Published online Oct 26, 2021. doi: 10.4330/wjc.v13.i10.574
Peer-review started: April 21, 2021
First decision: May 13, 2021
Revised: May 24, 2021
Accepted: July 15, 2021
Article in press: July 15, 2021
Published online: October 26, 2021
Processing time: 182 Days and 21.4 Hours
Radial artery obstruction is the most common complication of coronary angiography performed via transradial access. Patent hemostasis can significantly reduce the risk of radial artery occlusion. Previous studies utilized sophisticated methods to evaluate radial artery patency. Simplified and easily applicable methods for successful patent hemostasis are currently lacking.
To determine which method (pulse oximeter vs the traditional radial artery palpation) is better to achieve patent hemostasis.
This prospective, single center study included 299 consecutive patients who underwent coronary angiography or percutaneous coronary intervention between November 2017 and July 2019. Patients less than 18 years old, with a history of radial artery disease, or no palpable artery pulse were excluded from the study. Patients were randomly assigned to two groups. In the first group, radial artery flow was assessed by palpation of the artery during hemostasis (traditional method). In the second group, radial artery patency was estimated with the use of a pulse oximeter. Two different compression devices were used for hemostasis (air chamber and pressure valve). The primary study endpoint was the achievement of successful patent hemostasis.
The two groups (pulse oximeter vs artery palpation) had no significant differences in age, sex, body mass index, risk factors, or comorbidities except for supraven
Patent hemostasis with the use of pulse oximeter is a simple, efficient, and safe method that is worthy of further investigation. Larger randomized studies are required to consider its clinical implications.
Core Tip: This was a prospective, single center study with 299 consecutive patients who underwent coronary angiography or percutaneous coronary intervention. It aimed to evaluate the best method (pulse oximeter vs the traditional radial artery palpation) for successful patent hemostasis. The use of a pulse oximeter increased the probability of achieving patent hemostasis compared with artery palpation, and was associated with lower rates of artery spasm. In the multivariate analysis, the use of pulse oximeter and advanced age were independently associated with an increased probability of successful patent hemostasis.
- Citation: Kyriakopoulos V, Xanthopoulos A, Papamichalis M, Skoularigkis S, Tzavara C, Papadakis E, Patsilinakos S, Triposkiadis F, Skoularigis J. Patent hemostasis of radial artery: Comparison of two methods. World J Cardiol 2021; 13(10): 574-584
- URL: https://www.wjgnet.com/1949-8462/full/v13/i10/574.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i10.574
Transradial access is increasingly used in coronary angiography vs transfemoral access as it has several advantages. Transradial access is associated with fewer vascular complications, lower bleeding complications, and reduced mortality in specific high-risk populations[1]. Furthermore, the technique offers earlier mobilization after the procedure, and the patient usually has a shorter hospital stay. The European guidelines for coronary angiography in patients with acute coronary syndrome favor transradial over transfemoral access with a Class IA indication[2,3].
Radial artery obstruction (RAO) is a frequent complication of coronary angiography performed via transradial access[4]. RAO may prevent radial artery access for future coronary angiography or as a conduit for coronary artery bypass grafting. At the same time, patients requiring hemodialysis lose an artery that can be used to create an arteriovenous fistula. Therefore, prevention of RAO is of particular clinical importance in patients undergoing coronary angiography via transradial access. Experts emphasize the need for the adoption of novel techniques that may reduce the incidence of RAO to less than 5%[5].
Complete obstruction of blood flow in the radial artery during hemostasis is a strong predictor of RAO occurrence[6]. On the contrary, maintaining circulatory anterograde flow in the radial artery during hemostasis, known as patent or nonobstructive hemostasis, reduces the risk of RAO[7,8]. Various methods of patent hemostasis have been described, but there is no current consensus on the optimal method. Of note, the application of nonobstructive hemostasis is technically difficult as it requires intense staff mobilization, multiple evaluations of radial artery blood flow, and adjustment of hemostatic pressure in order to maintain patency. That is why patent hemostasis is not fully adopted in most laboratories[9]. The goal of this study was to evaluate a simplified and easily applicable method to achieve patent hemostasis in patients undergoing diagnostic coronary angiography or percutaneous coronary intervention (PCI).
A total of 299 consecutive patients undergoing cardiac catheterization between November 2017 and July 2019 and considered eligible for radial access were included in the study. Patients underwent a Barbeau test precatheterization, to assess collateral palmar arch sufficiency. Patients were randomly assigned to two groups. In the first group, radial artery flow was assessed by artery pulse palpation during hemostasis (traditional method). In the second group, radial artery patency was estimated with the use of a pulse oximeter. Two different compression devices were used for hemostasis, one with an air chamber and another with a pressure valve. The primary study endpoint was the achievement of successful patent hemostasis.
Randomization did not affect coronary angiography, either diagnostic or invasive, and operators were unaware of the patient allocation. RAO was assessed at 24 h and 30 d after the procedure. Patients younger than 18 years of age with a history of radial artery disease or absence of radial artery pulse were excluded from the study. Patients participating in the study provided written informed consent, and the study protocol was approved by the hospital’s ethics review board.
Radial artery catheterization was performed using the Seldinger technique. The catheter diameter was 5/6-French.
The introducer sheath was removed immediately after the procedure. The sheath was pulled out by 4 to 5 cm and a hemostatic bandage was applied around the wrist. The bandage was then tightened and the catheter was removed. In group 1, radial artery patency was assessed by radial artery pulse palpation. The bandage remained in place for 4 h and then was slowly removed. A light dressing was applied at the entry site after the procedure.
The sheath was pulled out by 4 to 5 cm and a hemostatic bandage was applied over the entry site. In group 2 a pulse oximeter sensor was placed on the index finger, the bandage was tightened, and the sheath was removed. The ulnar artery was compressed and the hemostatic bandage gradually began to relax. Radial artery patency was confirmed by plethysmographic signal reoccurrence. In case of bleeding before plethysmographic signal appearance, the hemostatic bandage compression was increased. If radial artery flow was confirmed by the oximeter and no bleeding complications occurred, then a bandage remained in place for 4 h. Radial artery patency was assessed on an hourly basis.
Radial artery flow was evaluated with a Barbeau test. The pulse oximeter sensor was placed on the index finger and the plethysmographic signal was observed. Ulnar and radial artery compression led to signal loss. Radial artery pressure was then removed while maintaining ulnar artery compression. Appearance of the plethysmographic signal was proof of radial artery patency, while absence of a signal indicated RAO. The test was performed precatheterization, at 24 h and at 30 d after coronary angiography. Radial artery patency was also assessed at 30 d by vascular ultrasonography with Doppler assessment.
Hemorrhagic complications that resulted in blood loss from the puncture site and judged capable of causing hemodynamic instability, blood transfusion, or death were regarded as significant. Hematomas at the puncture site were considered clinically significant when their diameter exceeded 3 cm.
Quantitative variables were expressed as means ± SD or as medians and interquartile range (IQR). Qualitative variables were reported with absolute and relative frequencies. Chi-square and Fisher’s exact tests were used to compare proportions. Student’s t-tests were used to compare mean values that were normally distributed. Mann-Whitney tests were used to compare median values when the distribution was not normal. Logistic regression analyses in a stepwise method (P for entry 0.05, P for removal 0.10) were performed in order to identify factors associated with the presence of specific outcomes. Unadjusted and adjusted odds ratios with 95%CI were computed from the results of the logistic regression analyses. Statistical significance was set at 0.05. The analyses were conducted using SPSS statistical software (version 22.0).
Radial artery patency during hemostasis was assessed by artery palpation (control group) in 147 patients (49.2%) and by pulse oximeter sensor in 152 patients (50.8%). The demographic characteristics of the study population are shown in Table 1. The study population consisted mainly of men (75%) with a mean age of 60.8 years. Dyslipidemia was the most common comorbidity followed by hypertension, coronary artery disease, and diabetes mellitus. One out of three patients (35%) had previously undergone PCIs and 6% had previously undergone coronary bypass surgery. The two groups of patients did not have significant differences in their baseline clinical characteristics (Table 1). Patients in the control group had a higher rate of supraventricular arrhythmia, mainly atrial fibrillation (26.5% vs 13.2%, P = 0.004).
| Baseline characteristics | Control group (conventional hemostasis), n = 147 | Oximetry – plethysmography group, n = 152 | P value |
| Age (mean ± SD, yr) | 61.5 ± 9.8 | 60.1 ± 11.6 | 0.2731 |
| Male sex | 109 (74.1) | 115 (75.7) | 0.7642 |
| Body mass index (mean ± SD, kg/m2) | 0.3432 | ||
| Normal (18.5-24.9) | 31 ± 21.1 | 35 ± 23 | |
| Overweight (25-29.9) | 73 ± 49.7 | 63 ± 41.4 | |
| Obese (> 30) | 43 ± 29.3 | 54 ± 35.5 | |
| Risk factors/Comorbidities, n (%) | |||
| Hypertension | 89 (60.5) | 92 (60.5) | 0.9972 |
| Diabetes mellitus | 29 (19.7) | 40 (26.3) | 0.1762 |
| Insulin | 6 (20.7) | 14 (35.0) | 0.1963 |
| Dyslipidemia | 112 (76.2) | 114 (75.0) | 0.8112 |
| Smoking | 69 (46.9) | 74 (48.7) | 0.8502 |
| History of coronary artery disease | 46 (31.3) | 52 (34.2) | 0.5912 |
| Supraventricular arrhythmia | 39 (26.5) | 20 (13.2) | 0.0042 |
| History of interventions, n (%) | |||
| Percutaneous coronary intervention | 49 (33.3) | 56 (36.8) | 0.5252 |
| Coronary artery bypass grafting | 11 (7.5) | 7 (4.6) | 0.2963 |
Table 2 shows the procedural characteristics of the two study groups. PCI was performed in 30% of the patients. There were no differences in the number of coronary vessels that received intervention. Half the patients underwent coronary angiography using a 5-french introducer sheath and the other half using a 6-french sheath. The two groups of patients did not differ in several other procedural characteristics (e.g., right or left hand, duration of procedure, radiation time). Patients received similar doses of anticoagulants (heparin) and did not differ in the type of device used for hemostasis (Table 2).
| Procedural data | Control group (conventional hemostasis) (n = 147) | Oximetry – plethysmography group (n = 152) | P value |
| PCI, n (%) | 44 (29.9) | 58 (38.2) | 0.1341 |
| Primary PCI, n (%) | 12 (8.2) | 15 (9.9) | 0.6071 |
| Heparin dose, median (IQR) | 5000 (5000-7000) | 5000 (5000-7000) | 0.1132 |
| INR, mean ± SD | 1.1 ± 0.3) | 1.1 ± 0.3) | 0.9583 |
| Significant coronary artery lesions, n (%) | |||
| Left anterior descending artery | 32 (21.8) | 36 (23.7) | 0.6931 |
| Circumflex | 10 (6.8) | 12 (7.9) | 0.7181 |
| Right coronary artery | 17 (11.6) | 20 (13.2) | 0.6761 |
| Number of vessels4, n (%) | 0.7073 | ||
| PCI in 1 vessel (%) | 35 (81.4) | 50 (87.7) | |
| PCI >1 vessels (%) | 8 (18.6) | 7 (12.3) | |
| Hemostatic device | 0.2231 | ||
| Air chamber, n (%) | 80 (54.4) | 72 (47.4) | |
| Valve with pressure plate, n (%) | 67 (45.6) | 80 (52.6) | |
| Right hand, n (%) | 104 (70.7) | 115 (75.7) | 0.3621 |
| Left hand, n (%) | 43 (29.3) | 37 (24.3) | |
| Puncture attempts, median (IQR) | 1 (1-1) | 1 (1-1) | 0.3542 |
| Puncture duration (min), median (IQR) | 2.25 (1.42-3.3) | 2.22 (1.44-3.37) | 0.6602 |
| Procedure time (min), median (IQR) | 13 (8.4-27.3) | 13.8 (9.2-26.9) | 0.4482 |
| Fluoro time (min), median (IQR) | 3.1 (1.3-9.1) | 3.4 (1.4-7.7) | 0.6632 |
| Sheath, n (%) | 0.2573 | ||
| 5F | 74 (50.3) | 70 (46.1) | |
| 6F | 73 (49.7) | 79 (52.0) | |
| 7F | 0 (0.0) | 3 (2.0) | |
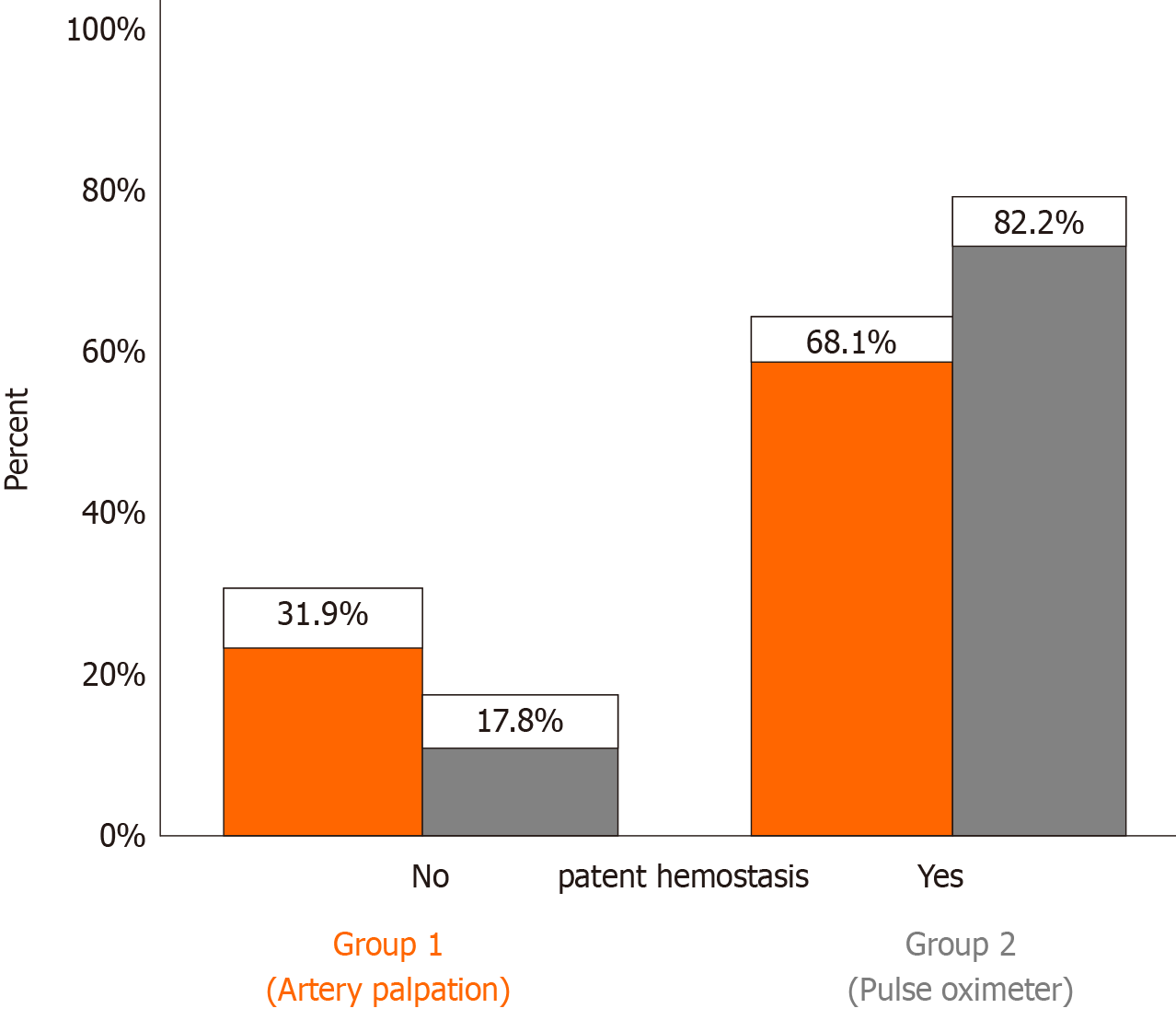
| Patent hemostasis, n (%) | 94 (68.1) | 125 (82.2) | 0.0051 |
Table 3 shows the coronary angiography complications. The group of patients in whom the radial artery patency was assessed with the traditional method (artery palpation) had a higher rate of radial artery spasm (P = 0.024). The two groups had similar rates of vagotonia, hematoma, bleeding, edema, local complications, and pain.
| Coronary angiography complications | Control group (Conventional Hemostasis) | Oximetry – plethysmography group | P value |
| Spasm | 28 (19.0) | 15 (9.9) | 0.0241 |
| Vagotonia | 24 (16.3) | 23 (15.1) | 0.7771 |
| Hematoma | 25 (17.0) | 15 (9.9) | 0.0701 |
| Hematoma diameter, median value (IQR) | 0 (0 - 3) | 0 (0 - 3) | 0.4622 |
| Bleeding | 7 (4.8) | 5 (3.3) | 0.5171 |
| Edema | 26 (17.7) | 29 (19.1) | 0.7561 |
| Local complication | 18 (12.2) | 24 (15.8) | 0.3781 |
| Pain | 28 (19.0) | 22 (14.4) | 0.2891 |
The group of patients whose radial artery patency was assessed using the pulse oximeter achieved significantly higher rates of patent hemostasis than those in the control group, using radial artery palpation (82.2% vs 68.1%, P = 0.005; Figure 1). The type of hemostatic device (air chamber or pressure valve device) did not affect patent hemostasis (P = 0.450). Radial artery flow was restored in a significant percentage of patients at 24 h and at 30 d after coronary angiography (Table 4).
Multivariate logistic regression analysis revealed that pulse oximeter use (OR: 2.35, 95%CI: 1.34-4.13, P = 0.003) and patient age (per 1 year increase; OR: 1.04, 95%CI: 1.01-1.07, P = 0.006) as independent predictors of patent hemostasis (Table 5).
| OR (95%CI) | Ρ value | |
| Patent hemostasis | ||
| Age (per 1 yr increase) | 1.04 (1.01-1.07) | 0.006 |
| Control group (reference) | ||
| Oximetry-plethysmography group | 2.35 (1.34-4.13) | 0.003 |
The main study findings were: (1) Successful patent hemostasis was significantly more frequent in the pulse oximeter group vs the radial artery palpation group; (2) A lower percentage of complications (i.e. spasm) was recorded in the pulse oximeter group; and (3) Advanced age and the use of a pulse oximeter were independent predictors of successful patent hemostasis.
Many studies have reported the safety and efficacy of performing coronary angiography via the transradial access. Transradial access is preferred over trans
RAO is a potential complication of coronary angiography using the transradial approach[5,10]. In randomized trials, RAO incidence ranged up to 10%[5]. However, in daily clinical practice RAO frequency is much higher[4,14-16]. Radial artery patency should be routinely checked before discharge of any patient who has undergone coronary angiography via transradial access[5]. Radial artery palpation is the most common technique used[17]. However, artery palpation may be misleading as the presence of collateral circulation from palmar arches in the upper extremity is likely to lead to a palpable pulse from the distal stump even in the presence of RAO[5]. RAO is more common at the end of hemostasis and thereafter gradually decreases in the first 24 h and even further in the 30 d after the procedure. In a meta-analysis of 112 studies including 46.631 patients, late revascularization occurred in a significant proportion of patients with RAO[18]. In this study, radial artery flow was restored in a significant percentage of patients at 24 h and at 30 d after coronary angiography.
Measures to reduce RAO incidence include smaller catheters, adequate anticoagulation, the adoption of patent hemostasis strategies with or without ulnar artery compression, and the reduction of hemostasis time to ≤ 120 min[5,16,18-24]. Patent hemostasis is the technique of maintaining radial artery forward flow through guided artery compression during hemostasis after coronary angiography[7]. In patients undergoing coronary angiography, complete absence of radial artery flow during hemostatic compression is a strong predictor of RAO[6,24]. On the contrary, maintaining radial artery antegrade flow during hemostasis, known as patent or nonocclusive hemostasis, is an important factor in preventing RAO, but its complexity has limited adoption[5,7,25]. Maintaining radial artery antegrade flow during hemostatic compression constitutes part of the recommended best practice after transradial access for coronary angiography[25].
The best technique to achieve patent hemostasis is a subject of ongoing research. Previous studies utilized relatively sophisticated methods to evaluate radial artery patency. In the landmark prevention of radial artery occlusion-patent hemostasis evaluation trial (the PROPHET study), 436 patients were randomized to undergo either conventional hemostasis or patent hemostasis after diagnostic coronary angiography via the transradial approach. Twelve percent of patients who underwent conventional hemostasis experienced RAO at 24 h. The corresponding rate for patients in the patent hemostasis group was 5%[7]. The use of an oximetry-plethysmography test was the strongest predictor of achieving patent hemostasis. In the prophylactic hyperperfusion evaluation trial (PROPHET-II), ipsilateral ulnar artery compression during radial artery hemostatic compression increased the rate of patent hemostasis and reduced the incidence of RAO from 3.0% to 0.9%[25]. In the randomized radial compression guided by mean artery pressure vs standard compression with a pneumatic device (RACOMAP) trial, a significant reduction in RAO rates from 12.0% to 1.1% was observed in patients following the patent hemostasis protocol compared with traditional arterial obstructive compression[8]. In the RACOMAP trial, nonobstructive hemostasis was performed by compressing the radial artery during hemostasis, guided by the mean blood pressure[8].
In a study by Edris et al[26], patent hemostasis was achieved with rapid deflation of the compression band. The technique increased patent hemostasis rates from 40% to 95% and reduced RAO rates from 14.9% to 2.0% without bleeding complications. A study comparing nonobstructive hemostasis to conventional hemostasis reported reduced RAO at 24 h in the patent hemostasis group, whereas the difference at 7 d between the two groups was not statistically significant[27]. The result is similar to that observed in this study in which RAO rates at 30 d did not differ between the two groups. The rates of patent hemostasis in this study are similar to those in previous studies (68.1% to 82.2%)[7,24]. Furthermore, in previous studies, nonocclusive hemostasis did not increase bleeding complications compared with conventional hemostasis[7,8]. Plethysmographic evaluation of radial artery flow allows easier achievement of patent hemostasis without adversely affecting the method safety. Similarly, in the present study, no difference was observed in the hemorrhagic events that occurred in the two treatment groups. Interestingly, in the current study, manual compression was not required to achieve hemostasis in the pulse oximeter group. In the PROPHET study manual compression was required in a small percentage of patients (3.6%) to achieve hemostasis[7]. Lastly, the rates of spasm in our study, which were lower in the oximeter vs the artery palpation group, were in accord with those reported in the literature[4].
In this study, we observed an association between increased age and successful patent hemostasis. Several speculations can be made regarding that finding. Firstly, radial artery spasm is more frequent in younger than in older patients undergoing PCI via radial access and therefore older adults are more likely to have a successful patent hemostasis[28-31]. Secondly, increased arterial stiffness in elderly patients produces a steeper increase in radial artery flow, resulting in reopening of the occlusion in the early period and maintaining vessel patency in the long-term[32]. Lastly, increased arterial stiffness in older patients may preclude the total interruption of flow during manual compression and therefore facilitate patent hemostasis[32].
The current study has several limitations that need to be addressed. Firstly, the study population was not large, but it was comparable to previous studies in the field. Secondly, at 30 d, radial artery patency was assessed with duplex ultrasonography in 204 out of 299 patients. We performed a telephone follow-up of the patients who did not return at 30 d. The three main reasons cited for follow-up interruptions were lack of understanding regarding the necessity of follow-up, social reasons (e.g., distant hometown, financial barriers, relocation) and unawareness of the appointment schedule. Nevertheless, radial artery flow was restored in a significant percentage of patients who presented at follow-up, which is in accord with the current literature. Thirdly, patent hemostasis achieved in the current study by the use of pulse oximetry is relatively more simple than the techniques described in previous studies, and can be more widely implemented in everyday clinical practice.
Oximetry-plethysmography is an efficient and safe method to achieve patent hemostasis after coronary angiography via transradial access. Larger randomized control trials are urgently needed.
Radial artery obstruction is a frequent complication of coronary angiography performed via transradial access. Maintaining circulatory anterograde flow in the radial artery during hemostasis (patent or nonobstructive hemostasis) reduces the risk of radial artery obstruction.
Simplified and easily applicable methods for successful patent hemostasis are currently lacking.
To determine which method, pulse oximeter vs the traditional radial artery palpation, is better to achieve patent hemostasis.
This a prospective, single center study included 299 consecutive patients who underwent coronary angiography or percutaneous coronary intervention between November 2017 and July 2019. The exclusion criteria were: (1) Age of < 18 years; (2) History of radial artery disease; and (3) No palpable arterial pulse. Patients were randomly assigned to two groups. In the first group, radial artery flow was assessed by palpation of the artery during hemostasis (traditional method). In the second group, radial artery patency was estimated with a pulse oximeter. Two different compression devices were used for hemostasis (air chamber and pressure valve). The primary study endpoint was the successful achievement of patent hemostasis.
The two groups (pulse oximeter vs artery palpation) had no significant differences in age, sex, body mass index, risk factors, or comorbidities except for supraventricular arrhythmias. The percentage of patients with successful patent hemostasis was significantly higher in the pulse oximeter group (82.2% vs 68.1%, P = 0.005). A lower percentage of patients with spasm was recorded in the pulse oximeter group (9.9% vs 19.0%, P = 0.024). Multivariate analysis found that the use of pulse oximeter (OR: 2.35, 95%CI: 1.34-4.13, P = 0.003) and advanced age (OR: 1.04, 95%CI: 1.01-1.07, P = 0.006), were independently associated with an increased probability of successful patent hemostasis. The type of hemostatic device did not affect patent hemostasis (P = 0.450).
Patent hemostasis with the use of pulse oximeter is a simple, efficient, and safe method, and is worthy of further investigation.
Larger randomized studies are required to consider its clinical implications.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ito S S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Ferrante G, Rao SV, Jüni P, Da Costa BR, Reimers B, Condorelli G, Anzuini A, Jolly SS, Bertrand OF, Krucoff MW, Windecker S, Valgimigli M. Radial Versus Femoral Access for Coronary Interventions Across the Entire Spectrum of Patients With Coronary Artery Disease: A Meta-Analysis of Randomized Trials. JACC Cardiovasc Interv. 2016;9:1419-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 354] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 2. | Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM; ESC Scientific Document Group. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2569] [Cited by in RCA: 3076] [Article Influence: 769.0] [Reference Citation Analysis (0)] |
| 3. | Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7073] [Cited by in RCA: 6548] [Article Influence: 935.4] [Reference Citation Analysis (0)] |
| 4. | Coghill EM, Johnson T, Morris RE, Megson IL, Leslie SJ. Radial artery access site complications during cardiac procedures, clinical implications and potential solutions: The role of nitric oxide. World J Cardiol. 2020;12:26-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Bernat I, Aminian A, Pancholy S, Mamas M, Gaudino M, Nolan J, Gilchrist IC, Saito S, Hahalis GN, Ziakas A, Louvard Y, Montalescot G, Sgueglia GA, van Leeuwen MAH, Babunashvili AM, Valgimigli M, Rao SV, Bertrand OF; RAO International Group. Best Practices for the Prevention of Radial Artery Occlusion After Transradial Diagnostic Angiography and Intervention: An International Consensus Paper. JACC Cardiovasc Interv. 2019;12:2235-2246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Sanmartin M, Gomez M, Rumoroso JR, Sadaba M, Martinez M, Baz JA, Iniguez A. Interruption of blood flow during compression and radial artery occlusion after transradial catheterization. Catheter Cardiovasc Interv. 2007;70:185-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Pancholy S, Coppola J, Patel T, Roke-Thomas M. Prevention of radial artery occlusion-patent hemostasis evaluation trial (PROPHET study): a randomized comparison of traditional vs patency documented hemostasis after transradial catheterization. Catheter Cardiovasc Interv. 2008;72:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 367] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 8. | Cubero JM, Lombardo J, Pedrosa C, Diaz-Bejarano D, Sanchez B, Fernandez V, Gomez C, Vazquez R, Molano FJ, Pastor LF. Radial compression guided by mean artery pressure vs standard compression with a pneumatic device (RACOMAP). Catheter Cardiovasc Interv. 2009;73:467-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Shroff AR, Fernandez C, Vidovich MI, Rao SV, Cowley M, Bertrand OF, Patel TM, Pancholy SB. Contemporary transradial access practices: Results of the second international survey. Catheter Cardiovasc Interv. 2019;93:1276-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Aldoori JS, Mohammed AI. Transradial approach for coronary angiography and percutaneos coronary intervention: personal experience. Egypt Heart J. 2019;71:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (2)] |
| 11. | Di Santo P, Simard T, Wells GA, Jung RG, Ramirez FD, Boland P, Marbach JA, Parlow S, Kyeremanteng K, Coyle D, Fergusson D, Russo JJ, Chong AY, Froeschl M, So DY, Dick A, Glover C, Labinaz M, Hibbert B, Le May M. Transradial Versus Transfemoral Access for Percutaneous Coronary Intervention in ST-Segment-Elevation Myocardial Infarction: A Systematic Review and Meta-Analysis. Circ Cardiovasc Interv. 2021;14:e009994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Agostoni P, Biondi-Zoccai GG, de Benedictis ML, Rigattieri S, Turri M, Anselmi M, Vassanelli C, Zardini P, Louvard Y, Hamon M. Radial vs femoral approach for percutaneous coronary diagnostic and interventional procedures; Systematic overview and meta-analysis of randomized trials. J Am Coll Cardiol. 2004;44:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 710] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 13. | Spaulding C, Lefèvre T, Funck F, Thébault B, Chauveau M, Ben Hamda K, Chalet Y, Monségu H, Tsocanakis O, Py A, Guillard N, Weber S. Left radial approach for coronary angiography: results of a prospective study. Cathet Cardiovasc Diagn. 1996;39:365-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Uhlemann M, Möbius-Winkler S, Mende M, Eitel I, Fuernau G, Sandri M, Adams V, Thiele H, Linke A, Schuler G, Gielen S. The Leipzig prospective vascular ultrasound registry in radial artery catheterization: impact of sheath size on vascular complications. JACC Cardiovasc Interv. 2012;5:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 188] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 15. | Rao SV. Observations from a transradial registry: our remedies oft in ourselves do lie. JACC Cardiovasc Interv. 2012;5:44-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Rashid M, Kwok CS, Pancholy S, Chugh S, Kedev SA, Bernat I, Ratib K, Large A, Fraser D, Nolan J, Mamas MA. Radial Artery Occlusion After Transradial Interventions: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 260] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 17. | Bertrand OF, Rao SV, Pancholy S, Jolly SS, Rodés-Cabau J, Larose E, Costerousse O, Hamon M, Mann T. Transradial approach for coronary angiography and interventions: results of the first international transradial practice survey. JACC Cardiovasc Interv. 2010;3:1022-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 300] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 18. | Hahalis G, Aznaouridis K, Tsigkas G, Davlouros P, Xanthopoulou I, Koutsogiannis N, Koniari I, Leopoulou M, Costerousse O, Tousoulis D, Bertrand OF. Radial Artery and Ulnar Artery Occlusions Following Coronary Procedures and the Impact of Anticoagulation: ARTEMIS (Radial and Ulnar ARTEry Occlusion Meta-AnalysIS) Systematic Review and Meta-Analysis. J Am Heart Assoc. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Pacchioni A, Bellamoli M, Mugnolo A, Ferro J, Pesarini G, Turri R, Ribichini F, Saccà S, Versaci F, Reimers B. Predictors of patent and occlusive hemostasis after transradial coronary procedures. Catheter Cardiovasc Interv. 2021;97:1369-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Hahalis GN, Leopoulou M, Tsigkas G, Xanthopoulou I, Patsilinakos S, Patsourakos NG, Ziakas A, Kafkas N, Koutouzis M, Tsiafoutis I, Athanasiadis I, Koniari I, Almpanis G, Anastasopoulou M, Despotopoulos S, Kounis N, Dapergola A, Aznaouridis K, Davlouros P. Multicenter Randomized Evaluation of High Versus Standard Heparin Dose on Incident Radial Arterial Occlusion After Transradial Coronary Angiography: The SPIRIT OF ARTEMIS Study. JACC Cardiovasc Interv. 2018;11:2241-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Pancholy SB. Comparison of the effect of intra-arterial vs intravenous heparin on radial artery occlusion after transradial catheterization. Am J Cardiol. 2009;104:1083-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Pancholy SB, Ahmed I, Bertrand OF, Patel T. Frequency of radial artery occlusion after transradial access in patients receiving warfarin therapy and undergoing coronary angiography. Am J Cardiol. 2014;113:211-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Plante S, Cantor WJ, Goldman L, Miner S, Quesnelle A, Ganapathy A, Popel A, Bertrand OF. Comparison of bivalirudin vs heparin on radial artery occlusion after transradial catheterization. Catheter Cardiovasc Interv. 2010;76:654-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Pancholy SB, Bertrand OF, Patel T. Comparison of a priori vs provisional heparin therapy on radial artery occlusion after transradial coronary angiography and patent hemostasis (from the PHARAOH Study). Am J Cardiol. 2012;110:173-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Pancholy SB, Bernat I, Bertrand OF, Patel TM. Prevention of Radial Artery Occlusion After Transradial Catheterization: The PROPHET-II Randomized Trial. JACC Cardiovasc Interv. 2016;9:1992-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 151] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 26. | Edris A, Gordin J, Sallam T, Wachsner R, Meymandi S, Traina M. Facilitated patent haemostasis after transradial catheterisation to reduce radial artery occlusion. EuroIntervention. 2015;11:765-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Roghani F, Tajik MN, Khosravi A. Compare Complication of Classic vs Patent Hemostasis in Transradial Coronary Angiography. Adv Biomed Res. 2017;6:159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Varenne O, Jégou A, Cohen R, Empana JP, Salengro E, Ohanessian A, Gaultier C, Allouch P, Walspurger S, Margot O, El Hallack A, Jouven X, Weber S, Spaulding C. Prevention of arterial spasm during percutaneous coronary interventions through radial artery: the SPASM study. Catheter Cardiovasc Interv. 2006;68:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 29. | Jia DA, Zhou YJ, Shi DM, Liu YY, Wang JL, Liu XL, Wang ZJ, Yang SW, Ge HL, Hu B, Yan ZX, Chen Y, Gao F. Incidence and predictors of radial artery spasm during transradial coronary angiography and intervention. Chin Med J (Engl). 2010;123:843-847. [PubMed] |
| 30. | Rathore S, Stables RH, Pauriah M, Hakeem A, Mills JD, Palmer ND, Perry RA, Morris JL. Impact of length and hydrophilic coating of the introducer sheath on radial artery spasm during transradial coronary intervention: a randomized study. JACC Cardiovasc Interv. 2010;3:475-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 31. | Ho HH, Jafary FH, Ong PJ. Radial artery spasm during transradial cardiac catheterization and percutaneous coronary intervention: incidence, predisposing factors, prevention, and management. Cardiovasc Revasc Med. 2012;13:193-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Buturak A, Gorgulu S, Norgaz T, Voyvoda N, Sahingoz Y, Degirmencioglu A, Dagdelen S. The long-term incidence and predictors of radial artery occlusion following a transradial coronary procedure. Cardiol J. 2014;21:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |