Published online Oct 26, 2021. doi: 10.4330/wjc.v13.i10.526
Peer-review started: April 1, 2021
First decision: July 6, 2021
Revised: July 12, 2021
Accepted: September 8, 2021
Article in press: September 8, 2021
Published online: October 26, 2021
Processing time: 201 Days and 15.4 Hours
More than twenty years ago, knowledge about the importance of cholesterol absorption and the potential therapeutic effect of its inhibition led to the discovery and clinical application of the first and only cholesterol absorption inhibitor to date – ezetimibe. Since then, ezetimibe has become a well-recognized player in lipid-lowering therapy. Recent findings of IMPROVE-IT and EWTOPIA 75 imply that elderly patients over the age of 75 years in particular benefit from ezetimibe. This review summarizes the evidence, discusses the possible underlying pathophysiological mechanisms and calls for a change in future dyslipidemia guidelines.
Core Tip: The review summarizes the evidence of lipid-lowering therapies in patients 75 years and older, and discusses the possible underlying pathophysiological mechanisms and calls for a change in future dyslipidemia guidelines.
- Citation: Makhmudova U, Schulze PC, Davis HR, Weingärtner O. Lipid lowering in patients 75 years and older. World J Cardiol 2021; 13(10): 526-532
- URL: https://www.wjgnet.com/1949-8462/full/v13/i10/526.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i10.526
Aging is a risk factor for cardiovascular morbidity, as the prevalence of atherosclerosis, myocardial infarction, and stroke increases with age[1]. Among all the physiological alterations that occur during the lifespan of a human being, changes in cholesterol metabolism are among the most important. Total cholesterol levels increase beginning from age 18-19 in men and age 20-21 in women and reach their climax at 50-51 and 56-57 years of age, respectively[2]. Although the concentration of endogenously synthesized cholesterol exceeds the amount of exogenous dietary cholesterol in the bloodstream, the inhibition of cholesterol absorption by ezetimibe reduces both low density lipoprotein (LDL)-c levels and the occurrence of cardiovascular events[3-5]. The particular benefit of ezetimibe treatment in regard to hard cardiovascular outcomes in patient populations over 75 years of age was only recently demonstrated in large cardiovascular outcome trials, such as IMPROVE-IT[6] (secondary prevention) and EWTOPIA 75[7] (primary prevention).
Cholesterol is one of the key components of the cell membrane and a precursor of steroid hormones, bile acids and vitamin D. Plasma cholesterol levels are regulated by three factors: Dietary absorption, endogenous biosynthesis in the liver and bile acids that are reabsorbed in the small intestine. Endogenous cholesterol synthesis begins with the 18-step formation of mevalonate through its conversion by HMG (3-hydroxy-3-methyl-glutaryl-) CoA reductase, which is a target of statin therapy, and ends with the 19-step synthesis of cholesterol from lanosterol. This cascade of reactions occurs in all nucleated cells and is catalyzed by a diversity of enzymes. Late precursors of cholesterol, such as squalene, cholestanol, desmosterol and lathosterol, are traditionally used as cholesterol synthesis markers. Due to its structure, cholesterol cannot be easily transported to tissues. Therefore, it is transported by lipoproteins, which contain triglycerides and cholesterol in their cores and apolipoproteins on their surface. Very low-density lipoprotein is synthesized in the liver and hydrolyzed to LDL in two steps. LDL is the major transporter of cholesterol in the plasma.
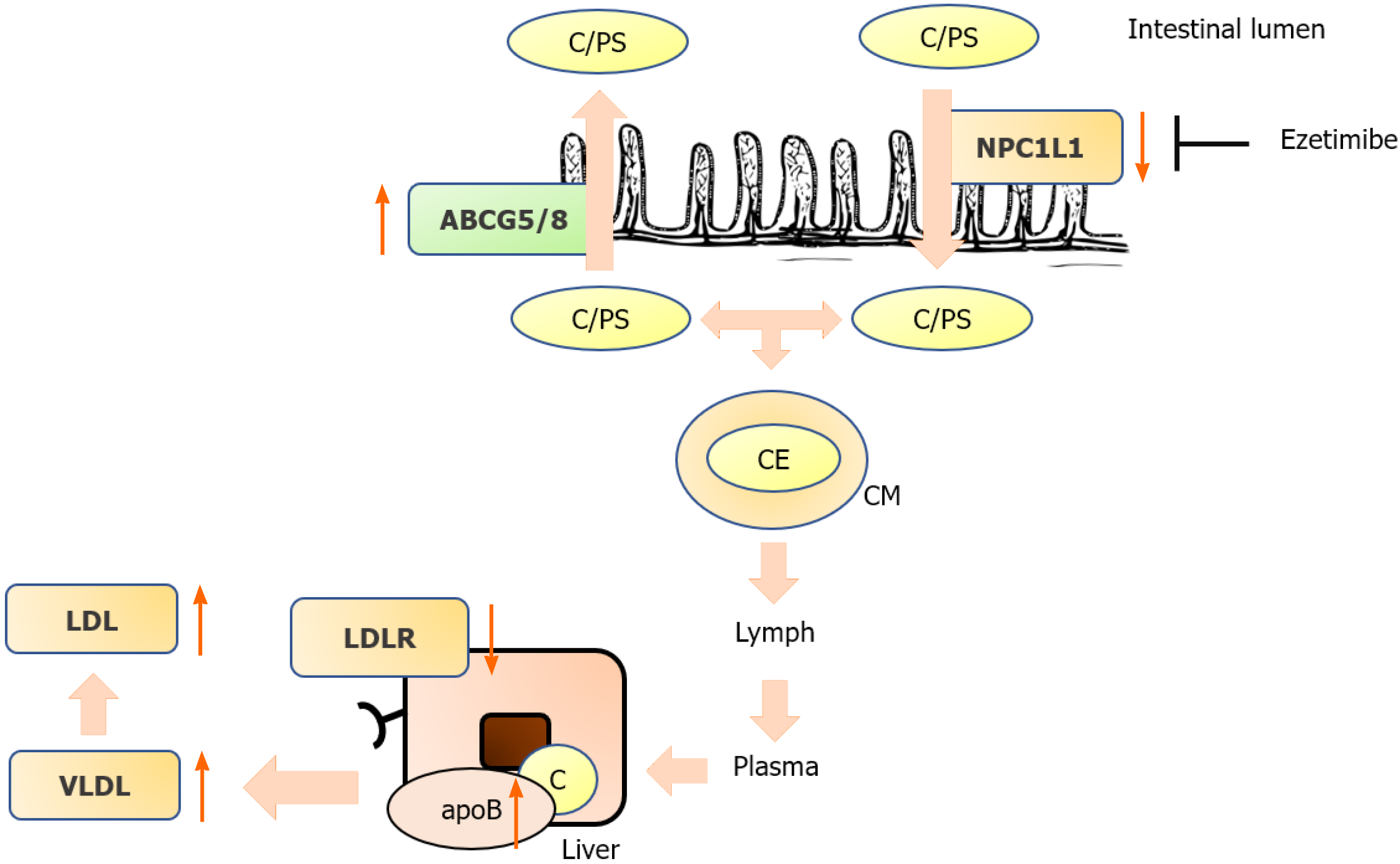
In addition to be endogenously synthesized, cholesterol is absorbed from the diet. In the small intestine, esterified cholesterol is converted to free cholesterol, which is absorbed via transport and endocytosis by NPC1L1 (Niemann-Pick C1-Like 1). In enterocytes, chylomicrons are formed from cholesterol, phospholipids, and triacylglycerol by apoprotein B48. Chylomicrons are transported via the lymphatic system to the bloodstream, where they deliver fatty acids to peripheral tissues and are eventually degraded by the liver. Moreover, nonesterified plant sterols and “excess cholesterol” are excreted back into the small intestine via the ATP-binding cassette transporter (ABCG5/G8) heterodimer. This results in large differences between cholesterol and plant sterol concentrations in the bloodstream, with plasma cholesterol levels being approximately 1000-fold higher than plant sterol levels.
The increase in LDL-c levels with age can be explained by several factors: A decrease in LDL receptor levels, an increase in the levels of apoB-100 in the liver and serum[8], an increase in cholesterol absorption, and a decrease in bile acid synthesis[9].
Animal studies have demonstrated that the cholesterol absorption rate increases with age[10,11]. According to Duan et al[11], cholesterol absorption in older mice (measured by the plasma dual isotope ratio method) is higher than that in younger mice, which is mechanistically explained by an increase in NPC1L1 mRNA expression with aging. Interestingly, the expression of ABCG5/G8, a transporter that pumps plant sterols back into the intestinal lumen and thus decreases plant sterol concentrations in the plasma, is negatively correlated with aging[11].
Another important age-related alteration is a reduction in CYP7A1 expression, an enzyme involved in bile acid synthesis. This leads to a decrease in bile acid synthesis, which in turn results in a lower cholesterol utilization rate[9].
Unfortunately, the number of human studies in this context is very limited. One study reported a positive correlation between cholesterol synthesis marker levels and aging in a Northern Italian population. In that study, cholesterol absorption marker levels were also elevated in elderly individuals but not in patients with gallstones[12] (Figure 1).
Methods to quantify cholesterol absorption were established as early as 1960[13,14]. Almost all of the early methods were based on isotope labeling of plasma and fecal probes: Cholesterol balance, single-dose isotopic feeding, dual isotope plasma ratio, continuous isotope feeding, and intestinal perfusion[13]. Another validated method that does not require isotope feeding is the measurement of the ratio of plant sterol levels (campesterol and sitosterol) and cholestanol levels (metabolite of cholesterol), which is performed mainly via high-performance liquid chromatography. Plant sterol concentrations have been shown in several studies to be markers of cholesterol absorption[15].
A landmark study addressing the importance of cholesterol metabolism in elderly patients was the DEBATE-Study[16]. Strandberg and colleagues conducted a prospective cohort study of home-dwelling elderly individuals to assess the prognostic value of markers of cholesterol metabolism (absorption vs synthesis). They found that low cholesterol absorption in individuals older than 75 years of age was associated with fewer cardiovascular events and better survival. In that study, the levels of markers of cholesterol synthesis were negatively associated with cardiovascular outcomes[16]. Strandberg and colleagues speculated that lower cholesterol absorption was associated with better prognosis since these individuals had a lower cholesterol burden during their lifespan. On the other hand, the worse prognosis may have also been due to increased plant sterol absorption. Similar results were reported by our group in elderly patients with aortic stenosis and patients with diabetes mellitus[17,18].
Statins inhibit 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase in the liver, thus preventing the formation of mevalonate, which determines the rate of endogenous cholesterol synthesis[19]. Statins reduce LDL-c levels by up to 60% depending on the specific drug and dose (rosuvastatin is the most potent statin, as 40 mg of rosuvastatin reduces LDL-c levels by 55%)[20]. Although statins are the cornerstone of lipid-lowering therapy, target goal attainment with statin monotherapy is often unsatisfactory. In JUPITER (Justification for the Use of Statin in Prevention: An Intervention Trial Evaluating Rosuvastatin), approximately 10% of the patients showed no change in LDL-c levels, and over 40% had a LDL-c level reduction below 50%[21]. Several loci were identified by genome-wide association studies to be responsible for “nonresponsiveness” to statin therapy[21,22]. On the other hand, ezetimibe treatment is particularly effective in these patients since low endogenous synthesis is associated with high cholesterol absorption[23,24].
The secondary analysis of ALLHAT-LTT demonstrated that statins (pravastatin) conveyed no benefit in a primary prevention setting. Moreover, there was a nonsignificant trend toward increased all-cause mortality in the pravastatin group vs. the placebo group. PROSPER (PROspective Study of Pravastatin in the Elderly at Risk) evaluated hard cardiovascular outcomes in pravastatin- vs placebo-treated patients. Pravastatin reduced primary endpoints (a composite of coronary death, nonfatal myocardial infarction, and nonfatal stroke occurrence) after 3 years of follow-up. The subgroup analysis in the study revealed that this effect was stronger among those with established cardiovascular disease than among those without cardiovascular diseases, implying that statins are less effective in a primary prevention setting. However, no significant difference was observed in the interaction test between groups. Moreover, in pravastatin-treated patients, the incidence of newly diagnosed cancer was 25% higher than in the placebo group, although a meta-analysis did not show any significant association with cancer[25].
Ezetimibe targets NPC1L1 in the small intestine, resulting in the inhibition of cholesterol and plant sterol absorption. In humans, ezetimibe also inhibits hepatic NPC1L1 to prevent reabsorption of cholesterol and plant sterols from the bile and increases their excretion. Ezetimibe is metabolized to ezetimibe-glucuronide, which is also a potent inhibitor of NPC1L1. The maximal concentration is reached 1–2 h after oral administration, and it has a terminal half-life of 22 h[3]. Ezetimibe lowers LDL-c levels by approximately 20%[3,4]. PRECISE-IVUS demonstrated that ezetimibe in combination with atorvastatin resulted in greater coronary plaque regression than atorvastatin monotherapy in patients with coronary artery disease[26]. The SHARP trial demonstrated that the combination of statins and ezetimibe reduced LDL-c levels and cardiovascular outcomes in patients with chronic kidney disease[27]. According to the current ESC/EAS guidelines, ezetimibe is recommended as an adjuvant to statin therapy when LDL-c goals cannot be achieved by statin monotherapy[28].
A post hoc analysis of the Scandinavian Simvastatin Survival Study (4S) suggested that patients with high cholesterol absorption and low cholesterol synthesis did not benefit from statins in terms of cardiovascular event reduction[29]. HIJ-Proper, on the other hand, demonstrated that patients with high cholesterol absorption benefited from the addition of ezetimibe in terms of cardiovascular event reduction, whereas patients with low cholesterol absorption did not[30].
IMPROVE-IT demonstrated for the first time that ezetimibe, a nonstatin drug, reduces LDL-c levels and hard cardiovascular outcomes[5]. In a recent secondary analysis, the outcomes of IMPROVE-IT were analyzed according to age. Interestingly, in patients older than 75 years of age, ezetimibe treatment was much more effective in reducing cardiovascular events. In patients over 75 years of age, the number “needed to treat” was 11, whereas it was 125 in patients younger than 75 years of age[6]. These findings illustrate the preventive capacity of adding a cholesterol absorption inhibitor in elderly individuals over 75 years of age[31].
The EWTOPIA 75 study is a prospective, double-blind, placebo-controlled randomized trial that was conducted in Japan and demonstrated the benefit of ezetimibe in elderly patients without a history of coronary artery disease[7]. In this primary prevention trial, ezetimibe reduced both LDL-C levels and hard cardiovascular outcomes. This further adds to the notion that individuals over 75 years of age with elevated cholesterol levels benefit in particular from ezetimibe treatment[32]. This is of particular interest since all statin trials of primary prevention in patients over 75 years of age failed to reduce cardiovascular events[25,33].
In a recently published large-scale genetic analysis of over 1 million individuals with and without cardiovascular diseases, Helgadottir and colleagues reported genetic variants of ABCG5/G8 and NPC1L1 that increase plasma plant sterol levels, and plasma cholesterol levels have a greater impact on cardiovascular risk than the levels of other lipid genes that increase only serum cholesterol levels (LDL-c receptor, HMG-CoA-reductase, apoB, etc.). Genes that increased non-HDL cholesterol levels by 1 mmol/L and also increased serum plant sterol levels increased cardiovascular risk 2.0-fold. However, genes that increased serum non-HDL cholesterol levels by 1 mmol/L but had no impact on serum plant sterol levels increased cardiovascular risk only 1.5-fold[34]. These findings are important since they demonstrate that plant sterols “per se” are atherogenic. On the basis of these findings, determination of the levels of markers of cholesterol metabolism, such as campesterol, sitosterol and lathosterol, should play a more important role in future cardiovascular risk stratification and a more personalized and individualized treatment approach for hyperlipidemia[24,35]. As early as 2016, we suggested evaluating the ratio of cholesterol synthesis and absorption in IMPROVE-IT[36]. This analysis is under way, as assured by the TIMI Study group, and will provide important mechanistic insights[37].
Evidence from animal studies and human trials indicates that cholesterol and plant sterol absorption increase with age. Findings from large-scale prospective, randomized trials on primary and secondary prevention demonstrate that patients over 75 years of age benefit in particular from the addition of a cholesterol absorption inhibitor, such as ezetimibe. Therefore, ezetimibe should be the lipid-lowering drug of choice in patients 75 years and older.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wierzbicka A S-Editor: Ma YJ L-Editor: A P-Editor: Li JH
| 1. | Rodgers JL, Jones J, Bolleddu SI, Vanthenapalli S, Rodgers LE, Shah K, Karia K, Panguluri SK. Cardiovascular Risks Associated with Gender and Aging. J Cardiovasc Dev Dis. 2019;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 520] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 2. | Yi SW, Yi JJ, Ohrr H. Total cholesterol and all-cause mortality by sex and age: a prospective cohort study among 12.8 million adults. Sci Rep. 2019;9:1596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 3. | Kosoglou T, Statkevich P, Johnson-Levonas AO, Paolini JF, Bergman AJ, Alton KB. Ezetimibe: a review of its metabolism, pharmacokinetics and drug interactions. Clin Pharmacokinet. 2005;44:467-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 332] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 4. | Morrone D, Weintraub WS, Toth PP, Hanson ME, Lowe RS, Lin J, Shah AK, Tershakovec AM. Lipid-altering efficacy of ezetimibe plus statin and statin monotherapy and identification of factors associated with treatment response: a pooled analysis of over 21,000 subjects from 27 clinical trials. Atherosclerosis. 2012;223:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 5. | Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM; IMPROVE-IT Investigators. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med. 2015;372:2387-2397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3427] [Cited by in RCA: 2991] [Article Influence: 299.1] [Reference Citation Analysis (0)] |
| 6. | Bach RG, Cannon CP, Giugliano RP, White JA, Lokhnygina Y, Bohula EA, Califf RM, Braunwald E, Blazing MA. Effect of Simvastatin-Ezetimibe Compared With Simvastatin Monotherapy After Acute Coronary Syndrome Among Patients 75 Years or Older: A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2019;4:846-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 7. | Ouchi Y, Sasaki J, Arai H, Yokote K, Harada K, Katayama Y, Urabe T, Uchida Y, Hayashi M, Yokota N, Nishida H, Otonari T, Arai T, Sakuma I, Sakabe K, Yamamoto M, Kobayashi T, Oikawa S, Yamashita S, Rakugi H, Imai T, Tanaka S, Ohashi Y, Kuwabara M, Ito H. Ezetimibe Lipid-Lowering Trial on Prevention of Atherosclerotic Cardiovascular Disease in 75 or Older (EWTOPIA 75): A Randomized, Controlled Trial. Circulation. 2019;140:992-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 8. | Higuchi K, Kitagawa K, Kogishi K, Takeda T. Developmental and age-related changes in apolipoprotein B mRNA editing in mice. J Lipid Res. 1992;33:1753-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Morgan AE, Mooney KM, Wilkinson SJ, Pickles NA, Mc Auley MT. Cholesterol metabolism: A review of how ageing disrupts the biological mechanisms responsible for its regulation. Ageing Res Rev. 2016;27:108-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 10. | Hollander D, Morgan D. Increase in cholesterol intestinal absorption with aging in the rat. Exp Gerontol. 1979;14:201-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Duan LP, Wang HH, Ohashi A, Wang DQ. Role of intestinal sterol transporters Abcg5, Abcg8, and Npc1 L1 in cholesterol absorption in mice: gender and age effects. Am J Physiol Gastrointest Liver Physiol. 2006;290:G269-G276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Bertolotti M, Mussi C, Pellegrini E, Magni A, Del Puppo M, Ognibene S, Carulli L, Anzivino C, Baldelli E, Loria P, Carulli N. Age-associated alterations in cholesterol homeostasis: evidence from a cross-sectional study in a Northern Italy population. Clin Interv Aging. 2014;9:425-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Matthan NR, Lichtenstein AH. Approaches to measuring cholesterol absorption in humans. Atherosclerosis. 2004;174:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Wang DQ, Carey MC. Measurement of intestinal cholesterol absorption by plasma and fecal dual-isotope ratio, mass balance, and lymph fistula methods in the mouse: an analysis of direct vs indirect methodologies. J Lipid Res. 2003;44:1042-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Tilvis RS, Miettinen TA. Serum plant sterols and their relation to cholesterol absorption. Am J Clin Nutr. 1986;43:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 252] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Strandberg TE, Tilvis RS, Pitkala KH, Miettinen TA. Cholesterol and glucose metabolism and recurrent cardiovascular events among the elderly: a prospective study. J Am Coll Cardiol. 2006;48:708-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Weingärtner O, Weingärtner N, Scheller B, Lütjohann D, Gräber S, Schäfers HJ, Böhm M, Laufs U. Alterations in cholesterol homeostasis are associated with coronary heart disease in patients with aortic stenosis. Coron Artery Dis. 2009;20:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Weingärtner O, Lütjohann D, Vanmierlo T, Müller S, Günther L, Herrmann W, Böhm M, Laufs U, Herrmann M. Markers of enhanced cholesterol absorption are a strong predictor for cardiovascular diseases in patients without diabetes mellitus. Chem Phys Lipids. 2011;164:451-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Endo A. The discovery and development of HMG-CoA reductase inhibitors. J Lipid Res. 1992;33:1569-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 520] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 20. | Hou R, Goldberg AC. Lowering low-density lipoprotein cholesterol: statins, ezetimibe, bile acid sequestrants, and combinations: comparative efficacy and safety. Endocrinol Metab Clin North Am. 2009;38:79-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Ridker PM, Mora S, Rose L; JUPITER Trial Study Group. Percent reduction in LDL cholesterol following high-intensity statin therapy: potential implications for guidelines and for the prescription of emerging lipid-lowering agents. Eur Heart J. 2016;37:1373-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 169] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 22. | Postmus I, Trompet S, Deshmukh HA, Barnes MR, Li X, Warren HR, Chasman DI, Zhou K, Arsenault BJ, Donnelly LA, Wiggins KL, Avery CL, Griffin P, Feng Q, Taylor KD, Li G, Evans DS, Smith AV, de Keyser CE, Johnson AD, de Craen AJ, Stott DJ, Buckley BM, Ford I, Westendorp RG, Slagboom PE, Sattar N, Munroe PB, Sever P, Poulter N, Stanton A, Shields DC, O'Brien E, Shaw-Hawkins S, Chen YD, Nickerson DA, Smith JD, Dubé MP, Boekholdt SM, Hovingh GK, Kastelein JJ, McKeigue PM, Betteridge J, Neil A, Durrington PN, Doney A, Carr F, Morris A, McCarthy MI, Groop L, Ahlqvist E; Welcome Trust Case Control Consortium, Bis JC, Rice K, Smith NL, Lumley T, Whitsel EA, Stürmer T, Boerwinkle E, Ngwa JS, O'Donnell CJ, Vasan RS, Wei WQ, Wilke RA, Liu CT, Sun F, Guo X, Heckbert SR, Post W, Sotoodehnia N, Arnold AM, Stafford JM, Ding J, Herrington DM, Kritchevsky SB, Eiriksdottir G, Launer LJ, Harris TB, Chu AY, Giulianini F, MacFadyen JG, Barratt BJ, Nyberg F, Stricker BH, Uitterlinden AG, Hofman A, Rivadeneira F, Emilsson V, Franco OH, Ridker PM, Gudnason V, Liu Y, Denny JC, Ballantyne CM, Rotter JI, Adrienne Cupples L, Psaty BM, Palmer CN, Tardif JC, Colhoun HM, Hitman G, Krauss RM, Wouter Jukema J, Caulfield MJ. Pharmacogenetic meta-analysis of genome-wide association studies of LDL cholesterol response to statins. Nat Commun. 2014;5:5068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 197] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 23. | Weingärtner O, Lütjohann D, Böhm M, Laufs U. Relationship between cholesterol synthesis and intestinal absorption is associated with cardiovascular risk. Atherosclerosis. 2010;210:362-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Lütjohann D, Stellaard F, Mulder MT, Sijbrands EJG, Weingärtner O. The emerging concept of "individualized cholesterol-lowering therapy": A change in paradigm. Pharmacol Ther. 2019;199:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2375] [Cited by in RCA: 2305] [Article Influence: 100.2] [Reference Citation Analysis (0)] |
| 26. | Tsujita K, Sugiyama S, Sumida H, Shimomura H, Yamashita T, Yamanaga K, Komura N, Sakamoto K, Ono T, Oka H, Nakao K, Nakamura S, Ishihara M, Matsui K, Sakaino N, Nakamura N, Yamamoto N, Koide S, Matsumura T, Fujimoto K, Tsunoda R, Morikami Y, Matsuyama K, Oshima S, Kaikita K, Hokimoto S, Ogawa H; PRECISE-IVUS study investigators. Plaque REgression with Cholesterol absorption Inhibitor or Synthesis inhibitor Evaluated by IntraVascular UltraSound (PRECISE-IVUS Trial): Study protocol for a randomized controlled trial. J Cardiol. 2015;66:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellström B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Grönhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181-2192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1946] [Cited by in RCA: 1754] [Article Influence: 125.3] [Reference Citation Analysis (0)] |
| 28. | Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 5301] [Article Influence: 1060.2] [Reference Citation Analysis (0)] |
| 29. | Miettinen TA, Gylling H, Strandberg T, Sarna S. Baseline serum cholestanol as predictor of recurrent coronary events in subgroup of Scandinavian simvastatin survival study. Finnish 4S Investigators. BMJ. 1998;316:1127-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 185] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 30. | Hagiwara N, Kawada-Watanabe E, Koyanagi R, Arashi H, Yamaguchi J, Nakao K, Tobaru T, Tanaka H, Oka T, Endoh Y, Saito K, Uchida T, Matsui K, Ogawa H. Low-density lipoprotein cholesterol targeting with pitavastatin + ezetimibe for patients with acute coronary syndrome and dyslipidaemia: the HIJ-PROPER study, a prospective, open-label, randomized trial. Eur Heart J. 2017;38:2264-2276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 31. | Weingärtner O, Sijbrands EJG, Lütjohann D. Interpreting the Benefit of Simvastatin-Ezetimibe in Patients 75 Years or Older. JAMA Cardiol. 2020;5:234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Weingärtner O, Sijbrands E, Lütjohann D. Letter by Weingärtner et al Regarding Article, "Ezetimibe Lipid-Lowering Trial on Prevention of Atherosclerotic Cardiovascular Disease in 75 or Older (EWTOPIA 75): A Randomized, Controlled Trial". Circulation. 2020;141:e65-e66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Han BH, Sutin D, Williamson JD, Davis BR, Piller LB, Pervin H, Pressel SL, Blaum CS; ALLHAT Collaborative Research Group. Effect of Statin Treatment vs Usual Care on Primary Cardiovascular Prevention Among Older Adults: The ALLHAT-LLT Randomized Clinical Trial. JAMA Intern Med. 2017;177:955-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 34. | Helgadottir A, Thorleifsson G, Alexandersson KF, Tragante V, Thorsteinsdottir M, Eiriksson FF, Gretarsdottir S, Björnsson E, Magnusson O, Sveinbjornsson G, Jonsdottir I, Steinthorsdottir V, Ferkingstad E, Jensson BÖ, Stefansson H, Olafsson I, Christensen AH, Torp-Pedersen C, Køber L, Pedersen OB, Erikstrup C, Sørensen E, Brunak S, Banasik K, Hansen TF, Nyegaard M, Eyjolfssson GI, Sigurdardottir O, Thorarinsson BL, Matthiasson SE, Steingrimsdottir T, Bjornsson ES, Danielsen R, Asselbergs FW, Arnar DO, Ullum H, Bundgaard H, Sulem P, Thorsteinsdottir U, Thorgeirsson G, Holm H, Gudbjartsson DF, Stefansson K. Genetic variability in the absorption of dietary sterols affects the risk of coronary artery disease. Eur Heart J. 2020;41:2618-2628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 35. | Weingärtner O, Patel SB, Lütjohann D. It's time to personalize and optimize lipid-lowering therapy. Eur Heart J. 2020;41:2629-2631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Weingärtner O, Lütjohann D, Elsässer A. Personalize and Optimize Lipid-Lowering Therapies. J Am Coll Cardiol. 2016;68:325-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Murphy SA, Cannon CP, Blazing MA, Giugliano RP, Tershakovec AM, Braunwald E. Reply: Personalize and Optimize Lipid-Lowering Therapies. J Am Coll Cardiol. 2016;68:326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |