fat-free mass and muscle strength. Among several amino acid supplementation types, branched-chain amino acids (BCAAs) were beneficial in forming skeletal muscles because they account for a large part of the essential amino acids that form these skeletal muscles[6]. Ottestad et al[7] reported that BCAA levels decreased by 10% in sarcopenic adults, whereas nonessential amino acid levels did not change, suggesting the importance of BCAAs in skeletal muscle maintenance.
Several reports about BCAA’s effect on cardiopulmonary performance in other populations exist (Table 1). Chang et al[8] demonstrated that BCAA and arginine supplementation improved performance in intermittent sprints by reducing perceived exertion. Other reports on experimental and clinical conditions, according to the effect of improvement in exercise capacity by BCAA supplementation, were also presented[9-11]. Additionally, BCAA supplementation also reduced the muscle damage associated with endurance exercise[12]. Therefore, BCAA supplementation might have favorable effects on improving and maintaining exercise capacity, which might help patients with heart failure and reduced exercise capacity. Furthermore, several reports about the efficacy of BCAA supplementation for the improvement of sarcopenia also exist. Ko et al[13] demonstrated that BCAA administration for five weeks improved several parameters, including bioelectrical-impedance-analysis-derived skeletal mass index by approximately 10% and grip strength by about 10%. BCAA supplementation before and after exercise has shown beneficial effects in decreasing exercise-induced muscle damage and promoting muscle-protein synthesis[14]. Leucine supplementation also enhances myofibrillar protein synthesis, leading to increased muscle strength[15,16]. These effects could be partly explained by the shift to anabolic signaling of the skeletal muscle through the mammalian target of rapamycin complex 1 pathway[17]. Indeed, the anabolic pathway decreased because of alterations in the insulin-like growth factor 1/growth hormone axis and increased catabolism, induced by proinflammatory cytokines, in the presence of heart failure with sarcopenia[18]. There were several reports of the impact of BCAA on the treatment of sarcopenia.
| Ref. | Study design | Sample size | Subjects | Dose | Length | Outcome |
| Chang et al[8] | Double-blind, randomized | 22 | Well-trained handball players | 0.17 g/kg BCAA and 0.04 g/kg arginine together | 1 d | Improve the performance in intermittent sprint |
| Watson et al[11] | Double-blind, randomized | 8 | Healthy male | 12 g/L BCAA | Every 15 mins during exercise | Exercise capacity change observed between subjects in response to BCAA ingestion |
| Coombes and McNaughton [12] | Prospective, assigned to one of two groups | 16 | Males | 12 g/d BCAA | 14 d | Supplementary BCAA decreased serum concentrations of the intramuscular enzymes |
| Ko et al [13] | Quasi-experimental single-arm intervention | 33 | Middle-aged and elderly | Leucine 0.54 g, isoleucine 0.43 g, valine 0.36 g, glutamine 0.65 g, arginine 0.61 g and other amino acids 1.01 g | Twice daily for 5 wk | Short-term positive effects on sarcopenic parameters |
| Komar et al[15] | Systematic review and meta-analysis | 999 | - | Each reference | Each reference | Beneficial effects on body weight, body mass index, and lean body mass in older persons |
| Murphy et al[16] | Randomized, single-blind, parallel-group, placebo-controlled crossover study | 20 | Men, 65-85 yr of age, BMI (in kg/m2) from 20 to 35, nonsmokers, and generally healthy | Higher protein intake group (1.2 g/kg/d) or lower protein intake group (0.8 g/kg/d) | 9 d | Enhances the anabolic effect of resistance exercise |
| Glynn et al[17] | Prospective | 14 | Young participants (6 men, 8 women) | 10 g essential amino acids | 180 min post ingestion | Induce a maximal skeletal muscle protein anabolic response |
| Nichols et al[19] | Systematic review and meta-analysis | 167 | - | Each reference | Each reference | Increase lean body mass and 6-minute walk test distance in patients with heart failure |
| Uchino et al[21] | Randomized, controlled trial | 18 | In-hospital heart failure patients with serum albumin < 3.5 g/dL | One pack of BCAA granules containing 1144 mg of l-valine, 1904 mg of l-leucine, and 952 mg of l-isoleucine | 28 d, 3 time a day | Increased serum albumin and decreased ctr in-hospital hf patients with hypoalbuminemia |
Nichols et al[19] performed a systematic review of the effect of amino acid supplementation in heart failure. They demonstrated that essential amino acid supplementation could improve important outcome measures related to sarcopenia. For instance, amino acid supplementation increased the six-minute walk test distance by approximately 20%. In contrast, few reports demonstrated BCAA efficacy in the improvement of heart failure[20,21]. Oral intake of AAs is presumed to improve exercise capacities through its beneficial effect on the skeletal muscle in patients with heart failure. Furthermore, BCAA treatment decreased the heart rate, preserved cardiac function, and prolonged survival in heart failure with reduced ejection fraction model rats[20]. Uchino et al[21] reported that in-hospital heart failure patients with hypoalbuminemia showed increased serum albumin, decreased cardiothoracic ratio (CTR), and increased cholinesterase after BCAA supplementation. Another beneficial effect of BCAA is that it activates rapamycin’s mammalian target (mTOR), promoting albumin synthesis[22]. The increase in serum albumin might favorably affect the clinical course of heart failure. The improvement in CTR could be due to decongestion efficiently induced by BCAA administration.
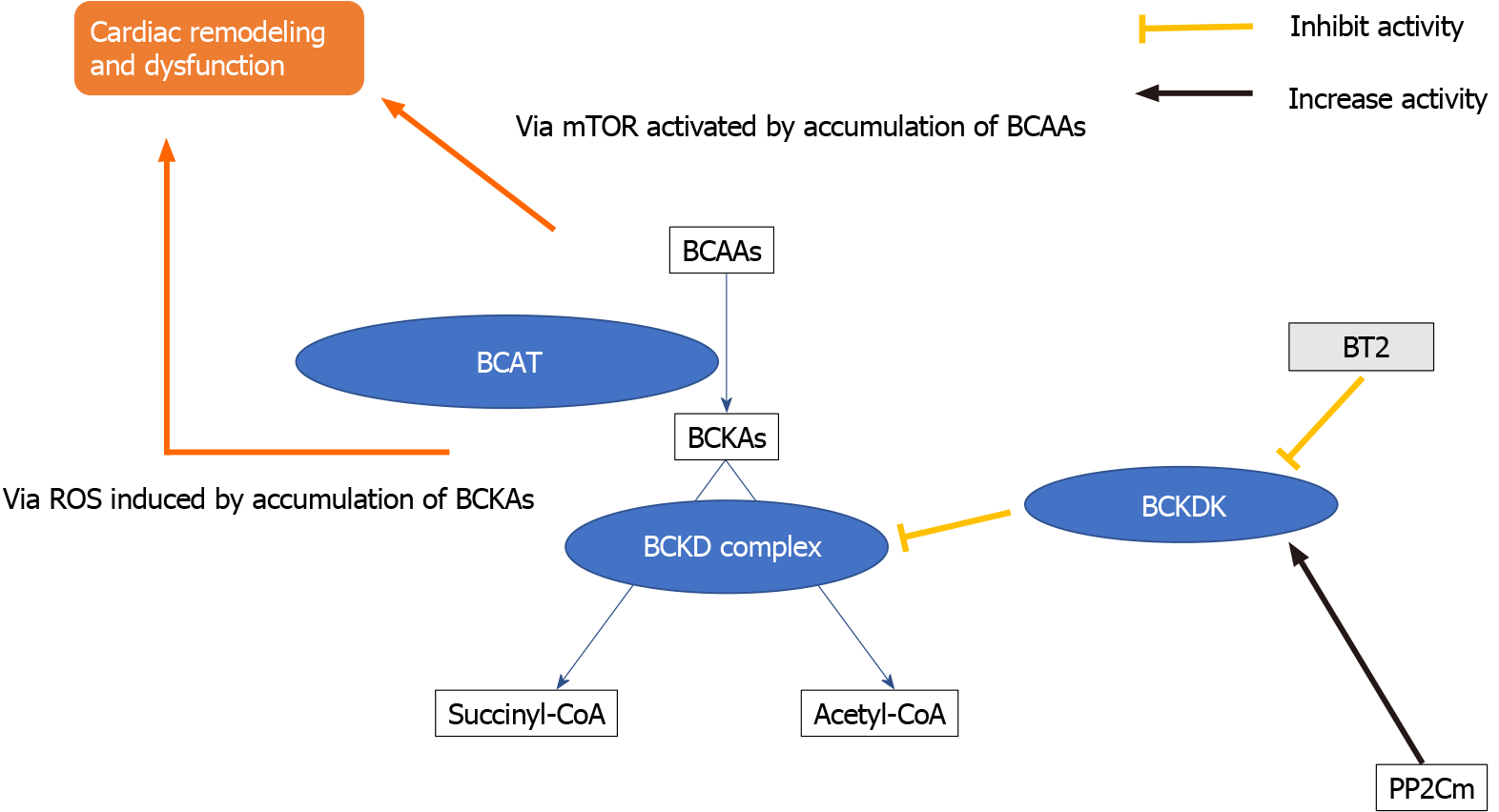
A clinical trial on the efficacy of BCAA supplementation in cardiac rehabilitation was conducted[23]. However, the issue might be more complex. Conversely, there are reports of BCAA’s pathological role in heart failure. In clinical studies, several reports about the link between the high level of circulating BCAA and the risk of cardio
By contrast, some hopeful hints about the BCAA metabolic pathway in heart failure therapy might exist. In BCKDK regulation, 3,6-dichlorobenzo[b]thiophene-2-carboxylic acid (BT2), a small-molecule BCKDK inhibitor, blocks BCKD phosphory
The transcriptional factor Kruppel-like factor 15 (KLF15) also has a critical role in cardiac BCAA catabolic regulation[28]. KLF15-deficient hearts displayed reduced BCAT2 expression, another critical step in BCAA catabolism, whereas intramyocardial BCKA levels were elevated in KLF15-null hearts. KLF15 is reportedly a direct transcriptional activator of BCAT2[36]. KLF15 expression is lower in human cardiomyopathy. Therefore, the loss of KLF15 is a critical molecular mechanism underlying stress-induced BCAA catabolic defects in the diseased heart[37,38]. The modification of the KLF15 pathway could help the diseased heart in the BCAA metabolic pathway; however, its overexpression evoked arrhythmia due to its regulatory role in the potassium channel[39].
Additionally, the mitochondrial matrix-targeted 2C-type ser/thr protein phos
Studies have shown that BCAAs are beneficial in heart failure. Conversely, BCAAs could act as exacerbators of heart failure. Nevertheless, improving BCAA metabolism might lead to an effective treatment strategy for the disease. In conclusion, the pros and cons of BCAA supplementation could vary depending on the patient and their specific conditions. Particularly, BCAA supplementation for patients with cardiac dysfunction, who could easily be presumed to have metabolic dysfunction, should be carefully considered.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kharlamov AN S-Editor: Liu M L-Editor: A P-Editor: Yuan YY
| 1. | Christensen HM, Kistorp C, Schou M, Keller N, Zerahn B, Frystyk J, Schwarz P, Faber J. Prevalence of cachexia in chronic heart failure and characteristics of body composition and metabolic status. Endocrine. 2013;43:626-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Emami A, Saitoh M, Valentova M, Sandek A, Evertz R, Ebner N, Loncar G, Springer J, Doehner W, Lainscak M, Hasenfuß G, Anker SD, von Haehling S. Comparison of sarcopenia and cachexia in men with chronic heart failure: results from the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF). Eur J Heart Fail. 2018;20:1580-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 162] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 3. | Curcio F, Testa G, Liguori I, Papillo M, Flocco V, Panicara V, Galizia G, Della-Morte D, Gargiulo G, Cacciatore F, Bonaduce D, Landi F, Abete P. Sarcopenia and Heart Failure. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 4. | von Haehling S, Garfias Macedo T, Valentova M, Anker MS, Ebner N, Bekfani T, Haarmann H, Schefold JC, Lainscak M, Cleland JGF, Doehner W, Hasenfuss G, Anker SD. Muscle wasting as an independent predictor of survival in patients with chronic heart failure. J Cachexia Sarcopenia Muscle. 2020;11:1242-1249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 5. | Rondanelli M, Klersy C, Terracol G, Talluri J, Maugeri R, Guido D, Faliva MA, Solerte BS, Fioravanti M, Lukaski H, Perna S. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am J Clin Nutr. 2016;103:830-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 280] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 6. | Shimomura Y, Yamamoto Y, Bajotto G, Sato J, Murakami T, Shimomura N, Kobayashi H, Mawatari K. Nutraceutical effects of branched-chain amino acids on skeletal muscle. J Nutr. 2006;136:529S-532S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 140] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Ottestad I, Ulven SM, Øyri LKL, Sandvei KS, Gjevestad GO, Bye A, Sheikh NA, Biong AS, Andersen LF, Holven KB. Reduced plasma concentration of branched-chain amino acids in sarcopenic older subjects: a cross-sectional study. Br J Nutr. 2018;120:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Chang CK, Chang Chien KM, Chang JH, Huang MH, Liang YC, Liu TH. Branched-chain amino acids and arginine improve performance in two consecutive days of simulated handball games in male and female athletes: a randomized trial. PLoS One. 2015;10:e0121866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Calders P, Pannier JL, Matthys DM, Lacroix EM. Pre-exercise branched-chain amino acid administration increases endurance performance in rats. Med Sci Sports Exerc. 1997;29:1182-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Calders P, Matthys D, Derave W, Pannier JL. Effect of branched-chain amino acids (BCAA), glucose, and glucose plus BCAA on endurance performance in rats. Med Sci Sports Exerc. 1999;31:583-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Watson P, Shirreffs SM, Maughan RJ. The effect of acute branched-chain amino acid supplementation on prolonged exercise capacity in a warm environment. Eur J Appl Physiol. 2004;93:306-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Coombes JS, McNaughton LR. Effects of branched-chain amino acid supplementation on serum creatine kinase and lactate dehydrogenase after prolonged exercise. J Sports Med Phys Fitness. 2000;40:240-246. [PubMed] |
| 13. | Ko CH, Wu SJ, Wang ST, Chang YF, Chang CS, Kuan TS, Chuang HY, Chang CM, Chou W, Wu CH. Effects of enriched branched-chain amino acid supplementation on sarcopenia. Aging (Albany NY). 2020;12:15091-15103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Shimomura Y, Murakami T, Nakai N, Nagasaki M, Harris RA. Exercise promotes BCAA catabolism: effects of BCAA supplementation on skeletal muscle during exercise. J Nutr. 2004;134:1583S-1587S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 240] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Komar B, Schwingshackl L, Hoffmann G. Effects of leucine-rich protein supplements on anthropometric parameter and muscle strength in the elderly: a systematic review and meta-analysis. J Nutr Health Aging. 2015;19:437-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 16. | Murphy CH, Saddler NI, Devries MC, McGlory C, Baker SK, Phillips SM. Leucine supplementation enhances integrative myofibrillar protein synthesis in free-living older men consuming lower- and higher-protein diets: a parallel-group crossover study. Am J Clin Nutr. 2016;104:1594-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 17. | Glynn EL, Fry CS, Drummond MJ, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr. 2010;140:1970-1976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 18. | Anker SD, Chua TP, Ponikowski P, Harrington D, Swan JW, Kox WJ, Poole-Wilson PA, Coats AJ. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation. 1997;96:526-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 566] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 19. | Nichols S, McGregor G, Al-Mohammad A, Ali AN, Tew G, O'Doherty AF. The effect of protein and essential amino acid supplementation on muscle strength and performance in patients with chronic heart failure: a systematic review. Eur J Nutr. 2020;59:1785-1801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Tanada Y, Shioi T, Kato T, Kawamoto A, Okuda J, Kimura T. Branched-chain amino acids ameliorate heart failure with cardiac cachexia in rats. Life Sci. 2015;137:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Uchino Y, Watanabe M, Takata M, Amiya E, Tsushima K, Adachi T, Hiroi Y, Funazaki T, Komuro I. Effect of Oral Branched-Chain Amino Acids on Serum Albumin Concentration in Heart Failure Patients with Hypoalbuminemia: Results of a Preliminary Study. Am J Cardiovasc Drugs. 2018;18:327-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Gopal DM, Kalogeropoulos AP, Georgiopoulou VV, Tang WW, Methvin A, Smith AL, Bauer DC, Newman AB, Kim L, Harris TB, Kritchevsky SB, Butler J; Health ABC Study. Serum albumin concentration and heart failure risk The Health, Aging, and Body Composition Study. Am Heart J. 2010;160:279-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Takata M, Amiya E, Watanabe M, Hosoya Y, Nakayama A, Fujiwara T, Taya M, Oguri G, Hyodo K, Takayama N, Takano N, Mashiko T, Uemura Y, Komuro I. An exploratory study on the efficacy and safety of a BCAA preparation used in combination with cardiac rehabilitation for patients with chronic heart failure. BMC Cardiovasc Disord. 2017;17:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Du X, Li Y, Wang Y, You H, Hui P, Zheng Y, Du J. Increased branched-chain amino acid levels are associated with long-term adverse cardiovascular events in patients with STEMI and acute heart failure. Life Sci. 2018;209:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 25. | Paynter NP, Balasubramanian R, Giulianini F, Wang DD, Tinker LF, Gopal S, Deik AA, Bullock K, Pierce KA, Scott J, Martínez-González MA, Estruch R, Manson JE, Cook NR, Albert CM, Clish CB, Rexrode KM. Metabolic Predictors of Incident Coronary Heart Disease in Women. Circulation. 2018;137:841-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 178] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 26. | Fan Y, Li Y, Chen Y, Zhao YJ, Liu LW, Li J, Wang SL, Alolga RN, Yin Y, Wang XM, Zhao DS, Shen JH, Meng FQ, Zhou X, Xu H, He GP, Lai MD, Li P, Zhu W, Qi LW. Comprehensive Metabolomic Characterization of Coronary Artery Diseases. J Am Coll Cardiol. 2016;68:1281-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 185] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 27. | Lim LL, Lau ESH, Fung E, Lee HM, Ma RCW, Tam CHT, Wong WKK, Ng ACW, Chow E, Luk AOY, Jenkins A, Chan JCN, Kong APS. Circulating branched-chain amino acids and incident heart failure in type 2 diabetes: The Hong Kong Diabetes Register. Diabetes Metab Res Rev. 2020;36:e3253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Peterson MB, Mead RJ, Welty JD. Free amino acids in congestive heart failure. J Mol Cell Cardiol. 1973;5:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Li R, He H, Fang S, Hua Y, Yang X, Yuan Y, Liang S, Liu P, Tian Y, Xu F, Zhang Z, Huang Y. Time Series Characteristics of Serum Branched-Chain Amino Acids for Early Diagnosis of Chronic Heart Failure. J Proteome Res. 2019;18:2121-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Sun H, Olson KC, Gao C, Prosdocimo DA, Zhou M, Wang Z, Jeyaraj D, Youn JY, Ren S, Liu Y, Rau CD, Shah S, Ilkayeva O, Gui WJ, William NS, Wynn RM, Newgard CB, Cai H, Xiao X, Chuang DT, Schulze PC, Lynch C, Jain MK, Wang Y. Catabolic Defect of Branched-Chain Amino Acids Promotes Heart Failure. Circulation. 2016;133:2038-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 421] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 31. | Guo X, Huang C, Lian K, Wang S, Zhao H, Yan F, Zhang X, Zhang J, Xie H, An R, Tao L. BCKA down-regulates mTORC2-Akt signal and enhances apoptosis susceptibility in cardiomyocytes. Biochem Biophys Res Commun. 2016;480:106-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Li Y, Xiong Z, Yan W, Gao E, Cheng H, Wu G, Liu Y, Zhang L, Li C, Wang S, Fan M, Zhao H, Zhang F, Tao L. Branched chain amino acids exacerbate myocardial ischemia/reperfusion vulnerability via enhancing GCN2/ATF6/PPAR-α pathway-dependent fatty acid oxidation. Theranostics. 2020;10:5623-5640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 33. | Wang W, Zhang F, Xia Y, Zhao S, Yan W, Wang H, Lee Y, Li C, Zhang L, Lian K, Gao E, Cheng H, Tao L. Defective branched chain amino acid catabolism contributes to cardiac dysfunction and remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol. 2016;311:H1160-H1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 155] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 34. | Chen M, Gao C, Yu J, Ren S, Wang M, Wynn RM, Chuang DT, Wang Y, Sun H. Therapeutic Effect of Targeting Branched-Chain Amino Acid Catabolic Flux in Pressure-Overload Induced Heart Failure. J Am Heart Assoc. 2019;8:e011625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 35. | Uddin GM, Zhang L, Shah S, Fukushima A, Wagg CS, Gopal K, Al Batran R, Pherwani S, Ho KL, Boisvenue J, Karwi QG, Altamimi T, Wishart DS, Dyck JRB, Ussher JR, Oudit GY, Lopaschuk GD. Impaired branched chain amino acid oxidation contributes to cardiac insulin resistance in heart failure. Cardiovasc Diabetol. 2019;18:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 36. | Liu Y, Dong W, Shao J, Wang Y, Zhou M, Sun H. Branched-Chain Amino Acid Negatively Regulates KLF15 Expression via PI3K-AKT Pathway. Front Physiol. 2017;8:853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Fisch S, Gray S, Heymans S, Haldar SM, Wang B, Pfister O, Cui L, Kumar A, Lin Z, Sen-Banerjee S, Das H, Petersen CA, Mende U, Burleigh BA, Zhu Y, Pinto YM, Liao R, Jain MK. Kruppel-like factor 15 is a regulator of cardiomyocyte hypertrophy. Proc Natl Acad Sci USA. 2007;104:7074-7079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 38. | Leenders JJ, Wijnen WJ, Hiller M, van der Made I, Lentink V, van Leeuwen RE, Herias V, Pokharel S, Heymans S, de Windt LJ, Høydal MA, Pinto YM, Creemers EE. Regulation of cardiac gene expression by KLF15, a repressor of myocardin activity. J Biol Chem. 2010;285:27449-27456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Jeyaraj D, Haldar SM, Wan X, McCauley MD, Ripperger JA, Hu K, Lu Y, Eapen BL, Sharma N, Ficker E, Cutler MJ, Gulick J, Sanbe A, Robbins J, Demolombe S, Kondratov RV, Shea SA, Albrecht U, Wehrens XH, Rosenbaum DS, Jain MK. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature. 2012;483:96-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 271] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 40. | Lu G, Ren S, Korge P, Choi J, Dong Y, Weiss J, Koehler C, Chen JN, Wang Y. A novel mitochondrial matrix serine/threonine protein phosphatase regulates the mitochondria permeability transition pore and is essential for cellular survival and development. Genes Dev. 2007;21:784-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 41. | Sun H, Lu G, Ren S, Chen J, Wang Y. Catabolism of branched-chain amino acids in heart failure: insights from genetic models. Pediatr Cardiol. 2011;32:305-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |