Published online Oct 26, 2021. doi: 10.4330/wjc.v13.i10.585
Peer-review started: March 29, 2021
First decision: June 25, 2021
Revised: July 8, 2021
Accepted: September 10, 2021
Article in press: September 10, 2021
Published online: October 26, 2021
Processing time: 205 Days and 14.3 Hours
Dipeptidyl peptidase-4 (DPP-4) inhibitors are a generally safe and well tolerated antidiabetic drug class with proven efficacy in type 2 diabetes mellitus (T2DM). Recently, a series of large, randomized controlled trials (RCTs) addressing cardiovascular outcomes with DPP-4 inhibitors have been published.
To pool data from the aforementioned trials concerning the impact of DPP-4 inhibitors on surrogate cardiovascular efficacy outcomes and on major cardiac arrhythmias.
We searched PubMed and grey literature sources for all published RCTs assessing cardiovascular outcomes with DPP-4 inhibitors compared to placebo until October 2020. We extracted data concerning the following “hard” efficacy outcomes: fatal and non-fatal myocardial infarction, fatal and non-fatal stroke, hospitalization for heart failure, hospitalization for unstable angina, hospitalization for coronary revascularization and cardiovascular death. We also extracted data regarding the risk for major cardiac arrhythmias, such as atrial fibrillation, atrial flutter, ventricular fibrillation and ventricular tachycardia.
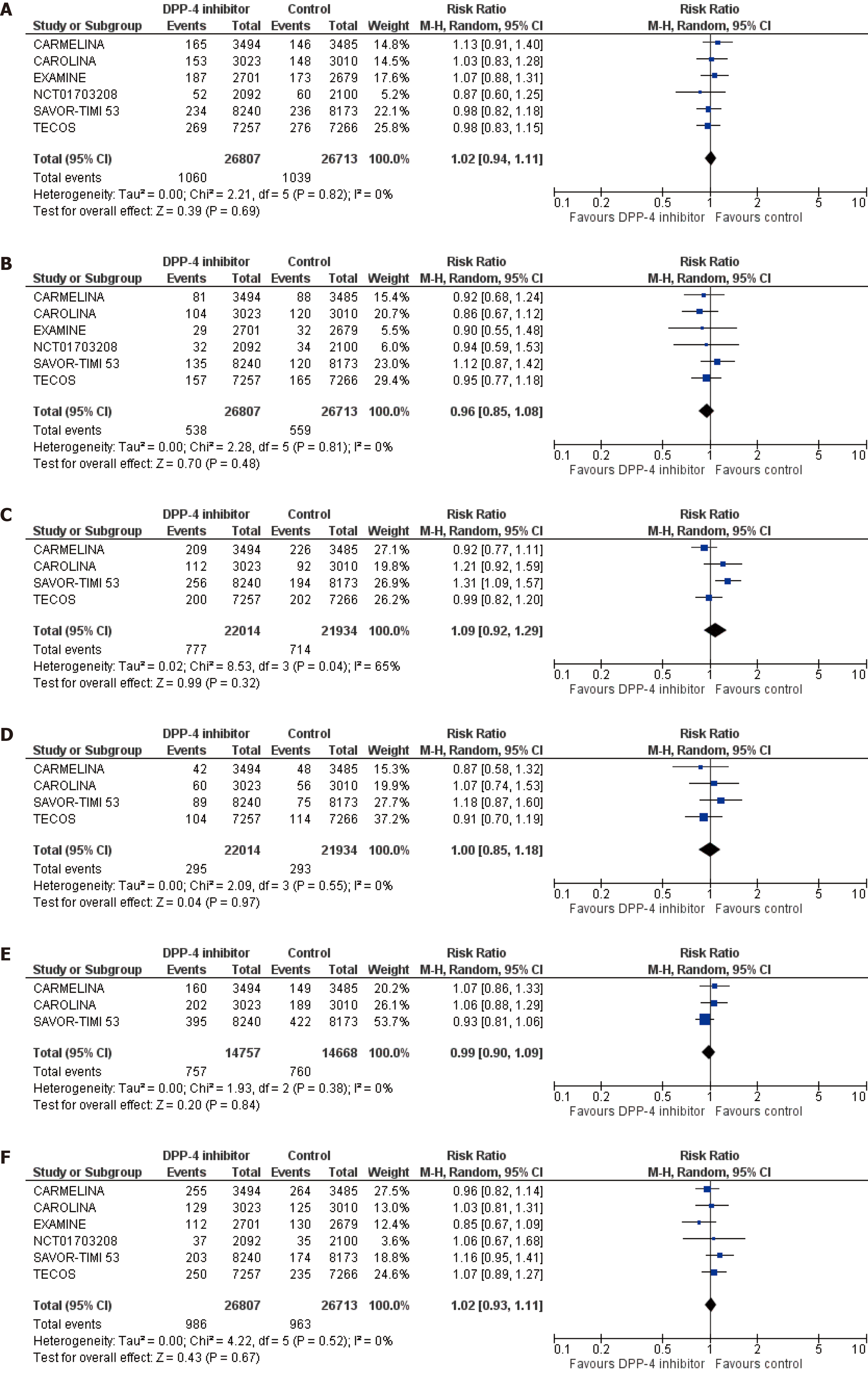
We pooled data from 6 trials in a total of 52520 patients with T2DM assigned either to DPP-4 inhibitor or placebo. DPP-4 inhibitors compared to placebo led to a non-significant increase in the risk for fatal and non-fatal myocardial infarction [risk ratio (RR) = 1.02, 95%CI: 0.94-1.11, I2 = 0%], hospitalization for heart failure (RR = 1.09, 95%CI: 0.92-1.29, I2 = 65%) and cardiovascular death (RR = 1.02, 95%CI: 0.93-1.11, I2 = 0%). DPP-4 inhibitors resulted in a non-significant decrease in the risk for fatal and non-fatal stroke (RR = 0.96, 95%CI: 0.85-1.08, I2 = 0%) and coronary revascularization (RR = 0.99, 95%CI: 0.90-1.09, I2 = 0%), Finally, DPP-4 inhibitors demonstrated a neutral effect on the risk for hospitalization due to unstable angina (RR = 1.00, 95%CI: 0.85-1.18, I2 = 0%). As far as cardiac arrhythmias are concerned, DPP-4 inhibitors did not significantly affect the risk for atrial fibrillation (RR = 0.95, 95%CI: 0.78-1.17, I2 = 0%), while they were associated with a significant increase in the risk for atrial flutter, equal to 52% (RR = 1.52, 95%CI: 1.03-2.24, I2 = 0%). DPP-4 inhibitors did not have a significant impact on the risk for any of the rest assessed cardiac arrhythmias.
DPP-4 inhibitors do not seem to confer any significant cardiovascular benefit for patients with T2DM, while they do not seem to be associated with a significant risk for any major cardiac arrhythmias, except for atrial flutter. Therefore, this drug class should not be the treatment of choice for patients with established cardiovascular disease or multiple risk factors, except for those cases when newer antidiabetics (glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter-2 inhibitors) are not tolerated, contraindicated or not affordable for the patient.
Core Tip: The antidiabetic efficacy of dipeptidyl peptidase-4 (DPP-4) inhibitors has already been proven in recently published large randomized controlled trials. The purpose of the present meta-analysis was to clarify the impact of antidiabetic therapy with DPP-4 inhibitors on surrogate cardiovascular outcomes, and to elucidate the effect of these drugs on major cardiac arrhythmias. According to our analysis, this drug class does not significantly affect the risk for any of the addressed cardiovascular outcomes; however, it increases the risk for atrial flutter compared to placebo.
- Citation: Patoulias DI, Boulmpou A, Teperikidis E, Katsimardou A, Siskos F, Doumas M, Papadopoulos CE, Vassilikos V. Cardiovascular efficacy and safety of dipeptidyl peptidase-4 inhibitors: A meta-analysis of cardiovascular outcome trials. World J Cardiol 2021; 13(10): 585-592
- URL: https://www.wjgnet.com/1949-8462/full/v13/i10/585.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i10.585
It is well-established that type 2 diabetes mellitus (T2DM) represents an independent risk factor for the development of cardiovascular disease, which accounts for half of deaths among diabetic patients[1]. Patients with T2DM experience higher incidence of vascular interventions compared to high-risk patients without T2DM or cardio
Dipeptidyl peptidase-4 (DPP-4) inhibitors constitute a safe treatment option with adequate glycemic efficacy in T2DM. However, their cardiovascular efficacy has been doubted over recent years, after the publication of relevant cardiovascular outcome trials. Previous meta-analyses failed to show any cardiovascular benefit with their use in patients with T2DM[5-7]. Since then, additional randomized controlled trials addressing “hard” cardiovascular outcomes with DPP-4 inhibitors have been published. Therefore, we sought to update and extend these meta-analyses, by incorporating all relevant data from published cardiovascular outcome trials until October 2020. In addition, we planned to assess the effect of DPP-4 inhibitors on major cardiac arrhythmias, since there are no relevant studies published in the literature so far.
Our meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. We searched PubMed database and grey literature sources form inception to October 2020, in order to identify relevant cardiovascular outcome trials assessing the cardiovascular efficacy and safety of DPP-4 inhibitors in patients with T2DM. Our inclusion criteria were: (1) Randomized controlled trials; (2) Enrollment of patients with T2DM; (3) Enrollment of adult patients; and (4) Assessment of at least one cardiovascular outcome of interest. Our exclusion criteria were: (1) Observational studies; (2) Studies enrolling patients with type 1 diabetes mellitus; and (3) Studies enrolling children or adolescents.
We utilized the following search terms: “DPP-4 inhibitor”, “dipeptidyl peptidase-4 inhibitor”, “vildagliptin”, “sitagliptin”, “alogliptin”, “linagliptin”, “saxagliptin”, “omarigliptin”, “tenegliptin”, “evogliptin”, “gliptin”, “cardiovascular outcome”, “cardiac arrhythmia”, “atrial fibrillation” combined with the use of Boolean operators “AND” and “OR”. We used both free-text words and MeSH terms. We did not imply any filter regarding study setting, study sample, language or publication date. Unfortunately, we did not registered prospectively our protocol in a publicly available repository.
After de-duplication and assessment of eligible studies at title and abstract level for potential inclusion, two independent reviewers (D.P. and E.T.) extracted the data from the eligible reports, by using a pilot tested, data extraction form. We assessed the following cardiovascular efficacy outcomes: fatal and non-fatal myocardial infarction, fatal and non-fatal stroke, hospitalization for heart failure, hospitalization for unstable angina, hospitalization for coronary revascularization and cardiovascular death. We also assessed the risk for the following cardiac arrhythmias with DPP-4 inhibitor treatment compared to placebo or active comparator: atrial fibrillation, atrial flutter, atrial tachycardia, ventricular fibrillation, ventricular tachycardia, ventricular extrasystoles, supraventricular tachycardia, sinus node dysfunction, second degree atrioventricular block, complete atrioventricular block.
As we assessed only dichotomous variables, differences were calculated with the use of risk ratio (RR), with 95% confidence interval (CI), after implementation of the Mantel-Haenszel random effects formula. Statistical heterogeneity among studies was assessed by using I2 statistics. Heterogeneity was considered to be low if I² was between 0% and 25%, moderate if I² was between 25% and 50%, or high if I² was greater than 75%[8]. All analyses were performed at the 0.05 significance level, while they were undertaken with RevMan 5.3 software.
Two independent reviewers (D.P. and A.B.) assessed the quality of the included RCTs, by using the Revised Cochrane risk of bias tool for randomized trials (RoB 2.0) for the primary efficacy outcome[9]. Discrepancies between reviewers were solved by discussion, consensus or arbitration by a third senior reviewer (V.V.).
We finally pooled data from six trials in a total of 52520 patients[10-15]. Overall risk of bias was considered as low across all selected trials.
DPP-4 inhibitor treatment did not significantly affect any of the prespecified cardiovascular efficacy outcomes. More specifically, DPP-4 inhibitors compared to control led to a non-significant increase in the risk for fatal and non-fatal myocardial infarction (RR = 1.02, 95%CI: 0.94-1.11, I2 = 0%), hospitalization for heart failure (RR = 1.09, 95%CI: 0.92-1.29, I2 = 65%) and cardiovascular death (RR = 1.02, 95%CI: 0.93-1.11, I2 = 0%), as shown in Figures 1A, 1C and 1F. In addition, DPP-4 inhibitors produced a non-significant decrease in the risk for fatal and non-fatal stroke (RR = 0.96, 95%CI: 0.85-1.08, I2 = 0%) and coronary revascularization (RR = 0.99, 95%CI: 0.90-1.09, I2 = 0%), as depicted in Figures 1B and 1E. Finally, DPP-4 inhibitors demonstrated a neutral effect on the risk for hospitalization due to unstable angina (RR = 1.00, 95%CI: 0.85-1.18, I2 = 0%), as shown in Figure 1D.
Regarding the risk for major cardiac arrhythmias, DPP-4 inhibitor treatment did not significantly affect the risk for atrial fibrillation (RR = 0.95, 95%CI: 0.78-1.17, I2 = 0%), as shown in Supplementary Figure 1A. Of note, DPP-4 inhibitors were associated with a significant increase in the risk for atrial flutter, equal to 52% (RR = 1.52, 95%CI: 1.03-2.24, I2 = 0%), as shown in Supplementary Figure 1B. Finally, DPP-4 inhibitors did not have a significant impact on the risk for any of the rest assessed major cardiac arrhythmias, as depicted in Supplementary Figures 1C-J.
To our knowledge, this is the first meta-analysis of recently published, large, placebo-controlled cardiovascular outcome trials broadly assessing the cardiovascular efficacy and safety of DPP-4 inhibitors in T2DM. Our meta-analysis demonstrates a rather neutral effect of DPP-4 inhibitors on the risk for myocardial infarction, hospitalization for heart failure, stroke, urgent coronary revascularization and cardiovascular death; in parallel, we highlighted the absence of a significant effect of DPP-4 inhibitors on different types of cardiac arrhythmias, except for atrial flutter, for which corresponding risk increased by 52% compared to placebo. Our results are in accordance with previous meta-analyses in the field[6,16]; nevertheless, the impact of DPP-4 inhibitors on the arrhythmic burden across patients with T2DM has not been previously evaluated.
To date, a series of previous reports have indicated some cardioprotective effects of antidiabetic treatment with DPP-4 inhibitors; these generally safe and well-tolerated regimens have been associated with a significant reduction in blood pressure and with a rather low risk for hypoglycemia compared to other categories of antidiabetic drugs, while they do not increase body weight[17,18]. It has also been shown that they reduce arterial stiffness, whereas no significant effect on endothelial function was documented[19]. Additionally, in animal models, DPP-4 inhibitors have been shown to stabilize cardiac electrophysiology by decreasing the total number of premature ventricular contractions and demonstrating an antiapoptotic effect, significantly reducing the infarct size in experimental myocardial ischemia[20,21]. However, the above cardioprotective effects were not clearly translated into clinically significant results in relevant cardiovascular outcome trials and in our meta-analysis, as well.
Of particular interest is the finding of our analysis that DPP-4 inhibitors are associated with a significant increase in the risk for atrial flutter. Underlying pathophysiologic mechanisms remain largely unknown, since there are no relevant published data. However, it is well-established that diabetes mellitus increases the odds for atrial flutter development, almost by two times, as derived from epidemiological data two decades before[22]. In addition, it is known that atrial flutter at baseline is strongly associated with a significant increase in the 10-year risk for myocardial infarction, stroke, heart failure and all-cause death among affected subjects, constituting this arrhythmia as a prognostic marker of future adverse cardiovascular outcomes[23]. Therefore, the observation that DPP-4 inhibitors actually increase the risk for atrial flutter is of utmost importance that may influence decision-making concerning high-risk patients, such as those suffering from T2DM.
The importance of documenting a neutral effect on a surrogate, prespecified endpoint for a drug class is as important as demonstrating a positive or negative effect, since knowledge about the risk to benefit profile of each different class plays a crucial role in decision-making process in daily clinical routine[24]. Furthermore, considering CVOTs demonstrating significant cardiovascular and renal benefits of other classes of glucose-lowering agents, namely sodium glucose cotransporter-2 inhibitors and glucagon-peptide-1 receptor agonists[25,26], in patients with T2DM, it is important that all such information is incorporated into the clinical guidelines, which have already incorporated these results in their latest recommendations[27,28].
In conclusion, DPP-4 inhibitors do not confer any significant cardiovascular benefit for patients with T2DM. In addition, they are not associated with a significant risk for any major cardiac arrhythmias, except for atrial flutter. However, this drug class should not be the treatment of choice for patients with established cardiovascular disease or multiple risk factors, except for those cases when newer antidiabetics (glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter-2 inhibitors) are not tolerated, contraindicated or not affordable for the patient.
Dipeptidyl peptidase-4 (DPP-4) inhibitors are a safe and efficacious treatment option in type 2 diabetes mellitus (T2DM).
Recently, several large, cardiovascular-outcome, randomized controlled trials (RCTs) with DPP-4 inhibitors in patients with T2DM have been published, raising some doubts on the cardiovascular efficacy and safety of this drug class.
Herein the authors provide the most updated and broad relevant meta-analysis by pooling data of interest from the available cardiovascular-outcome RCTs, addressing the cardiovascular efficacy and safety of this drug class.
The authors searched PubMed and grey literature sources for all published RCTs assessing cardiovascular outcomes with DPP-4 inhibitors compared to placebo until October 2020.
Overall, DPP-4 inhibitors seem to have a neutral effect on most surrogate cardio
DPP-4 inhibitors do not provide any clear cardiovascular benefit in patients with T2DM.
Notably, DPP-4 inhibitors are not associated with a significant effect on the risk for major cardiac arrhythmias, except for atrial flutter, increasing the risk by 52% compared to placebo.
DPP-4 inhibitors do not provide any clear cardiovascular benefit in patients with T2DM.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kawanami D, Wierzbicka A S-Editor: Ma YJ L-Editor: A P-Editor: Li JH
| 1. | Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol. 2018;17:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1295] [Cited by in RCA: 1330] [Article Influence: 190.0] [Reference Citation Analysis (2)] |
| 2. | Engelen SE, van der Graaf Y, Stam-Slob MC, Grobbee DE, Cramer MJ, Kappelle LJ, de Borst GJ, Visseren FLJ, Westerink J; SMART study group. Incidence of cardiovascular events and vascular interventions in patients with type 2 diabetes. Int J Cardiol. 2017;248:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (2)] |
| 3. | Einarson TR, Acs A, Ludwig C, Panton UH. Economic Burden of Cardiovascular Disease in Type 2 Diabetes: A Systematic Review. Value Health. 2018;21:881-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 199] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 4. | Grisanti LA. Diabetes and Arrhythmias: Pathophysiology, Mechanisms and Therapeutic Outcomes. Front Physiol. 2018;9:1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 5. | Fei Y, Tsoi MF, Cheung BMY. Cardiovascular outcomes in trials of new antidiabetic drug classes: a network meta-analysis. Cardiovasc Diabetol. 2019;18:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 6. | Sinha B, Ghosal S. Meta-analyses of the effects of DPP-4 inhibitors, SGLT2 inhibitors and GLP1 receptor analogues on cardiovascular death, myocardial infarction, stroke and hospitalization for heart failure. Diabetes Res Clin Pract. 2019;150:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 7. | Alfayez OM, Almutairi AR, Aldosari A, Al Yami MS. Update on Cardiovascular Safety of Incretin-Based Therapy in Adults With Type 2 Diabetes Mellitus: A Meta-Analysis of Cardiovascular Outcome Trials. Can J Diabetes. 2019;43:538-545.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Higgins JPT GS, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Collaboration. Cochrane Database Syst Rev. 2010;6:143-148. |
| 9. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 15045] [Article Influence: 2507.5] [Reference Citation Analysis (0)] |
| 10. | Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, Lachin JM, McGuire DK, Pencina MJ, Standl E, Stein PP, Suryawanshi S, Van de Werf F, Peterson ED, Holman RR; TECOS Study Group. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015;373:232-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1831] [Cited by in RCA: 1873] [Article Influence: 187.3] [Reference Citation Analysis (0)] |
| 11. | Rosenstock J, Kahn SE, Johansen OE, Zinman B, Espeland MA, Woerle HJ, Pfarr E, Keller A, Mattheus M, Baanstra D, Meinicke T, George JT, von Eynatten M, McGuire DK, Marx N; CAROLINA Investigators. Effect of Linagliptin vs Glimepiride on Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes: The CAROLINA Randomized Clinical Trial. JAMA. 2019;322:1155-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 398] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 12. | Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N, Alexander JH, Pencina M, Toto RD, Wanner C, Zinman B, Woerle HJ, Baanstra D, Pfarr E, Schnaidt S, Meinicke T, George JT, von Eynatten M, McGuire DK; CARMELINA Investigators. Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults With Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. JAMA. 2019;321:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 785] [Article Influence: 130.8] [Reference Citation Analysis (0)] |
| 13. | Gantz I, Chen M, Suryawanshi S, Ntabadde C, Shah S, O'Neill EA, Engel SS, Kaufman KD, Lai E. A randomized, placebo-controlled study of the cardiovascular safety of the once-weekly DPP-4 inhibitor omarigliptin in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2017;16:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 14. | Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2804] [Cited by in RCA: 2574] [Article Influence: 214.5] [Reference Citation Analysis (0)] |
| 15. | White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F; EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1927] [Cited by in RCA: 1896] [Article Influence: 158.0] [Reference Citation Analysis (0)] |
| 16. | Liu D, Jin B, Chen W, Yun P. Dipeptidyl peptidase 4 (DPP-4) inhibitors and cardiovascular outcomes in patients with type 2 diabetes mellitus (T2DM): a systematic review and meta-analysis. BMC Pharmacol Toxicol. 2019;20:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Karagiannis T, Paschos P, Paletas K, Matthews DR, Tsapas A. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ. 2012;344:e1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 301] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 18. | Papagianni M, Tziomalos K. Cardiovascular effects of dipeptidyl peptidase-4 inhibitors. Hippokratia. 2015;19:195-199. [PubMed] |
| 19. | Batzias K, Antonopoulos AS, Oikonomou E, Siasos G, Bletsa E, Stampouloglou PK, Mistakidi CV, Noutsou M, Katsiki N, Karopoulos P, Charalambous G, Thanopoulou A, Tentolouris N, Tousoulis D. Effects of Newer Antidiabetic Drugs on Endothelial Function and Arterial Stiffness: A Systematic Review and Meta-Analysis. J Diabetes Res. 2018;2018:1232583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 20. | Chinda K, Palee S, Surinkaew S, Phornphutkul M, Chattipakorn S, Chattipakorn N. Cardioprotective effect of dipeptidyl peptidase-4 inhibitor during ischemia-reperfusion injury. Int J Cardiol. 2013;167:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Kubota A, Takano H, Wang H, Hasegawa H, Tadokoro H, Hirose M, Kobara Y, Yamada-Inagawa T, Komuro I, Kobayashi Y. DPP-4 inhibition has beneficial effects on the heart after myocardial infarction. J Mol Cell Cardiol. 2016;91:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Movahed MR, Hashemzadeh M, Jamal MM. Diabetes mellitus is a strong, independent risk for atrial fibrillation and flutter in addition to other cardiovascular disease. Int J Cardiol. 2005;105:315-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 273] [Article Influence: 13.7] [Reference Citation Analysis (1)] |
| 23. | Rahman F, Wang N, Yin X, Ellinor PT, Lubitz SA, LeLorier PA, McManus DD, Sullivan LM, Seshadri S, Vasan RS, Benjamin EJ, Magnani JW. Atrial flutter: Clinical risk factors and adverse outcomes in the Framingham Heart Study. Heart Rhythm. 2016;13:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Chi C, Snaith J, Gunton JE. Diabetes Medications and Cardiovascular Outcomes in Type 2 Diabetes. Heart Lung Circ. 2017;26:1133-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1634] [Cited by in RCA: 1910] [Article Influence: 318.3] [Reference Citation Analysis (0)] |
| 26. | Giugliano D, Maiorino MI, Bellastella G, Longo M, Chiodini P, Esposito K. GLP-1 receptor agonists for prevention of cardiorenal outcomes in type 2 diabetes: An updated meta-analysis including the REWIND and PIONEER 6 trials. Diabetes Obes Metab. 2019;21:2576-2580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 27. | American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S98-S110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 683] [Article Influence: 136.6] [Reference Citation Analysis (0)] |
| 28. | Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, D'Alessio DA, Davies MJ. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43:487-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 856] [Cited by in RCA: 815] [Article Influence: 163.0] [Reference Citation Analysis (0)] |