Published online Jul 26, 2020. doi: 10.4330/wjc.v12.i7.351
Peer-review started: February 29, 2020
First decision: April 7, 2020
Revised: May 11, 2020
Accepted: June 17, 2020
Article in press: June 17, 2020
Published online: July 26, 2020
Processing time: 146 Days and 5.9 Hours
Patients undergoing cardiac surgery particularly those with comorbidities and frailty, experience frequently higher rates of post-operative morbidity, mortality and prolonged hospital length of stay. Muscle mass wasting seems to play important role in prolonged mechanical ventilation (MV) and consequently in intensive care unit (ICU) and hospital stay.
To investigate the clinical value of skeletal muscle mass assessed by ultrasound early after cardiac surgery in terms of duration of MV and ICU length of stay.
In this observational study, we enrolled consecutively all patients, following their admission in the Cardiac Surgery ICU within 24 h of cardiac surgery. Bedside ultrasound scans, for the assessment of quadriceps muscle thickness, were performed at baseline and every 48 h for seven days or until ICU discharge. Muscle strength was also evaluated in parallel, using the Medical Research Council (MRC) scale.
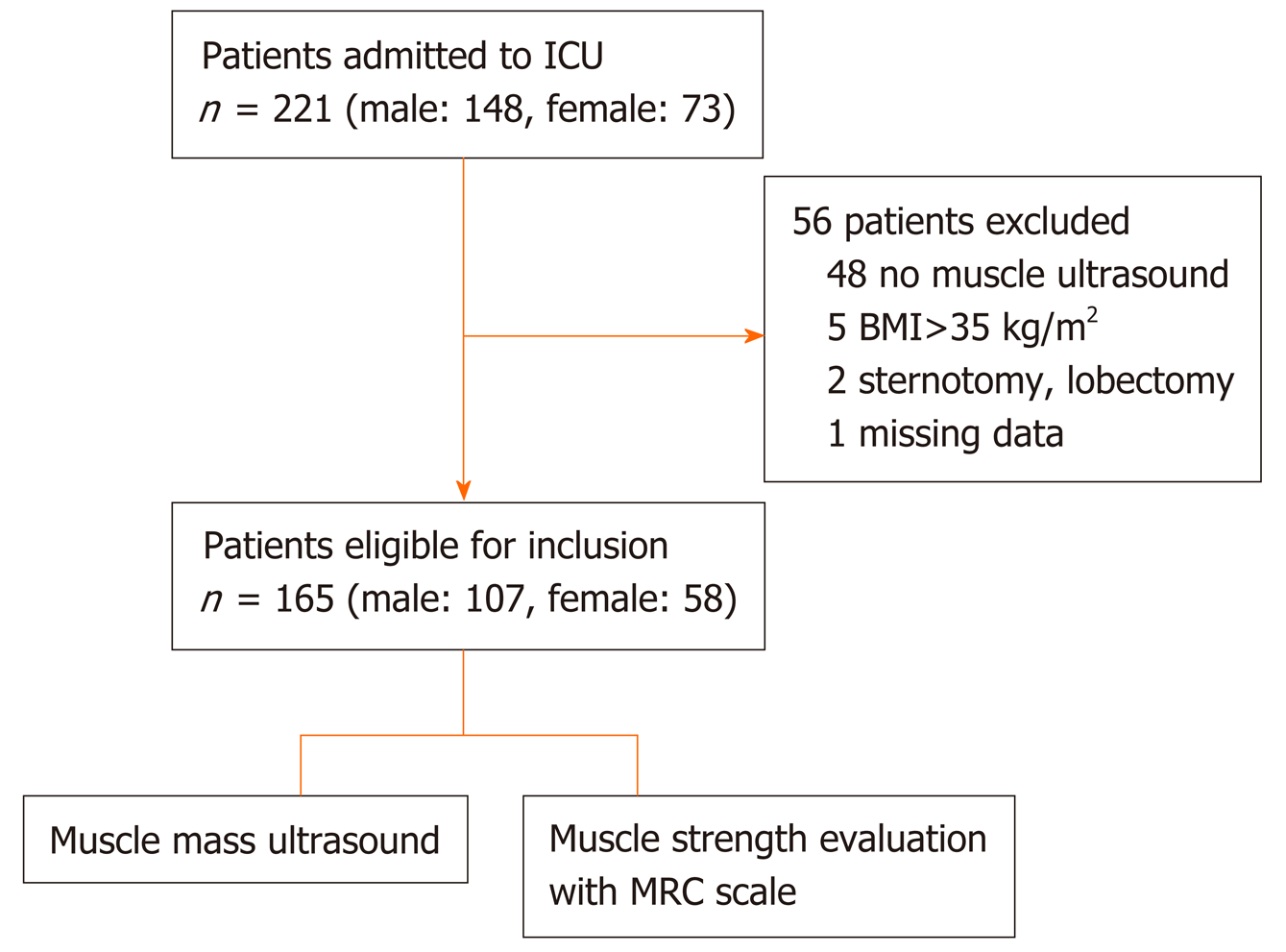
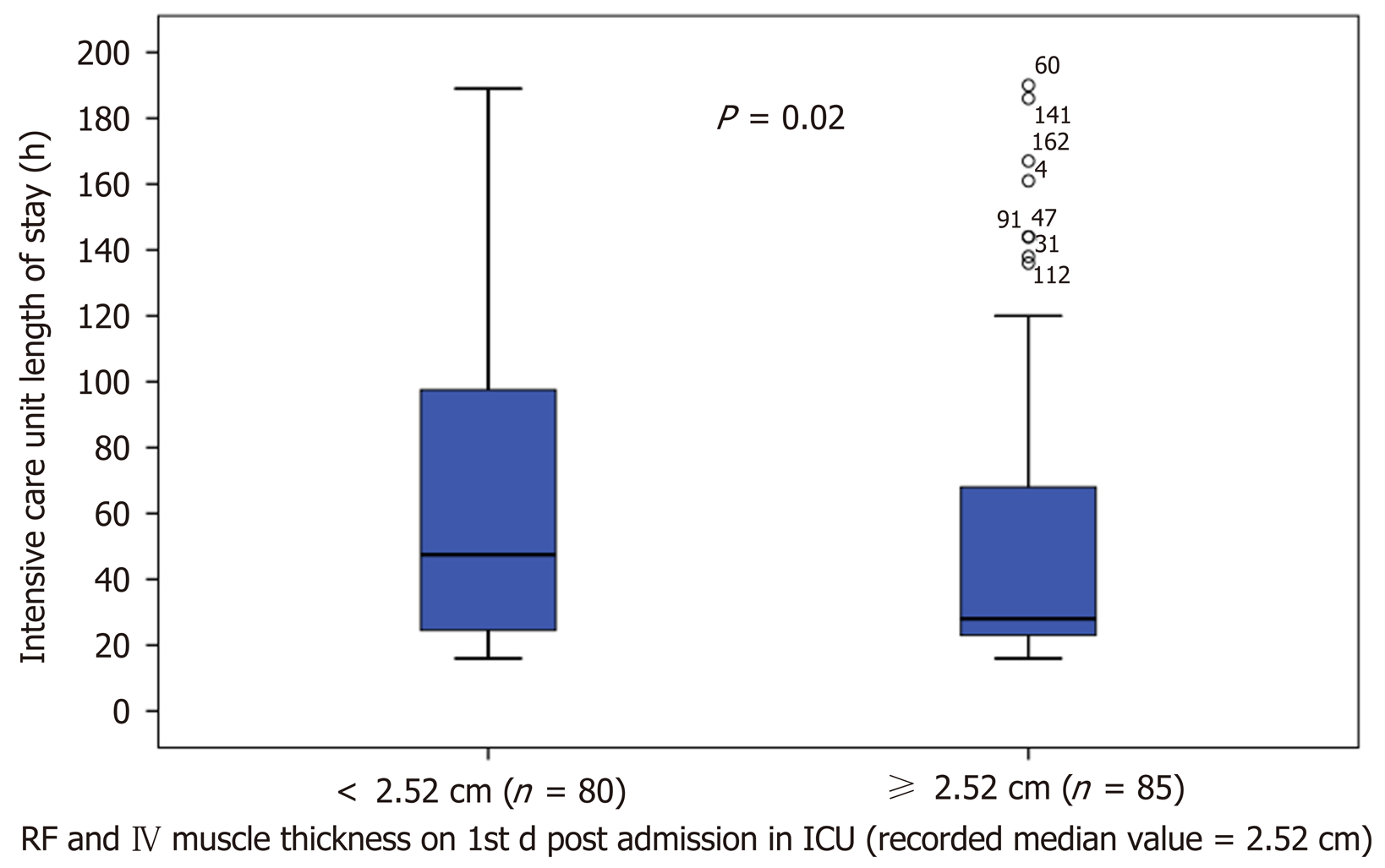
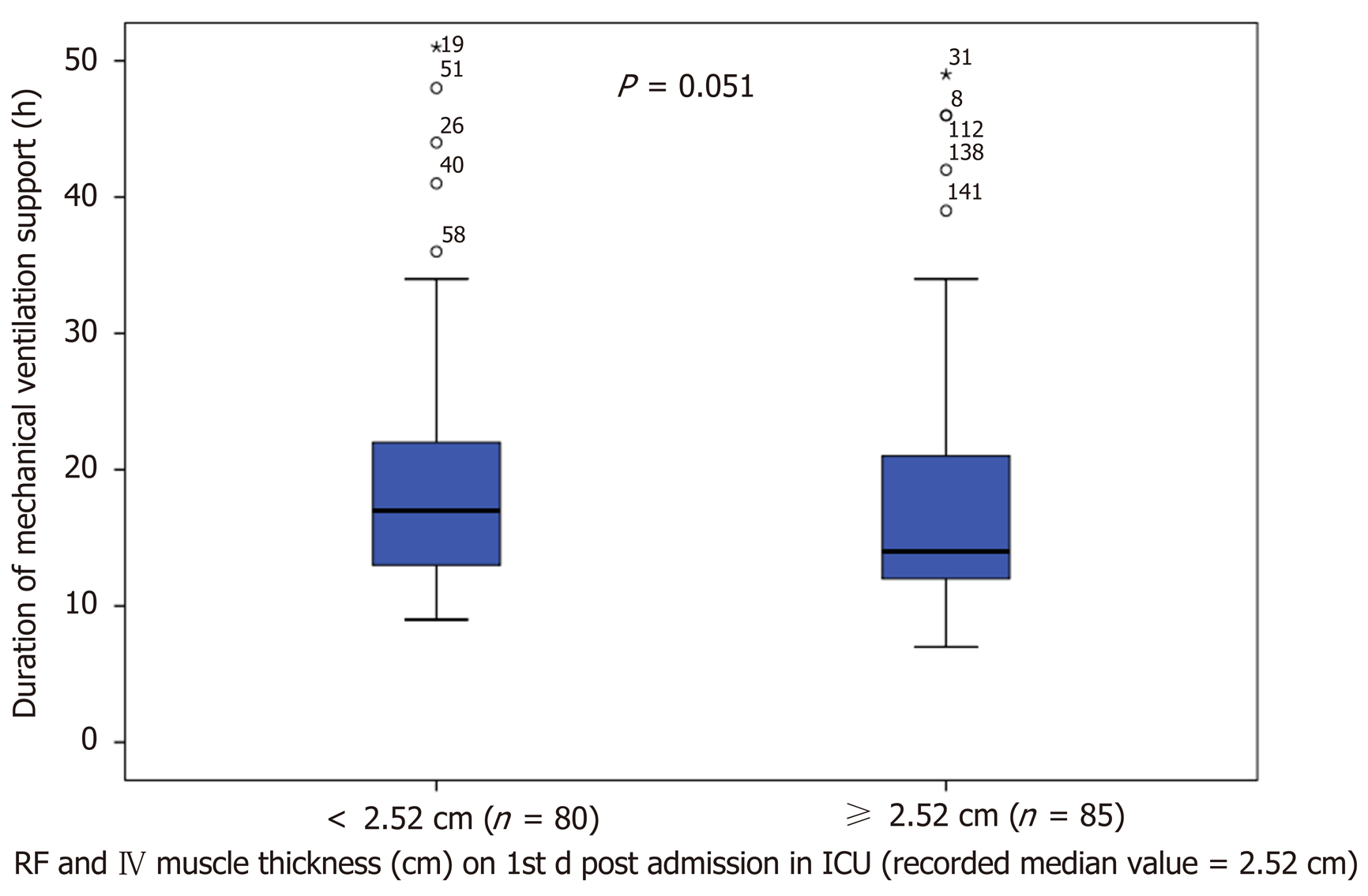
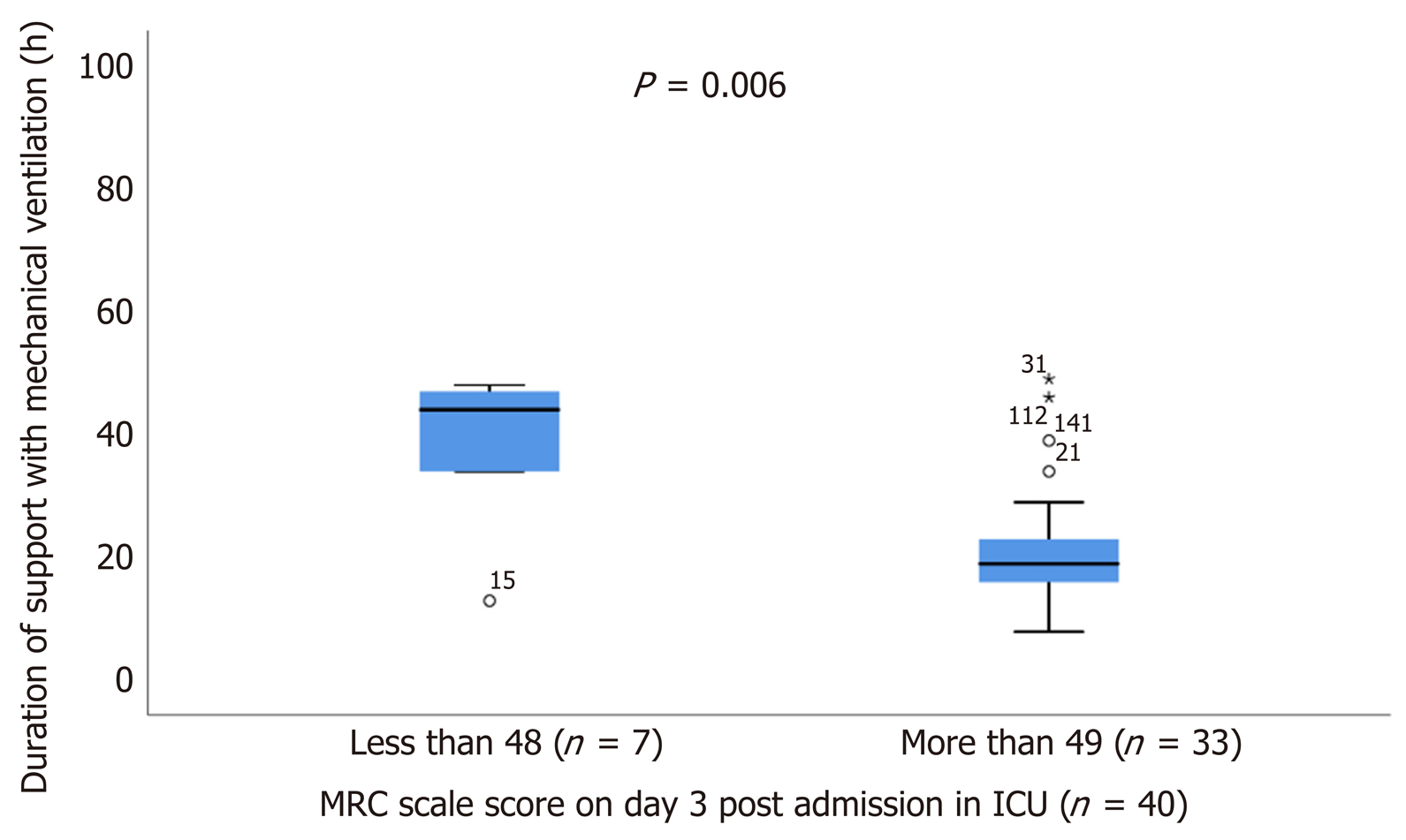
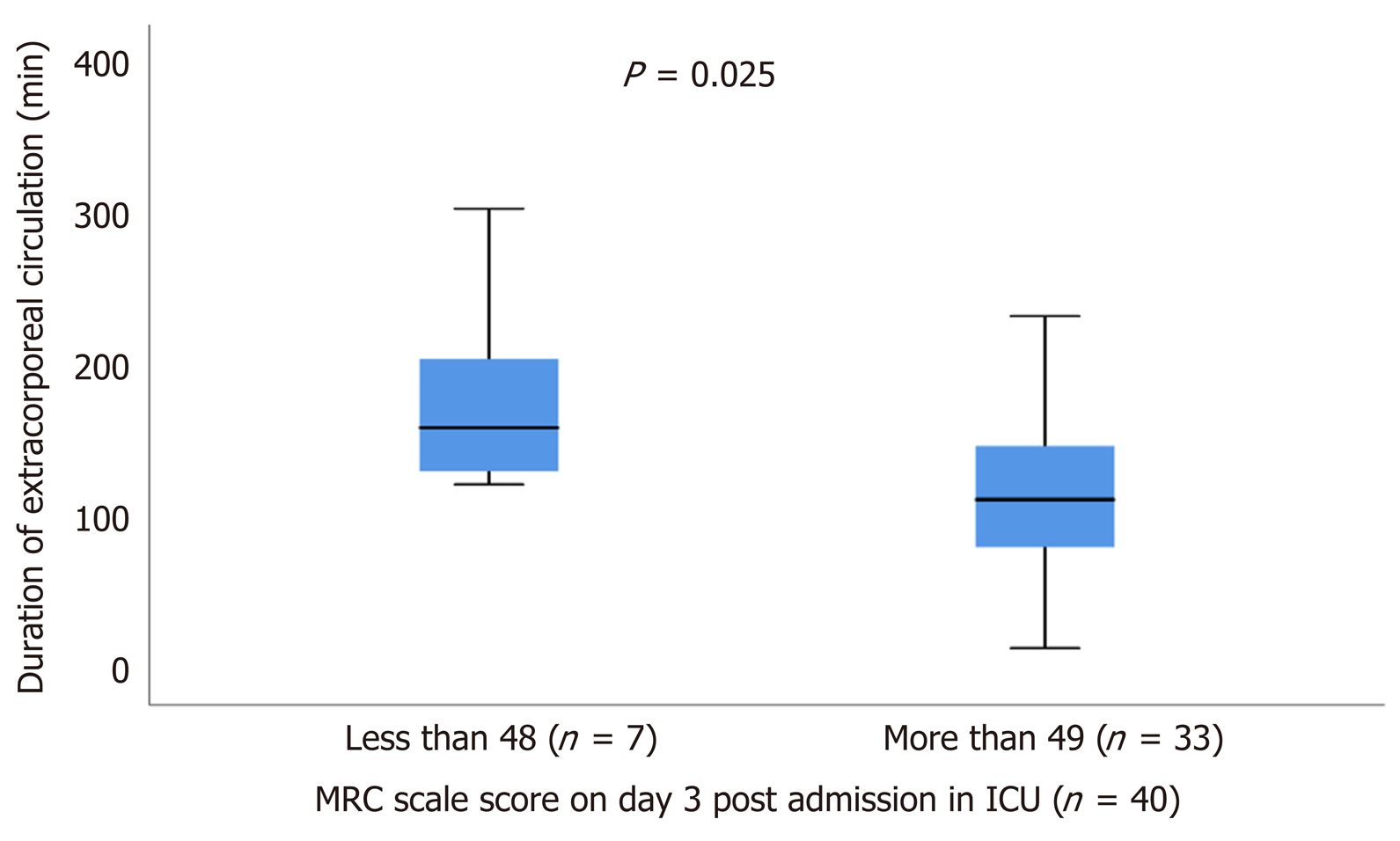
Of the total 221 patients enrolled, ultrasound scans and muscle strength assessment were finally performed in 165 patients (patients excluded if ICU stay < 24 h). The muscle thickness of rectus femoris (RF), was slightly decreased by 2.2% [(95% confidence interval (CI): - 0.21 to 0.15), n = 9; P = 0.729] and the combined muscle thickness of the vastus intermedius (VI) and RF decreased by 3.5% [(95%CI: - 0.4 to 0.22), n = 9; P = 0.530]. Patients whose combined VI and RF muscle thickness was below the recorded median values (2.5 cm) on day 1 (n = 80), stayed longer in the ICU (47 ± 74 h vs 28 ± 45 h, P = 0.02) and remained mechanically ventilated more (17 ± 9 h vs 14 ± 9 h, P = 0.05). Moreover, patients with MRC score ≤ 48 on day 3 (n = 7), required prolonged MV support compared to patients with MRC score ≥ 49 (n = 33), (44 ± 14 h vs 19 ± 9 h, P = 0.006) and had a longer duration of extracorporeal circulation was (159 ± 91 min vs 112 ± 71 min, P = 0.025).
Skeletal quadriceps muscle thickness assessed by ultrasound shows a trend to a decrease in patients after cardiac surgery post-ICU admission and is associated with prolonged duration of MV and ICU length of stay.
Core tip: Muscle mass wasting may occur in post-cardiac surgery patients affecting outcome. We assessed the clinical significance of muscle mass in post-cardiac surgery after intensive care unit (ICU) admission. Sonographic assessment of quadriceps muscle thickness was performed to 165 post-cardiac surgery patients for 7 d or until ICU discharge. The results of the study showed a trend to a decreased muscle mass in post-cardiac surgery patients. There was also an association between muscle mass andduration of mechanical ventilation support and ICU length of stay. Sonographic assessment seems to be a valid method to quantify quadriceps muscle mass in patients after cardiac surgery.
- Citation: Dimopoulos S, Raidou V, Elaiopoulos D, Chatzivasiloglou F, Markantonaki D, Lyberopoulou E, Vasileiadis I, Marathias K, Nanas S, Karabinis A. Sonographic muscle mass assessment in patients after cardiac surgery. World J Cardiol 2020; 12(7): 351-361
- URL: https://www.wjgnet.com/1949-8462/full/v12/i7/351.htm
- DOI: https://dx.doi.org/10.4330/wjc.v12.i7.351
Factors such as immobilization, prolonged mechanical ventilation (MV) and sedation elicit the development of neuromuscular complications to patients admitted in the intensive care unit (ICU)[1]. These functional and structural alterations of muscle and nerve fibers contribute to muscle weakness and atrophy[2]. Muscle mass wasting is detected mainly to lower limbs in critically ill ventilated patients early after their admission in ICU[3]. Low skeletal muscle area is a risk factor for mortality[4] and has been linked to long term disability[5,6] and prolonged hospitalization[7].
Previous studies have also described that patients after cardiothoracic surgery exhibit muscle mass loss[7-9], as assessed by computed tomography scanning[10,11]. This muscle weakness is associated with frailty, morbidity, prolonged hospital length of stay and low quality of life after hospital discharge. In addition, low skeletal muscle density before cardiac surgery is related to decreased muscle function post-operatively and increased mortality[11].
Muscle ultrasound is a reliable and noninvasive diagnostic tool in evaluating muscle architecture changes. It has been used widely in the ICU during the last few years. It can be performed at the bedside providing real-time quantitative and qualitative data for muscle tissue[12,13]. Recent systematic reviews have confirmed its clinical and prognostic value to the measurement of peripheral skeletal muscle alterations[14] and to the early detection of intensive care unit acquired weakness (ICUAW)[15]. However, its clinical and prognostic value in patients after cardiac surgery has not been thoroughly investigated yet.
We hypothesized that cardiac surgery patients would present a decrease in sonographically quantified quadriceps muscle mass during their ICU stay and that decreased quadriceps muscle mass would be associated with prolonged MV support and ICU stay.
Aim of the present study is to evaluate sonographically quadriceps muscle mass in cardiac surgery patients during their ICU stay and assess its clinical and prognostic value.
This observational study was conducted at the Cardiac Surgery ICU of Onassis Cardiac Surgery Center from February 1, 2018 to May 15, 2018. The research was approved by Ethics Committee of the Onassis Cardiac Surgery Center (No. 607/17.11.17) with obtained patients’ informed consent and carried out in accordance with the ethical standards set by the Declaration of Helsinki.
Inclusion criteria were consecutive patients, over 18 years old, following their admission in the Cardiac Surgery ICU within 24 h of cardiac surgery.
Participants who were unable to get a muscle ultrasound assessment within 24 h of admission in the ICU were excluded from the study. Other exclusion criteria were severe obesity [body mass index (BMI) > 35kg/m2], patients with open chest-sternotomy, lobectomy or Central Extracorporeal Membrane Oxygenation, extensive peripheral thigh edema and preexisting neuromuscular disease. Moreover, patients who were re-admitted to the ICU were excluded from the study.
This was a prospective observational study conducted in a single center Cardiac Surgery ICU. All patients enrolled to the study were subjected to ultrasound measurement of quadriceps muscle mass thickness and muscle strength evaluation using the Medical Research Council (MRC) scale. Ultrasound scans and MRC scale assessment were performed, every 48 h for seven days or until ICU discharge, by previously experienced ICU staff and study researchers in muscle ultrasound. Routine physiotherapy intervention was also recorded in parallel.
The duration of MV, ICU stay and the ICU outcome of enrolled patients were also recorded.
The primary outcome of the present study was the prognostic assessment of quadriceps muscle mass thickness after cardiac surgery with regards to the duration of MV, ICU stay and the ICU outcome
The secondary outcomes were: (1) The quadriceps muscle mass thickness difference after cardiac surgery from baseline to 7th day of ICU stay or discharge; and (2) The muscle strength assessment using the MRC scale from baseline to 7th day of ICU stay or discharge.
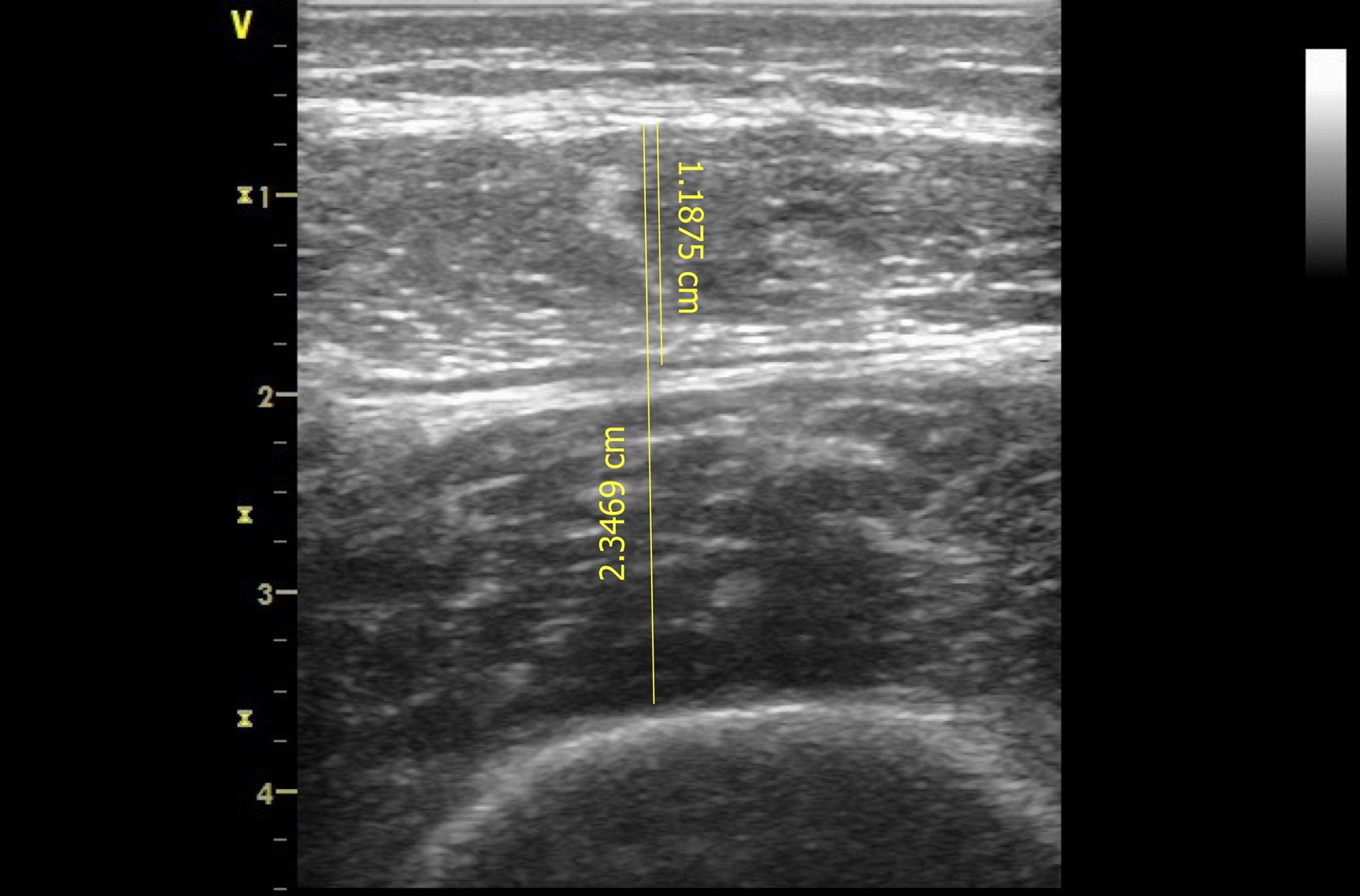
A portable GE Healthcare Vivid-Ι; Wauwatosa, Wisconsin, United States. Device was used with a 7.5-MHz linear transducer to obtain scans on days 1 (within 24 h from admission), 3, 5 and 7 subjects were positioned in supine position with their right thigh in neutral position, knee extended and muscles relaxed. Transducer was placed perpendicular to the thigh, at a standardized anatomical point, in the middle distance between anterior lower iliac crest and the upper pole of the patella of the thigh. Prior to the assessment, the located point was marked to enable repeated measurements on the subjects’ limb. To avoid muscle compression, a water-soluble transmission gel was applied to the ultrasound transducer. All underlying tissues as scanned with the ultrasound, were displayed in “B” mode and saved to the ultrasound hard drive for further analysis to a computer using an ultrasound imaging software.
Two sonographic images were acquired in each measurement illustrating the subcutaneous tissue, rectus femoris muscle, muscle fascia, vastus intermedius muscle and femur (Figure 1). Analyses performed on each image included measurements of rectus femoris muscle thickness (RF mass) in centimeters (cm) and combined muscle thickness of rectus femoris and vastus intermedius (RF_VI mass) in cm and averaged values were calculated. Sonographic assessment and image acquisition analyses were performed by an experienced operator and intra-rater variability was tested.
The MRC scale was used to evaluate muscle strength. Patients proceeded to assessment as soon as were awake and cooperative. Evaluation included the measurement of six muscle groups bilaterally: Shoulder abductors, elbow flexors and wrist dorsiflexors for the upper limbs as well as hip flexors, knee extensors and ankle dorsiflexors for the lower limbs. Test was performed in the same order each time. Each muscle group scored from 0 indicating no contraction to 5 indicating normal power. Total maximum score was 60 whilst MRC score ≤ 48 defined as ICUAW.
Descriptive statistics analysis was performed to describe the baseline data. Distribution‘s normality was checked with Kolmogorov-Smirnov test. Normally distributed continuous variables were expressed as mean ± SD and non-normally distributed variables as median with interquartile range, and categorical variables as proportions with percentages and absolute numbers. The sample size was estimated based on feasibility for a predefined certain period. Differences between the same patients were analyzed with the Paired t test for continuous variables and χ2 test for categorical variables. To analyze continuous variables between patient groups, Mann Whitney test was used for those with non-normal distribution and t test for those with normal distribution. Reliability analysis was performed and intra-class correlation coefficient (ICC) was calculated for intra-rater variability of quadriceps muscle mass thickness measured by ultrasound. Level of significance was set at P value < 0.05. All statistical analyses were performed with SPSS v.25 software.
In this study, we included 221 consecutive patients (148 men and 73 women) post-Cardiac Surgery ICU admission. Fifty-six patients were excluded from the study; 48 patients due to missing ultrasound scan within 24 h from admission, 5 patients due to high BMI (> 35 kg/m2), 2 patients due to open chest-sternotomy or lobectomy, and one patient due to missing data. Study population consisted of 165 patients, 107 males (64.8) and 58 females (35.2), median age 71 (64-77) years (Figure 2). All patients underwent ultrasound scans of quadriceps muscle mass and muscle strength evaluation. Baseline clinical characteristics of the 165 included subjects are summarized in Table 1. ICU length of stay was 41 (24-77) h, duration of sedation until awakening was 672 (553-896) min and duration of MV was 15 (12-21) h (Table 2).
| Characteristics | Values |
| Demographic data | |
| Sex (male, female) | 107 (65)/58 (35) |
| Age (yr) | 71 (64-77) |
| Weight (kg) | 77 ± 11.84 |
| Height (m) | 1.67 ± 0.09 |
| Body mass index (kg/m2) | 27.55 ± 3.69 |
| Clinical characteristics | |
| Hypertension | 126 (76) |
| Diabetes | 57 (34) |
| Dyslipidemia | 97 (59) |
| Smoker | 34 (21) |
| Former smoker | 38 (23) |
| Coronary heart disease | 89 (54) |
| Chronic heart Failure | 10 (6) |
| Chronic pulmonary disease | 22 (13) |
| Chronic kidney failure | 13 (8) |
| Thoracic aortic aneurysm | 11 (7) |
| Valve disease | 10 (6) |
| Other disease | 65 (39.4) |
| Apache II score | 2 (1.2) |
| SOFA score | 5 (3-6) |
| Characteristics | Values |
| Type of surgery | |
| Coronary artery bypass grafting | 86 (52) |
| Heart valve repair or replacement surgery | 79 (48) |
| Other cardiac surgery | 6 (4) |
| Medication | |
| Propofol | 111 (67) |
| Dobutamine | 103 (62) |
| Noradrenaline | 40 (24) |
| Morphine | 13 (8) |
| Adrenaline | 11 (7) |
| Other characteristics | |
| Duration of extracorporeal circulation (min) | 104 (81-135) |
| Duration of aortic cross-clamp (min) | 75 (56-99) |
| Duration of mechanical ventilation (h) | 15 (12-21) |
| Duration of surgery anesthesia (min) | 246 (202-292) |
| Duration of sedation (min) | 672 (553-896) |
| Duration of intensive care unit stay (h) | 41 (24-77) |
Assessment of ultrasound measurements has shown excellent reliability results with a high ICC calculated for intra-rater variability of quadriceps muscle mass thickness [ICC: 0.99, 95% confidence interval (CI): 0.97-0.99, P < 0.001].
Muscle ultrasound assessment showed that RF mass was 1.34 cm (1.15-1.65) within the first 24 h post admission (n = 165), 1.2 ± 0.5 cm on day 3 (n = 15), and 1.25 ± 0.52 cm on day 5 (n = 10). RF_VI mass within the first 24 h was 2.52 cm (2.16-3.12), (n = 165), on day 3 was 2.41 ± 0.94 cm (n = 15), and on day 5 was 2.37 ± 0.8 cm (n = 10). During the first five days, RF mass presented a trend to decrease by 2.2% [(95%CI: - 0.21 to 0.15), n = 9; P = 0.729] and RF_VI mass by 3.5% [(95%CI: - 0.4 to 0.22), n = 9; P = 0.530].
Patients remained more in the ICU (47 ± 74 h vs 28 ± 46 h, P = 0.02), (Figure 3) and greater time on ventilator (17 ± 9 h vs 14 ± 9 h, P = 0.05), (Figure 4), if presented a combined RF_VI mass below the median value (2.5 cm) on day 1 (n = 80).
Patients with ICUAW on day 3 (n = 7), stayed more intubated on ventilator compared to patients with no ICUAW (n = 33), (44 ± 14 h vs 19 ± 9 h, P = 0.006), (Figure 5) and had also greater duration of extracorporeal circulation, (159 ± 91 min vs 112 ± 71 min, P = 0.025), (Figure 6).
This study investigated quadriceps muscle mass, as assessed by ultrasound, in patients who underwent cardiac surgery as well as factors associated with their ICU length of stay. The main results of the study demonstrated a trend to a decrease of quadriceps muscle mass thickness over the first wk post-admission in the ICU and that quadriceps muscle mass was associated with prolonged MV support and ICU stay.
The quadriceps muscle mass has shown a trend to decrease in the present study which reflects acute muscle mass wasting occurring early in cardiac surgery patients, post-ICU admission. Our results are in line with a recent observational study[3] and another pilot study[16] which reported a significant muscle loss of lower limbs within the first week of ICU hospitalization. A previous study[17] has shown similar results and it is notable that the largest muscle wasting is on average 10% and occurs during the first week of hospitalization[18].
The results of these studies confirm our finding that muscle mass wasting occurs during the first week of hospitalization. However, we reported a low rate of muscle mass loss in our study without statistical significance. Main explanation for these differentiate results is possibly due to our study population characteristics compared to general ICU patients. Cardiac surgery ICU patients mostly have a short ICU stay with less severity scores as shown from our study, making the ICUAW syndrome less pronounced. Interestingly, in a small subgroup of patients we observed a relative “increase” of muscle mass thickness during the first week, reflecting possibly muscle mass edema. We believe that this finding occurs probably due to the fluid overload necessary to preserve hemodynamic stability of post-operative cardiac surgery patients. However further studies are needed to elucidate this possible mechanism.
Another significant finding from the present study was that the size of muscle mass affected the length of ICU stay and the duration of MV. The prolonged hospitalization of patients with reduced muscle mass thickness on the first day post-cardiac ICU admission emphasizes the prognostic and clinical value of quadriceps muscle mass. Muscle mass wasting of psoas muscle has been previously associated with prolonged ICU stay in patients after cardiac surgery[7]. A recent study[19] stated that the muscle mass is an independent factor of mortality and serious morbidity in patients undergoing heart transplantation. Similar results have been reported by Yamashita et al[11], where skeletal muscle density seems to affect muscle function and mortality after cardiovascular surgery. However not all studies are in concordance; in a retrospective cohort study[10] they found that low psoas muscle mass is not related to the survival outcome of patients with asymptomatic abdominal aortic aneurysm.
A secondary finding in our study was that patients who developed ICU acquired weakness post-cardiac surgery had also greater duration of extracorporeal circulation and prolonged MV support. Kraft et al[20] reported that extracorporeal circulation is related to the development of systematic inflammatory response to patients undergoing cardiac surgery. However, a single-centre prospective randomized study[21], has shown that minimally invasive extracorporeal circulation in patients undergoing coronary artery bypass grafting improves postoperative health-related quality of life compared to conventional cardiopulmonary bypass. According to our findings, patients with muscle weakness had longer duration of mechanical ventilation support. Other previous studies[5,22] confirm our finding showing also that muscle weakness is associated with weaning failure and ICU mortality[23].
Assessing quadriceps muscle mass by ultrasound might help identify those patients at high risk of developing ICU acquired weakness post-cardiac surgery, but also patients that would stay longer in MV and in ICU. Frailty is an independent predictor of hospital mortality, prolonged ICU stay and mid-term survival for patients undergoing cardiac interventions[24]. Early mobilization and rehabilitation on the first postoperative days are beneficial in terms of increasing muscle strength and functional capacity even after ICU discharge and reducing ICU length of stay[25]. In particular those ICU patients with decreased quadriceps muscle thickness would possibly require earlier passive mobilization and intensified rehabilitation and if possibly, pre-habilitation prior to cardiac surgery to avoid ICUAW and post-cardiac surgery worse outcome. Previous studies have shown that neuromuscular electrical stimulation has local and systemic effects in critical ill patients[26,27] and might prevent muscle atrophy and reduce mechanical MV and ICU stay[28,29].
This observational study represents one of the first prospective studies investigating the clinical value of sonographically muscle mass assessment of patients after cardiac surgery and consists of the first study from a Greek cardiac surgery ICU. However, the present study has several limitations. This is an explorative study and the sample size was estimated based mainly on feasibility for a predefined certain period. For this reason, the study might have been underpowered to demonstrate quadriceps muscle thickness changes during ICU and association with ICU outcome. Although the number of patients enrolled to the study was large, the sample size for the observed effect size was small. Most patients remained in the ICU for a short time period, which did not allow the assessment of a sufficient number of patients until the seventh day post admission. However, results from the present study will allow future studies to perform power analysis and calculate sample size. The presence of muscle edema during the first postoperative days might have affected the ultrasound measurements too. Ultrasound scans are operator-dependent that may limit accuracy of results. In our study ultrasound measurements analyses were done by an experienced researcher with excellent intra-rater reproducibility results. This is consistent with previous intra- and inter-rater variability studies that have demonstrated high diagnostic accuracy results[30,31]. We were not be able to associate the grade of muscle mass decrease with long-term outcome after cardiac surgery due to short-term follow-up period of the present study; however, we did found an important association with duration of MV and ICU length of stay.
In conclusion, quadriceps muscle mass assessed by ultrasound presented with a trend to decrease during the first week post-ICU admission in patients after cardiac surgery. Quadriceps muscle mass is associated with the duration of MV support and ICU length of stay. Quadriceps muscle mass sonography seems to be a valid tool to assess preventive and therapeutic measures efficacy.
Intensive care unit (ICU) acquired weakness (ICUAW) remains a major cause of mortality and morbidity in critically ill patients. Ultrasonography is a valid diagnostic tool in critical ill patients who present muscle weakness. Muscle wasting may occur in cardiac surgery patients’ post-ICU admission affecting outcome. Early detection of muscle wasting may benefit interventions to decrease the duration of mechanical ventilation, increase muscle strength and improve their quality of life.
Sonography is a diagnostic method that allows the assessment of muscle mass in bedridden. It has been introduced recently as a valid and reliable to measure quantity and quality of skeletal muscle. It's a non-invasive, low-cost method offering real-time imaging without radiation exposure.
The clinical value of ultrasound-assessed muscle mass in patients post-cardiac surgery ICU admission.
An observational study was conducted to 221 consecutive patients after cardiac surgery at the Cardiac Surgery ICU of Onassis Cardiac Surgery Center from February 1, 2018 to May 15, 2018. Sonographic assessment of quadriceps muscle thickness and evaluation of muscle strength using the Medical Research Council (MRC) scale were performed until 7th day post-ICU admission or ICU discharge.
Among the 165 patients finally included in the analysis [median age: 71 (64-77) years], there was a decrease of femoris muscle thickness by 2.2% [(95% confidence interval (CI): - 0.21 to 0.15), n = 9; P = 0.729] and vastus intermedius mass (RF_VI mass) decreased by 3.5% [(95%CI: - 0.4 to 0.22), n = 9; P = 0.530]. Patients with RF_VI mass below the recorded median values (2.5 cm) on day 1 (n = 80) had a longer ICU length of stay compared to those patients with RF_VI mass above than 2.5 cm (n = 85), (47 ± 74 h vs 28 ± 45 h, P = 0.02) and remained to MV more time, (17 ± 9 h vs 14 ± 9 h, P = 0.05). Patients with ICUAW on day 3 (n = 7) had prolonged ventilation (44 ± 14 h vs 19 ± 9 h, P = 0.006) compared to patients with no ICUAW (n = 33). Moreover, the duration of extracorporeal circulation was greater for patients with low MRC scale score on day 3 (n = 7) compared with patients with higher MRC scale score (n = 33), (159 ± 91 min vs 112 ± 71 min, P = 0.025).
The results of the study have shown that there is a trend to a decreased muscle mass in patients after cardiac surgery post-ICU admission. Patients with decreased muscle mass remained more on ventilator and stayed longer in ICU. Sonographic assessment seems to be a valid method to quantify quadriceps muscle mass in patients after cardiac surgery.
We advocate further research to investigate muscle wasting in patients after cardiac surgery in order to implement preventive measures for ICU acquired weakness. Furthermore, it is recommended to identify a standardized protocol for sonographic muscle mass assessment to be implemented in research studies and intervention protocols.
We would like to thank Aggeliki Dorkofiti, professional English translator and editor for her contribution editing our manuscript and all ICU staff of Cardiac Surgery ICU of Onassis Cardiac Surgery Center for their continuous support throughout the whole study period.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El Amrousy D, Sicari R S-Editor: Liu M L-Editor: A E-Editor: Zhang YL
| 1. | Hermans G, Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care. 2015;19:274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 450] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 2. | Friedrich O, Reid MB, Van den Berghe G, Vanhorebeek I, Hermans G, Rich MM, Larsson L. The Sick and the Weak: Neuropathies/Myopathies in the Critically Ill. Physiol Rev. 2015;95:1025-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 254] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 3. | Turton P, Hay R, Taylor J, McPhee J, Welters I. Human limb skeletal muscle wasting and architectural remodeling during five to ten days intubation and ventilation in critical care - an observational study using ultrasound. BMC Anesthesiol. 2016;16:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Sekine H. [An immunohistochemical study of various breast tissues using CA15-3 (MAb 115D8 and MAb DF3)]. Gan No Rinsho. 1987;33:913-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 313] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 5. | Matthews EK, Petersen OH, Williams JA. Analysis of tissue amylase output by an automated method. Anal Biochem. 1974;58:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 357] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 6. | Moisey LL, Mourtzakis M, Cotton BA, Premji T, Heyland DK, Wade CE, Bulger E, Kozar RA; Nutrition and Rehabilitation Investigators Consortium (NUTRIC). Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit Care. 2013;17:R206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 367] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 7. | Zuckerman J, Ades M, Mullie L, Trnkus A, Morin JF, Langlois Y, Ma F, Levental M, Morais JA, Afilalo J. Psoas Muscle Area and Length of Stay in Older Adults Undergoing Cardiac Operations. Ann Thorac Surg. 2017;103:1498-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 8. | Hansen D, Linsen L, Verboven K, Hendrikx M, Rummens JL, van Erum M, Eijnde BO, Dendale P. Magnitude of muscle wasting early after on-pump coronary artery bypass graft surgery and exploration of aetiology. Exp Physiol. 2015;100:818-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | van Venrooij LM, Verberne HJ, de Vos R, Borgmeijer-Hoelen MM, van Leeuwen PA, de Mol BA. Postoperative loss of skeletal muscle mass, complications and quality of life in patients undergoing cardiac surgery. Nutrition. 2012;28:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Indrakusuma R, Zijlmans JL, Jalalzadeh H, Planken RN, Balm R, Koelemay MJW. Psoas Muscle Area as a Prognostic Factor for Survival in Patients with an Asymptomatic Infrarenal Abdominal Aortic Aneurysm: A Retrospective Cohort Study. Eur J Vasc Endovasc Surg. 2018;55:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Yamashita M, Kamiya K, Matsunaga A, Kitamura T, Hamazaki N, Matsuzawa R, Nozaki K, Tanaka S, Nakamura T, Maekawa E, Masuda T, Ako J, Miyaji K. Prognostic Value of Psoas Muscle Area and Density in Patients Who Undergo Cardiovascular Surgery. Can J Cardiol. 2017;33:1652-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Mourtzakis M, Parry S, Connolly B, Puthucheary Z. Skeletal Muscle Ultrasound in Critical Care: A Tool in Need of Translation. Ann Am Thorac Soc. 2017;14:1495-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 13. | Paris MT, Mourtzakis M, Day A, Leung R, Watharkar S, Kozar R, Earthman C, Kuchnia A, Dhaliwal R, Moisey L, Compher C, Martin N, Nicolo M, White T, Roosevelt H, Peterson S, Heyland DK. Validation of Bedside Ultrasound of Muscle Layer Thickness of the Quadriceps in the Critically Ill Patient (VALIDUM Study). JPEN J Parenter Enteral Nutr. 2017;41:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 14. | Connolly B, MacBean V, Crowley C, Lunt A, Moxham J, Rafferty GF, Hart N. Ultrasound for the assessment of peripheral skeletal muscle architecture in critical illness: a systematic review. Crit Care Med. 2015;43:897-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Bunnell A, Ney J, Gellhorn A, Hough CL. Quantitative neuromuscular ultrasound in intensive care unit-acquired weakness: A systematic review. Muscle Nerve. 2015;52:701-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Ten Haaf D, Hemmen B, van de Meent H, BovendʼEerdt TJH. The Magnitude and Time Course of Muscle Cross-section Decrease in Intensive Care Unit Patients. Am J Phys Med Rehabil. 2017;96:634-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, Hopkinson NS, Phadke R, Dew T, Sidhu PS, Velloso C, Seymour J, Agley CC, Selby A, Limb M, Edwards LM, Smith K, Rowlerson A, Rennie MJ, Moxham J, Harridge SD, Hart N, Montgomery HE. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1118] [Cited by in RCA: 1346] [Article Influence: 112.2] [Reference Citation Analysis (0)] |
| 18. | Joskova V, Patkova A, Havel E, Najpaverova S, Uramova D, Kovarik M, Zadak Z, Hronek M. Critical evaluation of muscle mass loss as a prognostic marker of morbidity in critically ill patients and methods for its determination. J Rehabil Med. 2018;50:696-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Bibas L, Saleh E, Al-Kharji S, Chetrit J, Mullie L, Cantarovich M, Cecere R, Giannetti N, Afilalo J. Muscle Mass and Mortality After Cardiac Transplantation. Transplantation. 2018;102:2101-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Kraft F, Schmidt C, Van Aken H, Zarbock A. Inflammatory response and extracorporeal circulation. Best Pract Res Clin Anaesthesiol. 2015;29:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Anastasiadis K, Antonitsis P, Kostarellou G, Kleontas A, Deliopoulos A, Grosomanidis V, Argiriadou H. Minimally invasive extracorporeal circulation improves quality of life after coronary artery bypass grafting. Eur J Cardiothorac Surg. 2016;50:1196-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Dres M, Dubé BP, Mayaux J, Delemazure J, Reuter D, Brochard L, Similowski T, Demoule A. Coexistence and Impact of Limb Muscle and Diaphragm Weakness at Time of Liberation from Mechanical Ventilation in Medical Intensive Care Unit Patients. Am J Respir Crit Care Med. 2017;195:57-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 306] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 23. | Dres M, Jung B, Molinari N, Manna F, Dubé BP, Chanques G, Similowski T, Jaber S, Demoule A. Respective contribution of intensive care unit-acquired limb muscle and severe diaphragm weakness on weaning outcome and mortality: a post hoc analysis of two cohorts. Crit Care. 2019;23:370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Reichart D, Rosato S, Nammas W, Onorati F, Dalén M, Castro L, Gherli R, Gatti G, Franzese I, Faggian G, De Feo M, Khodabandeh S, Santarpino G, Rubino AS, Maselli D, Nardella S, Salsano A, Nicolini F, Zanobini M, Saccocci M, Bounader K, Kinnunen EM, Tauriainen T, Airaksinen J, Seccareccia F, Mariscalco G, Ruggieri VG, Perrotti A, Biancari F. Clinical frailty scale and outcome after coronary artery bypass grafting. Eur J Cardiothorac Surg. 2018;54:1102-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 25. | Tipping CJ, Harrold M, Holland A, Romero L, Nisbet T, Hodgson CL. The effects of active mobilisation and rehabilitation in ICU on mortality and function: a systematic review. Intensive Care Med. 2017;43:171-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 395] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 26. | Angelopoulos E, Karatzanos E, Dimopoulos S, Mitsiou G, Stefanou C, Patsaki I, Kotanidou A, Routsi C, Petrikkos G, Nanas S. Acute microcirculatory effects of medium frequency versus high frequency neuromuscular electrical stimulation in critically ill patients - a pilot study. Ann Intensive Care. 2013;3:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Stefanou C, Karatzanos E, Mitsiou G, Psarra K, Angelopoulos E, Dimopoulos S, Gerovasili V, Boviatsis E, Routsi C, Nanas S. Neuromuscular electrical stimulation acutely mobilizes endothelial progenitor cells in critically ill patients with sepsis. Ann Intensive Care. 2016;6:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Gerovasili V, Stefanidis K, Vitzilaios K, Karatzanos E, Politis P, Koroneos A, Chatzimichail A, Routsi C, Roussos C, Nanas S. Electrical muscle stimulation preserves the muscle mass of critically ill patients: a randomized study. Crit Care. 2009;13:R161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 221] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 29. | Routsi C, Gerovasili V, Vasileiadis I, Karatzanos E, Pitsolis T, Tripodaki E, Markaki V, Zervakis D, Nanas S. Electrical muscle stimulation prevents critical illness polyneuromyopathy: a randomized parallel intervention trial. Crit Care. 2010;14:R74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 192] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 30. | Pardo E, El Behi H, Boizeau P, Verdonk F, Alberti C, Lescot T. Reliability of ultrasound measurements of quadriceps muscle thickness in critically ill patients. BMC Anesthesiol. 2018;18:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 31. | Kumar R, Shah TH, Hadda V, Tiwari P, Mittal S, Madan K, Khan MA, Mohan A. Assessment of quadriceps muscle thickness using bedside ultrasonography by nurses and physicians in the intensive care unit: Intra- and inter-operator agreement. World J Crit Care Med. 2019;8:127-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |