Published online Jun 26, 2020. doi: 10.4330/wjc.v12.i6.291
Peer-review started: February 17, 2020
First decision: March 15, 2020
Revised: March 26, 2020
Accepted: May 15, 2020
Article in press: May 15, 2020
Published online: June 26, 2020
Processing time: 130 Days and 16.4 Hours
Sarcoidosis is a rare multisystem disease characterized histologically by non-caseating granuloma formation in the affected organ. While cardiac sarcoidosis is found on autopsy in up to 25% of sarcoidosis cases, it is still underdiagnosed and is associated with a poor prognosis. Although the etiology of sarcoidosis remains unclear, an antigen triggered exaggerated immune response has been hypothesized. Early detection and prompt management of cardiac sarcoidosis remains pivotal.
A 60-year-old female, with pulmonary sarcoidosis in remission, presented to the cardiology outpatient clinic for evaluation of weeks-long dyspnea on moderate exertion (New York Heart Association class II) that was relieved by rest. Submaximal exercise stress test showed multifocal ventricular extrasystoles, followed by a self-limiting torsades de pointes. Cardiac magnetic resonance imaging showed nondilated and normotrophic left ventricle with basoseptal and mid-septal dyskinesis. The magnetic resonance imaging-derived left ventricular ejection fraction was 45%. Delayed enhancement showed patchy transmural fibrosis of the septum and hyperenhancement of the papillary muscles, all in favor of extensive cardiac involvement of sarcoidosis. A double-chamber implantable cardiac defibrillator was implanted, and methylprednisolone (12 mg/d) and methotrexate (12.5 mg/wk) treatment was initiated. Follow-up and implantable cardiac defibrillator interrogation showed episodes of asymptomatic nonsustained ventricular tachycardia and an asymptomatic episode of nonsustained ventricular tachycardia ending by the first antitachycardia pacing run.
Along an extensive review of the literature, this unusual case report highlights the importance of early detection of cardiac involvement of sarcoidosis, in order to avoid potential complications and increase survival.
Core tip: Cardiac sarcoidosis (CS) remains an underdiagnosed illness bearing a poor prognosis. While a number of reviews in the literature have tackled the treatment of CS, no published guidelines and only consensus publications of global experts’ opinions are available for the diagnosis. Our objective with this case report and literature review was to consolidate the available literature for a better delineation of the diagnosis and treatment of CS.
- Citation: Ghafari C, Vandergheynst F, Parent E, Tanaka K, Carlier S. Exercise-induced torsades de pointes as an unusual presentation of cardiac sarcoidosis: A case report and review of literature. World J Cardiol 2020; 12(6): 291-302
- URL: https://www.wjgnet.com/1949-8462/full/v12/i6/291.htm
- DOI: https://dx.doi.org/10.4330/wjc.v12.i6.291
In 1869, Dr. Jonathan Hutchinson introduced the modern description of cutaneous sarcoidosis[1]. The name “sarcoidosis” was bestowed in 1899 by Dr. Boeck, for its sarcoid nature according to its derivation from sarcoma cells[2]. Dr. Bernstein was the first to recognize cardiac involvement in 1929 [known as cardiac sarcoidosis (CS)], and in 1952, Drs. Longcope and Freiman reported myocardial involvement in 20% of 92 autopsy cases of sarcoidosis[3].
Today, sarcoidosis is known to be more common in younger adults, with African-Americans having 3- to 4-fold increased risk for the disease (as compared to Caucasian); the overall prevalence ranges between 4.7 and 64 per 100000 population[4,5]. While the etiology of sarcoidosis remains unclear, it has been hypothesized that it is possibly precipitated by an antigen triggering an exaggerated immune response, leading to granuloma formation in different organs[6]. This response mainly affects – but is not limited to – the lungs, skin, eyes, and lymph nodes.
Cardiac involvement is diagnosed clinically in as few as 5% of sarcoidosis cases[7], and most of those cases come from Japan. CS is clinically silent in 20%-25% of those diagnosed and isolated in two-thirds of patients. For all, it bears a poor prognosis[5,8]; accounting for up to 85% of deaths from sarcoidosis[9], mainly secondary to ventricular arrhythmias[10]. Unfortunately, to date, there are no existing reliable standard references to diagnose CS.
The guidelines for the diagnosis of CS published by the Japanese Ministry of Health and Welfare are not systematically endorsed and, compared to imaging techniques, have reduced sensitivity and specificity[11]. The Heart Rhythm Society (HRS) released a more contemporary expert consensus statement for the diagnosis of CS[10], which was revised in 2017 by Terasaki et al[11]. We report herein the diagnosis and treatment of an unusual presentation of CS via an exercise-induced torsades de pointes (TdP) in a patient with known pulmonary sarcoidosis.
A 60-year-old Caucasian female patient consulted our cardiology outpatient clinic for complaint of dyspnea on moderate exertion (New York Heart Association class II) which had lasted for the past few weeks, and which she reported was relieved by rest.
The patient estimated that her symptoms started a couple of weeks prior to presentation and reported increasing frequency in the last couple of days. She denied any chest pain, palpitations, orthopnea, lower leg edema, paroxysmal nocturnal dyspnea, change in weight, or syncope.
The patient is a nonsmoker, known to have a 10-year history of type 2 diabetes mellitus, essential arterial hypertension, dyslipidemia, and untreated asymptomatic stable pulmonary sarcoidosis (diagnosed 5 years prior, according to mediastinal lymph node biopsy findings). Her past medical history also included resected epidermoid carcinoma of the tongue. A coronary angiography given 5 years prior showed a 40%-50% mid-left anterior descending artery stenosis.
Her routine medications included bisoprolol (2.5 mg/d), acetylsalicylic acid (80 mg/d), atorvastatine (10 mg/d), metformin (850 mg twice/d), gliquidone (15 mg/d), and dulaglutide (1.5 mg/wk).
There was no family history of sudden cardiac death, and the patient denied any recent severe illness or respiratory symptoms. A recent abdominal and thoracic computed tomography (CT) scan revealed several infracentimetric mediastinal and hilar lymph nodes.
On physical examination, the patient showed no signs of distress. The vital signs displayed temperature of 37.2 °C, blood pressure of 130/70 mmHg, heart rate of 50 beats/min, and oxygen saturation of 97% on room air. There was no jugular vein distention nor carotid bruit. Peripheral pulses were present and equal. No skin lesions were noted. The heart rate was regular, with an occasional premature beat. The first and second heart sounds were heard, and no murmurs, rubs or gallops were noted. The lung and abdomen exams were unremarkable. There was no lower leg edema.
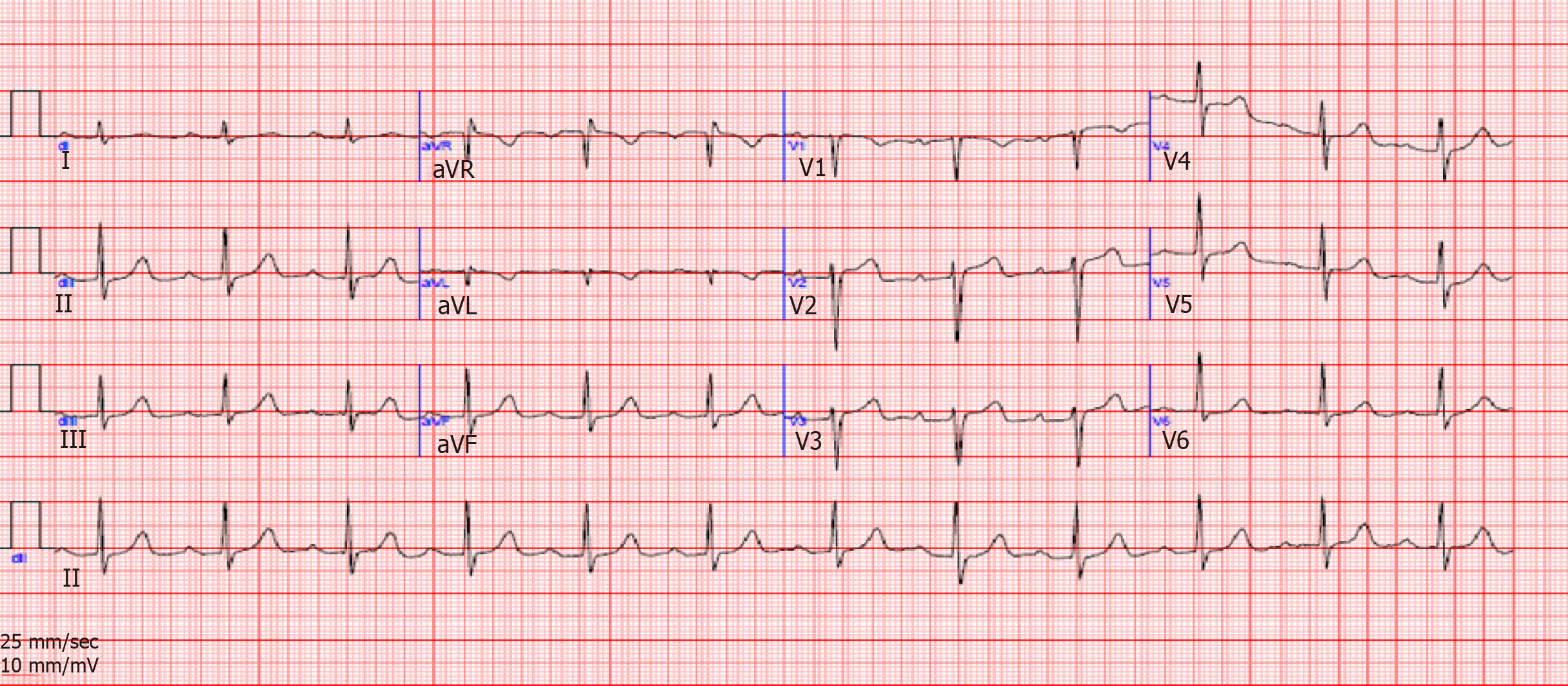
Laboratory work-up (Table 1) was remarkable for an elevated level of glycated hemoglobin, normal levels of potassium and cardiac ultrasensitive troponin, and normal thyroid findings. Angiotensin converting enzyme levels were also within normal range. Notable finding on electrocardiogram was a regular sinus rhythm with prolonged PR interval and a QTc at 450 ms (Figure 1).
| Test | Results | Reference range |
| Hematology | ||
| White blood cells | 6.6 | 4.2-11.4 × 103/mm3 |
| Hemoglobin | 12.7 | 11.8-15.6 g/dL |
| Hematocrit | 40.5 | 35.3%-46.1% |
| Platelets | 275 | 174-402 × 103/mm3 |
| Biochemistry | ||
| Sodium | 140 | 132-145 mmol/L |
| Potassium | 4.3 | 3.5-5.1 mmol/L |
| Magnesium | 0.94 | 0.6-1.1 mmol/L |
| Calcium | 2.27 | 2.1-2.55 mmol/L |
| Urea | 25 | 15-50 mg/dL |
| Creatinine | 0.68 | 0.2-1.2 mg/dL |
| HbA1c | 6.7 | 4.0%-6.0% |
| Angiotensin converting enzyme | 35 | 20-70 U/L |
| Cardiac markers | ||
| Creatinine kinase MB | 2.8 | 0.0-5.0 ng/mL |
| Troponin I (HS) | 3 | 0-15 pg/mL |
| Cytometry markers | ||
| CD4/CD8 ratio | 2.78 | 1.20-2.40 |
| CD3-16+56+ lymphocytes | 8.3 | 5%-15% |
| CD19 (pan B) lymphocytes | 13.40 | 5%-20% |
| Thyroid panel | ||
| TSH | 1.45 | 0.35-4.95 mU/L |
| Free T4 | 1.03 | 0.70-1.48 ng/dL |
Initial cardiac ultrasound showed basal-septal akinesia, with a globally preserved left ventricular systolic ejection fraction by the modified Simpson method, normal left and right chamber sizes, and a normal tricuspid aortic valve associated with a trace insufficiency. The ascending aorta measured 41 mm. No other abnormalities were found.
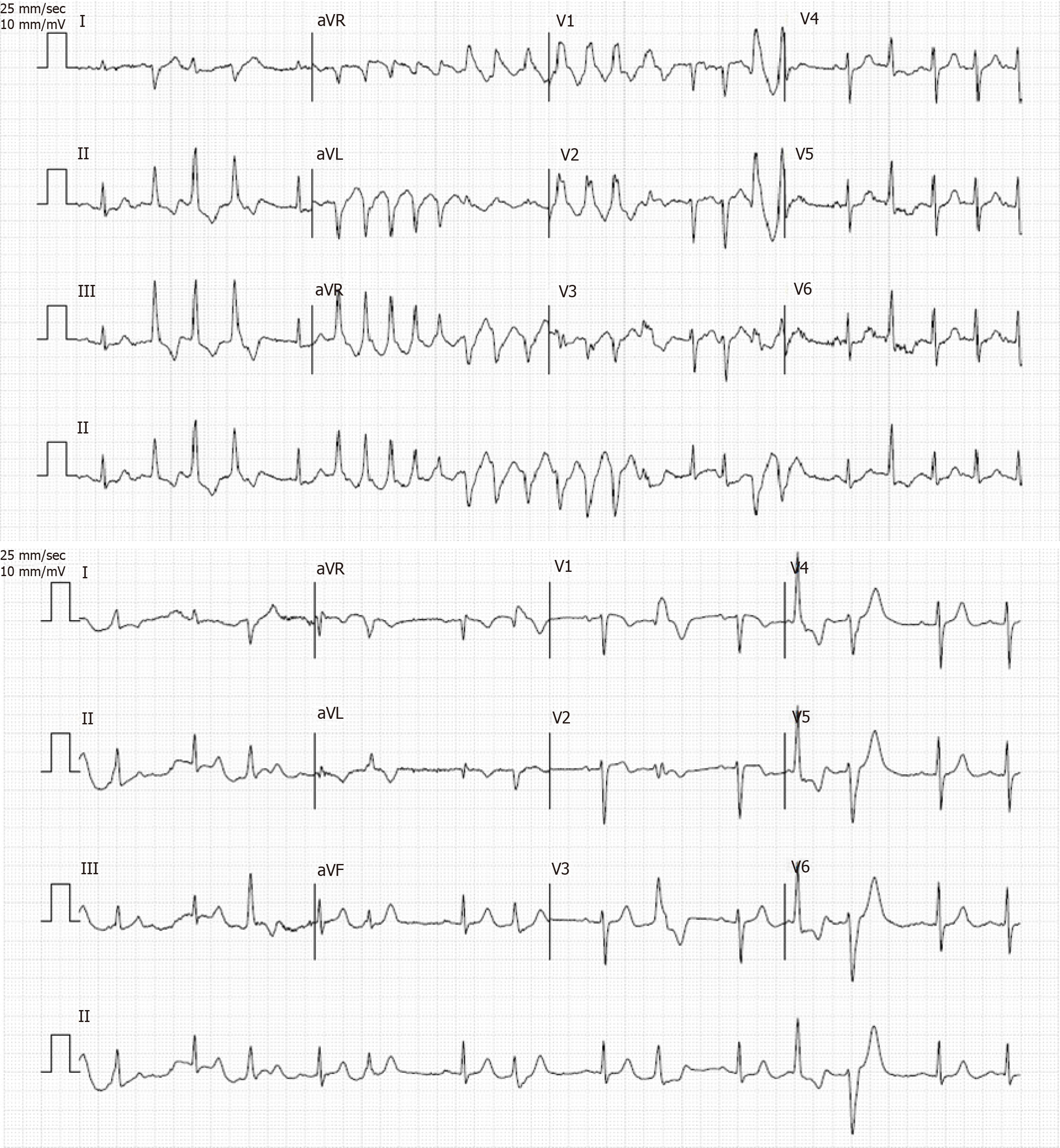
A submaximal exercise stress test reaching 67% maximal predicted heart rate for the patient’s age stopped due to multifocal ventricular extrasystoles followed by a self-limiting TdP at 2 min, with no syncope or chest pain (Figure 2). The maximal blood pressure was 152/70 mmHg, and the recovery was notable for multiple multifocal ventricular extrasystoles.
We ordered an increase in the patient’s bisoprolol (from 2.5 mg to 5 mg) and stopped the dulaglutide. The patient was admitted to the hospital for a diagnostic coronary angiography, which showed a stable 40%-50% mid-left anterior descending plaque. Cardiac continuous monitoring showed several ectopic supraventricular beats along abundant polymorphic ventricular extrasystoles and intermittent type I second degree atrioventricular block (Mobitz I).
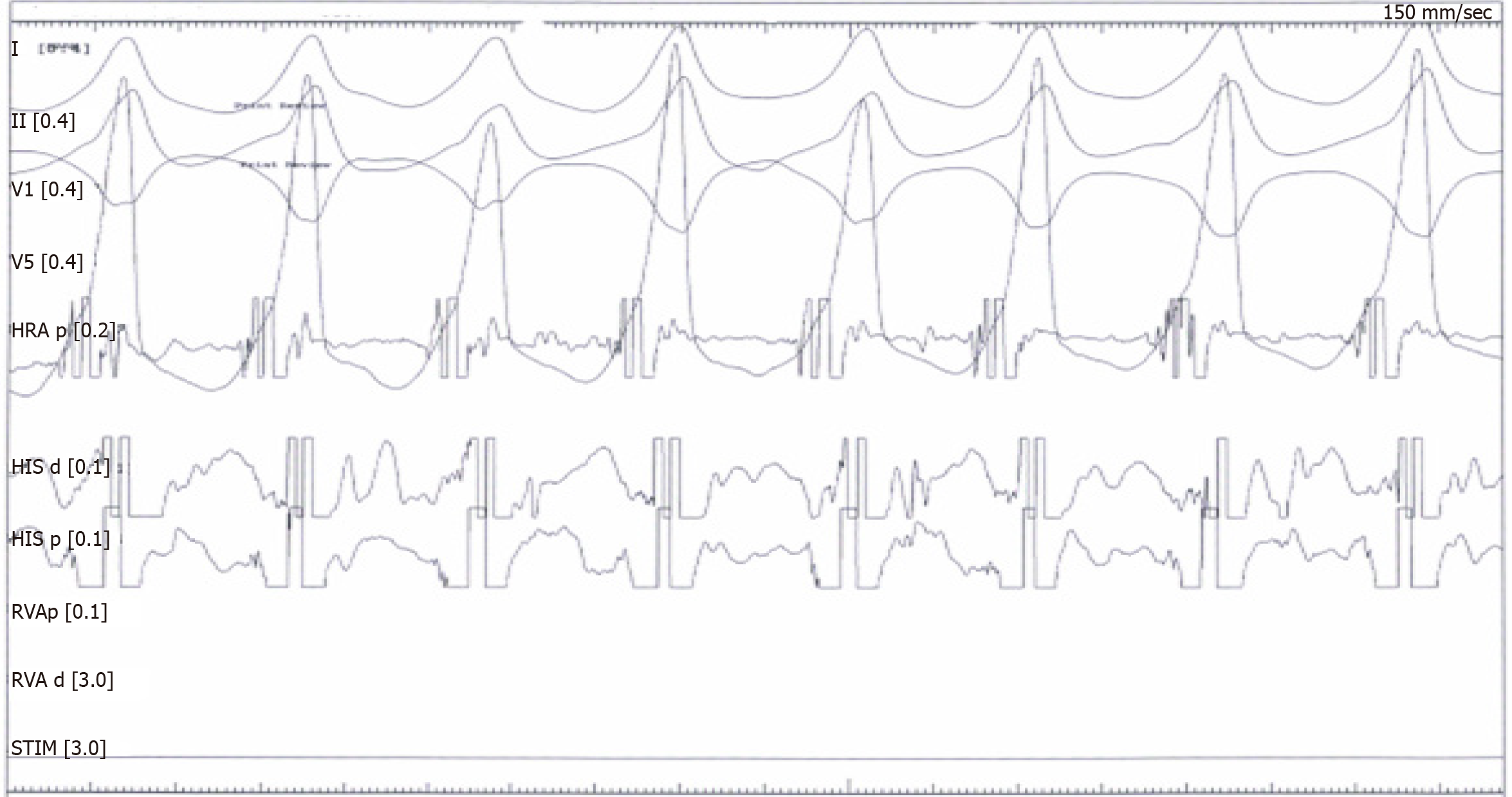
A cardiac electrophysiology study was undertaken, inducing a poorly-tolerated, sustained monomorphic ventricular tachycardia (at a rate of 240 beats/min) and terminated by a burst (Figure 3).
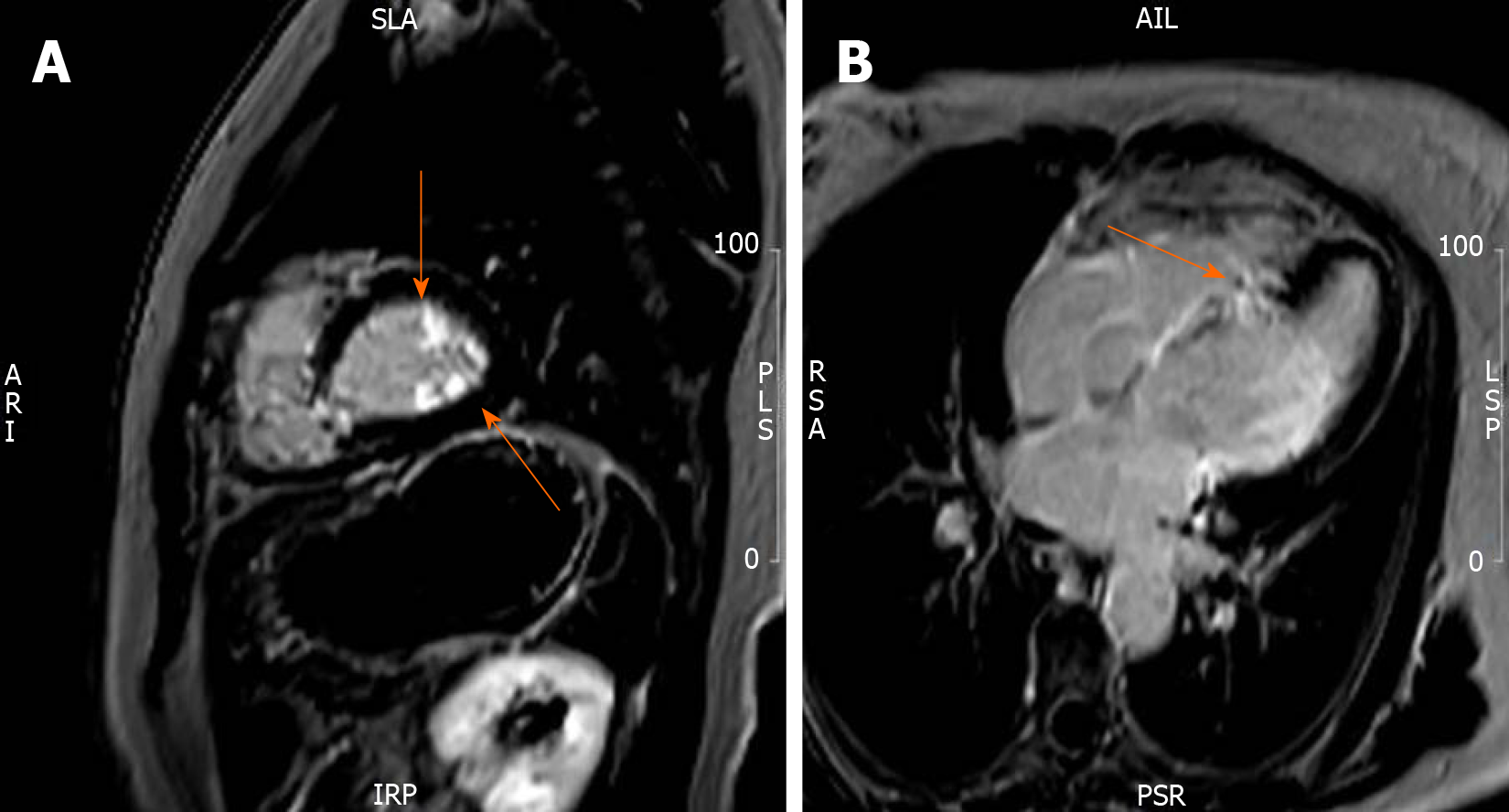
Cardiac magnetic resonance imaging (CMR) showed nondilated and normotrophic left ventricle with basoseptal and mid-septal dyskinesis. The MRI-derived left ventricular ejection fraction was 45%. Delayed enhancement showed patchy transmural fibrosis of the septum and hyperenhancement of the papillary muscles, all in favor of extensive cardiac involvement of sarcoidosis (Figure 4). A whole-body positron emission tomography (PET)/CT scan showed no myocardial uptake.
Based on CMR and malignant arrhythmia, a CS with pulmonary sarcoidosis in remission diagnosis was made.
A double-chamber implantable cardiac defibrillator (ICD) was implanted for secondary prevention despite an ejection fraction of 45%, and the patient was started on methylprednisolone (12 mg/d) and methotrexate (12.5 mg/wk).
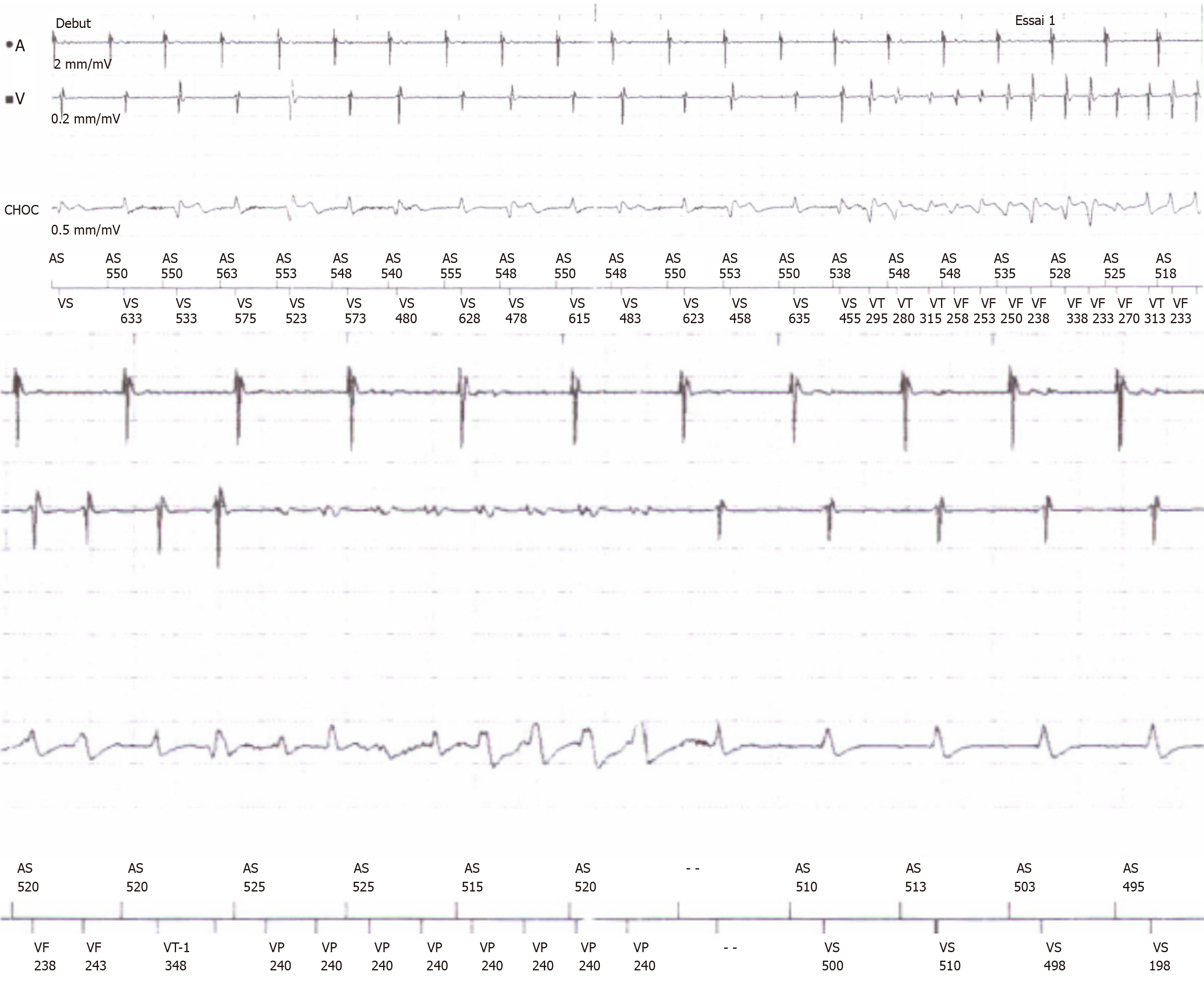
Regular ICD interrogation showed episodes of asymptomatic nonsustained ventricular tachycardia and an asymptomatic episode of nonsustained ventricular tachycardia ending by the first antitachycardia pacing run (Figure 5).
Although known to be a systemic inflammatory disease, the etiology of sarcoidosis remains unclear; infectious and environmental agents have been suggested as potential factors triggering a T helper (Th) cell-induced granuloma formation, which can later either resolve or progress to fibrosis[10]. Studies have begun to define the pathogenic processes of sarcoidosis. Early in the course of the disease, exposure to the culprit antigen activates phagocytes and CD4-positive T cells, with a Th1 profile secreting interleukin-2 and interferon-γ. Later on, the cytokine profile shifts towards a Th2 cell response, exerting an anti-inflammatory effect and creating scarring[12-14].
Three successive histological stages lead to CS - edema, granulomatous infiltration, and fibrosis, the latter of which is responsible for the characteristic post-inflammatory scarring[15]. These scaring zones can involve any portion of the heart (the pericardium, the myocardium, and the endocardium), with the myocardium being the most frequently affected by far. The left ventricular free wall is the predominant site of involvement, followed by the basal part of the ventricular septum and the right ventricular free wall[3,16]. From a histological standpoint, the typical granulomas characterizing sarcoidosis are noncaseating and consist of aggregates of epithelioid histiocytes, with minimal inflammation and large multinucleated giant cells. Advanced cases, however, develop a fibrotic reaction, causing permanent tissue damage.
Genetic factors have also been implicated in CS, with monozygotic twins being more likely to develop the disease. A Case Controlled Etiologic Sarcoidosis Study also concluded that first-degree relatives with sarcoidosis had a 5 times higher relative risk of developing the disease than control groups[17]. Finally, the HLA–DQB1*0601 type has been reported as associated with CS[18].
CS presentation ranges from asymptomatic conduction abnormalities and congestive heart failure to fatal ventricular arrhythmias. While the most important predictor of mortality is the left ventricular ejection fraction[12], the severity of the disease is not proportional to the number of granulomas[15]. In a retrospective study by Chapelon-Abric et al[19], CS was observed most commonly in the setting of severe multivisceral disease, presenting unusual clinical and imaging cardiac signs. Complete heart block is one of the most common findings in patients with CS, occurring mostly at a younger age and in 30% of patients[3], with either normal or reduced left ventricular ejection fraction. Complete atrioventricular block and bundle branch block occur in 23%-30% and 12%-32% of CS cases respectively[13]. These manifestations are caused by the involvement of the basal septum affected by scar tissue, granulomas, or ischemia in the conduction system secondary to involvement of the nodal artery.
Sudden death caused by ventricular arrhythmias or complete heart block account for up to 65% of CS deaths[3] and represent the initial presentation in 40% of patients. As is the case with our patient, ventricular tachycardia is also the most common reported arrhythmia noted in CS[3,20,21], with an incidence of 23%[9]. To the best of our knowledge, there has been no report of TdP. Arrhythmia mechanisms are postulated to be secondary to sarcoid granulomas becoming foci for abnormal automaticity or serving to disperse ventricular activation[14,22]. The healing of granulomas prompted by corticotherapy provides a substrate for reentrant arrhythmias to create a slow conduction zone in and around the scar area[23]. Active inflammation may also play a role in promoting monomorphic ventricular tachycardia due to reentry, either by triggering it with ventricular ectopy or by slowing conduction in diseased scarred tissue[23,24].
Supraventricular arrhythmias may also occur but are less common (15%-17%) and are mostly the result of atrial dilatation or pulmonary involvement. Congestive heart failure, with features of dilated cardiomyopathy, is another common presenting feature[14,25] and accounts for 25%-75% of cardiac deaths in patients with CS. Another rare presentation is acute sarcoid myocarditis, characterized by high-degree atrioventricular block, malignant ventricular arrhythmias, and congestive heart failure[26].
Poor outcome is also associated with pulmonary hypertension secondary to extrinsic compression of pulmonary arteries by enlarged lymph nodes or cor pulmonale (occurring in patients with pulmonary sarcoidosis and hypoxic vasoconstriction secondary to CS)[13].
Laboratory tests are generally nonspecific and, hence, nondiagnostic for sarcoidosis as illustrated in our case. A panel of tests, however, may support a clinical suspicion; these findings include anemia, elevation of sedimentation rate, and hypercalcemia secondary to the uncontrolled synthesis of 1,25-dihydroxyvitamin D3 by macrophages[27]. Serum angiotensin converting enzymes are often elevated (60%) and are useful for monitoring response to therapy[16,28,29]. Similarly, troponin levels, which might be elevated at presentation, usually normalize within 4 wk of steroid treatment initiation. Both levels were within normal ranges for our patient.
Nonspecific electrocardiogram changes are found in as many as 50% of patients with sarcoidosis, with or without clinical cardiac involvement[16]. QT dispersion on surface 12-lead electrocardiogram may be a predictor of sudden cardiac death[30]; carvedilol has been reported to significantly decrease the QT dispersion[31]. CS should be excluded in young patients with a high-degree atrioventricular block not explained by any coronary or hereditary cause[16] and in patients with a fragmented QRS or bundle-branch block pattern[32].
Transthoracic echocardiography, although useful for the assessment of the left ventricular systolic or diastolic function, lacks specificity in most of the CS cases. In CS, the spectrum of two-dimensional echocardiographic abnormalities include abnormal septal thickening or thinning, dilation of the left ventricle, systolic and diastolic dysfunction of the left ventricle, and regional wall motion abnormalities[5,16]. Thinning of the basal anterior septum in a young patient with dilated cardiomyopathy, although uncommon, is highly suggestive of CS[33]. Findings of left ventricular systolic dysfunction and left ventricular dilatation are predictors of mortality in CS and an ICD is recommended for primary prevention when left ventricular ejection fraction < 35%. When compared with age- and sex-matched groups, patients with CS were found to have an impaired global longitudinal strain[34,35].
Nonspecific cardiomegaly is often present on chest X-ray of patients with CS; if pulmonary involvement is also present, hilar adenopathy and/or pulmonary parenchymal changes can be noted, prompting performance of a CT scan. High-resolution CT scan is particularly sensitive for the detection of pulmonary involvement, whereas standard contrast-enhanced CT may be better for delineation of mediastinal and hilar lymphadenopathy[36]. Due to its high spatial and soft tissue resolution, CMR imaging allows for a noninvasive detection of scarring, biventricular dysfunction, edema, and myocardial perfusion defects. CMR relies on identifying areas of mid-wall and subepicardial late gadolinium enhancement, which corresponds to fibrosis as noted with our patient. Additional findings on CMR include thinning of the ventricular wall[5] and, on T2-weighted sequences, presence of edema and global or regional ventricular dysfunction[13]. According to Smedema et al[37], CMR sensitivity and specificity were respectively 100% and 78% in the diagnosis of cardiac involvement in patients with CS, who had been diagnosed using the Japanese Ministry of Health and Welfare guidelines[16].
Nuclear imaging provides an effective mean for assessing myocardial perfusion and inflammation. The fibro granulomatous lesions in the myocardium display segmental areas of decreased uptake. Thallium 201 or technetium 99m sestamibi are used most. Dipyridamole is able to differentiate between CS and coronary artery disease. This effect, termed “reverse distribution”, may be due to possible microvascular vasoconstriction in CS, where myocardial perfusion abnormalities are reversible after pharmacological dilation[13].
PET is also used to identify CS and assess its severity. As such, it has emerged as a particularly useful tool for the follow-up of patients with CS. PET has the advantage of being applicable to patients with pacemakers or ICDs. The 18F-fluorodeoxyglucose (FDG) radiopharmaceutical – a glucose analog that is generally useful in differentiating between normal and active inflammatory lesions – accumulates in inflammatory cells that have a higher metabolic rate and rate of glucose utilization[5]. FDG was also found to have higher binding ability than either thalium-201 or gallium-67[12].
Finally, endomyocardial biopsy in CS has low sensitivity due to the focal nature of the disease but may be necessary in cases of negative extracardiac biopsy yields. In order to increase sensitivity, electrophysiological or image-guided biopsy procedures are now recommended[38,39].
The prognosis of patients with symptomatic CS was found to be limited to 5 years[3,12], while more recent studies report up to 50% survival at 5 years[26,40]. Whether this is due to earlier detection of the disease or advances in therapy is still unknown. Independent predictors of mortality are New York Heart Association functional class, left ventricular end diastolic diameter, and sustained ventricular tachycardia.
In 2014, the first international guidelines for the diagnosis of CS were published by experts chosen by the HRS[10]. In 2017, Terasaki et al[11] published revised guidelines for the diagnosis of CS (Table 2).
| Diagnosis of cardiac sarcoidosis follows one of two pathways: |
| Histological diagnosis |
| Cardiac biopsy specimens demonstrating noncaseating epithelioid cell granuloma. |
| Clinical diagnosis |
| When extracardiac granulomas are found along with clinical findings strongly suggestive of cardiac involvement; or when the patient shows clinical findings strongly suggestive of pulmonary or ophthalmic sarcoidosis; at least two of the five characteristic laboratory findings of sarcoidosis; and clinical findings strongly suggestive of cardiac involvement |
| Clinical findings that satisfy the following strongly suggest the presence of cardiac involvement: |
| (1) More than two major criteria are met, OR |
| (2) One major criterion and two or more minor criteria are met |
| Major criteria: |
| Advanced atrioventricular block or malignant ventricular arrhythmia |
| Basal thinning of the ventricular septum or abnormal wall anatomy |
| Positive cardiac gallium uptake |
| Left ventricular contractile dysfunction |
| LGE on CMR showing delayed contrast enhancement of the myocardium |
| Minor criteria: |
| Abnormal ECG findings |
| Perfusion defects detected by myocardial perfusion scintigraphy |
| Interstitial fibrosis by endomyocardial biopsy |
| Laboratory findings |
| (1) Bilateral hilar lymphadenopathy |
| (2) High serum angiotensin-converting enzyme level or elevated serum lysozyme levels |
| (3) High serum soluble interleukin-2 receptor levels |
| (4) Significant tracer accumulation in 67Ga citrate scintigraphy or 18F-FDG PET |
| (5) A CD4/CD8 ratio of > 3.5 in broncho-alveolar lavage fluid |
Despite the paucity of data and controversy about its clinical efficacy, most experts recommend treatment of CS by corticosteroid therapy to control the inflammation, prevent fibrosis, and protect against any deterioration of the cardiac function[16,29]. The optimal doses of corticosteroids and how to best assess response to therapy also remain unknown, with no significant difference in survival curves for patients treated with a high initial dose vs a low initial dose[41,42]. Corticosteroid treatment may halt the progression of the disease but does not prevent ventricular arrhythmias[29]. Treatment was shown to be beneficial for CS patients with preserved left ventricular ejection fraction but did not show improvement of patients with a severely reduced left ventricular ejection fraction[43]. Ballul et al[44] recently suggested that the use of high-dose corticosteroids along with immunotherapeutic agents was associated with a better outcome. Treatments with methotrexate, azathioprine, cyclophosphamide, and infliximab have been studied but there is no evidence of superiority for any[19,45].
Antiarrhythmic treatment and β-blockers are also often needed in the management of CS. While β-blockers increase the risk of atrioventricular block, amiodarone increases the risk of restrictive lung disease; therefore, the use of these agents should be weighted. Class Ic drugs are usually avoided because of their inherent risk of structural heart disease[46].
Ablation of ventricular arrhythmias produces modest outcome, reflecting the extensive scarring of the myocardium in most CS. As such, ablation is considered as a final step in cases with refractory disease[16,29]. Recent studies have shown that catheter ablation of refractory ventricular tachycardia is a safe and effective approach and can decrease the arrhythmia burden by 88.4%[14,47].
Given these limitations and the fact that limited data is present for risk stratification for sudden cardiac death, implantation of an ICD is a class I indication for secondary prevention and should be considered as primary therapy for patients with CS with low ejection fraction and/or ventricular arrhythmias induced upon electrophy-siological study[29]. Inappropriate ICD shocks were reported in one-third of patients with CS and ICD implanted for primary or secondary prevention due to supraventricular arrhythmias[48]. The indications for permanent pacing are similar to those in patients without CS. There was a class I indication for ICD and the occurrence of VT during follow-up confirmed its need.
Cardiac transplantation is reserved for end-stage disease patients refractory to therapy. The major indications for cardiac transplantation are resistant ventricular tachyarrhythmias and severe heart failure in young patients. Recurrent disease in a transplanted patient, although rare under low-dose corticosteroids and immunosuppressive therapies, can occur[49].
Although all patients with extra-CS should be referred for cardiac evaluation, the routine use of advanced cardiac imaging remains limited to symptomatic patients. Although these imaging modalities appear to be lacking in diagnostic value for minor disease, the HRS expert consensus states that screening for CS by CMR and/or FDG-PET/CT is recommended for patients with biopsy-proven extra-CSA with signs or symptoms of cardiac involvement or patients with no prior history of sarcoidosis but with unexplained high-degree atrioventricular block or sustained ventricular tachyarrhythmia of unknown etiology.
To the best of our knowledge, the presentation of CS by TdP has not yet been reported in the literature. For our case, the work-up by cardiac echography was nondiagnostic. Given the high suspicion for CS, CMR and PET/CT were used to confirm the diagnosis. A sustained ventricular tachycardia was noted on cardiac electrophysiology, which led to the implantation of an ICD. Ultimately, the patient was treated by an intermediate dose of corticotherapy and methotrexate, a choice of treatment based on the patient’s co-morbidities. We hypothesized that the ventricular arrhythmia seen with our patient was secondary to a substrate due to myocardial inflammation and/or a scar-related reentry. The patient showed an advanced AV block, which could be attributed either to her underlying disease or her γ-blocker. The borderline QTc we observed was hypothesized to be secondary to her medications.
CS, although uncommon, should be considered in patients with extra-cardiac disease or unexplained cardiomyopathy, especially in young patients. Despite recent advances in cardiac imaging, CS still remains a challenge to diagnose and available guidelines are limited to expert’s recommendations. The clinical spectrum of CS is highly variable, ranging from conduction abnormalities and tachyarrhythmias to heart failure. Early diagnosis of the disease is crucial for maximizing survival following therapy. FDG-PET/CT and CMR are pivotal elements of the diagnosis, given that other modalities present a lower sensitivity. Due to the high risk of sudden cardiac death, ICD implantation must be considered early in the disease due to the high risk of tachyarrhythmias. Steroid therapy, although lacking randomized clinical trials, remains the cornerstone of the medical treatment. Alternative treatments include methotrexate, azathiorpine, infliximab, and antimalarial drugs. Close follow-up is mandatory during treatment.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Carlier Stéphane ESC (36015).
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Belgium
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D, D, D
Grade E (Poor): 0
P-Reviewer: Anan R, Barik R, Falconi M, Karatza AA, Kharlamov AN, Nurzynska D, Ueda H, Vidal-Perez R S-Editor: Dou Y L-Editor: A E-Editor: Qi LL
| 1. | James DG, Sharma OP. From Hutchinson to now: a historical glimpse. Curr Opin Pulm Med. 2002;8:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58:1204-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 655] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 3. | Roberts WC, McAllister HA, Ferrans VJ. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). Am J Med. 1977;63:86-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 571] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 4. | Rybicki BA, Major M, Popovich J, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 618] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 5. | Birnie DH, Kandolin R, Nery PB, Kupari M. Cardiac manifestations of sarcoidosis: diagnosis and management. Eur Heart J. 2017;38:2663-2670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Müller-Quernheim J. Sarcoidosis. Lancet. 2014;383:1155-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 766] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 7. | Hulten E, Aslam S, Osborne M, Abbasi S, Bittencourt MS, Blankstein R. Cardiac sarcoidosis-state of the art review. Cardiovasc Diagn Ther. 2016;6:50-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 90] [Reference Citation Analysis (0)] |
| 8. | Yazaki Y, Isobe M, Hiroe M, Morimoto S, Hiramitsu S, Nakano T, Izumi T, Sekiguchi M; Central Japan Heart Study Group. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88:1006-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 471] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 9. | Sekiguchi M, Numao Y, Imai M, Furuie T, Mikami R. Clinical and histopathological profile of sarcoidosis of the heart and acute idiopathic myocarditis. Concepts through a study employing endomyocardial biopsy. I. Sarcoidosis. Jpn Circ J. 1980;44:249-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 119] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J, Patel AR, Ohe T, Raatikainen P, Soejima K. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 1021] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 11. | Terasaki F, Yoshinaga K. New Guidelines for Diagnosis of Cardiac Sarcoidosis in Japan. Ann Nucl Cardiol. 2017;3:42-45. [RCA] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 12. | Doughan AR, Williams BR. Cardiac sarcoidosis. Heart. 2006;92:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 190] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 13. | Sekhri V, Sanal S, Delorenzo LJ, Aronow WS, Maguire GP. Cardiac sarcoidosis: a comprehensive review. Arch Med Sci. 2011;7:546-554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 14. | Muser D, Santangeli P, Pathak RK, Castro SA, Liang JJ, Magnani S, Hayashi T, Garcia FC, Hutchinson MD, Supple GE, Frankel DS, Riley MP, Lin D, Schaller RD, Desjardins B, Dixit S, Callans DJ, Zado ES, Marchlinski FE. Long-Term Outcomes of Catheter Ablation of Ventricular Tachycardia in Patients With Cardiac Sarcoidosis. Circ Arrhythm Electrophysiol. 2016;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Dubrey SW, Falk RH. Diagnosis and management of cardiac sarcoidosis. Prog Cardiovasc Dis. 2010;52:336-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Timmers M, Claeys MJ, Vanhauwaert B, Rivero-Ayerza M, De Hondt G. Cardiac sarcoidosis: a diagnostic and therapeutic challenge. Acta Cardiol. 2018;73:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H, Bresnitz EA, DePalo L, Hunninghake G, Iannuzzi MC, Johns CJ, McLennan G, Moller DR, Newman LS, Rabin DL, Rose C, Rybicki B, Weinberger SE, Terrin ML, Knatterud GL, Cherniak R; Case Control Etiologic Study of Sarcoidosis (ACCESS) research group. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1094] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 18. | Burstow DJ, Tajik AJ, Bailey KR, DeRemee RA, Taliercio CP. Two-dimensional echocardiographic findings in systemic sarcoidosis. Am J Cardiol. 1989;63:478-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 129] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Chapelon-Abric C, Sene D, Saadoun D, Cluzel P, Vignaux O, Costedoat-Chalumeau N, Piette JC, Cacoub P. Cardiac sarcoidosis: Diagnosis, therapeutic management and prognostic factors. Arch Cardiovasc Dis. 2017;110:456-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Fleming HA. Sarcoid heart disease. Br Heart J. 1974;36:54-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 21. | Bashour FA, McConnell T, Skinner W, Hanson M. Myocardial sarcoidosis. Dis Chest. 1968;53:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Kumar S, Barbhaiya C, Nagashima K, Choi EK, Epstein LM, John RM, Maytin M, Albert CM, Miller AL, Koplan BA, Michaud GF, Tedrow UB, Stevenson WG. Ventricular tachycardia in cardiac sarcoidosis: characterization of ventricular substrate and outcomes of catheter ablation. Circ Arrhythm Electrophysiol. 2015;8:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 23. | Tselentakis EV, Woodford E, Chandy J, Gaudette GR, Saltman AE. Inflammation effects on the electrical properties of atrial tissue and inducibility of postoperative atrial fibrillation. J Surg Res. 2006;135:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Stees CS, Khoo MS, Lowery CM, Sauer WH. Ventricular tachycardia storm successfully treated with immunosuppression and catheter ablation in a patient with cardiac sarcoidosis. J Cardiovasc Electrophysiol. 2011;22:210-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Lewin RF, Mor R, Spitzer S, Arditti A, Hellman C, Agmon J. Echocardiographic evaluation of patients with systemic sarcoidosis. Am Heart J. 1985;110:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Okura Y, Dec GW, Hare JM, Kodama M, Berry GJ, Tazelaar HD, Bailey KR, Cooper LT. A clinical and histopathologic comparison of cardiac sarcoidosis and idiopathic giant cell myocarditis. J Am Coll Cardiol. 2003;41:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 200] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 27. | Ackermann D. [Hypercalcemia in sarcoidosis--case report, prevalence, pathophysiology and therapeutic options]. Ther Umsch. 2007;64:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Ainslie GM, Benatar SR. Serum angiotensin converting enzyme in sarcoidosis: sensitivity and specificity in diagnosis: correlations with disease activity, duration, extra-thoracic involvement, radiographic type and therapy. Q J Med. 1985;55:253-270. [PubMed] |
| 29. | Kusano KF, Satomi K. Diagnosis and treatment of cardiac sarcoidosis. Heart. 2016;102:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 30. | Uyarel H, Uslu N, Okmen E, Tartan Z, Kasikcioglu H, Dayi SU, Cam N. QT dispersion in sarcoidosis. Chest. 2005;128:2619-2625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Tatli E, Aktoz M, Gul C, Buyuklu M, Kurum T. Does carvedilol therapy reduce QT dispersion in patients with heart failure? Arch Med Sci. 2008;4:404-408. |
| 32. | Schuller JL, Olson MD, Zipse MM, Schneider PM, Aleong RG, Wienberger HD, Varosy PD, Sauer WH. Electrocardiographic characteristics in patients with pulmonary sarcoidosis indicating cardiac involvement. J Cardiovasc Electrophysiol. 2011;22:1243-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Uemura A, Morimoto S, Kato Y, Hiramitsu S, Ohtsuki M, Kato S, Sugiura A, Miyagishima K, Iwase M, Hishida H. Relationship between basal thinning of the interventricular septum and atrioventricular block in patients with cardiac sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2005;22:63-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Joyce E, Ninaber MK, Katsanos S, Debonnaire P, Kamperidis V, Bax JJ, Taube C, Delgado V, Ajmone Marsan N. Subclinical left ventricular dysfunction by echocardiographic speckle-tracking strain analysis relates to outcome in sarcoidosis. Eur J Heart Fail. 2015;17:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 35. | Schouver ED, Moceri P, Doyen D, Tieulie N, Queyrel V, Baudouy D, Cerboni P, Gibelin P, Leroy S, Fuzibet JG, Ferrari E. Early detection of cardiac involvement in sarcoidosis with 2-dimensional speckle-tracking echocardiography. Int J Cardiol. 2017;227:711-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Dubrey SW, Bell A, Mittal TK. Sarcoid heart disease. Postgrad Med J. 2007;83:618-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Smedema JP, Snoep G, van Kroonenburgh MP, van Geuns RJ, Dassen WR, Gorgels AP, Crijns HJ. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol. 2005;45:1683-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 392] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 38. | Leone O, Veinot JP, Angelini A, Baandrup UT, Basso C, Berry G, Bruneval P, Burke M, Butany J, Calabrese F, d'Amati G, Edwards WD, Fallon JT, Fishbein MC, Gallagher PJ, Halushka MK, McManus B, Pucci A, Rodriguez ER, Saffitz JE, Sheppard MN, Steenbergen C, Stone JR, Tan C, Thiene G, van der Wal AC, Winters GL. 2011 consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc Pathol. 2012;21:245-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 392] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 39. | Liang JJ, Hebl VB, DeSimone CV, Madhavan M, Nanda S, Kapa S, Maleszewski JJ, Edwards WD, Reeder G, Cooper LT, Asirvatham SJ. Electrogram guidance: a method to increase the precision and diagnostic yield of endomyocardial biopsy for suspected cardiac sarcoidosis and myocarditis. JACC Heart Fail. 2014;2:466-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 40. | Fleming HA, Bailey SM. The prognosis of sarcoid heart disease in the United Kingdom. Ann N Y Acad Sci. 1986;465:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Ho JSY, Chilvers ER, Thillai M. Cardiac sarcoidosis - an expert review for the chest physician. Expert Rev Respir Med. 2019;13:507-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Chiu CZ, Nakatani S, Zhang G, Tachibana T, Ohmori F, Yamagishi M, Kitakaze M, Tomoike H, Miyatake K. Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am J Cardiol. 2005;95:143-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 211] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 43. | Houston BA, Park C, Mukherjee M. A Diagnostic and Therapeutic Approach to Arrhythmias in Cardiac Sarcoidosis. Curr Treat Options Cardiovasc Med. 2016;18:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Ballul T, Borie R, Crestani B, Daugas E, Descamps V, Dieude P, Dossier A, Extramiana F, van Gysel D, Papo T, Sacre K. Treatment of cardiac sarcoidosis: A comparative study of steroids and steroids plus immunosuppressive drugs. Int J Cardiol. 2019;276:208-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 45. | Rosenthal DG, Bravo PE, Patton KK, Goldberger ZD. Management of Arrhythmias in Cardiac Sarcoidosis. Clin Cardiol. 2015;38:635-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 46. | Papageorgiou N, Providência R, Bronis K, Dechering DG, Srinivasan N, Eckardt L, Lambiase PD. Catheter ablation for ventricular tachycardia in patients with cardiac sarcoidosis: a systematic review. Europace. 2018;20:682-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 47. | Mohsen A, Jimenez A, Hood RE, Dickfeld T, Saliaris A, Shorofsky S, Saba MM. Cardiac sarcoidosis: electrophysiological outcomes on long-term follow-up and the role of the implantable cardioverter-defibrillator. J Cardiovasc Electrophysiol. 2014;25:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 48. | Perkel D, Czer LS, Morrissey RP, Ruzza A, Rafiei M, Awad M, Patel J, Kobashigawa JA. Heart transplantation for end-stage heart failure due to cardiac sarcoidosis. Transplant Proc. 2013;45:2384-2386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |