Published online Dec 26, 2021. doi: 10.4330/wjc.v13.i12.733
Peer-review started: March 30, 2021
First decision: June 17, 2021
Revised: July 1, 2021
Accepted: November 24, 2021
Article in press: November 24, 2021
Published online: December 26, 2021
Processing time: 272 Days and 14.4 Hours
Left atrial (LA) enlargement is a marker of increased risk in the general population undergoing stress echocardiography. African American (AA) patients with hypertension are known to have less atrial remodeling than whites with hypertension. The prognostic impact of LA enlargement in AA with hypertension undergoing stress echocardiography is uncertain.
To investigate the prognostic value of LA size in hypertensive AA patients undergoing stress echocardiography.
This retrospective outcomes study enrolled 583 consecutive hypertensive AA patients who underwent stress echocardiography over a 2.5-year period. Clinical characteristics including cardiovascular risk factors, stress and echocardiographic data were collected from the electronic health record of a large community hospital. Treadmill exercise and Dobutamine protocols were conducted based on standard practices. Patients were followed for all-cause mortality. The optimal cutoff value of antero-posterior LA diameter for mortality was assessed by receiver operating characteristic analysis. Cox regression was used to determine variables associated with outcome.
The mean age was 57 ± 12 years. LA dilatation was present in 9% (54) of patients (LA anteroposterior ≥ 2.4 cm/m2). There were 85 deaths (15%) during 4.5 ± 1.7 years of follow-up. LA diameter indexed for body surface area had an area under the curve of 0.72 ± 0.03 (optimal cut-point of 2.05 cm/m2). Variables independently associated with mortality included age [P = 0.004, hazard ratio (HR) 1.34 (1.10-1.64)], tobacco use [P = 0.001, HR 2.59 (1.51-4.44)], left ventricular hypertrophy [P = 0.001 , HR 2.14 (1.35-3.39)], Dobutamine stress [P = 0.003, HR 2.12 (1.29-3.47)], heart failure history [P = 0.031, HR 1.76 (1.05-2.94)], LA diameter ≥ 2.05 cm/m2 [P = 0.027, HR 1.73 (1.06-2.82)], and an abnormal stress echocardiogram [P = 0.033, HR 1.67 (1.04-2.68)]. LA diameter as a continuous variable was also independently associated with mortality but LA size ≥ 2.40 cm/m2 was not.
LA enlargement is infrequent in hypertensive AA patients when traditional reference values are used. LA enlargement is independently associated with mortality when a lower than “normal” threshold (≥ 2.05 cm/m2) is used.
Core Tip: In hypertensive African American patients referred for stress testing, left atrial (LA) enlargement was infrequent when using the established references values for the general population. Indexed LA Antero-posterior diameter has a superior area under the curve compared to LA diameter alone for discrimination of survivors and non-survivors. LA enlargement is an independent predictor of mortality on long-term follow-up when assessed as a continuous variable or when using a lower reference value derived from our population.
- Citation: Khemka A, Sutter DA, Habhab MN, Thomaides A, Hornsby K, Feigenbaum H, Sawada SG. Prognostic value of left atrial size in hypertensive African Americans undergoing stress echocardiography. World J Cardiol 2021; 13(12): 733-744
- URL: https://www.wjgnet.com/1949-8462/full/v13/i12/733.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i12.733
Left atrial (LA) enlargement is a known predictor of adverse cardiovascular events including atrial fibrillation, stroke and heart failure[1-3]. Hypertension can induce left ventricular (LV) remodeling resulting in increased LV mass and concentric hypertrophy, both of which are associated with LA enlargement[4,5]. In the general population of patients undergoing stress echocardiography, LA enlargement [defined by an anteroposterior (AP) dimension ≥ 2.4 cm/m2] has been shown to be predictive of myocardial infarction and death[6,7]. LA enlargement has also been shown to be predictive of an abnormal stress echocardiogram[8]. African Americans (AA) have a high burden of cardiovascular disease as well as risk factors including hypertension and diabetes mellitus. Although morbidity and mortality risk in this population is well established, pharmacotherapy is less commonly utilized and AA have higher mortality relative to other ethnicities[9]. The prognostic value of LA enlargement in AA patients undergoing stress echocardiography is less clear. Additionally, it is unclear if reference values for LA enlargement established in white populations should be applied in AA. In African American patients, LA remodeling appears reduced relative to whites even when controlling for risk factors such as obesity, age, increased LV mass, and hypertension[10-12]. The lower incidence of atrial fibrillation in AA may be attributed to their smaller LA size and may be related more to inter-racial differences in anteroposterior diameter rather than volume[10,13,14]. Despite less LA remodeling, AA are at increased for cardiovascular events and mortality compared to white patients[15]. However, LA size may also have prognostic value in this racial group[7,16]. The purpose of this study was to assess the prognostic value of LA size in hypertensive AA patients undergoing stress echocardiography and to determine a threshold value of LA enlargement associated with mortality.
The Indiana University Institutional Review Board approved this study. The study population comprised of 583 consecutive AA patients with a history of hypertension referred for stress echocardiography at an urban community hospital in Indianapolis over a 2.5-year period.
Clinical characteristics were extracted from the electronic health record. Patients were considered to have a smoking history if they were currently using tobacco or were a former smoker. Hypercholesterolemia was defined as total cholesterol greater than 200 mg/dL or if the patient was receiving lipid-lowering therapy. Obesity was defined as a body mass index ≥ 30 kg/m2. Patients were considered to have a history of coronary artery disease if they previously suffered a myocardial infarction, underwent a revascularization procedure, or had at least 50% diameter stenosis in one or more major epicardial coronary arteries by angiography. A history of heart failure was noted if there was a previous hospitalization for heart failure or a clinical diagnosis made in an outpatient setting with ongoing medical treatment for heart failure.
LA diameter was measured as the maximum end-systolic anterior-posterior diameter in the parasternal long- or short-axis views. LA enlargement was defined as a dimension ≥ 2.4 cm/m2 when indexed to body surface area (BSA) based on studies in the general population[7,17]. LA volume index was not routinely assessed because only a minority of subjects had apical views visualizing the entire LA. In a small subset of patients with apical views that included the entire left atrium, LA volume index was also measured using the biplane Simpson’s method. LV diameters and wall thickness were obtained in the parasternal long- or short-axis views at the level of the mitral leaflet tips. LV mass was calculated using linear measurements with the following formula:
LV mass = 0.8 × {1.04 [(LVIDd + PWTd + SWTd)3 – (LVIDd)3]} + 0.6 g where:
LVIDd = maximum internal diameter at end-diastole.
PWTd = end-diastole posterior wall thickness.
SWTd = end-diastole septal wall thickness.
Left ventricular hypertrophy (LVH) was defined as an LV mass indexed to BSA greater than or equal to 96 g/m2 for women and 116 g/m2 for men[18,19]. Relative wall thickness (RWT) was calculated by the formula (2 × PWTd/LVIDd). LVH was further differentiated into concentric and eccentric hypertrophy if RWT was > 0.42 or ≤ 0.42, respectively. Concentric remodeling was defined as a normal LV mass with RWT > 0.42. Ejection fraction was calculated with either the area length method or with the modified Simpson’s method for patients with regional wall motion abnormalities.
Treadmill exercise was performed with protocols chosen based on the patient’s age and expected exercise ability. Standard end-points were used[20]. The Dobutamine protocol was conducted with a step-wise infusion using previously described methods and endpoints[21]. Images were obtained in the apical four- and two-chamber views and parasternal long- and short-axis views at rest, low-dose (5-10 µg/kg/min), peak dose and recovery in patients undergoing Dobutamine stress. Baseline and immediate post-stress images were obtained in patients undergoing exercise. Experienced echocardiographers blinded to the clinical data and follow-up interpreted the stress echocardiograms. An abnormal stress echocardiogram was defined by the presence of resting or stress-induced wall motion abnormalities in one or more of 16 myocardial segments[22].
Follow-up data was obtained retrospectively by review of the electronic health records and the Social Security Death Index database[23]. The end-point for the study was all-cause mortality.
Continuous variables were reported as mean ± SD. Patient groups were compared using the Student t-test for continuous variables and Chi-square test for categorical variables. A two sided P-value < 0.05 was considered significant. Receiver-operating characteristic (ROC) curve analysis was used to determine the best cut-point of LA diameter for predicting mortality. The area under the curve (AUC) was calculated for both LA diameter and LA diameter indexed to BSA. The difference between the two AUC values was compared using the correlated area test statistic. Kaplan-Meier analysis of survival was performed using the best cut-point from ROC analysis. Cox proportional hazards model was used to assess predictors of mortality. Variables with P value < 0.05 were included in a multivariate analysis employing a forward conditional method. LA diameter was tested on multivariate analysis both as a continuous variable and as a categorical variable using the cut-point of 2.4 cm/m2 previously established in the general population and the best cut-point determined from ROC analysis in our study population. The relationship between LA diameter index and LA volume index was assessed by linear regression.
Statistical analysis was performed using SPSS version 18 (SPS, Chicago, IL, United States) and the software package ROCKIT[24].
Table 1 shows the clinical and stress echocardiographic characteristics of the patient population. Of the 583 patients, 32% were male and the mean age was 57 ± 12 years. A history of heart failure or coronary atherosclerosis was present in 11% and 19%, respectively. Ninety percent of the patients were on anti-hypertensive therapy and the mean resting systolic blood pressure was 140 ± 17 mmHg. An abnormal stress echocardiogram was noted in 17% of patients. Eleven percent had an ejection fraction less than 50%. LVH was present in 25% and concentric remodeling was present in 52%. Only 9% of the study population had an elevated LA diameter index, using the cut-point of 2.4 cm/m2 as defined in the general population.
| Clinical | Echocardiographic | ||
| Age (yr) | 57 ± 12 | Ejection fraction (%) | 59 ± 10 |
| Male | 32% | Reduced EF | 11% |
| Tobacco | 60% | LA Diam (cm) | 3.7 ± 0.6 |
| Family history of CAD | 34% | LA Diam index (cm/m2) | 1.9 ± 0.4 |
| Hyperlipidemia | 50% | Abn LA Diam index | 9% |
| Diabetes mellitus | 38% | LV mass (g) | 172 ± 59 |
| Obesity | 45% | LV mass index (g/m2) | 89 ± 29 |
| CAD | 19% | LV hypertrophy | 25% |
| Heart failure | 11% | Relative wall thickness | 0.51 |
| Atrial fibrillation | 3% | LV remodeling pattern | |
| CKD (GFR < 60) | 17% | Normal geometry | 22% |
| Systolic BP (mmHg) | 140 ± 17 | Concentric remodeling | 52% |
| Hypertensive therapy | 90% | Concentric hypertrophy | 21% |
| Diuretic | 56% | Eccentric hypertrophy | 4% |
| Calcium channel blocker | 33% | Dobutamine study | 40% |
| ACE-I/ARB | 55% | Abnormal stress echo | 17% |
| Beta-blocker | 46% | ||
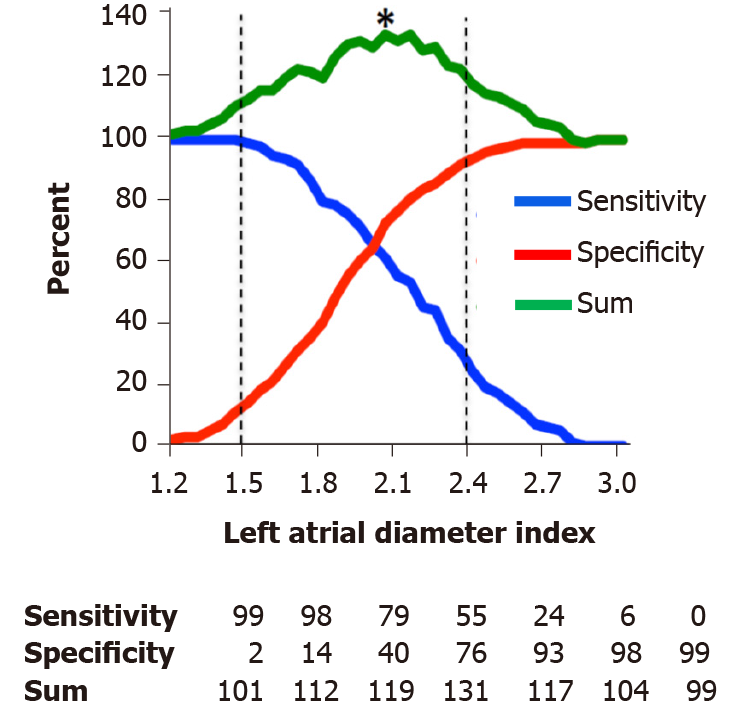
During follow-up of 4.5 ± 1.7 years (max 6.9 years), 85 patients (15%) died. ROC analysis showed that LA diameter referenced to body surface area had a larger AUC compared to LA diameter alone (AUC of 0.72 ± 0.03 vs 0.66 ± 0.03, P = 0.002) for distinguishing survivors and those who died. Figure 1 demonstrates a plot of sensitivity and specificity of LA diameter for death during follow-up at 0.05 cm/m2 intervals. LA size above the reference value (2.4 cm/m2) had sensitivity and specificity for mortality during follow-up of 24% and 93%, respectively. The best cut-point for predicting death during follow-up (maximum of sensitivity and specificity) was 2.05 cm/m2, which produced a sensitivity and specificity of 61% and 72%, respectively.
Figure 2 shows a Kaplan-Meier analysis of cumulative survival using the best cut-point of 2.05 cm/m2. Overall, survival was 92% if LA diameter index was ≤ 2.05 cm/m2 and 72% if LA diameter index was > 2.05 cm/m2 (P-value < 0.001).
Table 2 shows univariate predictors for all-cause mortality. There were six independent predictors of mortality by multivariate analysis using the reference cut-point for LA enlargement (2.4 cm/m2, see Table 3). These included age, smoking history, heart failure, the need for Dobutamine stress, an abnormal stress echocardiogram, and LVH (Chi-square 102). LA enlargement was not a predictor. In a second multivariate analysis using the ROC defined cut-point of 2.05 cm/m2 for LA size, LA enlargement was found to be an additional independent predictor (Chi-square 107). A third multivariate analysis considering LA diameter index as a continuous variable rather than a categorical variable also found LA diameter index to be independently predictive in addition to the other six predictors (Chi-square 109).
| Characteristic | Event | No event | Hazard ratio (95%CI) | P value |
| Clinical | ||||
| Age | 62.9 | 56.5 | 1.04 (1.02-1.06) | < 0.001 |
| Male sex | 40% | 32% | 1.43 (0.92-2.20] | 0.109 |
| Tobacco | 79% | 57% | 2.55 (1.51-4.29) | < 0.001 |
| Fam. History of CAD | 28% | 35% | 0.73 (0.45-1.28) | 0.194 |
| Hyperlipidemia | 48% | 51% | 0.91 (0.59-1.40) | 0.662 |
| Diabetes mellitus | 52% | 36% | 1.77 (1.16-2.71) | 0.008 |
| Obesity | 32% | 47% | 0.56 (0.35-0.88) | 0.010 |
| CAD | 33% | 17% | 2.15 (1.37-3.38) | 0.001 |
| Heart failure | 27% | 9% | 3.44 (2.13-5.56) | < 0.001 |
| Atrial fibrillation | 7% | 2% | 2.70 (1.18-6.19) | 0.019 |
| CKD (GFR < 60) | 29% | 15% | 2.37 (1.48-3.78) | < 0.001 |
| Systolic BP (mmHg) | 141.5 | 140.0 | 1.01 (0.999-1.02) | 0.438 |
| Echocardiographic | ||||
| Reduced EF | 25% | 9% | 2.89 (1.76-4.72) | < 0.001 |
| Abn. LA index (2.40 cut-point) | 24% | 7% | 3.16 (1.91-5.22) | < 0.001 |
| Abn. LA index (2.05 cut-point) | 61% | 28% | 3.35 (2.17-5.18) | < 0.001 |
| LV hypertrophy | 52% | 21% | 3.62 (2.36-5.54) | < 0.001 |
| Relative wall thickness | 0.51 | 0.51 | 0.94 (0.19-4.57) | 0.941 |
| LV diastolic diameter | 4.54 | 4.34 | 1.57 (1.15-2.13) | 0.005 |
| LV systolic diameter | 3.24 | 2.98 | 1.54 (1.20-1.99) | 0.001 |
| Fractional shortening | 0.30 | 0.32 | 0.05 (0.00-0.57) | 0.017 |
| IV septum thickness | 1.20 | 1.11 | 2.51 (1.19-5.33) | 0.016 |
| LV post. wall thickness | 1.13 | 1.07 | 2.48 (1.04-5.92) | 0.040 |
| Dobutamine study | 68% | 35% | 3.55 (2.25-5.60) | < 0.001 |
| Abnormal stress | 35% | 14% | 2.76 (1.77-4.31) | < 0.001 |
| Reference cut-point for Abn. LA Diam | Best cut-point for Abn. LA Diam | |||||
| Chi-square 102 | Chi-square 107 | |||||
| Predictor | Wald | HR (95%CI) | P value | Wald | HR (95%CI) | P value |
| Age (per 10 yr) | 11.4 | 1.40 (1.15-1.71) | 0.001 | 8.2 | 1.34 (1.10-1.64) | 0.004 |
| Tobacco | 11.9 | 2.61 (1.51-4.49) | 0.001 | 11.9 | 2.59 (1.51-4.44) | 0.001 |
| Heart failure | 6.4 | 1.92 (1.16-3.20) | 0.012 | 4.7 | 1.76 (1.05-2.94) | 0.031 |
| LVH | 17.6 | 2.54 (1.64-3.93) | <0.001 | 10.5 | 2.14 (1.35-3.39) | 0.001 |
| Abnormal stress | 5.9 | 1.79 (1.12-2.86) | 0.015 | 4.6 | 1.67 (1.04-2.68) | 0.033 |
| Dobutamine study | 8.5 | 2.09 (1.27-3.43) | 0.004 | 8.9 | 2.12 (1.29-3.47) | 0.003 |
| LA index ≥ 2.40 | – | – | NS | 1 | 1 | 1 |
| LA index ≥ 2.05 | 1 | 1 | 1 | 4.9 | 1.73 (1.06-2.82) | 0.027 |
In the 57 patients (10%) in whom LA volume index could be assessed, the R-value for the correlation of LA diameter index and LA volume index was 0.76. Fourteen subjects (25%) were identified as having LA enlargement by volume index based on a cut-point of 34 mL/m2 established in the general population[6].
Our study had three main findings. LA enlargement in the AP dimension was infrequent in AA with hypertension using reference values established in the general population. LA diameter indexed for BSA had a superior AUC to LA diameter alone. LA size was an independent predictor of mortality on long-term follow-up when assessed as a continuous variable or using the cut-point of 2.05 cm/m2 for enlargement derived from our population but not when using the cut-point of 2.4 cm/m2 derived from the general population.
In this study, only 9% of hypertensive AA were found to have LA enlargement. This is a lower than expected frequency of LA enlargement when compared to the general population. Among a broad sample of the Framingham study used to validate reference values of LA diameter, 22% of men and 29% of women had LA diameters that exceeded reference limits[2]. Compared to the Framingham study cohort, our population had a higher prevalence of hypertension (100% vs 33%), heart failure (11% vs 1%), and older age (mean age 57.4 years vs 50.8 years), which are all variables associated with LA enlargement[3,25,26]. Multiple studies have shown a higher prevalence of LA enlargement in white patients with hypertension (weighted average 37.3%)[27-30].
AA have a higher burden of hypertension and cardiovascular mortality with lower rates of pharmacologic interventions[9]. However, when traditional reference values for LA size are used, AA patient mortality risk may be underappreciated. Several studies have shown reduced LA remodeling in AA patients. In a cohort of men with hypertension (58% AA), investigators found that as age increased white patients had a greater mean LA diameter than AA patients[12]. Similarly, in a cohort of 3882 elderly subjects, AA men had significantly smaller mean LA diameter (1.9 mm LA dimension)[11]. Additionally, in a study evaluating the effect of race on the prevalence of atrial fibrillation, AA subjects were demonstrated to have significantly smaller LA diameters (2 mm smaller AP LA dimension)[10].
A more recent evaluation of 129 AA compared with 326 whites showed that in the presence of hypertension, the former had significantly smaller LA size despite similar ventricular relative wall-thickness, diastolic function, and 6-min walk test[31]. Why LA remodeling might be reduced in AA remains unclear although there is speculation that genetic and environmental factors influence the structure of the hearts of AA patients compared to hearts of white patients. Badertscher et al[31] found that AA have lower levels of collagen 1 telopeptide and higher levels of collagen 1 propeptide suggesting that different collagen homeostasis may contribute to atrial remodeling. While AA have a similar average LV mass index as whites, they have significantly smaller LV cavities and thicker LV walls, with a high percentage demonstrating the “concentric remodeling” pattern of cardiac structure[33-35]. This pattern was seen in a majority of our population with 52% displaying concentric remodeling. Similar genetic and environmental factors that produce differences in LV remodeling may also contribute to race related differences in LA remodeling. Gottdiener et al[12] proposed the possibility that in parallel with an increased LV wall thickness, there might also be a similar increase in LA wall thickness, which might reduce wall compliance and the resultant cavity size of the LA.
An additional possibility is that reduction of anterior-posterior LA dimension in AA is due to differences in chest and mediastinal structures rather than a consequence of true differences in LA remodeling. The LA is a relatively low-pressure chamber and its size and configuration is influenced by its surrounding structures. Manolio et al[11] reported that racial differences in LA dimensions were partially mitigated when accounting for chest dimensions and spirometric lung volumes. Given the close proximity of the ascending aorta to the LA, enlargement of the aortic root might limit the ability of the LA to expand in the antero-posterior direction. AA patients are known to have a higher than expected prevalence of aortic regurgitation, which was independently predicted by aortic root size[35]. In the small cohort of patients in our study who had LA volume measurements, the proportion (25%) that had enlargement remained lower than expected.
From ROC analysis, the optimal cut-point for an abnormal LA diameter that predicts mortality in AA was well within the normal reference range. In contrast, the guidelines-defined cut-point had very low sensitivity for predicting mortality in our study population. While LA dilation is infrequent in AA, LA diameter does hold prognostic significance in this population when a lower threshold for abnormal is used.
LA diameter indexed to BSA improved prediction of mortality over LA diameter alone. Indexing of echocardiographic measurements to BSA is currently recommended by the American Society of Echocardiography but it has been argued that correcting for body size inappropriately “forgives” for obesity[36]. Our population included a large proportion of AA females, a population known to have a high prevalence of obesity[37]. Forty-five percent of our population was obese. Therefore, use of indexed LA diameters raises the potential of overcorrection for obesity in our study. However, we found that indexed LA diameter had superior prognostic value over LA diameter alone suggesting that the correction is appropriate in our population. To our knowledge, this is the first study to demonstrate superiority of indexed LA diameter over LA diameter alone.
Our data found LA diameter index to be an independent predictor of all-cause mortality in addition to heart failure, age, smoking history, LVH, an abnormal stress echocardiogram, and the requirement for Dobutamine stress. Our study demonstrated that LA diameter predicted long-term outcome as survival curves continued to separate at 6 years of follow-up. Similar to our finding, data from the Framingham study found LA diameter to be predictive of death during 8 years of follow-up[2]. Within an AA cohort of the Atherosclerosis Risk in Communities study, those with the highest quintile of LA diameter had a higher risk of mortality during a median follow-up of 9.8 years[38]. Several investigations have suggested that LA enlargement serves as a marker of chronic diastolic dysfunction over time and thus accounts for the accumulated risk of elevated cardiac filling pressures for cardiovascular events[39]. Our results suggest different reference values are needed for AA patients to accurately evaluate their cardiovascular risk. This may also improve treatment in hypertensive AA patients which may translate to decreased mortality.
The primary limitation of this study is our use of LA diameter as opposed to LA volume index. LA volume is currently recommended by the American Society of Echocardiography as the most accurate measure of true LA size[6]. Unfortunately, majority of the patients in our study had truncated apical images utilized for stress echocardiography so we were unable to derive information on LA volume except in a minority of patients. In the small subset of patients there was a reasonable correlation between LA diameter and volume index. While LA volume is clearly a more accurate measure of true LA size, LA volume may be only marginally superior at identifying cardiovascular disease[26,40]. For patients undergoing stress echocardiography, LA diameter index has shown to offer adequate prognostic value and is probably acceptable for those with difficult visualization of the complete LA[7].
An additional limitation of our study was the large percentage of female subjects. Sixty-eight percent of our population was female. How this might affect the applicability of our data for predicting mortality in AA men is unknown, but previous data have suggested that indexing for body size nearly completely accounts for gender differences in LA dimensions[41].
LA enlargement is infrequent in AA with hypertension referred for stress testing when using the established references values for the general population. Indexed LA AP diameter has a superior AUC to LA diameter alone for discrimination of survivors and non-survivors. LA enlargement is an independent predictor of mortality on long-term follow-up when assessed as a continuous variable or when using a cut-point derived from our population.
African Americans (AA) have higher cardiovascular (CV) risk factors including hypertension and mortality compared to other races. Left atrial (LA) size has shown prognostic value in white patients.
Prior research has suggested AA have smaller LA volumes and standard references values may not apply.
We investigated the prognostic value of LA size in hypertensive AA patients undergoing stress echocardiography.
In this retrospective cohort study, we evaluated 583 consecutive AA patients with a history of hypertension referred for stress testing and evaluated LA diameter in the Antero-posterior window.
LA dilatation was present in 9% (54) of patients [LA anteroposterior (AP) ≥ 2.4 cm/m2]. There were 85 deaths (15%) during 4.5 ± 1.7 years of follow-up. LA diameter indexed for body surface area had an AUC of 0.72 ± 0.03 (optimal cut-point of 2.05 cm/m2). Variables independently associated with mortality included age (P = 0.004), tobacco use (P = 0.001), left ventricular hypertrophy (P = 0.001), need for pharmacologic dobutamine stress (P = 0.003), heart failure history (P = 0.031), LA diameter ≥ 2.05 cm/m2 (P = 0.027), and an abnormal stress echocardiogram (P = 0.033). LA diameter as a continuous variable was also independently associated with mortality but LA size ≥ 2.40 cm/m2 was not.
LA enlargement is infrequent in AA with hypertension referred for stress testing when using the established references values for the general population. Indexed LA AP diameter has a superior prognostic value to LA diameter alone for discrimination of survivors and non-survivors. LA enlargement is an independent predictor of mortality on long-term follow-up when assessed as a continuous variable or when using a cut-point derived from our population.
References values for LA size in AA patients may need to be adjusted to more accurately reflect CV risk and which may translate to more aggressive pharmacologic management.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pop TL S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89:724-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 711] [Cited by in RCA: 789] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 2. | Benjamin EJ, D'Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995;92:835-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 820] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 3. | Takemoto Y, Barnes ME, Seward JB, Lester SJ, Appleton CA, Gersh BJ, Bailey KR, Tsang TS. Usefulness of left atrial volume in predicting first congestive heart failure in patients > or = 65 years of age with well-preserved left ventricular systolic function. Am J Cardiol. 2005;96:832-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 240] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 4. | Cioffi G, Mureddu GF, Stefenelli C, de Simone G. Relationship between left ventricular geometry and left atrial size and function in patients with systemic hypertension. J Hypertens. 2004;22:1589-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Mureddu GF, Cioffi G, Stefenelli C, Boccanelli A. Relationships of the appropriateness of left ventricular mass to left atrial size and function in arterial hypertension. J Cardiovasc Med (Hagerstown). 2007;8:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1-39. e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6446] [Cited by in RCA: 9323] [Article Influence: 932.3] [Reference Citation Analysis (0)] |
| 7. | Bangalore S, Yao SS, Chaudhry FA. Role of left atrial size in risk stratification and prognosis of patients undergoing stress echocardiography. J Am Coll Cardiol. 2007;50:1254-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Alsaileek AA, Osranek M, Fatema K, McCully RB, Tsang TS, Seward JB. Predictive value of normal left atrial volume in stress echocardiography. J Am Coll Cardiol. 2006;47:1024-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA Jr, Willis M, Yancy CW; American Heart Association Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; and Stroke Council. Cardiovascular Health in African Americans: A Scientific Statement From the American Heart Association. Circulation. 2017;136:e393-e423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 813] [Article Influence: 101.6] [Reference Citation Analysis (0)] |
| 10. | Marcus GM, Olgin JE, Whooley M, Vittinghoff E, Stone KL, Mehra R, Hulley SB, Schiller NB. Racial differences in atrial fibrillation prevalence and left atrial size. Am J Med. 2010;123:375.e1-375.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Manolio TA, Gottdiener JS, Tsang TS, Gardin JM; Cardiovascular Health Study Collaborative Research Group. Left atrial dimensions determined by M-mode echocardiography in black and white older (> or =65 years) adults (The Cardiovascular Health Study). Am J Cardiol. 2002;90:983-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Gottdiener JS, Reda DJ, Williams DW, Materson BJ. Left atrial size in hypertensive men: influence of obesity, race and age. Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. J Am Coll Cardiol. 1997;29:651-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 101] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370-2375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4468] [Cited by in RCA: 4626] [Article Influence: 192.8] [Reference Citation Analysis (0)] |
| 14. | Ruo B, Capra AM, Jensvold NG, Go AS. Racial variation in the prevalence of atrial fibrillation among patients with heart failure: the Epidemiology, Practice, Outcomes, and Costs of Heart Failure (EPOCH) study. J Am Coll Cardiol. 2004;43:429-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Kamel H, Alwell K, Kissela BM, Sucharew HJ, Woo D, Flaherty M, Ferioli S, Demel SL, Moomaw CJ, Walsh K, Mackey J, Rios La Rosa Felipe L, Jasne A, Slavin S, Martini S, Adeoye O, Baig T, Chen ML, Levitan EB, Soliman EZ, Kleindorfer DO. Racial Differences in Atrial Cardiopathy Phenotypes in Ischemic Stroke Patients. Neurology. 2020;96:e1137-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Kochanek KD, Murphy SL, Xu J, Tejada-Vera B. Deaths: Final Data for 2014. Natl Vital Stat Rep. 2016;65:1-122. [PubMed] |
| 17. | Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ; Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8282] [Cited by in RCA: 8803] [Article Influence: 463.3] [Reference Citation Analysis (0)] |
| 18. | Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4462] [Cited by in RCA: 4711] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 19. | Ilercil A, O'Grady MJ, Roman MJ, Paranicas M, Lee ET, Welty TK, Fabsitz RR, Howard BV, Devereux RB. Reference values for echocardiographic measurements in urban and rural populations of differing ethnicity: the Strong Heart Study. J Am Soc Echocardiogr. 2001;14:601-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Yao SS, Bangalore S, Chaudhry FA. Prognostic implications of stress echocardiography and impact on patient outcomes: an effective gatekeeper for coronary angiography and revascularization. J Am Soc Echocardiogr. 2010;23:832-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Sawada SG, Segar DS, Ryan T, Brown SE, Dohan AM, Williams R, Fineberg NS, Armstrong WF, Feigenbaum H. Echocardiographic detection of coronary artery disease during dobutamine infusion. Circulation. 1991;83:1605-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 465] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 22. | Pellikka PA, Arruda-Olson A, Chaudhry FA, Chen MH, Marshall JE, Porter TR, Sawada SG. Guidelines for Performance, Interpretation, and Application of Stress Echocardiography in Ischemic Heart Disease: From the American Society of Echocardiography. J Am Soc Echocardiogr 2020; 33: 1-41. e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 301] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 23. | Ancestry.com. Social Security Death Index. [cited 2010 Aug 3]. Available from: http://ssdi.rootsweb.ancestry.com/. |
| 24. | Kurt Rossman Laboratories for Radiologic Image Research. Software package ROCKIT. [cited 2010 Dec 15]. Available from: http://www.radiology.uchicago.edu/krl/KRL_ROC/software_index6.htm. |
| 25. | Vaziri SM, Larson MG, Lauer MS, Benjamin EJ, Levy D. Influence of blood pressure on left atrial size. The Framingham Heart Study. Hypertension. 1995;25:1155-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 212] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol. 2003;41:1036-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 436] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 27. | Gerdts E, Oikarinen L, Palmieri V, Otterstad JE, Wachtell K, Boman K, Dahlöf B, Devereux RB; Losartan Intervention For Endpoint Reduction in Hypertension (LIFE) Study. Correlates of left atrial size in hypertensive patients with left ventricular hypertrophy: the Losartan Intervention For Endpoint Reduction in Hypertension (LIFE) Study. Hypertension. 2002;39:739-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 158] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 28. | Pearson AC, Gudipati C, Nagelhout D, Sear J, Cohen JD, Labovitz AJ. Echocardiographic evaluation of cardiac structure and function in elderly subjects with isolated systolic hypertension. J Am Coll Cardiol. 1991;17:422-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 92] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Dunn FG, Chandraratna P, deCarvalho JG, Basta LL, Frohlich ED. Pathophysiologic assessment of hypertensive heart disease with echocardiography. Am J Cardiol. 1977;39:789-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 217] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Piotrowski G, Banach M, Gerdts E, Mikhailidis DP, Hannam S, Gawor R, Stasiak A, Rysz J, Gawor Z. Left atrial size in hypertension and stroke. J Hypertens. 2011;29:1988-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Badertscher P, Gregg D, Baicu CF, Ramakrishnan V, Spinale FG, Zile MR, Gold MR. Racial difference in atrial size and extracellular matrix homeostatic response to hypertension: Is this a potential mechanism of reduced atrial fibrillation in African Americans? Heart Rhythm O2. 2021;2:37-45. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Meyers KJ, Mosley TH, Fox E, Boerwinkle E, Arnett DK, Devereux RB, Kardia SL. Genetic variations associated with echocardiographic left ventricular traits in hypertensive blacks. Hypertension. 2007;49:992-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Chaturvedi N, Athanassopoulos G, McKeigue PM, Marmot MG, Nihoyannopoulos P. Echocardiographic measures of left ventricular structure and their relation with rest and ambulatory blood pressure in blacks and whites in the United Kingdom. J Am Coll Cardiol. 1994;24:1499-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Gottdiener JS, Reda DJ, Materson BJ, Massie BM, Notargiacomo A, Hamburger RJ, Williams DW, Henderson WG. Importance of obesity, race and age to the cardiac structural and functional effects of hypertension. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. J Am Coll Cardiol. 1994;24:1492-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 115] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Fox ER, Wilson RS, Penman AD, King JJ, Towery JG, Butler KR, McMullan MR, Skelton TN, Mosley TH, Taylor HA. Epidemiology of pure valvular regurgitation in the large middle-aged African American cohort of the Atherosclerosis Risk in Communities study. Am Heart J. 2007;154:1229-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Lauer MS, Larson MG, Levy D. Gender-specific reference M-mode values in adults: population-derived values with consideration of the impact of height. J Am Coll Cardiol. 1995;26:1039-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291:2847-2850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2990] [Cited by in RCA: 2637] [Article Influence: 125.6] [Reference Citation Analysis (0)] |
| 38. | Nagarajarao HS, Penman AD, Taylor HA, Mosley TH, Butler K, Skelton TN, Samdarshi TE, Aru G, Fox ER. The predictive value of left atrial size for incident ischemic stroke and all-cause mortality in African Americans: the Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 2008;39:2701-2706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM. Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol. 2005;45:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 474] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 40. | Tsang TS, Abhayaratna WP, Barnes ME, Miyasaka Y, Gersh BJ, Bailey KR, Cha SS, Seward JB. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol. 2006;47:1018-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 575] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 41. | Knutsen KM, Stugaard M, Michelsen S, Otterstad JE. M-mode echocardiographic findings in apparently healthy, non-athletic Norwegians aged 20-70 years. Influence of age, sex and body surface area. J Intern Med. 1989;225:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 1.4] [Reference Citation Analysis (0)] |